Abstract
The intestinal barrier, which primarily consists of epithelial cells stitched together with connecting proteins called tight junctions, plays a critical role in health and disease. It is in close contact with the gut microbiota on its luminal side and with the enteric neurons on the tissue side. Both microbiota and the enteric nervous system are regulatory housekeepers of the intestinal barrier. Therefore, the recently observed enteric neuropathology along with gut dysbiosis in Parkinson’s disease have prompted research on intestinal permeability in this neurodegenerative disorder. In this mini-review we attempt to concisely summarize the current knowledge on intestinal barrier in Parkinson’s disease. We envision future direction research that should be pursued in order to demonstrate its possible role in disease development and progression.
Keywords: Intestinal barrier, tight junctions, enteric nervous system, Parkinson’s disease
INTRODUCTION
The epithelial lining of the intestine provides a protective barrier against the potentially hostile environment in the lumen of the intestine. It also separates the commensal microbes that reside at the apical side of the epithelium in the intestine lumen and the immune system and enteric nervous system at the basal side in the underlying tissue. The cells that make up this epithelial lining are packed closely together via so-called tight junctions. These ensure that nothing leaks through, while distinct compositions of receptors, pumps and channels at apical and basal surface domains allow regulated communications between luminal and tissue environments. A compromised intestinal barrier function has unequivocally been associated with inflammatory conditions in the gut. Recently, a compromised intestinal epithelial barrier has also been associated with Parkinson’s disease (PD), fueling the hypothesis that gut-derived factors may participate in the pathogenesis of PD. In this concise article we review the available evidence for a compromised intestinal barrier in PD patients. We discuss the possible contribution of intestinal barrier dysfunction to the pathogenesis of PD and its possible clinical implications, and eventually we propose directions for future research.
COMPROMISED INTESTINAL BARRIER FUNCTION IN PD: WHAT IS THE EVIDENCE?
Analyses of gut wall barrier function in human subjects are typically done via any of three assays. These include in vivo gut wall permeability tests, in situ immunolabeling of tight junction proteins, ex vivo mucosal permeability tests and analyses of proteins detectable in fecal samples (e.g., alpha-1-antitrypsin, zonulin) [1]. All assays have been used to investigate gut wall barrier function in PD patients.
In vivo gut wall permeability tests
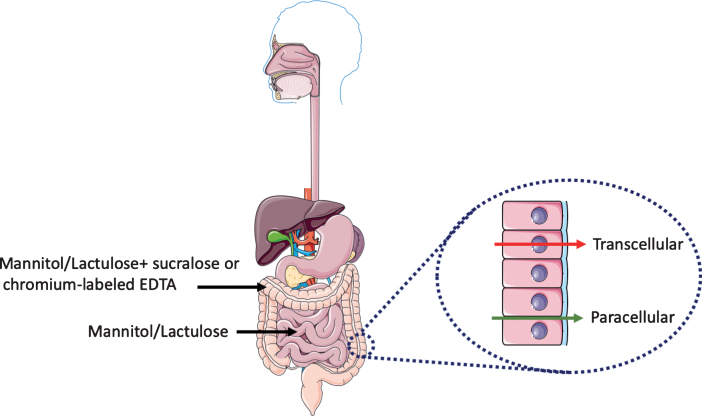
Urinary excretion of orally ingested non-metabolizable sugars of different sizes provides an easy and non-invasive in vivo read-out of intestinal barrier function [2, 3]. Measurement of urinary excretion as a function of time after sugar ingestion can be used to evaluate the barrier function along the horizontal axis of the intestine, i.e., from duodenum to colon. Most commonly used is a combination of a monosaccharide and disaccharide such as mannitol (or L-rhamnose) and lactulose, respectively, the urinary recovery of which is typically measured within 5 hours after ingestion. The relatively small sized mannitol easily moves from the gut lumen to the underlying tissue whereas the larger lactulose does not. An increase of urinary lactulose output in combination with an unchanged urinary mannitol output (which also serves to correct for differences in gastric emptying) gives rise to an increase in the lactulose/mannitol ratio. This is interpreted as a measure of increased permeability of the intestinal epithelium (Fig. 1).
