Abstract
Genome-wide association studies identified loci associated with polycystic ovary syndrome (PCOS), including those near the LH receptor gene (LHCGR), a clathrin-binding protein (DENND1A) that functions as a guanine nucleotide exchange factor, and the gene encoding RAB5B, a GTPase involved in vesicular trafficking. We proposed that these three PCOS loci could be assembled into a functional network that contributes to altered gene expression in theca cells, resulting in increased androgen synthesis. The functional significance of this network was supported by our discovery that a truncated protein splice variant of the DENND1A gene, termed DENND1A.V2, is elevated in PCOS theca cells, and that forced expression of DENND1A.V2 in normal theca cells increased CYP11A1 and CYP17A1 expression and androgen synthesis, a hallmark of PCOS. In this study, we demonstrate the colocalization of LHCGR, DENND1AV.2, and RAB5B proteins in various cellular compartments in normal and PCOS theca cells by immunofluorescence. Human chorionic gonadotropin and forskolin stimulation was shown to affect the cytoplasmic distribution of LHCGR, DENND1A.V2, and RAB5B. DENND1A.V2 accumulated in the nuclei of the theca cells. Moreover, PCOS theca cells, following forskolin treatment, had a significantly greater relative abundance of nuclear DENND1A.V2. RAB5B also accumulated in the nuclei of PCOS theca cells treated with forskolin. In contrast, LHCGR did not enter the nucleus. This cytological evidence, and the previously reported increase in androgen biosynthesis with forced expression of DENND1A.V2 in normal theca cells, raises the possibility that DENND1A.V2 and RAB5B participate in increasing transcription of genes involved in androgen synthesis.
Keywords: androgens, DENND1A, LHCGR, polycystic ovary syndrome, RAB5B, theca cells
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy of reproductive-age women and the leading cause of anovulatory infertility. PCOS is characterized by hyperandrogenism and the characteristic presence in the ovary of multiple small subcortical follicular cysts, detectable by transvaginal ultrasound. PCOS is often accompanied by metabolic disturbances, including insulin resistance and hyperinsulinemia [1, 2]. The etiology of PCOS is thought to be rooted in genetic and environmental factors [3].
Family-based and twin studies revealed a significant genetic contribution to PCOS [4, 5]. Genome-wide association studies (GWAS) have yielded >20 loci located near putative PCOS genes, including DENND1A, LHCGR, FSHR, ZNF217, YAP1, INSR, RAB5B, and C9orf3, among others [6–10]. A number of these loci have been replicated in GWAS or targeted genotyping studies in different populations [11, 12]. However, the specific variants associated with the PCOS phenotype and their functional significance remain to be identified for most of the PCOS candidates.
Among the candidate genes, the DENND1A locus at 9q22.32 was identified in both Asian and European ancestry populations in GWAS and replication studies [11, 12]. DENND1A, a member of the connecdenn family, encodes a clathrin-binding protein localized to coated pits, a clustering point for plasma membrane receptors [13]. The differentially expressed in normal and neoplastic cells (DENN) domain encodes a guanine nucleotide exchange function, which was previously shown to interact with RAB35, a small GTPase [13].
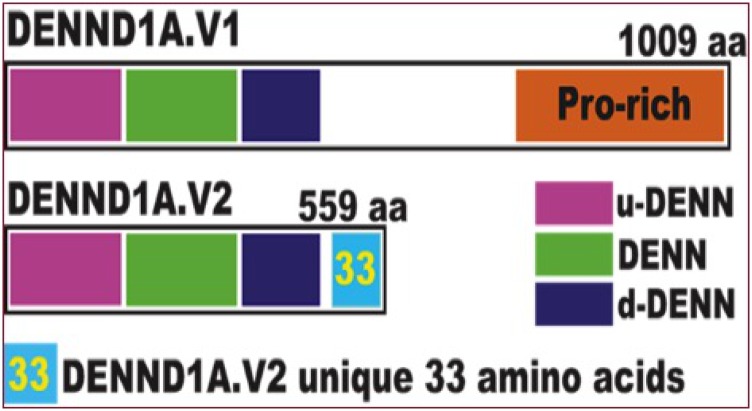
We discovered that a splice variant of DENND1A, termed DENND1A.V2, encodes a truncated molecule retaining the DENN and clathrin-binding domains, but lacking the proline-rich domain, which interacts with the adaptor protein, growth factor receptor–bound protein 2, and also undergoes posttranslational modification. DENND1A.V2 is produced by exonization of sequences in intron 20, generating a unique exon, 20A, which encodes the 33–amino acid C terminus of DENND1A.V2 that distinguishes it from DENND1A.V1, allowing production of antibodies specific to the DENND1A.V2 protein (Fig. 1) [14, 15].
Figure 1.
Schematic showing the differences between DENND1A.V1 and DENND1A.V2. The DENND1A gene yields two principal transcripts via alternative splicing: DENND1A variant 1 (DENND1A.V1), which encodes a 1009–amino acid (aa) protein with a large C-terminal proline-rich domain; and DENND1A.V2, a 559-aa protein that lacks the proline-rich domain but includes a distinct C-terminal 33-aa sequence that differs from DENND1A.V1. dDENN, downstream DENN domain; Pro, proline; uDENN, upstream DENN domain.
We found that DENND1A.V2 expression is elevated in theca cells of women with PCOS [16–18]. Moreover, forced expression of DENND1A.V2 in normal human theca cells in culture resulted in increased expression of CYP17A1 and production of androgens, whereas knockdown of DENND1A.V2 mRNA in PCOS theca cells reduced CYP17A1 expression and androgen secretion, supporting the notion that DENND1A.V2 contributes to hyperandrogenism in PCOS [16].
Among the other PCOS loci identified in GWAS are those near the LHCGR and RAB5B genes. LH stimulation is known to be required for the excess ovarian androgen levels in PCOS, whereas RAB5 proteins, particularly RAB5A, are known to play a role in endocytosis and gonadotropin receptor signaling [3, 19]. We have previously proposed that these PCOS GWAS loci/genes, along with DENND1A, form a network that contributes to the hyperandrogenemia of PCOS, with DENND1A.V2 being the link between LHCGR accumulated in coated pits and the trafficking of endocytic vesicles containing LHCGR and its downstream signaling molecules, including RAB5B [18, 20].
To explore this three gene network model, we examined the localization of DENND1A.V2-containing compartments and those containing LHCGR and RAB5B in normal and PCOS theca cells using immunofluorescence (IF) with specific antibodies. In this study, to our knowledge, we report the first localization of DENND1A.V2, evidence for interaction among compartments containing LHCGR and RAB5B, and the unexpected finding of translocation of DENND1A.V2 and RAB5B into the nucleus of human theca cells, raising the intriguing possibility that LHCGR may initiate alterations in gene expression via vesicular trafficking and nuclear regulation of transcription of genes involved in androgen synthesis.
