Abstract
Bromelain is dotted with anticancer properties on various cancer cell lines. Anticancer pathways of bromelain, as well related intervening signalization are under investigation. Investigating the inhibitory potential of bromelain on AGS, PC3, and MCF7 cells proliferation and colony formation. The bromelain inhibitory potential on AGS, PC3, and MCF7 cells proliferation at various bromelain concentrations was assessed by MTT; thereby, bromelain potency on colony formation impediment was evaluated using clonogenic assays at determined 50% inhibitory concentrations (IC50) on four different cell densities (10, 50, 100, and 200 cells per well). Bromelain inhibits AGS, PC3, and MCF7 cells proliferation in such a dose-dependent manner. Determined IC50 to AGS, PC3, and MCF7 cells were 65, 60 and 65μg/ml respectively. At IC50, bromelain significantly suppressed the AGS, PC3, and MCF7 cells colony formation at four treated densities (10, 50, 100 and 200 cells per well). Plating efficiency percentage and cell surviving fraction were decreased after bromelain treatment to AGS, PC3, and MCF7 human cancer cells as a function of initial cell density. The 50, 50 or 100, and 10 or 50 cells per well were considered to be optimum number of initial cell density for AGS, PC3, and MCF7 cells. Cell proliferative and colony formation inhibition are two pathways to in vitro bromelain anticancer effects. The current study displayed a dose-dependent inhibitory effect of bromelain, as well impeding colony formation AGS, PC3, and MCF7 human cancer cells.
Keywords: Bromelain, colony formation assay, human cancer cells
Introduction
Cancer is considered as uncontrolled dysregulated, and anarchic cell proliferation. Cancer is well known as one of the main leading causes of death in developed and developing countries. Several ways such as chemotherapy, surgery, radiotherapy, immunotherapy,[1] or electrochemotherapy have been developed to treat cancer.[2] Chemotherapy with single or a combination of two or more drugs is one of the mostly used treatments for treating different types of cancer.[3] However, chemotherapy can affect cancer cells as well as healthy cells, so its efficacy is often limited by side effects in normal tissues. Therefore, researches have been continued to find the effective and the less toxic anticancer drugs, which has created an increasing interest on natural products.[4]
Bromelain is an aqueous extract of pineapple (Ananas comosus) with a natural complex of proteolytic enzymes. Bromelain is widely used in traditional medicine in South America, China, and Asia, and it has an efficient role in activities such as digestion, wound healing, burnt debris, and enhancement of antibiotic absorption. Besides these activities, bromelain has been shown immunomodulatory, anti-inflammatory, and anticancer effects. Bromelain mostly contains protease components (80% stem bromelain, 10% fruit bromelain, and 5% ananain). Phosphatases, glucosidases, peroxidases, and cellulases constitute the nonprotease part of bromelain. Bromelain showed its anticancer feature in face of various human cancer cell lines such as leukemia lymphoma, sacroma, melanoma, colo-rectal carcinoma, lung carcinoma, gastric carcinoma, breast cancer, and glioma.[4] Bromelain can result in an increase in the expression of p53 and BAX genes and reduction of Akt signaling protein.[5]
Colony formation (clonogenic) assay is a commonly used technique to assay the survival and proliferation of cells under in vitro conditions.[6] Clonogenic assay can be used to estimate the reproductive death of cells before or after treatment such as irradiation therapy or cytotoxic drugs.[7] The anticancer effect of bromelain as a single therapy modality or in combination with other chemotherapeutic drugs has been shown in some human cancer cells.[8]
Breast cancer and prostate cancer are the most common malignancies, respectively, in women and men around the world.[9,10] In addition, gastric cancer is one of the most common cancers among the world and the fourth leading cause of cancer-related deaths worldwide.[11] Therefore, the aim of this study was to investigate the effect of bromelain as a single therapy on three common human cancer cells, namely PC3 (human prostate carcinoma), AGS (human gastric carcinoma), and MCF7 (human breast adenocarcinoma) cancer cells, using MTT and clonogenic assay under in vitro conditions, with different origin and phenotype.
