Abstract
Context
Adrenal crisis (AC) causes morbidity and mortality in patients with Addison disease [primary adrenal insufficiency (PAI)]. Patient-initiated stress dosing (oral or parenteral hydrocortisone) is recommended to avert ACs. Although these should be effective, the continued incidence of ACs remains largely unexplained.
Methods
Audit of all attendances between 2000 and 2017 of adult patients with treated PAI to one large regional referral center in New South Wales, Australia. Measurements were those taken on arrival at hospital.
Results
There were 252 attendances by 56 patients with treated PAI during the study period. Women comprised 60.7% (n = 34) of the patients. The mean age of attendees was 53.7 (19.6) years. Nearly half (45.2%, n = 114) of the patients had an infection. There were 61 (24.2%) ACs diagnosed by the treating clinician. Only 17.9% (n = 45) of the hospital presentations followed any form of stress dosing. IM hydrocortisone was used prior to presentation 7 (2.8%) attendances only. Among patients with a clinician-diagnosed AC, only 32.8% (n = 20) had used stress dosing before presentation. Vomiting was reported by 47.6% (n = 120) of the patients but only 33 (27.5%) of these attempted stress dosing and 5 patients with vomiting used IM hydrocortisone. The number of prior presentations was an independent predictor of use of stress doses [1.05 (1.01, 1.09)].
Conclusion
Dose-escalation strategies are not used universally or correctly by unwell patients with PAI; many patients do not use IM or subcutaneous hydrocortisone injections. Previous hospital treatment increases the likelihood of stress dosing, and hospital attendance offers the opportunity for reinforcement of prevention strategies.
Keywords: Addison disease, hydrocortisone, adrenal insufficiency, adrenal crisis, glucocorticoid
Addison disease or primary adrenal insufficiency (PAI) is a rare disorder that affects approximately 100 individuals per million in the population [1, 2]. In Western countries, PAI is largely the result of autoimmune adrenalitis, a condition that is more common in females and may be associated with other autoimmune endocrine disorders as part of the autoimmune polyglandular syndromes [1, 2]. Patients with PAI need lifelong glucocorticoid replacement therapy and the majority also need mineralocorticoid replacement. Despite modern approaches to management, patients are at increased risk of morbidity and mortality, some of which is attributable to adrenal crisis (AC) [3–7]. These acute episodes of severe adrenal insufficiency (AI), which occur during periods of physiological stress, have an estimated mortality rate of 0.5 per 100 patient-years [8].
Because ACs are infrequent emergency presentations, there may be uncertainty around the diagnosis and misclassifications of symptomatic AI as ACs and vice versa can occur [9]. ACs can be defined as episodes of acute AI with hypotension that may be associated with electrolyte abnormalities (hyponatremia, hyperkalemia), alterations in consciousness, and acute abdominal symptoms that improve rapidly after administration of IV hydrocortisone [9]. ACs occur at a rate of approximately 5 to 8 per 100 patient-years in treated PAI, may vary in incidence between patient subgroups, and appear to be increasing in frequency [8–12]. Comorbidities, particularly type 1 diabetes mellitus (T1DM), are thought to increase the risk of an AC [9, 12]. In patients with comorbid T1DM, this increase may be caused by difficulties managing the requirements of both conditions during an intercurrent illness [9, 12].
All patients with AI should be educated to increase their glucocorticoid replacement, i.e., stress dose, during an episode of physiological stress, most commonly an acute infection-related illness. Patients are advised to double or triple their oral glucocorticoid dose in these situations but when this cannot be taken or absorbed, for example when there is diarrhea or vomiting or when symptoms are severe or evolving rapidly, patients are advised to administer an IM [or subcutaneous (SC)] injection of hydrocortisone and seek medical attention promptly [2, 9, 13–15]. However, the persistent incidence of ACs in a number of populations suggest that management of AI during episodes of illness is not always successful, although the reasons for this are not clear. This study aims to identify factors that may explain the continued occurrence of ACs in patients with treated PAI.
1. Methods
The Hunter New England Local Health District provides health care to a large regional area, with a population of nearly one million people in New South Wales, Australia [16]. It comprises a large urban area with smaller semiurban and some rural areas. There is one main public referral hospital, another public hospital, a private hospital sector providing some tertiary services, and a number of smaller local hospitals providing limited services to a local community [16]. Data on all admissions to these hospitals are coded according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification [17] and stored securely at each health service.