Fig.1.
Evaluation of intestinal permeability. Urinary excretion of orally ingested non-metabolizable sugars of different sizes provides a reliable non-invasive in vivo read-out of intestinal barrier function. The mannitol/lactulose ratio evaluates the changes in permeability in the small intestine. Changes in the colon permeability is assessed with the addition of either sucralose or chromium-labeled EDTA. At the cellular level, there are two routes for transport of molecules and ions across the epithelium of the gut: across the plasma membrane of the epithelial cells (transcellular route) and across tight junctions between epithelial cells (paracellular route). This figure was created using Servier Medical Art, licensed under the Creative Commons Attribution 3.0 Unported License.
In 1996 Davies and colleagues [4] used the mannitol/lactulose test in 15 PD patients and found an increase in the lactulose/mannitol ratio in urinary samples taken 5 hours after ingestion of the sugar solution. However, they also found a 2-fold decrease urinary mannitol output (from 20% to 10% urinary recovery) when compared to control subjects, which by itself could have accounted for the increased ratio. Therefore, lactulose/mannitol ratios must be interpreted cautiously and analysis of the data for the individual sugars is required. In addition, possible differences in gastrointestinal motility between control and PD patients groups should be taken into account. In two studies published in 2011 [5] and 2019 [6], the mannitol/lactulose test was used with 9 and 6 PD patients, respectively, and no difference were found in the average lactulose/mannitol ratio in urinary samples taken 24 hours after ingestion of the sugars [5, 6]. The absence of an increase in urinary output of lactulose with a reduced or unchanged urinary output of mannitol in these three studies argues against an increased permeability of the small intestine in these small cohorts of PD patients.
Notably, mannitol and lactulose are most appropriate to study permeability changes in the small intestine. Mannitol and lactulose are fermented by colonic bacteria, which can make the interpretation of 24 hours measurements more difficult. This is particularly relevant for PD patients in which the composition of colonic bacteria (the microbiome) has been shown to be different from non-PD subjects [7]. In order to probe permeability changes in the large intestine or colon, the addition of an artificial disaccharide sucralose or chromium-labeled EDTA, which do not undergo fermentation by colonic bacteria, is more suitable [8, 9]. When applied to 6 PD patients, a significantly higher 24 hours—but not 5 hours—urinary excretion of sucralose between PD and control subjects was observed [6]. Together, the existing in vivo data on gut permeability in PD suggest that the colon, but not the small intestine of parkinsonian patients is hyperpermeant. It should however be kept in mind that because of the small sample size these studies are preliminary and larger independent surveys are needed to unequivocally demonstrate that the intestinal barrier is dysfunctional in PD.
An alternative approach to evaluate intestinal barrier function in vivo involves the measurement of alpha-1-antitrypsin and zonulin in the feces. Alpha-1-antitrypsin is a protein that is synthesized in the liver and secreted into the circulation. Detection of alpha-1-antitrypsin in the feces reflects its loss to the intestinal lumen and, indirectly, is a measure of mucosal barrier integrity. Zonulin is a tight junction-associated cytoplasmic protein and increased fecal concentrations have been associated with disruption of the mucosal barrier [10]. Schwiertz and colleagues [11] applied this approach to 36 PD patients and 28 control subjects and reported that significantly more PD patients (27 out of 36) than control subjects (8 out of 28) showed increased levels of alpha-1-antitrypsin in the feces. In addition, significantly more PD patients (16 out of 36) than control subjects (4 out of 28) showed increased levels of zonulin in the feces. The discrepancy between the alpha-1-antitrypsin and zonulin results and how this relates to mucosal integrity is not clear ad may reflect different aspects of gut wall permeability. Unfortunately, no direct comparisons between these fecal biomarkers and the sugar tests in PD patients have been reported, which may help in the further validation of these tests. Notably, the detection of elevated levels of alpha-1-antitrypsin and/or zonulin in feces, as well as detected changes in sugar test values, do not discriminate between a perturbation of the mucosal barrier due to damage to and loss of epithelial cells or due to dysfunctional tight junctions (discussed below). Thus far, no studies reported damage to the intestinal epithelium in PD patients.