Materials and Methods
Cell culture
AGS, PC3, and MCF7 cell lines were purchased from the Pasteur Institute (Tehran, Iran). All cells were cultured in RPMI-1640 (Gibco-Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) supplied with 10% fetal bovine serum (Gibco-Invitrogen) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a humidified 5% CO2 environment at 37°C. Cells were detached with trypsin/ethylenediaminetetraacetic acid (Gibco Laboratories) after reaching 80% confluence.
Cellular growth curves
The three human cancer cells were cultured in 12-well plates at 5 × 103 cells per well in triplicate to obtain doubling time (DT) of the cells. The cell culture medium was changed twice through 6 days. The cells were counted using trypan blue under a microscope after daily trypsinization to obtain cellular growth curves. Then, DTs were calculated using the following equation: DT = (duration × log2)/(log [final concentration] − log [initial concentration]).
Bromelain cytotoxicity
All the studied cells (AGS, PC3, and MCF7) were seeded in 96-well plates. The cells were incubated with bromelain at different concentrations (0, 5, 10, 20, 40, 75, 100, 200, 300, 400, and 600 μg/ml)[12] for 24 h at 37°C. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was conducted based on standard procedure. Briefly, MTT solution (12 mM) was added to each well and was incubated for 4 h. Then, the medium was removed, and formazan was solubilized in dimethyl sulfoxide (Merck, Darmstadt, Germany). A microplate reader (Stat Fax-2100, Awarness Technology Inc, Florida, USA) was used to measure the optical density of each well at 570 nm. The percentage of cytotoxicity was calculated using the following equation: ([1 − absorbance of treated well]/[absorbance of untreated well]) ×100. Then, the percentage of cytotoxicity was plotted over the bromelain concentrations to obtain the dose–response graph of bromelain for the three human cancer cells. Half-maximal inhibitory concentration (IC50) was extrapolated from the dose–response graph using GraphPad Prism software version 6 (GraphPad Software Inc., San Diego, CA, USA).
Clonogenic assay
The AGS, PC3, and MCF7 cancer cells were seeded in 6-well plates at different densities of 10, 50, 100, and 200 cells/well in triplicate. After overnight incubation, the AGS, PC3, and MCF7 cells were treated without or with bromelain at determined IC50 concentration for 24 h. Then, the cells were kept at 37°C in 5% CO2 for 14 days, and the culture medium was changed every 2 days. The cells were fixed, and colony morphologies were scored. Colonies were fixed with 70% ethanol and were stained with 0.5% crystal violet. A cell colony was defiend as a groupe formation of at least 50 cells and counted using Image J software (National Institutes of Health, Bethesda, Maryland, USA).
Data processing
All experiments were conducted three times. The results were presented as a cellular growth curve displaying the number of seeded cells in function of incubated time. Half-maximal inhibitory concentration (IC50) was obtained from the dose–response graph. Reproductive death of the three human cells was measured at their IC50 using colony number and presented as plating efficiency (PE) percentage (number of colonies/number of seeded cells × 100) and surviving fraction (SF) (number of colonies/number of seeded cells × PE).
Kruskal–Wallis and Dunn's tests were used to measure the statistical significance of the differences in colony numbers between the treated and the control groups in colony assay, as well cell viability between the treated and the control groups in MTT assay. All values were expressed as means ± standard errors. Results with P < 0.05 were considered statistically significant.
Results
Cellular growth curve
Figure 1 shows the growth curves of the three human cancer cells. Doubling time (DT) value was obtained as 22.11, 29.0, and 22.01 h for AGS, PC3, and MCF7 cell lines, respectively [Figure 1].
Figure 1.