For the purposes of this study, all patients with a diagnosis of an AC (E27.2) or PAI (E27.1) who had at least one admission to the referral hospital between 2000 and 2017 were eligible for inclusion. Hospital staff electronically screened the database for all eligible patient medical record numbers, which were extracted into a dataset. Using these, each electronic patient record was reviewed remotely using a password-protected access code. For each patient with a confirmed diagnosis of PAI, the first admission and all subsequent hospital attendances during the study period were reviewed. Where a presentation was because of an acute medical illness, the record was assessed in detail. Each patient was assigned a unique study number and each attendance was given an episode number linked to the study number. The following variables were extracted from each eligible presentation: age, sex, principal diagnosis, comorbid diagnoses, any procedures, whether the patient had used stress dosing and the type of stress dose, electrolytes, blood pressure on presentation, symptoms of AI, AC diagnosis, diabetic ketoacidosis (DKA), whether the patient was admitted, length of stay, and whether the patient was alive at discharge. Data on each patient was deidentified and entered into an Excel (Microsoft Excel Version 16.24) database.
For this analysis, only those patients who were receiving treatment of PAI were selected and episodes of hospital attendance prior to, or for, the initial diagnosis of PAI were excluded.
A. Data Management
Patient age was analyzed as both a continuous variable and according to 10-year age groups. The number of presentations, admissions, and episodes of treatment in which stress dosing had been reported were coded manually for each patient. For other variables, the following definitions were used: hyperkalemia: serum potassium > 5.0 mmol/L; hyponatremia: serum sodium < 135 mmol/L; hypoglycemia: glucose < 3.5 mmol/L; and hyperglycemia: glucose > 7.0 mmol/l. Hypotension was classified as a systolic blood pressure (sBP) of 100 mg Hg or below. An AC was considered present when this was recorded in the medical record by the treating clinician (Clin-AC). However, given the possibility of AC misclassification, ACs with hypotension were further classified as clinician-diagnosed AC with hypotension (Clin-AC-hypo). Patients with hypotension that did not have a recognizable non-PAI cause were also identified (hypo-non-Clin-AC). These two groups of patients were recategorized into a true AC group and were compared with the remaining patients, who were considered to have symptomatic AI.
B. Statistical Analysis
The data were analyzed using the SPSS software package version (SPSS Statistics version 25). Differences in continuous variables between groups were assessed using t-tests and, where the variances were unequal, appropriate significance tests were used. Categorical variables were evaluated using χ2 or Fisher exact tests. A logistic regression model was developed to assess the relation between demographic- and illness-related variables on the use of stress dosing in the study sample. Wald χ2 statistics were used to assess the significance of each variable in the model.
The study was approved by the St. Vincent’s Hospital, Sydney Human Resource Ethics Committee (HREC), the Hunter New England Health Service HREC, and the HREC of The University of Notre Dame, Australia.
2. Results
A. Patients
During the period 2000 to 2017, there were 252 attendances by 56 patients with PAI, who were receiving glucocorticoid replacement therapy, and who had at least one admission to the study hospital for an acute illness. This equates to an approximate incidence of one attendance per patient every three years. Of these, 49 (87.5%) patients had non-T1DM PAI and 7 (12.5%) had both PAI and T1DM. Comorbid thyroid disease was present in 52.0% (n = 131) of the attendees (Table 1). Women comprised 60.7% (n = 34) of the patients. Two-thirds (66.1%, n = 37) of the patients were found to have never used any kind of stress dosing prior to any attendance at the study hospital. Of those patients who had stress dosed, 11 (19.6%) had one recorded attempt at dose escalation, 5 (8.9%) had a record of dose escalation at 2 presentations; and there was 1 patient (1.8%) for each of 3, 6 and 16 separate presentations in which there was stress dosing before arrival.
Table 1.