1. Materials and Methods
A. Cell Culture
A-1. Chinese hamster ovary cells
Chinese hamster ovary (CHO) cells (16.4 CHO cell line, CRL-12023, American Type Culture Collection, Manassas, VA) were cultured in tissue culture dishes (60 by 15 mm, Corning, Corning, NY) for Western blot studies and a Falcon 8 well chamber tissue culture slide for IF studies. Cells were grown in DMEM, high-glucose pyruvate medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, catalog no. 10437028), 1% glutamine, and 1% penicillin/streptomycin (Thermo Fisher Scientific). At 60% to 70% confluency cells were transfected with pCMV6-XL5 (OriGene Technologies, Rockville, MD) for DENND1A.V2 expression (the vector has a CMV promoter and an ampicillin resistance marker for selection in a prokaryote host, and the DENND1A.V2 gene is inserted in a MCS between two BamHI sites), empty vector control (pCMV-BAM), and pGFP2-N2-myc-hLHCGR (provided by Dr. Deborah Segaloff, University of Iowa) [21] using Continuum (Gemini Bio-Products, West Sacramento, CA). The plasmid DNA/Continuum ratios were: DENND1A.V2 (5:1), LHCGR (2.5:1), and pCMV-BAM (5:1). Cells were harvested or fixed 48 hours after transfection. In some experiments, 48 hours after transfection cells were washed with DMEM with serum to remove the transfection medium. Cells were treated with human chorionic gonadotropin (hCG; Sigma-Aldrich, St. Louis, MO) at a concentration of 1 IU/mL for 0, 10, 30, 60, 90, 120, and 150 minutes prior to fixation.
A-2. Human theca cells
Human theca interna tissue was obtained from follicles of women undergoing hysterectomy, following informed consent under a protocol approved by the Institutional Review Board of the Pennsylvania State University College of Medicine. As a standard of care, oophorectomies were performed during the luteal phase of the cycle. Theca cells from normal cycling and PCOS follicles were isolated and grown as we have as previously reported in detail [22, 23].The theca cell preparations used in these studies have been described and characterized previously [22–30]. The steroidogenic phenotypes of the normal and PCOS theca cells have been reported to result from the inherent properties of the cells, rather than the cycle phase at the time that they were isolated [24, 26, 31]. PCOS and normal ovarian tissue came from age-matched women 38 to 40 years of age. The diagnosis of PCOS was made according to National Institutes of Health consensus guidelines [32, 33], which include hyperandrogenemia, oligoovulation, polycystic ovaries, and the exclusion of 21-hydroxylase deficiency, Cushing syndrome, and hyperprolactinemia. All of the PCOS theca cell preparations studied came from ovaries of women with fewer than six menses per year and elevated serum total testosterone or bioavailable testosterone levels [24, 26, 31]. Each of the PCOS ovaries contained multiple subcortical follicles of <10 mm in diameter. The control (normal) theca cell preparations came from ovaries of fertile women with normal menstrual histories, menstrual cycles of 21 to 35 days, and no clinical signs of hyperandrogenism. Neither PCOS nor normal subjects were receiving hormonal medications at the time of surgery. Indications for surgery were dysfunctional uterine bleeding, endometrial cancer, and pelvic pain. Experiments comparing PCOS and normal theca were performed using fourth-passage (31 to 38 population doublings) theca cells isolated from individual size-matched follicles obtained from age-matched subjects, in the absence of in vivo stimulation. The use of fourth-passage cells allowed us to perform multiple experiments from the same patient population, and they were propagated from frozen stocks of second-passage cells in the media described above. The passage conditions and split ratios for all normal and PCOS cells were identical. These studies were approved by the Human Subjects Protection Offices of Virginia Commonwealth University (HM10042) and Penn State College of Medicine (STUDY00007086).
For experiments, cells were transferred into serum-free medium containing DMEM/F12, 0.5 mg/mL BSA, 100 μg/mL transferrin, 20 pM insulin, 20 nM selenium, 1.0 μM vitamin E, and antibiotics. Sera and growth factors were obtained as follows: fetal bovine serum was obtained from Irvine Scientific (Irvine, CA), horse serum was obtained from Gibco BRL (Gaithersburg, MD), UltroSer G was from Reactifs IBF (Villeneuve-la-Garenne, France), and other compounds were from Sigma-Aldrich. In all experiments the gas phase used was 5% O2, 90% N2, and 5% CO2. Reduced oxygen tension and supplemental antioxidants (vitamin E and selenium) were employed to prevent oxidative damage to CYP17A1 and CYP11A1 [23, 24]. Cells were grown until subconfluent and treated with and without 20 μM forskolin for 16 hours in defined serum-free media. Similar treatments with 1 IU/mL hCG were performed for 0, 10, 30, 60, 90, 120, and 150 minutes prior to fixation.
B. Peptide Neutralization Experiments
A rabbit polyclonal antibody against a 20–amino acid peptide ([C]-QKSITHFAAKFPTRGWTSSSH) that is specific to DENND1A.V2 was generated by Thermo’s custom antibody service [34]. Primary antibody was neutralized by preincubation with the DENND1A.V2-specific peptide ([C]-QKSITHFAAKFPTRGWTSSSH; ChinaPeptides, Shanghai, China) for 30 minutes at room temperature. The peptide concentration was varied from 1 mg/mL to 0.1 μg/mL. Anti-RAB5B goat antibody (LifeSpan BioSciences, Seattle, WA [35]) (dilution 1:50) was neutralized by preincubation with 0.1 μg/mL human recombinant RAB5B protein (LifeSpan BioSciences) for 30 minutes at room temperature. Neutralized antibody was centrifuged at high speed and the supernatant passed through a 0.22-μm Millipore filter. Unneutralized antibody was treated the same way.
C. Immunofluorescence
Transfected cells were fixed with 4% formalin for 1 hour, washed with PBS twice, and blocked with a blocking serum containing 10% goat serum, 3% BSA, and 0.2% Triton X-100. The cells were then incubated with anti-DENND1A.V2 rabbit polyclonal antibody [34] (dilution 1:100). Other antibodies used for IF were anti-RAB5B goat antibody (LifeSpan BioSciences [35]) (dilution 1:50), which is a specific antibody for RAB5B and does not interact with RAB5A and RAB5C, and anti-LHCGR mouse antibody (Novus Biologicals, Centennial, CO [36]) (dilution 1:50). For detection of primary antibodies, the cells were incubated with secondary antibody (anti-mouse Alexa Fluor 488 labeled [37], anti-rabbit Cy3 labeled [38], anti-rabbit Alexa Fluor 488 labeled [39], or anti-goat Alexa Fluor 488 labeled [40]) for 1 hour. In some experiments, before mounting the slides, a Cy3-labeled Galanthus nivalis lectin/G. nivalis agglutinin (bioWORLD, Dublin, OH) was used to identify Golgi vesicles. The cells were then washed with PBS and mounted with VectaMount with 4′,6-diamidino-2-phenylindole (Vectasheild, Vector Laboratories, Burlingame, CA) and then sealed with nail polish.
D. Imaging
Images were captured with a Zeiss LSM 700 confocal laser-scanning microscope and processed by Fiji [41] and CellProfiler software [34, 42] (National Institutes of Health). To confirm the results, z-stacks were taken to span across the cell. High-resolution images were also captured by an N-SIM microscope and reconstructed by NIS-Elements AR software.
E. Image Analysis
The nuclear/cytoplasm staining ratios were calculated using CellProfiler version 3.1.5 software [42]. Composite images were split into RGB panels and saved individually using Fiji [41]. The “Identify Primary Objects” module was used to select the nuclear area from the images. The “Identify Secondary Objects” module was used to select the cells. This method finds dividing lines between clumped objects where the image stained for secondary objects shows a change in staining. A global threshold strategy, which calculates a single threshold value of the input image and uses that value to differentiate between foreground and background, was used to identify the secondary objects, as the background of the image was relatively uniform. Then, the “Identify Tertiary Objects” module was used to identify the cytoplasm of each cell. The inputs to this module were “Edited Nucleus” and “Edited Cells.” The area from the “Edited Cells” was subtracted from that of the “Edited Nucleus” to obtain the object cytoplasm. Fluorescence intensities of the selected nucleus and cytoplasm areas were determined and exported to a Microsoft Excel file using the module “Measure Object Intensity.” Finally, “Mean Intensity Data” was used to calculate the nucleus/cytoplasm ratio. Results were then expressed in a graph using InfoStat software [43].