Immunolabeling of biopsies
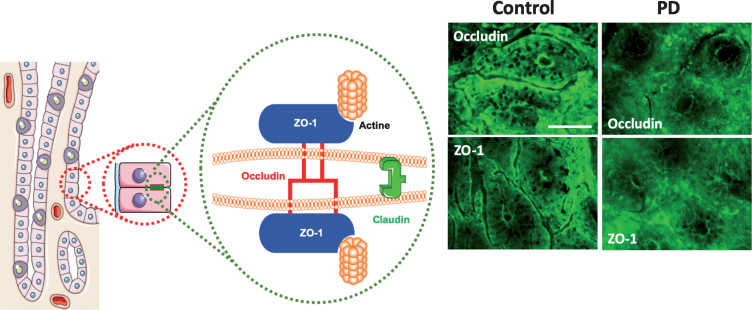
Tight junctions (also known as zona occludens) play a key role in intestinal barrier function and loss of tight junction integrity results in increased paracellular permeability of the intestinal epithelium [12]. Transmembrane protein components of tight junctions (e.g., claudins, occludin and junction-associated molecule (JAM)-A) of neighboring cells engage in homotypic interactions. These bring the cells in close proximity to each other while cytoplasmic protein components (e.g., zona occludens (ZO)-1, -2 and -3, and cingulin) connect tight junctions to intracellular machineries (Fig. 2). Comparative Western blot analyses and/or immunolabeling of intestinal biopsies with antibodies raised against tight junction proteins are often used to evaluate tight junction integrity (Fig. 2).
Fig.2.
(A) Composition of tight junctions. Tight junctions (TJs) of epithelial intestinal cells form selective barriers that regulate paracellular permeability. They consist of proteins including occludin, claudins and Zonula occludens-1 (ZO-1). (B) Representative photomicrographs of the colonic mucosa labeled with antibodies against ZO-1 and occludin in the colonic mucosa of one control subject and one PD patient; scale bar: 100 μm. A normal and typical reticular pattern of occludin and ZO-1 staining was observed in control, while TJs morphology is disrupted and irregularly distributed in the mucosa in PD. This figure was created using Servier Medical Art, licensed under the Creative Commons Attribution 3.0 Unported License.
Using western blot, Clairembault and colleagues [13] showed a 50% reduction in the expression level of occludin, but not of ZO-1, in lysates of sigmoid/descending colon biopsies of 31 PD patients when compared to 11 control subjects. Additional immunofluorescence microscopy experiments performed on biopsies from 31 PD patients and 8 control subjects, revealed aberrant subcellular distribution of occludin and ZO-1 in 22 out of 31 PD patients (∼70%) as opposed to 2 out of 8 control subjects (∼20%). Occludin but not ZO-1 showed a more cytoplasmic distribution. The severity of aberrant tight junction protein distribution varied greatly between patients and was not due to dopaminergic treatment as also observed in 5 drug-naïve PD patients. Perez-Pardo and colleagues [6] reported a reduction in the average intensity of ZO-1 immunolabeling in sigmoid colon biopsies from 6 PD patients when compared to control subjects. In both studies, only colonic biopsies and no small intestinal biopsies were studied. The aberrant subcellular distribution of tight junction-associated proteins may indicate impaired tight junction integrity in the colon of PD patients. This observation is in line with a recent report showing that the expression of occludin and ZO-1 is significantly reduced in PD patient brains [14]. However, a reduced expression or mislocalization of individual tight junction proteins does not necessarily correlate with perturbed barrier function [15, 16], and one should thus be cautious with the interpretation of tight junction protein immunolabeling without supporting functional data.