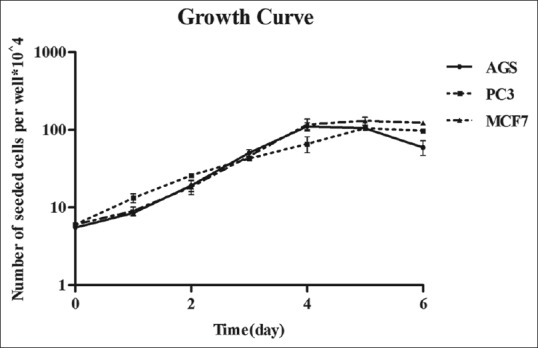
Cellular growth curves were determined for AGS, PC3, and MCF7 cells by trypan blue (counting dye technique). Data are mean ± standard error of the three independent experiments
Bromelain cytotoxicity
Bromelain inhibits the growth and proliferation of AGS, PC3, and MCF7 cells in a dose-dependent manner. Figure 2 shows that the percentage of cell viability remarkably decreased by incubation with bromelain at concentrations >75 μg/ml. The same results were found in the three human cell lines [Figure 2]. At low concentrations tested, cell growth was not inhibited by bromelain in AGS, PC3, and MCF7 cells using concentrations of 5, 10, and 20 μg/ml. However, bromelain inhibited cell growth in PC3 at low concentrations of 40 and 50 μg/ml and in AGS at 50 μg/ml partially. These results show that MCF7 seems to be more resistant to low concentrations of bromelain, due to which higher concentration is needed for inhibiting cell growth of MCF7.
Figure 2.
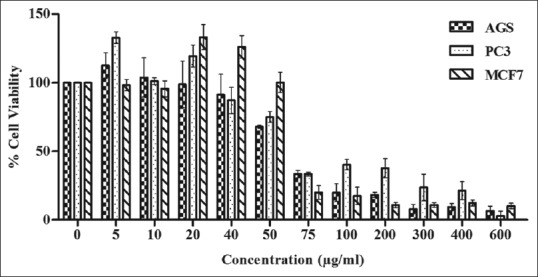
Changes in the percentage of viability of treated cells (AGS, PC3, and MCF7) with different concentrations (0, 5, 10, 20, 50, 75, 100, 200, 300, 400, and 600 μg/ml) of bromelain were measured by MTT (optical density technique). Cells were incubated for 24 h at 37°C. Data are mean ± standard error of at least three independent experiments
In this study, 50% inhibitory concentration (IC50) values were measured for all the three human cancer cells from concentration–response curve [Figure 2]. Similar value of IC50 was found in both AGS and MCF7 (65 μg/ml) cells, but in PC3 cells, IC50 occurred at 60 μg/ml. However, there was no significant difference in IC50 value of used cancer cells in this study. Previous studies showed that the value of IC50 depends on the type of cancer cell and drug treatment.
Clonogenic assay
Bromelain at IC50 concentration significantly suppressed the clonogenic formation potential of AGS, PC3, and MCF7 cells invitro. The number of colonies of all the three human cancer cells decreased after treatment with bromelain at IC50 concentration [Figures 3-5]. In this study, the percentage of plating efficiency and SF decreased after treatment with the three human cancer cells (AGS, PC3, and MCF7).
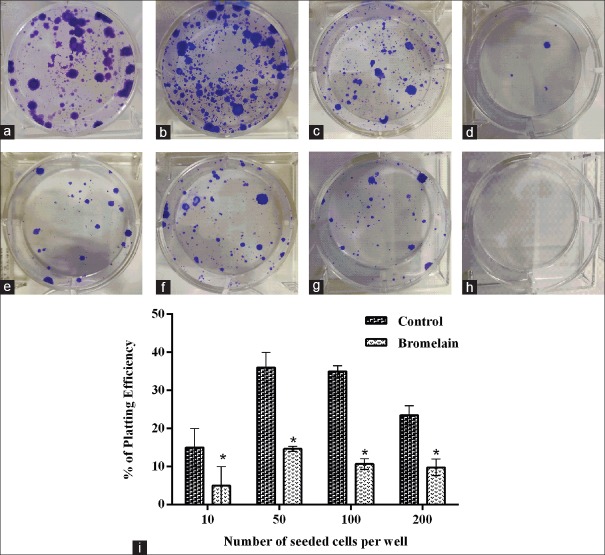
Figure 3.