The “Coloc” module of the Zeiss Zen Black edition 2012 was used to estimate colocalization. The cellular areas were selected manually for this purpose, and the colocalization coefficients were used for data analysis. The colocalization analysis in this software is done on a pixel-by-pixel basis, and thresholds (called as crosshairs in this software) were set according to the guidance given in the “Acquiring and Analyzing Data for Co-localization Experiments in AIM or ZEN Software” manual.
F. Statistical Methods
Statistical analysis was performed using InfoStat [43] software. Two-way ANOVA and a Newman–Keuls post hoc test were used to determine whether there was any significant difference between the nucleus/cytoplasm ratios in normal and PCOS theca cells, with/without the forskolin treatment.
2. Results
A. Specificity of the Polyclonal Anti-DENND1A.V2 Antibody for IF Studies
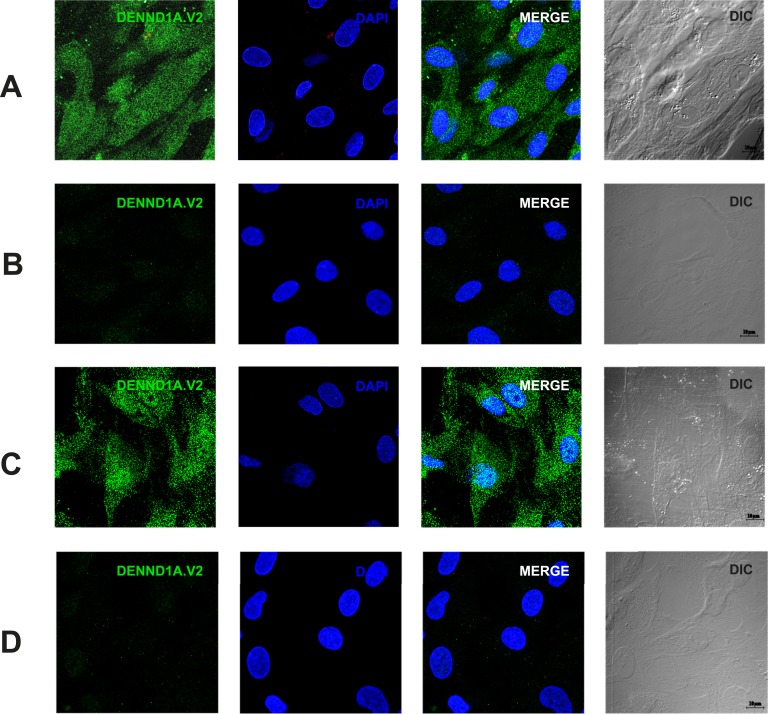
We previously described the specificity of the polyclonal antibody generated against the unique C-terminal peptide of DENND1A.V2 for western blotting [16] (also see the online repository [44]). This antibody did not react with DENND1A.V1 in western blotting. The localization of DENND1A.V2 by IF was examined in CHO cells transfected with a plasmid expressing human DENND1A.V2. As shown in an online repository [45], DENND1A.V2 was localized in the cytoplasm of transfected cells, but no signal was detected in nontransfected or empty vector–transfected cells. The anti-DENND1A.V2 signal was completely suppressed when IF was carried out with peptide-neutralized antibody [45].
IF of normal human theca cells and PCOS theca cells revealed DENND1A.V2 present in a punctate pattern in the cytoplasm and the nucleus. The IF signals were completely suppressed when studies were carried out with neutralized antibody at peptide concentrations of 10 μg/mL and 1 μg/mL. The significant nuclear localization of DENND1A.V2 in human theca cells (Fig. 2A and 2B) differs from the pattern seen in transfected CHO cells [45].
Figure 2.
Characterization of the polyclonal antibody used to detect DENND1A.V2. (A) IF detection of DENND1A.V2 in normal theca cells. (B) Absence of signal when antibody was neutralized with immunogenic peptide. (C) IF detection of DENND1A.V2 in PCOS theca cells. (D) Absence of signal when antibody was neutralized with immunogenic peptide. Scale bars, 10 μm. Representative images from three different experiments using different cell lines are shown. 3D, three-dimensional.
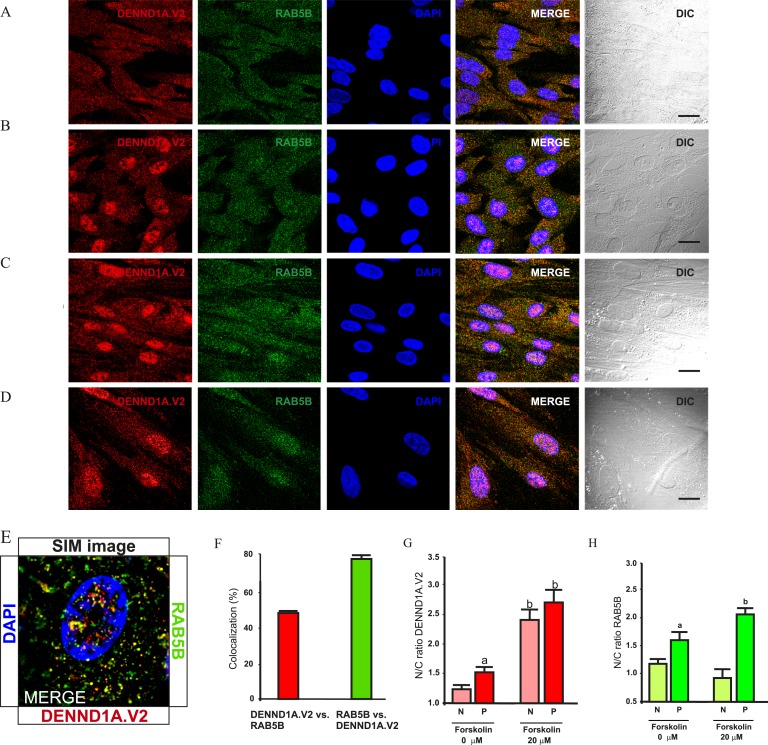
B. Colocalization of DENND1A.V2 and RAB5B in Human Theca Cells
As noted previously, DENND1A.V2 retains the guanine nucleotide exchange factor domain, and because RAB5B was identified as a PCOS GWAS candidate, studies were performed to determine whether DENND1A.V2 colocalizes with RAB5B in normal and/or PCOS theca cells. Figure 3A and 3C show that some DENND1A.V2 colocalizes with RAB5B in both normal and PCOS theca cells. High-resolution images were captured and analyzed with an N-SIM microscope. Figure 3E shows colocalization of DENND1A.V2 and RAB5B in vesicle-like structures, with a high colocalization coefficient of ∼80% for RAB5B and ∼50% for DENND1A.V2 (Fig. 3F).
Figure 3.