Demographic and Disease Characteristics of Hospital Attendances for Patients With PAI to a Regional Hospital, New South Wales 2000–2017
| Total N (%) | PAI only N (%) | PAI & T1DM N (%) | P | |
|---|---|---|---|---|
| Total Presentations | 252 | 215 (85.3) | 37 (14.7) | |
| Sex | ||||
| Female | 174 (69.0) | 148 (68.8) | 26 (70.3) | |
| Male | 78 (31.0) | 67 (31.2) | 11 (29.7) | NS |
| Age group | ||||
| 18–29 | 41 (16.3) | 35 (16.3) | 6 (16.2) | |
| 30–39 | 29 (11.5) | 22 (10.2) | 7 (18.9) | |
| 40–49 | 36 (14.3) | 35 (16.3) | 1 (2.7) | |
| 50–59 | 25 (9.9) | 21 (9.8) | 4 (10.8) | |
| 60–69 | 48 (19.0) | 39 (18.1) | 9 (24.3) | |
| 70+ | 73 (29.0) | 63 (29.3) | 10 (27.0) | NS |
| Ambulance | 122 (48.4) | 94 (43.7) | 28 (75.7) | <0.001 |
| AC recorded | 63 (25.0) | 56 (26.0) | 7 (18.9) | NS |
| Admissions | 211 (83.7) | 181 (84.2) | 30 (81.1) | NS |
| Principal diagnosis | ||||
| Infection | 114 (45.2) | 103 (47.9) | 11 (29.7) | <0.05 |
| Trauma | 19 (7.5) | 14 (6.5) | 5 (13.5) | <0.05 |
| AI | 86 (34.1) | 80 (37.2) | 6 (16.2) | <0.05 |
| Gastroenteritis | 31 (12.3) | 31 (14.4) | 0 (0.0) | <0.05 |
| Comorbid conditions | ||||
| Asthma/COPD | 36 (14.3) | 32 (17.5) | 4 (12.1) | NS |
| Cardiovascular disease | 40 (15.9) | 38 (21.5) | 2 (5.7) | <0.05 |
| Thyroid | 131 (52.0) | 104 (48.4) | 27 (73.0) | <0.01 |
| T2DM | 82 (32.5) | 82 (38.1) | 0 (0.0) | <0.001 |
| Management | ||||
| Oral stress dosing | 40 (15.9) | 35 (16.3) | 5 (13.5) | NS |
| IM stress dosing | 7 (2.8) | 7 (3.3) | 0 (0.0) | NS |
| Any stress dosing | 45 (17.9) | 40 (18.6) | 5 (13.5) | NS |
| IV hydrocortisone | 185 (73.4) | 162 (75.3) | 23 (62.2) | NS |
| Surgical intervention | 14 (5.6) | 9 (4.2) | 5 (13.5) | <0.05 |
| DKA recorded | 11 (4.4) | 0 (0.0) | 11 (29.7) | <0.001 |
| Mortality | 5 (2.0) | 3 (1.4) | 2 (5.4) | NS |
Abbreviations: COPD, chronic obstructive pulmonary disease; NS, not significant.
B. Attendances at Hospital
The majority (83.7%, n = 211) of the 252 attendances resulted in an admission (Table 1). Females comprised two-thirds [174 (69.0%)] of the attendees. The mean age of patients was 53.7 (19.6) years and 29.0% (n = 73) were aged 70 or more years. The median length of stay was 2 (interquartile range 1 to 5) days and 99 (39.3%) admissions were for one day only. Nearly three-quarters (73.4%, n = 185) of the patients were treated with IV hydrocortisone (Table 1).
Any form of dose escalation was used by 17.9% (n = 45) of the patients prior to attendance. Oral stress dosing was used before 40 presentations (15.9%) and in 7 (2.8%) there was a record of prior IM hydrocortisone administration (one of these was administered by a family doctor). There was no record of SC hydrocortisone administration. Nearly half (48.4%, n = 182) the attendees arrived by ambulance.
C. Deaths in Hospital
There were 5 (2.0%) in-hospital deaths but in only one had an AC been diagnosed but four had had IV hydrocortisone administered. Of the five deaths, three were patients who had PAI only and two had comorbid PAI/T1DM. Of the PAI-only patients, none attempted stress-dosing, none had an AC recorded, and all three received IV hydrocortisone. Causes of death were complex and the result of extensive comorbid disease. The first was caused by a fractured femoral neck leading to cardiorespiratory failure. The second had many diagnoses listed as contributing to the cause of death including hepatic encephalopathy, liver failure, aspiration pneumonia, small bowel obstruction, and sepsis. The third was the result of multisystem failure (hepatic, renal, respiratory) as a result of sepsis. Of the two deaths of PAI/T1DM patients, none attempted stress-dosing, one patient had an AC recorded, and received IV hydrocortisone, the other did not receive IV hydrocortisone. Causes of death were listed as sepsis from a pressure sore and cerebral anoxia.