Effects of bromelain on clonogenic formation of AGS cells were evaluated by a clonogenic assay, using IC50: 65 μg/ml. Clonogenic assay was conducted 14 days after treatment. (a-d) Untreated cells, (e-h) treated cells with IC50 of 65 μg/ml as a function of seeded cell number per well; (a and e) 200; (b and f) 100; (c and g) 50; and (d and h) 10. (i) The percentage of plating efficiency was plotted as the number of seeded cells per well which were treated with/without bromelain. Data are presented as mean ± standard error of at least three independent experiments
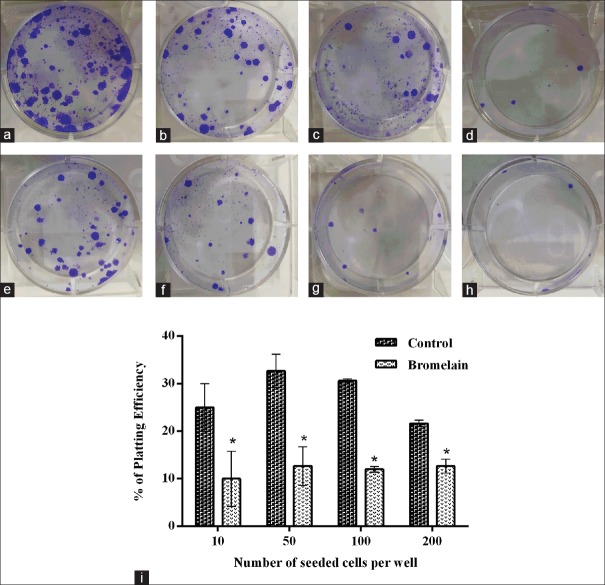
Figure 5.
MCF7 cell response to bromelain after 14 days of treatment (IC50 = 65 μg/ml) on clonogenic formation evaluated by a clonogenic assay. (a-d) Untreated cells and (e-h) treated cells. The function of seeded cell number per well is as follows: (a and e): 200; (b and f): 100; (c and g): 50; and (d and h) 10. (i) The percentage of plating efficiency was plotted as the number of seeded cells per well which were treated with/without bromelain. Data are presented as mean ± standard error of at least three independent experiments
In this study, the clonogenic formation potential of three human cancer cells was evaluated as a function of initial cell density in the absence or presence of bromelain. It was observed that the number of colonies increased with increasing number of seeded cells per well in untreated cells as well as in treated cells. Similar results were obtained on AGS, PC3, and MCF7 cell lines, in which the number of colonies was equal to the initial cell density. MCF7 [Figure 5] showed to be more prone to be clonogenic compared to AGS [Figure 3] and PC3 [Figure 4] cell lines for all conducted initial cell density.
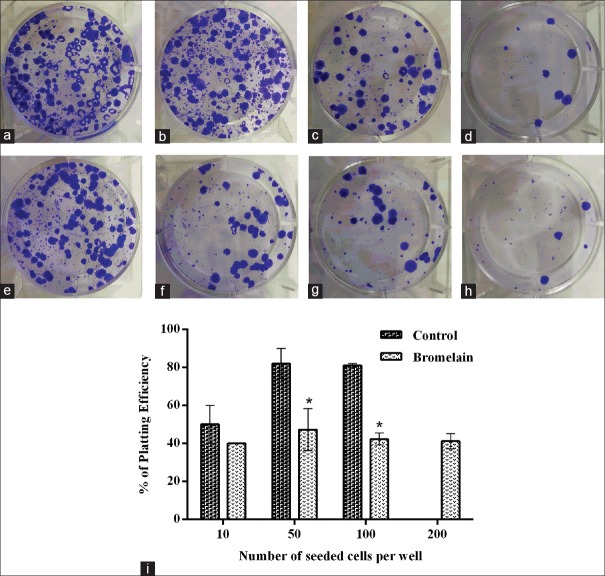
Figure 4.