Ex vivo analyses of barrier function
Epithelial barrier function in mucosal biopsies can be investigated using Ussing chambers [17]. Clairembault and colleagues [13] used this approach to study barrier function in colonic tissues of 31 PD patients. Immunolabeling of the sigmoid/descending colon tissues of this group of PD patients revealed aberrant subcellular distribution of tight junction proteins (see above). However, no change in the average paracellular flux of two independent tracers was observed over a 3-hour time period when compared to controls. Significant variability however was noted among individual PD patients. No in vivo gut permeability assays were performed in this group of PD patients preventing a direct comparison between in vivo and ex vivo mucosal permeability.
It should be however kept in mind that mucosal biopsies only imperfectly reflect the in vivo intestinal wall. Indeed, these typically lose intrinsic neural input (e.g., from submucosal neurons) and the luminal content of the intestine which includes the microbiome. As both can influence mucosal permeability, it can be speculated that the increase in colonic permeability as possibly observed in vivo in PD patients may not be fully intestinal epithelial cell autonomous, but the net result of a combination of alterations in intestinal epithelial tight junction integrity, gut lumen/colonic bacteria composition and/ or submucosal neurons.
HOW COULD A COMPROMISED INTESTINAL BARRIER FUNCTION CONTRIBUTE TO PD PATHOGENESIS?
Based on the topographic distribution of Lewy bodies and neurites established after autopsy from PD patients, Heiko Braak hypothesized that PD pathology may start in the gastrointestinal tract subsequently reaching the brain via the vagus nerve. This is supported by animal studies. Holmqvist and colleagues [18] demonstrated that different α-synuclein forms when injected into the intestinal wall of mice could propagate from the gut to the brain. More recently, Kim et al. [19] demonstrated gut-to-brain spread of pathological alpha-synuclein fibrils following their injection into the mouse muscularis layer of the pylori and duodenum but not when the mice were subjected to truncal vagotomy following alpha-synuclein fibril injection. Finally, also in a bacterial artificial chromosome transgenic rat model that overexpressed the human alpha-synuclein encoding gene, gut-to-brain propagation of injected alpha-synuclein fibrils via the vagus nerve was demonstrated [20]. Conceivably, the Braak model requires that the intestinal epithelial barrier should be porous enough to give way to a hitherto unknown neurotropic pathogen to trigger alpha-synuclein misfolding and aggregation in the nearby enteric neurons [21]. However, it is also possible that the initial alpha-synuclein aggregation could take place in the nerve terminals of the ENS or autonomic nerves in the gut lining for some other reason unrelated to the microbiome, pathogens, or a compromised intestinal barrier. A correlation between gut wall permeability and the presence of alpha-synuclein aggregates was proposed in a mouse model of PD [22] and in human PD samples [5]. Future studies with larger patient cohorts should further validate the correlation between tight junction abnormalities and the presence of alpha-synuclein aggregates in patient samples. Even in the absence of a specific pathogen, it might be speculated that the mere increase in intestinal permeability could be sufficient to expose enteric neurons to bacterial derived pro-inflammatory products, such as lipopolysaccaharide (LPS). These may then induce local inflammation and oxidative stress and in turn neuronal pathological α-synuclein aggregates. This relatively straightforward scenario is supported by experimental findings, which showed that local (intestinal) or systemic administration of LPS in mice is associated with more alpha-synuclein expression [6, 22] or aggregation [23]. However, the underlying molecular mechanisms by which LPS increases enteric alpha-synuclein levels in these animal models remain to be elucidated. Indeed, the direct application of LPS or proinflammatory cytokines to primary enteric neurons does not increase alpha-synuclein but instead transcriptionally downregulates alpha-synuclein expression via a p38 pathway [24].
Aside from the vagal neuronal pathway, a putative mechanism by which gut hyperpermeability may influence the brain in PD include bacterial products that gain access to the brain via the bloodstream. Systemic inflammation has been demonstrated in PD patients [25] and evidence from animal models supports a role for systemic inflammation in the exacerbation of neurodegeneration [26].