DENND1A.V2 colocalizes with the endosome protein RAB5B in normal and PCOS theca cells. (A) Normal untreated theca cells. (B) Normal theca cells treated with 20 μM forskolin. (C) Untreated PCOS theca cells. (D) PCOS theca cells treated with 20 μM forskolin. Scale bars, 10 μm. (E) Representative image taken using a SIM microscope, showing colocalization at higher magnification. (F) Graph showing percentage colocalization between DENND1A.V2 and RAB5B. (G) Quantification of nucleus/cytoplasm ratio for DENND1A.V2 expression. Bars with a letter are significantly different from unlettered bars and bars with a different letter (two-way ANOVA and a Newman–Keuls post hoc test). aStatistically significant difference between PCOS theca cells (P) vs normal theca cells (N) without forskolin treatment (P < 0.05). bStatistically significant difference between 0 μM vs 20 μM forskolin treatment (P < 0.05). (H) Quantification of nucleus/cytoplasm ratio for RAB5B expression. aStatistically significant difference between P vs N without forskolin treatment (P < 0.05). bStatistically significant difference between 0 μM vs 20 μM forskolin treatment (P < 0.05). Representative images from three different experiments using different theca cell preparations are shown. Data are presented as means ± SE.
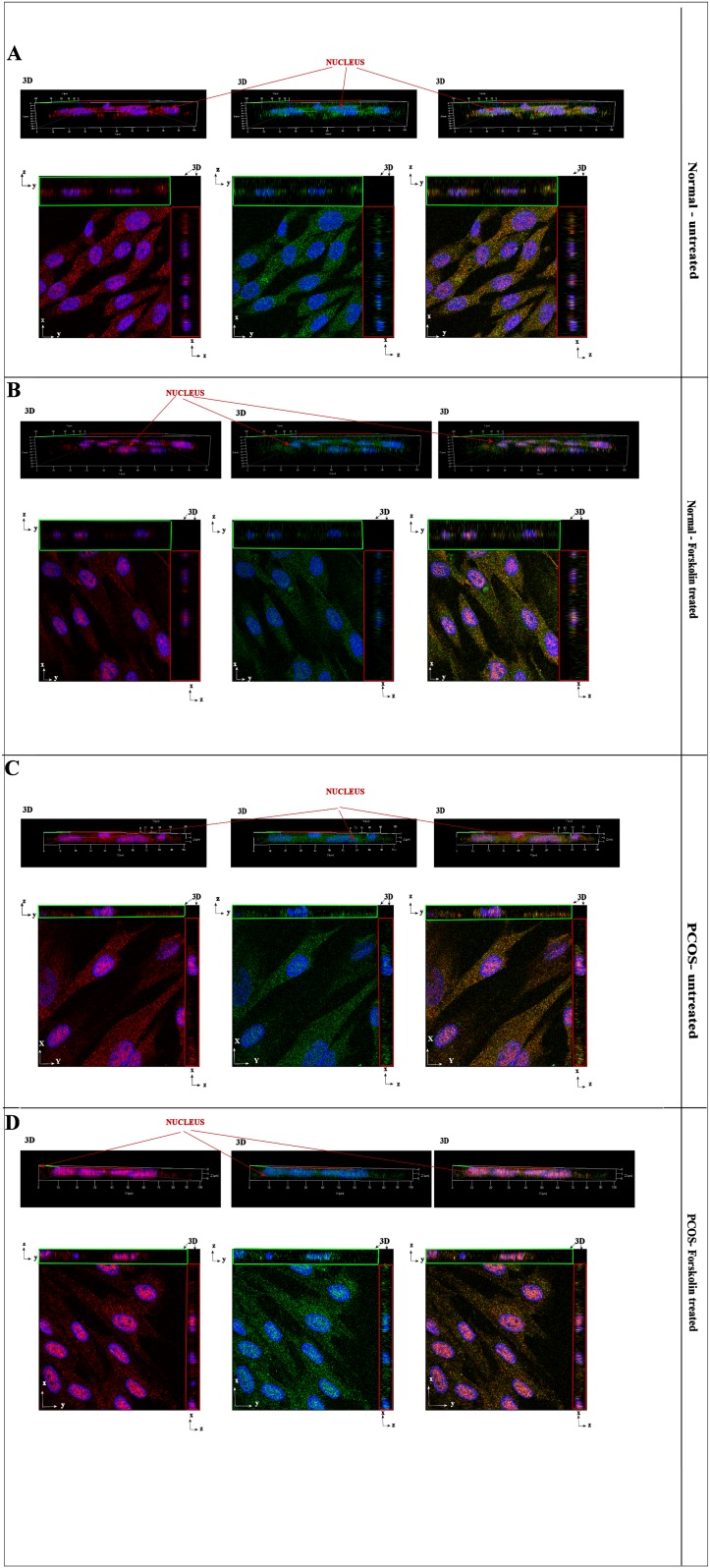
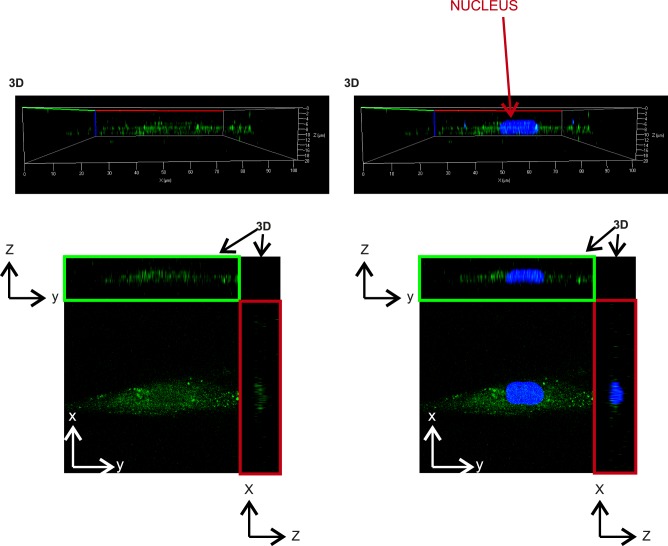
Figure 4 shows the three-dimensional and orthogonal images of theca cells stained using anti-DENND1A.V2 polyclonal antibody (in red) and goat polyclonal anti-RAB5B (in green). Greater nuclear staining for DENND1A.V2 and RAB5B was detected in PCOS theca cells as compared with normal theca cells, as indicated by the orthogonal z-stack (Fig. 4).
Figure 4.
3D reconstruction image showing colocalization of DENND1A.V2 and RAB5B in the cytoplasm as well as the nucleus of normal and PCOS theca cells. Top panels show 3D side view and bottom panels show 2D front and z-stack view for x and y coordinates. (A) Normal untreated cells. (B) Normal forskolin-treated cells. (C) PCOS theca cells. (D) Forskolin-treated PCOS cells. DENND1A.V2 is labeled in red and RAB5B is labeled in green. Blue represents nuclear staining with 4′,6-diamidino-2-phenylindole. Representative images from three experiments using different normal and PCOS theca cell preparations are shown. 2D, two-dimensional; 3D, three-dimensional.