Response of PC3 cells 14 days after bromelain treatment (IC50 = 60 μg/ml). (a-d) Untreated cells and (e-h) treated cells. The function of seeded cell number per well is as follows: (a and e) 200; (b and f) 100; (c and g) 50; and (d and h): 10. The percentage of plating efficiency (i) was defined as the number of seeded cells per well (treated and untreated cells). Data are presented as mean ± standard error of at least three independent experiments
The optimal cell density to bromelain anticancer effect was determined for each of PC3, AGS, and MCF7 cell lines. In AGS cells, 50 seemed to be the convenient number for obtaining reproducible results in this study. Whereas in PC3 cells, 10 or 50 had to be considered as the convenient number of seeded cells, which is the same result as found with AGS cells. However, in MCF7 cells, the convenient number was reached at 10 or 50. This result shows that MCF7 cells seem to have more clonogenic potentials with respect to AGS and PC3 cells.
Discussion
In the present study, we evaluated the anticancer effect of bromelain on three human cancer cell lines, namely, human gastric carcinoma (AGS), human prostate carcinoma (PC3), and human breast adenocarcinoma (MCF7). These three various cell lines do be driven by different tumorigenic and biochemical signalization evolving in different tumor microenvironements. The results showed that bromelain inhibits the growth and proliferation of AGS, PC3, and MCF7 cells in a concentration-dependent manner. Figure 2 depicts that the percentage of cell viability remarkably decreased by incubation with bromelain at all concentrations above 75 μg/ml. Similar results were found in the three human cell lines [Figure 2]. At lower concentrations tested (5, 10, and 20 μg/ml), cell growth was not inhibited by bromelain in AGS, PC3, and MCF7 cells. However, bromelain inhibited cell growth in PC3 at lower concentrations of 40 and 50 μg/ml and in AGS at a concentration of 50 μg/ml partially. These results showed that MCF7 seems to be more resistant to the lower concentration of bromelain, and hence higher concentration is essential to inhibit cell growth. These results are in agreement with those of previous studies that evaluated the anticancer effect of bromelain on different types of cancer cells under in vitro conditions.[12,13,14,15] Bromelain induced the cytotoxic effects on murine lung carcinoma, mammary adenocarcinoma, leukemia, lymphoma, sarcoma, melanoma and ascetic tumor cell lines, human gastrointestinal carcinoma cells (MKN45, KATO-III, HT29-5F12, and HT29-5M21), glioma, breast cancer, epidermoid carcinoma, melanoma, malignant peritoneal mesothelioma,[16] human skin fibroblasts 1184, human cervical cancer cells (HeLa), human breast cancer cells (MDA-MB-468 and MDA-MB-231), and murine breast cancer cells (4T1).[8,13,14,16,17]
Previous studies reported on different bromelain IC50 in respect to various cancer cell types. Using concentration-response curve, calculated bromelain IC50 did not differ significantly among PC3, AGS, and MCF7 by the current investigation [Table 1].
Table 1.
Surviving fraction was calculated as the ratio of colony number in treated wells to the colony number in untreated wells at different initial cell densities, for three studied human cancer cell lines, namely, human gastric carcinoma, human prostate carcinoma, and human breast adenocarcinoma cell lines
| Cells | Percentage of SF various initial cell densities | |||
|---|---|---|---|---|
| 10 | 50 | 100 | 200 | |
| AGS | 19.23 | 53.8 | 50 | 51.9 |
| PC3 | 33.56 | 40.2 | 41.9 | 42.5 |
| MCF7 | 62.11 | 59.6 | 82 | 81 |
SF – Surviving fraction
Furthermore, we have described the effect of bromelain on clonogenic formation potential of three cancer cell lines used in this study, namely AGS, PC3, and MCF7 cells. Bromelain at IC50 concentration significantly suppressed the clonogenic formation potential of AGS, PC3, and MCF7 cells invitro. A study by Nasiri et al.[8] showed that bromelain as a single or combination treatment agent had clonogenic inhibitory effect on human cervical cancer cell (HeLa), human breast cancer (MDA-MB-231), and murine breast cancer (4T1). In addition, they found that the survival rate decreased as a consequence of pretreatment with bromelain. Bromelain at IC50 concentration reduced the size and number of colonies of HeLa, MDA-MB-231, and 4T1 cells.