FUTURE PERSPECTIVES
Preliminary results suggest an increase in barrier permeability of the colon in PD patients. Considering that a leaky gut may allow factors from the gut lumen to elicit negative effects on the nerve cells in the intestine and brain, fortification of the intestinal barrier may provide a novel therapeutic approach in PD. Such approach has been proposed in other gastrointestinal disorders [27]. However, the group sizes in the existing studies were relatively small and follow-up studies with larger and treatment-naïve PD patient cohorts are definitely warranted to substantiate these findings. Such studies would benefit from the parallel inclusion of multiple permeability tests (e.g., in vivo triple sugar test and fecal biomarkers, ex vivo permeability test and tissue staining) which allows for the comparison and further validation of these different methodologies and for elucidation of the underlying pathology and mechanisms. They would also benefit from a multivariate and personalized approach to provide the necessary contextual relevance of increased gut permeability in the face of an altered gut microbiome, enteric alpha-synuclein pathology, inflammation and neurological/motor symptoms.
Reductionist model systems may help to elucidate the order of events and causalities between these parameters, and identify microbes or microbial factors that may promote alpha-synuclein pathology in enteric neurons. Particularly promising is the use of patient fibroblast-derived induced pluripotent stem (iPS) cells. The iPSC can be differentiated into three-dimensional intestinal organoids (mini guts) [28, 29] as well as to enteric neurons [30, 31]. Three-dimensional epithelial cultures are suitable for epithelial permeability analyses [32, 33]. Such model system thus allows for co-culture of mini guts with enteric nerve cells on the basal side and microbes in their lumen [34, 35] from the same patient and/ or control subjects. As with all models, the iPSC model system has its limitations. For example, iPSC-derived organoids typically display immature, perinatal characteristics [34] and lack the aging aspect of PD. iPSC also lack epigenetic aspects that may contribute to PD. On the other hand, the organoid model system also takes into account the patient’s genetic background, a parameter the contribution of which to mucosal barrier integrity in PD has not been explored.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM, Mercenier A, Nauta A, Garcia-Rodenas CL (2017) Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol 312, G171–G193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. van Elburg RM, Uil JJ, Kokke FT, Mulder AM, van de Broek WG, Mulder CJ, Heymans HS (1995) Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars. J Pediatr Gastroenterol Nutr 20, 184–188. [DOI] [PubMed] [Google Scholar]
- [3]. Uil JJ, van Elburg RM, van Overbeek FM, Mulder CJ, VanBerge-Henegouwen GP, Heymans HS (1997) Clinical implications of the sugar absorption test: Intestinal permeability test to assess mucosal barrier function. Scand J Gastroenterol Suppl 223, 70–78. [PubMed] [Google Scholar]
- [4]. Davies KN, King D, Billington D, Barrett JA (1996) Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad Med J 72, 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6, e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ, Kordower JH, Shannon KM, Garssen J, Kraneveld AD, Keshavarzian A (2019) Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut 68, 829–843. [DOI] [PubMed] [Google Scholar]
- [7]. Chiang H-L, Lin C-H (2019) Altered gut microbiome and intestinal pathology in Parkinson’s disease. J Mov Disord 12, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Jenkins AP, Nukajam WS, Menzies IS, Creamer B (1992) Simultaneous administration of lactulose and 51Cr-ethylenediaminetetraacetic acid. A test to distinguish colonic from small-intestinal permeability change. Scand J Gastroenterol 27, 769–773. [DOI] [PubMed] [Google Scholar]
- [9]. Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A (2003) Gas chromatographic method for detection of urinary sucralose: Application to the assessment of intestinal permeability. J Chromatogr B Analyt Technol Biomed Life Sci 784, 145–154. [DOI] [PubMed] [Google Scholar]
- [10]. Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartení M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A (2006) Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55, 1443–1449. [DOI] [PubMed] [Google Scholar]
- [11]. Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, Schäfer K-H, Unger MM (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50, 104–107. [DOI] [PubMed] [Google Scholar]