D. AC Diagnoses and Hypotension
In 63 (25.0%) of the presentations a clinician-diagnosed AC (Clin-AC) was recorded, and in 21 (33.3%) of these, stress dosing had been used prior to attendance. Almost all (93.7%, n = 59) patients with Clin-AC were given IV hydrocortisone (Table 1). Nearly half (47.6%, n = 30) of these patients had hypotension (Clin-AC-hypo) and the remainder (52.4%, n = 33) were normotensive. There were 26 (10.3%) patients who were not diagnosed with an AC but who, on arrival, had hypotension that appeared attributable to PAI (hypo-non-Clin-AC). These patients were combined with the Clin-AC-hypo group as the true AC category of presentations (n = 56, 22.2% of all attendances).
Among the patients in the true AC category, 31 (55.4%) had hyponatremia; 13 (23.2%) had hyperkalemia; 3 (5.4%) had hypoglycemia; 9 (16.1%) had reduced consciousness; 15 (26.8%) had used any form of stress dosing; 12 (21.4%) had been stress dosed orally; and 5 (8.9%) had used IM hydrocortisone. Patients in this group were younger than the remaining patients [43.5 (18.3) and 56.6 (19.0) years, respectively, P < 0.001]. Patients in the true AC group also had a higher burden of vomiting than the remaining patients [n = 40 (71.4%) and n = 80 (40.8%), respectively, P < 0.001]. Infection (n = 32, 57.1%) was more common in the true AC group than in those with symptomatic AI (n = 82, 41.8%) (P < 0.05) as was a diagnosis of gastroenteritis [n = 14 (25.0%) and n = 17 (8.7%), respectively, P < 0.01].
E. Vomiting and Diarrhea
There were 120 (47.6%) presentations in which the patient had vomiting but in only 33 (27.5%) of these had the patient used any form of dose escalation and in only 5 cases was IM hydrocortisone used. Diarrhea was less frequently reported, affecting 53 (21.0%) attendees. Of these, only 5 (9.4%) used any form of stress dosing (2 used IM and 3 used oral stress dosing). Most of the patients (90.6%, n = 48) who had diarrhea also had vomiting. Almost all (95%, n = 114) patients who had vomiting were given IV hydrocortisone, as were all patients with diarrhea. Of those who reported vomiting, 35.8% (n = 43) were classified as having a clin-AC and 40.8% (n = 49) were hypotensive on admission.
F. Comorbid Disease
The 7 patients with T1DM and PAI contributed 37 presentations to the study hospital. Among these attendances were 7 (18.9%) in whom a Clin-AC was recorded; in one-quarter (27.0%, n = 10) of all the attendances the patient was hypotensive on arrival (Table 2). After reclassification, 9 (24.3%) of the T1DM patients were considered to have had a true AC. Nearly two-thirds (64.9%, n = 24) had hyperglycemia; 18.9%, (n = 7) had hypoglycemia, both of which were higher than those found in non-T1DM PAI patients (Table 2). DKA was diagnosed in 11 (29.7%) presentations. Patients with both T1DM and PAI had no record of IM hydrocortisone use, and only 5 (13.5%) had used oral glucocorticoid dose escalation prior to attendance at hospital; in 1 presentation the patient had increased their insulin and 2 had increased their glucose, with another 5 patients being given glucose in the ambulance.
Table 2.
Signs, Symptoms, and Biochemistry of Hospital Attendances for Patients With PAI to a Regional Hospital, New South Wales 2000–2017
| Total N (%) | PAI only N (%) | PAI & T1DM N (%) | P | |
|---|---|---|---|---|
| Signs and symptoms | ||||
| Hypotension | 77 (30.6) | 67 (31.2) | 10 (27.0) | NS |
| Fever | 60 (23.8) | 50 (23.3) | 10 (27.0) | NS |
| Vomiting | 120 (47.6) | 105 (48.8) | 15 (40.5) | NS |
| Diarrhea | 53 (21.0) | 51 (23.7) | 2 (5.4) | <0.05 |
| Abdominal pain | 96 (38.1) | 86 (40.0) | 10 (10.4) | NS |
| Reduced level of consciousness | 39 (15.5) | 29 (13.5) | 10 (27.0) | <0.05 |
| Confusion | 16 (6.3) | 15 (7.0) | 1 (2.7) | NS |
| Lethargy | 132 (52.4) | 118 (54.9) | 14 (37.8) | NS |
| Dehydration | 94 (37.3) | 82 (38.1) | 12 (32.4) | NS |
| Biochemistry | ||||
| Hypoglycemia | 23 (9.1) | 9 (4.2) | 14 (37.8) | <0.001 |
| Hyperglycemia | 88 (34.9) | 64 (29.8) | 24 (64.9) | <0.001 |
| Hyponatremia | 99 (39.3) | 85 (39.5) | 14 (37.8) | NS |
| Hyperkalemia | 37 (14.7) | 28 (13.0) | 9 (24.3) | NS |
| Acidosis | 39 (15.5) | 26 (12.1) | 13 (35.1) | <0.001 |
Abbreviation: NS, not significant.