C. The Effect of hCG Treatment on DENND1A.V2, RAB5B, and LHCGR Localization in Normal and PCOS Theca Cells
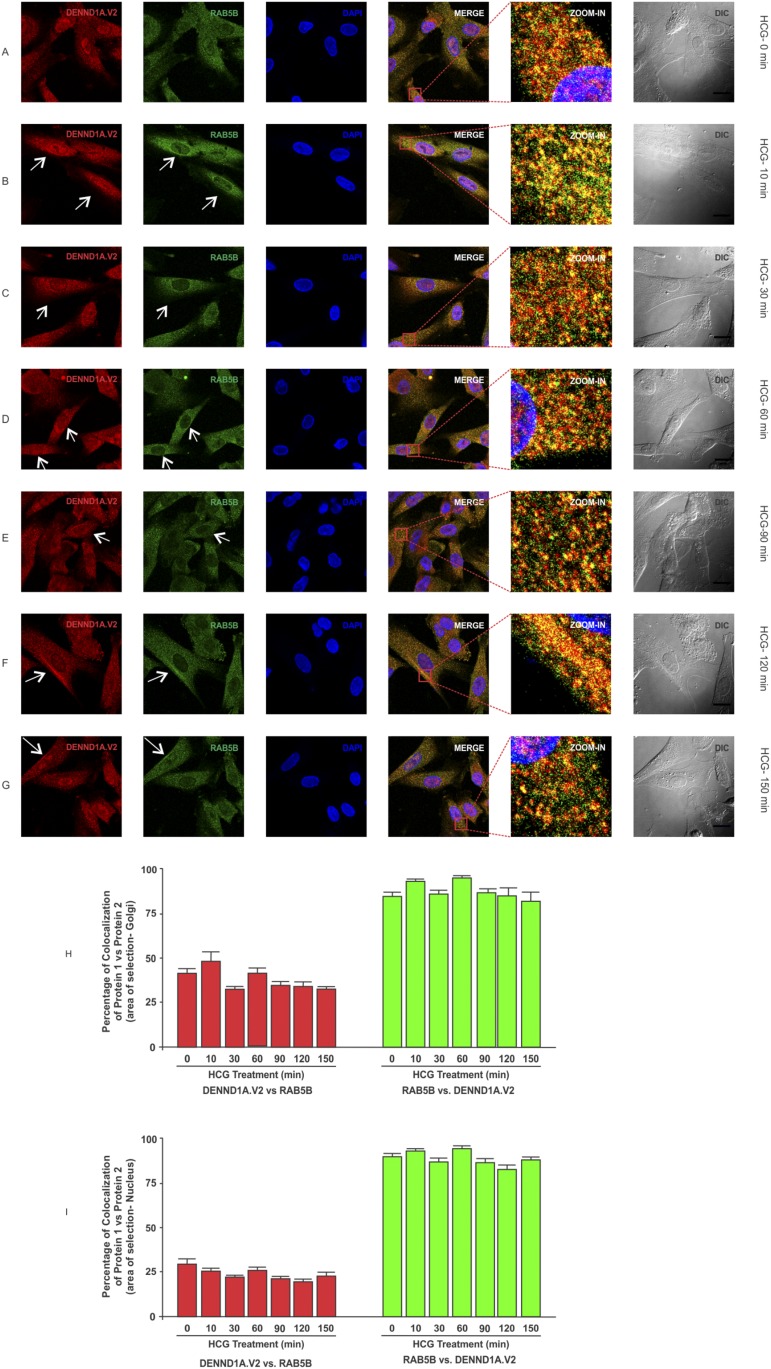
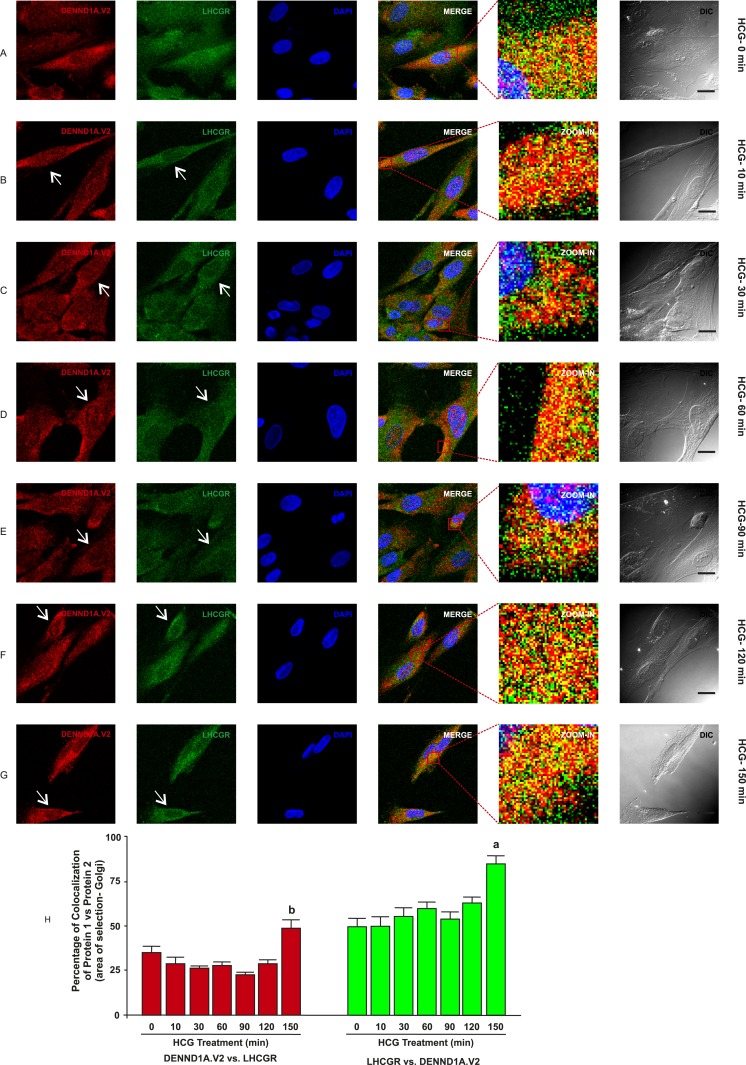
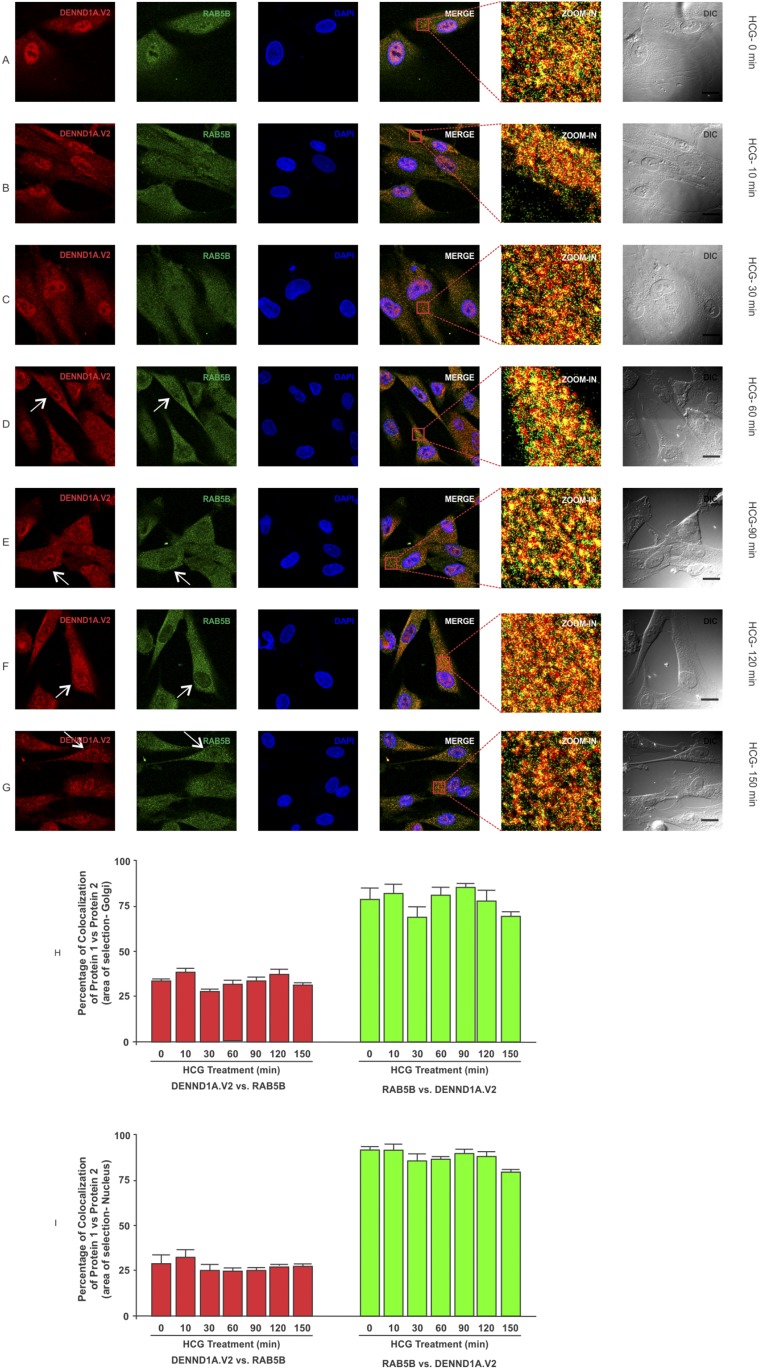
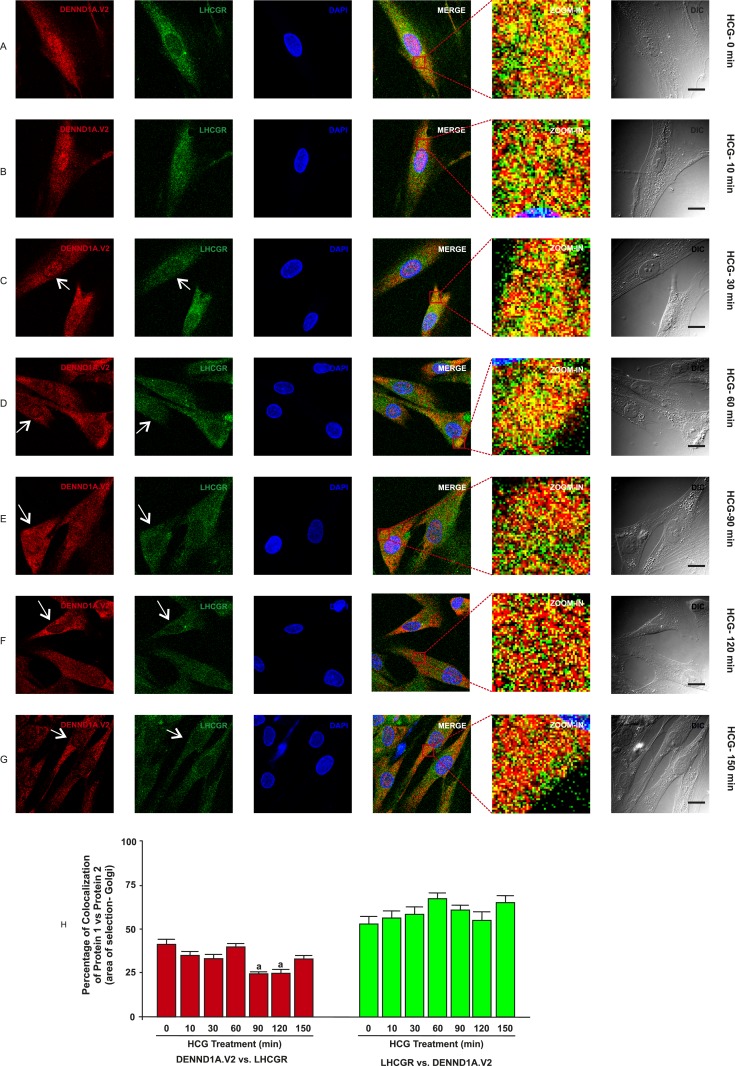
To determine whether there was any change in localization of DENND1A.V2, RAB5B and LHCGR in normal and PCOS theca cells treated with hCG at various time points, normal and PCOS theca cells were treated with hCG at a concentration of 1 IU/mL for 0, 10, 30, 60, 90, 120, and 150 minutes and were fixed at the appropriate time points. In normal theca cells, we observed that DENND1A.V2 and RAB5B colocalize, with relatively more of the RAB5B colocalizing with DENND1A.V2, but the percentage of colocalization was unaffected in response to hCG treatment (Fig. 5H and 5J). However, DENND1A.V2 and LHCGR showed increases in the percentage of colocalization at 150 minutes of hCG treatment (Fig. 6H). Additionally, hCG treatment had an effect on translocation of DENND1A.V2, RAB5B, and LHCGR towards the Golgi area, with increasing time of hCG treatment (indicated by arrows in Figs. 5 and 7) [46, 47]. Similar results were observed with PCOS theca cells; however, DENND1A.V2, RAB5B, and LHCGR movement was delayed to a later time point than that observed in normal theca cells (Figs. 6 and 8) [48, 49]. The percentage of colocalization of DENND1A.V2 and LHCGR was slightly but significantly lower at 90 and 120 minutes of hCG treatment (Fig. 8H), perhaps reflecting different kinetics of protein/vesicle compartment movement.
Figure 5.
Time course of hCG treatment of normal theca cells on the localization of DENND1A.V2 and RAB5B. Cells were treated with 1 IU/mL hCG for the time points of (A) 0 min, (B) 10 min, (C) 30 min, (D) 60 min, (E) 90 min, (F) 120 min, and (G) 150 min. White arrows point to perinuclear areas. Scale bars, 10 μm. (H) Graph showing