- [12]. Giepmans BNG, van Ijzendoorn SCD (2009) Epithelial cell-cell junctions and plasma membrane domains. Biochim Biophys Acta 1788, 820–831. [DOI] [PubMed] [Google Scholar]
- [13]. Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann M-F, Neunlist M, Derkinderen P (2015) Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Kuan W-L, Bennett N, He X, Skepper JN, Martynyuk N, Wijeyekoon R, Moghe PV, Williams-Gray CH, Barker RA (2016) α-Synuclein pre-formed fibrils impair tight junction protein expression without affecting cerebral endothelial cell function. Exp Neurol 285, 72–81. [DOI] [PubMed] [Google Scholar]
- [15]. Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M (2005) Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta 1669, 34–42. [DOI] [PubMed] [Google Scholar]
- [16]. Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A, Tsukita S (2009) Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann N Y Acad Sci 1165, 44–52. [DOI] [PubMed] [Google Scholar]
- [17]. Herrmann JR, Turner JR (2016) Beyond Ussing’s chambers: Contemporary thoughts on integration of transepithelial transport. Am J Physiol Cell Physiol 310, C423–C431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang Z-Y, Roybon L, Melki R, Li J-Y (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128, 805–820. [DOI] [PubMed] [Google Scholar]
- [19]. Kim S, Kwon S-H, Kam T-I, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS (2019) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Van Den Berge N, Ferreira N, Gram H, Mikkelsen TW, Alstrup AKO, Casadei N, Tsung-Pin P, Riess O, Nyengaard JR, Tamgüney G, Jensen PH, Borghammer P (2019) Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. doi: 10.1007/s00401-019-02040-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396, 67–72. [DOI] [PubMed] [Google Scholar]
- [22]. Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RAE, Kordower JH (2014) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29, 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Choi JG, Kim N, Ju IG, Eo H, Lim S-M, Jang S-E, Kim D-H, Oh MS (2018) Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci Rep 8, 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Prigent A, Gonzales J, Durand T, Le Berre-Scoul C, Rolli-Derkinderen M, Neunlist M, Derkinderen P (2019) Acute inflammation down-regulates alpha-synuclein expression in enteric neurons. J Neurochem 148, 746–760. [DOI] [PubMed] [Google Scholar]
- [25]. Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M (2009) Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23, 55–63. [DOI] [PubMed] [Google Scholar]
- [26]. Hernández-Romero MC, Delgado-Cortés MJ, Sarmiento M, de Pablos RM, Espinosa-Oliva AM, Argüelles S, Bández MJ, Villarán RF, Mauriño R, Santiago M, Venero JL, Herrera AJ, Cano J, Machado A (2012) Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology 33, 347–360. [DOI] [PubMed] [Google Scholar]
- [27]. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, Tilg H, Watson A, Wells JM (2014) Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol 14, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro . Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. McCracken KW, Howell JC, Wells JM, Spence JR (2011) Generating human intestinal tissue from pluripotent stem cells in vitro . Nat Protoc 6, 1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang C-F, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM (2017) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Schlieve CR, Fowler KL, Thornton M, Huang S, Hajjali I, Hou X, Grubbs B, Spence JR, Grikscheit TC (2017) Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue-engineered small intestine. Stem Cell Rep 9, 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Juuti-Uusitalo K, Klunder LJ, Sjollema KA, Mackovicova K, Ohgaki R, Hoekstra D, Dekker J, van Ijzendoorn SCD (2011) Differential effects of TNF (TNFSF2) and IFN-γ on intestinal epithelial cell morphogenesis and barrier function in three-dimensional culture. PLoS One 6, e22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Hill DR, Huang S, Tsai Y-H, Spence JR, Young VB (2017) Real-time measurement of epithelial barrier permeability in human intestinal organoids. J Vis Exp. doi: 10.3791/56960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Hill DR, Huang S, Nagy MS, Yadagiri VK, Fields C, Mukherjee D, Bons B, Dedhia PH, Chin AM, Tsai Y-H, Thodla S, Schmidt TM, Walk S, Young VB, Spence JR (2017) Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife 6, e29132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Hill DR, Spence JR (2017) Gastrointestinal organoids: Understanding the molecular basis of the host-microbe interface. Cell Mol Gastroenterol Hepatol 3, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]