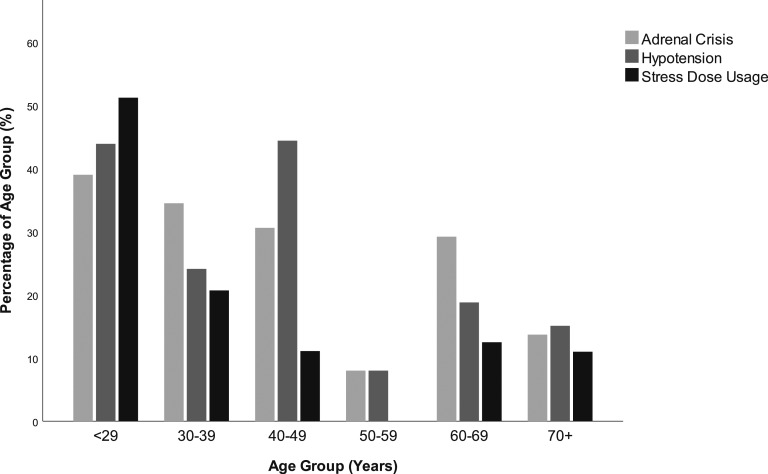
Comorbid cardiovascular disease (CVD) was more prevalent in older patients [mean age (SD) with CVD: 71.0 (10.5) years and without: 50.4 (19.2) years, P < 0.0001], as was type 2 diabetes mellitus (T2DM) [mean age with T2DM: 63.6 (48.9) years and without: 48.9 (21.0) years, P < 0.0001] and thyroid disease [mean age with thyroid disease: 61.4 (17.5) and without: 45.3 (18.2) years, P < 0.0001]. In addition, among older patients, the acute symptoms of confusion [mean age (SD) with confusion: 72.1 (14.2) and without: 52.4 (19.3) years, P < 0.0001] and reduced consciousness [mean age (SD) with reduced consciousness: 63.9 (16.4) and without: 51.8 (19.6), P < 0.0001] were more common. By comparison, patients who were hypotensive were younger than normotensive patients [47.5 (19.9) and 56.4 (18.8) years, respectively, P < 0.01]. Similarly, patients who had vomiting were younger than patients who did not [46.8 (18.7) years and 59.9 (18.2) years, respectively, P < 0.0001], as were patients who had abdominal pain on presentation [with pain: mean age (SD) 50.0 (19.7) years and without 55.9 (19.2) years, P < 0.05]. In addition, patients who had used any form of stress dosing were younger than those who had not [41.2 (21.0) years and 56.4 (18.2) years respectively, P < 0.0001]. The age distribution of patients with Clin-AC, hypotension, and who had used stress dosing is shown in Figure 1.
Figure 1.
Adrenal crisis, hypotension, and stress dose usage among hospital attendees with PAI to a regional hospital, New South Wales 2000–2017.
G. Predictors of Stress Dosing
Logistic regression modeling was used to predict which demographic or disease-related factors were associated with the use of stress dosing in this population. Logistic models showed that three factors were independent predictors of the use of dose escalation. These were patient age: OR = 0.96 (0.94, 0.98), for each year of increasing age; vomiting: OR = 2.76 (1.28, 5.98); and the number of presentations: OR = 1.05 (1.01, 1.09) for each extra presentation.
3. Discussion
This comprehensive study of all presentations by patients with PAI to a referral hospital in a large regional area demonstrated that pre-emergent domiciliary management of AI by stress dosing was attempted by only a minority (33.9%) of patients and, that among those who used dose escalation, the recommended strategies were used inconsistently across the illness episodes. When all attendances at hospital by these patients were considered, oral stress dosing was used in fewer than one in five presentations and in only 2.8% of the total attendances did a patient use IM hydrocortisone prior to arrival. Nearly one-quarter of the presentations included a clinician diagnosis of an AC. Patient age was strongly associated with stress dosing attempts, which were made more frequently by younger rather than older patients. Use of dose escalation was more likely to have occurred with an increasing number of prior presentations to hospital.