high colocalization coefficient of ∼90% for RAB5B with DENND1A.V2 and ∼45% for DENND1A.V2 with RAB5B in the Golgi area. (I) Graph showing high colocalization coefficient of ∼90% for RAB5B with DENND1A.V2 and ∼30% for DENND1A.V2 with RAB5B in the nucleus. Representative images from three experiments using three different normal theca cell preparations are shown.
Figure 6.
Time course of hCG treatment of normal theca cells on localization of DENND1A.V2 and LHCGR. Cells were treated with 1 IU/mL hCG for the time points of (A) 0 min, (B) 10 min, (C) 30 min, (D) 60 min, (E) 90 min, (F) 120 min, and (G) 150 min. White arrows point to perinuclear areas. Scale bars, 10 μm. (H) Graph showing high colocalization coefficient of ∼70% for LHCGR with DENND1A.V2 and ∼35% for DENND1A.V2 with RAB5B in the perinuclear area. Representative images from three experiments using different theca cell preparations are shown. Letters indicate significant difference (P < 0.05) from unlettered bars (one-way ANOVA and Newman–Keuls post hoc test). Data are presented as means ± SE.
Figure 7.
Time course of hCG treatment of PCOS theca cells and the localization of DENND1A.V2 and RAB5B. Cells were treated with 1 IU/mL hCG for the time points of (A) 0 min, (B) 10 min, (C) 30 min, (D) 60 min, (E) 90 min, (F) 120 min, and (G) 150 min. Increasing concentrations of hCG drive DENND1A.V2 and RAB5B toward the Golgi area (white arrows), but at a later time point than that with normal theca cells. Scale bars, 10 μm. (H) Graph showing high colocalization coefficient of ∼80% for RAB5B with DENND1A.V2 and ∼30% for DENND1A.V2 with RAB5B in Golgi area. (I) Graph showing high colocalization coefficients of ∼80% for RAB5B with DENND1A.V2 and ∼25% for DENND1A.V2 with RAB5B in the nucleus. Representative images from three experiments using three different PCOS theca cell preparations are shown. Data are presented as means ± SE.
Figure 8.