[8] Our results are in agreement with those of a previous study. The number of colonies of all the three human cancer cells decreased after treatment with bromelain at IC50 concentration [Figures 3-5]. In this study, the percentage of plating efficiency and SF decreased after treatment with the three human cancer cells (AGS, PC3, and MCF7). We found results similar to those obtained in previous studies, showing the reduction in plating efficiency and survival rate of treated human cancer cells with bromelain, platinum nanoparticles, phospholipase D inhibitor, and flavonoid quercetin.[8,18,19,20]
When it came to the relation of initial seeded cell number with colony formation, MCF7 cells formed most of the colonies at all studied seeding densities [Figure 5] and were different from AGS [Figure 3] and PC3 [Figure 4] cells, while AGS and PC3 cells were not different from each other. Our results in untreated cells are in agreement with previous in vitro studies that measured the clonogenicity of human ovarian cancer cell lines (CAOV3, COV362, Kuramochi, OVCAR4, OVCAR5, OVCAR8, OVSAHO, and SNU119) and human prostate cancer cells (PC3, DU-145, and LNCap),[20] showing significant differences in the colony-forming ability of human cancer cells. Similar relation between initial seeded cell number and colony formation was found even with bromelain treatment; although, the ensuing pattern needs to be further elucidated.
The latter pointed out an insightful research direction as to determine the optimal cell density to confer optimal in vitro bromelain anticancer effect in respect to each cell line. MCF7 cell line showed to be of stronger clonogenic potency compared to PC3 and AGS cell lines.
Conclusion
In light of the current results, bromelain proved to be of in vitro inhibitory impeding on cell proliferation and colony formation to three AGS, PC3, and MCF7 human cancer cell lines. The advantage of bromelain anticancer feature can be integrated in the frame of ongoing combinational treatment with chemotherapeutic agents.
Financial support and sponsorship
This study was founded by the research deputy of Shahrekord University of Medical Sciences, Iran (Grant No.1059 and Grant No.1060 with corresponding registration IR.SKUMS.RIC.1395.148/149).
Conflicts of interest
There are no conflicts of interest.
BIOGRAPHIES

Farzane Raeisi obtained her BSc in Physics from Shahrekord University in 2014 and her MSc in Medical Physics from Isfahan University of Medical Sciences in 2016. Her research interests include radiationtherapy, image processing, and molecular genetics.
Email: farzaneraisi@yahoo.com

Elham Raeisi received her BSc degree from Shahid Beheshti Unuiversity, Tehran, Iran, MSc degree in Medical Physics from Tarbiat Modares University, Tehran, Iran in 2005 and PhD degree in Medical Physics/Interdisciplinary in 2011, 2013. Her research interest is Cancer Research.
Email: elhamraeisi@gmail.com

Esfandiar Heidarian received his PhD degree in clinical biochemistry in 2005 from Isfahan University of Medical Sciences, Isfahan, Iran. He is currently a professor at Shahrekord University of Medical Sciences, Shahrekord, Iran. His research interests are focused on oxidative stress and antioxidants. He has participated in several scientific and technical committees.
Email: heidariyan46@yahoo.com

Daryoush Shahbazi-Gahroui Professor of Medical Physics, BSc in Physics (School of Science, Isfahan University, Iran, 1987), MSc in Medical Physics (Tarbiat Modarres University, Tehran, Iran, 1991), PhD in Medical Physics (University of Western Sydney and St. George Cancer Care Centre, Sydney, Australia, 2000).
Email: shahbazi@med.mui.ac.ir

Yves Lemoigne received his “Doctorat d‘Etat” in 1987 from the Université Paris XI-Orsay (now Paris-Saclay University). Doctorat d‘Etat is equivalent to PhD with habilitation to conduct research. During 16 years (1997 – 2013) he was professor and director of the European School of Medical Physics in Archamps near Geneva (Ch). He is now Director of the Institute For Medical Physics, Ambilly, France. (http://www.ifmp.eu
Associated Physicist at TERA foundation Novarra, Italy
Associated Physicist at CERN, Geneva, Switzerland.