A high proportion of presentations (25.0%) were associated with a clinician-derived AC diagnosis and, although there appeared to be some misclassification of the syndrome of symptomatic AI as an actual AC among these, the proportion of presentations that included symptoms indicative of acute AI/AC was considerable. Half the patients diagnosed with an AC by their treating clinician had hypotension (sBP < 100 mm Hg), suggesting that there may still be confusion about the diagnostic criteria that are used to define an AC [9, 15] and highlighting the value and importance of ongoing education about AI and its management [18]. When the presenting features were reviewed and patients were reclassified based on the presence of hypotension, there were 56 patients (18.8%) who were considered to have experienced an actual AC. Hypotension was more common in younger patients; hyponatremia was diagnosed in 39.3% and hyperkalemia in 14.7%. Both absolute (sBP < 100 mm Hg) and relative (>20 mm Hg lower than usual sBP) are considered indicative of an AC but, in this analysis, we were unable to assess relative hypotension [9]. This may have affected the estimation of hypotension in older patients especially, given the elevation in blood pressure with increasing age.
Infection has been shown to be a major cause of episodes of AC in a number of studies and was found to be associated with 45.2% of attendances in this population [8, 19]. Vomiting and diarrhea are particularly hazardous in AI because patients may not absorb either their usual glucocorticoid replacement or any oral stress doses and thus are at increased risk of an AC. In these situations, IM or even SC use of hydrocortisone is recommended [9, 13, 14]. However, in this population, self-management strategies were either not executed at all or implemented in a way that would be potentially ineffective. This is consistent with the levels of preparedness reported in other populations [20, 21], but is in contrast to the higher use of IM hydrocortisone by children with congenital adrenal hyperplasia attending the pediatric hospital on the same campus for treatment of an acute illness [22]. In the current study, among patients who were vomiting, only a minority (27.5%) reported some form of stress dosing and, among those who did, only five used IM hydrocortisone. Hypotension on arrival to hospital was found in 40.8% of patients with vomiting in this study, highlighting the risks posed by nonabsorption of oral glucocorticoids and illustrating the importance of parenteral glucocorticoid administration when there is vomiting and/or diarrhea [9, 15].
Younger patients were found to be more likely to have some of the acute symptoms of an AC (vomiting, abdominal pain, and hypotension) than older patients in this study. In addition, they were found to be more likely to use oral stress dosing strategies than the older patients, although these were likely to be ineffective or only partially effective because of the higher incidence of acute gastrointestinal symptoms in the younger patients. By comparison, older patients had a greater burden of chronic comorbid disease such as CVD and T2DM and were more likely to present with confusion or reduced consciousness. Although ACs were diagnosed in all age groups and stress dosing was underused, the underlying causes may differ between the age groups and, therefore, any interventions to reduce AC risk may need to be tailored accordingly [9, 23]. In younger people, particularly those who have been managed in pediatric settings, factors such as missing medications and medical appointments and poor adherence to prevention strategies may contribute, especially during the transition from pediatric to adult care arrangements [9, 24–26]. In contrast, among older patients, higher levels of comorbid conditions, social isolation and cognitive issues may make preventive strategies more difficult to implement [21, 23].
Those patients with PAI and comorbid T1DM had a comparable proportion of admissions with true AC diagnoses (24.3%) to patients with PAI only but there were also 11 (29.7%) patients with T1DM who had a diagnosis of DKA, meaning that half of these patients attended hospital with an acute metabolic disorder. Hypoglycemia was also identified in 18.9% of the T1DM patients on arrival at hospital. Patients with combined disease have been found to be at increased risk of AC events [12], which may be the result of the complexities of managing the demands of both disorders, especially during an acute illness. The patients in this study with combined disease were no more likely to use oral glucocorticoid dose escalation than patients with PAI alone and none used IM hydrocortisone. In addition, only 1 patient had increased their insulin dose and 2 had given themselves glucose, highlighting the need for improved self-management in patients with combined disease.
Whereas the results from this study represent an evaluation of the illness experience of all patients attending the regional referral hospital, they are likely to represent an underestimate of the true range of PAI-related morbidity in this population, because some patients may have had other episodes treated elsewhere and others may have managed their AI at home without the need for hospitalization. In addition, given that the patients in this study were drawn from one large regional area, the generalizability of these results to other populations is uncertain. ACs in this analysis were those identified and diagnosed as such by the treating clinician and may be affected by misclassification bias. To address this, the incidence of AC was also assessed based on the presence of hypotension without an obvious non-AI cause. Furthermore, although the records were assessed thoroughly, there may have been episodes of stress dosing that were unreported or undocumented, resulting in an underestimate of the true rate of dose escalation in this population.