Time course of hCG treatment of PCOS theca cells and the localization of DENND1A.V2 and LHCGR. Cells were treated with 1 IU/mL hCG for the time points of (A) 0 min, (B) 10 min, (C) 30 min, (D) 60 min, (E) 90 min, (F) 120 min, and (G) 150 min. Increasing concentrations of hCG drive DENND1A.V2 and LHCGR movement toward the Golgi area (white arrows), but at a later time point than that with normal theca cells. Scale bars, 10 μm. (H) Graph showing high colocalization coefficients of ∼70% for LHCGR with DENND1A.V2 and ∼40% for DENND1A.V2 with RAB5B in the perinuclear area. Representative images from three experiments using three different theca cell preparations are shown. Data are presented as means ± SE.
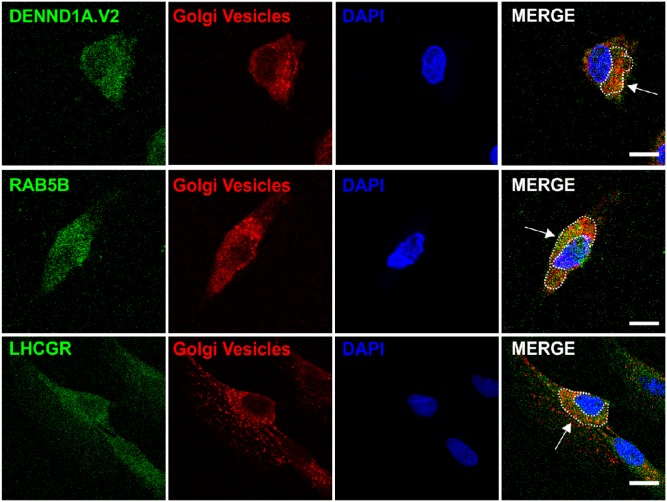
Analysis of z-stacks was performed to determine whether LHCGR entered into the nuclei of human theca cells, but no nuclear receptor was detected (Fig. 9). To verify that DENND1A.V2, RAB5B, and LHCGR were localizing in the Golgi, cells were stained with Cy3-labeled lectin specific for Golgi and Golgi vesicles. Figure 10 shows some colocalization of all three proteins with a lectin Golgi vesicle marker.
Figure 9.
Representative 3D reconstruction image showing that LHCGR does not enter into the nucleus. Top panels show 3D side view, and bottom panels show 2D front and z-stack view for x and y coordinates for untreated PCOS thecal cells. 2D, two-dimensional; 3D, three-dimensional.
Figure 10.
Representative images of DENND1A.V2, RAB5B, and LHCGR localization in Golgi vesicles. Top panel: DENND1A.V2 and Golgi vesicles, identified by staining with Cy3-labeled Galanthus nivalis lectin/G. nivalis agglutinin. Middle panel: RAB5B and Golgi vesicles. Bottom panel: LHCGR and Golgi vesicles. Scale bars, 10 μm.
D. Effect of Forskolin on Localization of DENND1A.V2 and RAB5B in Human Theca Cells
The hCG-triggered movement of DENND1A.V2 and the increased localization of DENND1A.V2 in the nuclei of PCOS theca cells raised the possibility that the nuclear accumulation of DENND1A.V2 might be the result of altered signal transduction involving cAMP, which is known to stimulate thecal androgen production. To evaluate this possibility, normal and PCOS theca cells were treated with or without 20 μM forskolin, an activator of adenyl cyclase, for 24 hours prior to fixation to determine whether there is a difference in localization of DENND1A.V2 and RAB5B (Fig. 3B and 3D). We previously showed that forskolin treatment increases theca cell androgen biosynthesis and expression of genes encoding enzymes involved in androgen production [16]. In the presence of forskolin, the PCOS theca cells showed significantly more nuclear accumulation of DENND1A.V2 and RAB5B (higher nucleus/cytoplasm ratio) (Fig. 3G and 3H). However, in normal theca cells increased nuclear accumulation was observed only for DENND1A.V2 after forskolin treatment.
3. Discussion
During the past decade convincing evidence has accumulated regarding genetic factors that contribute to PCOS. The studies of genetic and molecular factors involved in the pathophysiology of the PCOS accelerated after milestone GWAS identified PCOS candidate loci, including those near DENND1A, INSR, YAP1, C9orf3, RAB5B, HMGA2, TOX3, SUMO1P1/ZNF217, THADA, and LHCGR [6–10]. In our previous studies, we established that a truncated splice variant of PCOS GWAS candidate gene, DENND1A, termed DENND1A.V2, showed increased expression in PCOS theca cells [16–18]. DENND1A has been described as a clathrin-binding protein that most likely is related to gonadotropin receptor signaling as part of the clustering and endocytosis of plasma membrane receptors via the involvement of RAB5 proteins [50, 51].
The functional importance of DENND1A.V2 in the PCOS phenotype of human theca cells was established by our previously published studies demonstrating that increased DENND1A.V2 stimulates androgen synthesis by normal theca cells and that knockdown of DENND1A.V2 diminishes androgen production in PCOS theca cells [16]. Although these studies provided evidence for a direct role for DENND1A.V2 in production of the ovarian PCOS phenotype, the mechanisms of DENND1A.V2 action and the relationship of DENND1A.V2 to other PCOS candidate genes were unknown. Moreover, little was known about DENND1A.V2 and its cellular location in normal and PCOS theca cells until the present studies.
Based on the available information on the function of DENND1A, its clathrin-binding domain, the role of the DENN domain as a guanine nucleotide exchange factor, the critical role of LH action in theca cell androgen production, as well as the existing literature on the role of RAB5 proteins in gonadotropin receptor function, we proposed that LHCGR, DENND1A.V2, and RAB5B participate in a network to create the ovarian theca cell PCOS phenotype [20]. Consistent with this hypothesis, to our knowledge, we have provided the first cytological evidence to demonstrate interactions of cellular compartments containing LHCGR, DENND1A.V2, and RAB5B using transfected CHO cells as well as well-characterized cultured theca cells obtained from the ovaries of normal cycling women and women with PCOS. Based on these combined observations, we have demonstrated that LHCGR and DENND1A.V2 are colocalized on the plasma membrane; and that DENND1A.V2 is also colocalized with RAB5B in endocytic vesicles and perinuclear area. Furthermore, following hCG/forskolin stimulation, DENND1A.V2 translocates to the perinuclear region and nucleus to a larger extent in PCOS theca cells as compared with normal cells, and may subsequently mediate increased expression of CYP17A1 and CYP11A, leading to hyperandrogenism in PCOS theca cells. Our studies also suggest that DENND1A.V2 and RAB5B localize to endocytotic compartments in the cytoplasm, and particularly the perinuclear region (in some cases colocalized with Golgi vesicles), where they may mediate changes in signaling in PCOS theca cells that may contribute to alterations in gene expression. Importantly, note that although our IF studies in human theca cells document overlapping signals from the specific antibodies, which strongly suggests close geographical proximity, IF cannot establish direct physical interaction of these proteins.
The IF studies conducted on transfected CHO cells helped establish the specificity of the DENND1A.V2 antibody, but they yielded results that were not unexpectedly different from those obtained from the study of human theca cells, although they did support the colocalization of DENND1A.V2 and LHCGR in some compartments. There were significant differences in the extent of colocalization of LHCGR and DENND1A.V2 and nuclear accumulation of DENND1A.V2. This is not surprising because CHO cells, although derived from ovaries, are not steroidogenic. The study of overexpressed human proteins, including one with a tag (LHCGR tagged with green fluorescent protein and myc) in a hamster-derived cell line may have also significantly affected the patterns of protein localization. These are major limitations of the CHO cell model system we used, as compared with the study of endogenous proteins in their physiological and pathophysiological context in the human theca cell system.