Email: yv.lemoigne@gmail.com
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Raeisi E, Aghamiri SM, Bandi A, Rahmatpour N, Firoozabadi SM, Kafi-Abad SA, et al. The antitumor efficiency of combined electrochemotherapy and single dose irradiation on a breast cancer tumor model. Radiol Oncol. 2012;46:226–32. doi: 10.2478/v10019-012-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaliberov SA, Buchsbaum DJ. Chapter seven – Cancer treatment with gene therapy and radiation therapy. Adv Cancer Res. 2012;115:221–63. doi: 10.1016/B978-0-12-398342-8.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chobotova K, Vernallis AB, Majid FA. Bromelain's activity and potential as an anti-cancer agent: Current evidence and perspectives. Cancer Lett. 2010;290:148–56. doi: 10.1016/j.canlet.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Menyhart O, Harami-Papp H, Sukumar S, Schafer R, Magnani L, de Barrios O, et al. Guidelines for the selection of functional assays to evaluate the hallmarks of cancer. Biochim Biophys Acta. 2016;1866:300–19. doi: 10.1016/j.bbcan.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC. Clonogenic assay: Adherent cells. J Vis Exp. 2011 doi: 10.3791/2573. pii: 2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasiri R, Almaki JH, Idris A, Nasiri M, Irfan M, Majid FA, et al. Targeted delivery of bromelain using dual mode nanoparticles: Synthesis, physicochemical characterization, in vitro and in vivo evaluation. RSC Adv. 2017;7:40074–94. [Google Scholar]
- 9.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–79. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AV, Oesterreich S, Davidson NE. MCF-7 cells – Changing the course of breast cancer research and care for 45 years. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv073. pii: djv073. [DOI] [PubMed] [Google Scholar]
- 11.Choi ES, Kim H, Kim HP, Choi Y, Goh SH. CD44v8-10 as a potential theranostic biomarker for targeting disseminated cancer cells in advanced gastric cancer. Sci Rep. 2017;7:4930. doi: 10.1038/s41598-017-05247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL. Bromelain and N-acetylcysteine inhibit proliferation and survival of gastrointestinal cancer cells in vitro: Significance of combination therapy. J Exp Clin Cancer Res. 2014;33:92. doi: 10.1186/s13046-014-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tysnes BB, Maurer HR, Porwol T, Probst B, Bjerkvig R, Hoover F. Bromelain reversibly inhibits invasive properties of glioma cells. Neoplasia. 2001;3:469–79. doi: 10.1038/sj.neo.7900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhandayuthapani S, Perez HD, Paroulek A, Chinnakkannu P, Kandalam U, Jaffe M, et al. Bromelain-induced apoptosis in GI-101A breast cancer cells. J Med Food. 2012;15:344–9. doi: 10.1089/jmf.2011.0145. [DOI] [PubMed] [Google Scholar]
- 15.Bhui K, Tyagi S, Prakash B, Shukla Y. Pineapple bromelain induces autophagy, facilitating apoptotic response in mammary carcinoma cells. Biofactors. 2010;36:474–82. doi: 10.1002/biof.121. [DOI] [PubMed] [Google Scholar]
- 16.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150–60. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 17.Amini A, Ehteda A, Masoumi Moghaddam S, Akhter J, Pillai K, Morris DL. Cytotoxic effects of bromelain in human gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21) Onco Targets Ther. 2013;6:403–9. doi: 10.2147/OTT.S43072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendale Y, Bendale V, Paul S. Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis. Integr Med Res. 2017;6:141–8. doi: 10.1016/j.imr.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble AR, Maitland NJ, Berney DM, Rumsby MG. Phospholipase D inhibitors reduce human prostate cancer cell proliferation and colony formation. Br J Cancer. 2018;118:189–99. doi: 10.1038/bjc.2017.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Diagn Lab Immunol. 2004;11:63–9. doi: 10.1128/CDLI.11.1.63-69.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]