The reason for the disappointing levels of stress dose utilization in this sample of patients could not be identified definitively in this analysis. Although there were no formal educational programs for patients with AI in this area over the period of the study, each patient had access to medical practitioners and specialist endocrinologists, although the extent to which patients accessed routine medical care could not be determined. Treatment guidelines for both PAI and T1DM have been published, with AI recommendations including the provision of education on oral stress dosing and IM injection of hydrocortisone by the patient and/or their caregiver and the use of medical identification jewelry and a steroid identification card. However, it has been shown in Australia and elsewhere that these are not used by all patients [21, 25, 27, 28]. In this study, a positive effect of cumulative previous presentations to hospital on the use of stress dosing was identified. This phenomenon has been found previously and indicates that there may be an experiential component to the development of stress dosing expertise among patients with AI [22].
In conclusion, the results of this study demonstrate clear shortfalls in the patient-initiated management of AI during acute illness in patients living in a large regional area with a comprehensive endocrine service. ACs are considered to be a cause of anxiety for many patients with AI, but in this population, it was found that a substantial number of patients appeared not to use any form of dose escalation and a proportion of those who did use oral stress dosing would have benefited from self-administered parenteral hydrocortisone. Increased efforts directed at understanding the barriers to the effective implementation of AC prevention strategies by different subgroups of patients may reduce the incidence of ACs.
Acknowledgments
Financial Support: T.G. received funding via a Summer Research Scholarship from The University of Notre Dame Australia to conduct data collection.
Glossary
Abbreviations:
- AC
adrenal crisis
- AI
adrenal insufficiency
- Clin-AC
clinician-diagnosed adrenal crisis
- Clin-AC-hypo
clinician-diagnosed adrenal crisis with hypotension
- CVD
cardiovascular disease
- DKA
diabetic ketoacidosis
- HREC
Human Resource Ethics Committee
- hypo-non-Clin-AC
no adrenal crisis with hypotension
- PAI
primary adrenal insufficiency
- sBP
systolic blood pressure
- SC
subcutaneous
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Additional Information
Disclosure Summary: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Barthel A, Benker G, Berens K, Diederich S, Manfras B, Gruber M, Kanczkowski W, Kline G, Kamvissi-Lorenz V, Hahner S, Beuschlein F, Brennand A, Boehm BO, Torpy DJ, Bornstein SR. An update on Addison's disease. Exp Clin Endocrinol Diabetes. 2019. Feb;127(2-03):165–175. [DOI] [PubMed] [Google Scholar]
- 2. Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erichsen MM, Løvås K, Fougner KJ, Svartberg J, Hauge ER, Bollerslev J, Berg JP, Mella B, Husebye ES. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol. 2009;160(2):233–237. [DOI] [PubMed] [Google Scholar]
- 4. Falhammar H, Frisén L, Norrby C, Hirschberg AL, Almqvist C, Nordenskjöld A, Nordenström A. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(12):E2715–E2721. [DOI] [PubMed] [Google Scholar]
- 5. Burman P, Mattsson AF, Johannsson G, Höybye C, Holmer H, Dahlqvist P, Berinder K, Engström BE, Ekman B, Erfurth EM, Svensson J, Wahlberg J, Karlsson FA. Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J Clin Endocrinol Metab. 2013;98(4):1466–1475. [DOI] [PubMed] [Google Scholar]
- 6. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab. 2006;91(12):4849–4853. [DOI] [PubMed] [Google Scholar]
- 7. Bensing S, Brandt L, Tabaroj F, Sjöberg O, Nilsson B, Ekbom A, Blomqvist P, Kämpe O. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf). 2008;69(5):697–704. [DOI] [PubMed] [Google Scholar]
- 8. Hahner S, Spinnler C, Fassnacht M, Burger-Stritt S, Lang K, Milovanovic D, Beuschlein F, Willenberg HS, Quinkler M, Allolio B. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. 2015;100(2):407–416. [DOI] [PubMed] [Google Scholar]
- 9. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises: perspectives and research directions. Endocrine. 2017;55(2):336–345. [DOI] [PubMed] [Google Scholar]
- 10. Rushworth RL, Torpy DJ. Modern hydrocortisone replacement regimens in adrenal insufficiency patients and the risk of adrenal crisis. Horm Metab Res. 2015;47(9):637–642. [DOI] [PubMed] [Google Scholar]
- 11. Rushworth RL, Torpy DJ. Adrenal insufficiency in Australia: is it possible that the use of lower dose, short-acting glucocorticoids has increased the risk of adrenal crises? Horm Metab Res. 2015;47(6):427–432. [DOI] [PubMed] [Google Scholar]
- 12. Meyer G, Badenhoop K, Linder R. Addison’s disease with polyglandular autoimmunity carries a more than 2.5-fold risk for adrenal crises: German Health insurance data 2010–2013. Clin Endocrinol (Oxf). 2016;85(3):347–353. [DOI] [PubMed] [Google Scholar]
- 13. Hahner S, Burger-Stritt S, Allolio B. Subcutaneous hydrocortisone administration for emergency use in adrenal insufficiency. Eur J Endocrinol. 2013;169(2):147–154. [DOI] [PubMed] [Google Scholar]
- 14. Rushworth RL, Bischoff C, Torpy DJ. Preventing adrenal crises: home-administered subcutaneous hydrocortisone is an option. Intern Med J. 2017;47(2):231–232. [DOI] [PubMed] [Google Scholar]
- 15. Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol. 2015;172(3):R115–R124. [DOI] [PubMed] [Google Scholar]
- 16. NSW Government. Hunter New England Health Group. Available at: http://www.hnehealth.nsw.gov.au/Pages/home.aspx. Accessed 1 July 2019.
- 17. National Centre for Classification in Health. International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM), Australian Classification of Health Interventions (ACHI), Australian Coding Standards (ACS), 5th ed.Sydney, Australia: National Centre for Classification in Health; 2006. [Google Scholar]
- 18. Kampmeyer D, Lehnert H, Moenig H, Haas CS, Harbeck B. A strong need for improving the education of physicians on glucocorticoid replacement treatment in adrenal insufficiency: An interdisciplinary and multicentre evaluation. Eur J Intern Med. 2016;33:e13–e15. [DOI] [PubMed] [Google Scholar]
- 19. Smans LC, Van der Valk ES, Hermus AR, Zelissen PM. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2016;84(1):17–22. [DOI] [PubMed] [Google Scholar]
- 20. Reisch N, Willige M, Kohn D, Schwarz HP, Allolio B, Reincke M, Quinkler M, Hahner S, Beuschlein F. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(1):35–42. [DOI] [PubMed] [Google Scholar]
- 21. Hahner S, Loeffler M, Bleicken B, Drechsler C, Milovanovic D, Fassnacht M, Ventz M, Quinkler M, Allolio B. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162(3):597–602. [DOI] [PubMed] [Google Scholar]
- 22. Chrisp GL, Maguire AM, Quartararo M, Falhammar H, King BR, Munns CF, Torpy DJ, Hameed S, Rushworth RL. Variations in the management of acute illness in children with congenital adrenal hyperplasia: An audit of three paediatric hospitals. Clin Endocrinol (Oxf). 2018;89(5):577–585. [DOI] [PubMed] [Google Scholar]
- 23. Rushworth RL, Torpy DJ. A descriptive study of adrenal crises in adults with adrenal insufficiency: increased risk with age and in those with bacterial infections. BMC Endocr Disord. 2014;14(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merke DP, Poppas DP. Management of adolescents with congenital adrenal hyperplasia. Lancet Diabetes Endocrinol. 2013;1(4):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rushworth RL, Chrisp GL, Torpy DJ. The use of medical identification jewellery in adults with adrenal insufficiency in Australia. Clin Endocrinol (Oxf). 2019;91(1):41–47. [DOI] [PubMed] [Google Scholar]
- 26. Vidmar AP, Weber JF, Monzavi R, Koppin CM, Kim MS. Improved medical-alert ID ownership and utilization in youth with congenital adrenal hyperplasia following a parent educational intervention. J Pediatr Endocrinol Metab. 2018;31(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burger-Stritt S, Kardonski P, Pulzer A, Meyer G, Quinkler M, Hahner S. Management of adrenal emergencies in educated patients with adrenal insufficiency—a prospective study. Clin Endocrinol (Oxf). 2018;89(1):22–29. [DOI] [PubMed] [Google Scholar]
- 28. Chapman SC, Llahana S, Carroll P, Horne R. Glucocorticoid therapy for adrenal insufficiency: nonadherence, concerns and dissatisfaction with information. Clin Endocrinol (Oxf). 2016;84(5):664–671. [DOI] [PubMed] [Google Scholar]