It was not possible for us to examine the localization of DENND1A.V1 and DENND1A.V2 simultaneously in human theca cells because the only available antibodies that specifically react with DENND1A.V1 have been generated in rabbits, precluding localization studies with our rabbit anti-DENNND1A.V2 antibody. Although we used a commercial antibody that recognized DENND1A.V1, but not DENND1A.V2, in western blotting of extracts of CHO cells transfected with plasmids expressing the two isoforms (data not shown), this antibody gave a pattern of staining identical to actin stress fibers in IF studies. The staining pattern overlapped with phalloidin staining, suggesting that it was not detecting DENND1A.V1 (data not shown).
Relatively little is known about the role of RAB5B in gonadotropin receptor trafficking, as most studies have focused on the highly related RAB5A protein, which is thought to participate in both FSHR and LHCGR endocytosis and signaling [50]. However, it is known that RAB5B is involved in phosphatidylinositol 3-kinase, protein kinase B/AKT, and MAPK/ERK signaling pathways, which could play important roles in LHCGR stimulation of steroidogenesis [19].
The most unexpected observation was the detection of DENND1A.V2 in the nuclei of theca cells, with PCOS theca cells showing a greater nuclear accumulation than normal theca cells. The nuclear translocation of DENND1A.V2 was stimulated by forskolin, suggesting that the translocation process is cAMP-dependent. RAB5B was colocalized with DENND1A.V2 in the nucleus of PCOS theca cells, with greater accumulation in the nuclei of forskolin-stimulated cells. It is noteworthy that in our earlier studies using immunoperoxidase staining of sections of normal and PCOS ovaries we detected both cytoplasmic and nuclear DENND1A.V2 staining, which is consistent with our IF studies on cultured theca cells [16]. Thus, the phenotype of PCOS theca cells includes increased DENND1A.V2 protein levels and greater DENND1A.V2 nuclear accumulation. Note that although DENND1A.V1 localization has been studied, we could not find evidence that this isoform enters the nucleus.
The mechanism underlying the translocation of DENND1A.V2 and RAB5B into the nucleus remains to be elucidated. Whether DENND1A.V2 and Rab5B enter the nucleus together or as individual components of endocytic vesicles that fuse with the nuclear membrane, or as nonvesicular proteins, is currently unknown. The latter seems unlikely because neither DENND1A.V2 or RAB5B have known nuclear localization signals.
The entry of DENND1A.V2 and RAB5B into the nucleus suggests that these proteins may participate in the regulation of steroidogenic enzyme genes beyond their roles in the plasma membrane and cytoplasmic endocytic vesicles, perhaps in part explaining the increased transcription of CYP11A1 and CYP17A1 associated with the elevated level of DENND1A.V2 protein in PCOS theca cells and the effects of forced DENND1A.V2 expression in normal theca cells. Future studies should explore the molecules that interact with DENND1A.V2 and RAB5B in the nucleus of PCOS theca cells and their relationship to chromatin. One possible mechanism of gene regulation involves APPL1, a multifunctional endosomal adaptor protein that interacts with RAB5 molecules. APPL1 is translocated into the nucleus, where it has been shown to modulate histone deacetylases and participate in nucleosome remodeling and gene transcription [52].
Our discovery that DENND1A.V2 and RAB5B both enter the nucleus, with more prominent nuclear localization in PCOS theca cells, raises the possibility that LHCGR signaling also occurs at multiple locations in theca cells and that quantitative differences in DENND1A.V2 and its nuclear translocation explain phenotypic differences between normal and PCOS theca cells. These findings also raise many unanswered questions regarding the translocation, including the nature of the nuclear localization signal and how the movement of DENND1A.V2 is increased by cAMP.
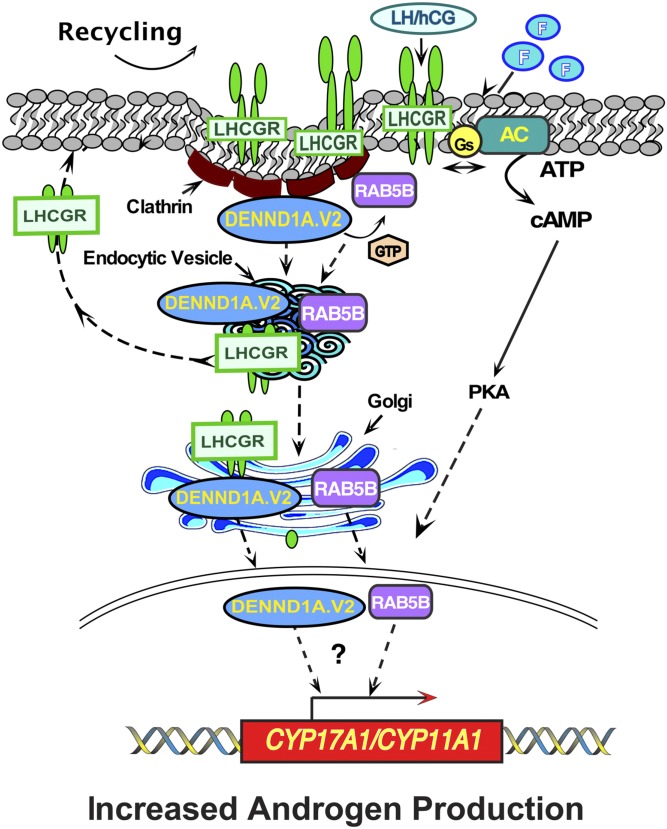
In summary, we have identified a network involving genes encoded by three different loci associated with PCOS. Figure 11 presents a schematic of how these three proteins and/or their compartments might be interacting with each other. This schematic is based on the present results as well as published work of others on LHCGR recycling. Note that this scheme is based on our “snapshot” images and not real-time movements. Therefore, we cannot provide details regarding the specific itineraries that each protein follows. The proteins studied undoubtedly traffic bidirectionally, and the scheme in Fig. 11 focuses only on the putative itinerary from the plasma membrane to the nucleus.
Figure 11.
Schematic showing the proposed dynamic changes in the localization of LHCGR, DENND1A.V2, and RAB5B in various cellular compartments. LH/hCG binding to the LH receptor (LHCGR), or forskolin stimulation (F), is predicted to promote the interaction between the guanine nucleotide exchange factor DENND1A.V2, in clathrin-coated pits, with the GTPase RAB5B. LHCGR, DENND1A.V2, and RAB5B move into early endocytic vesicles. LHCGR is subsequently recycled to the plasma membrane from the endocytic vesicles. Some LHCGR, DENND1A.V2, and RAB5B move to the Golgi, and some DENND1A.V2 and RAB5B, but not LHCGR, enter into the nucleus, where they could participate in regulating transcription of CY17A1 and CYP11A1, along with the cAMP signaling pathway, augmenting androgen production.
DENND1A.V2 and RAB5B are both involved in the early endocytic pathways. Thus, their proximity to each other, followed by the observation of their apparent movement into the nucleus after treatment by forskolin, suggests that they may be working together to increase the androgen production inside the cells. LHCGR, which is a receptor for both LH and hCG and is known to activate the cAMP pathway, increases androgen production in cells in the presence of hCG. The postulated network involves proteins including LHCGR, DENND1A.V2, and RAB5B that are plausible determinants of the theca cell phenotype of excess androgen production. We focused our attention on LHCGR in this study, and it remains to be determined whether DENND1A.V2 and RAB5B play roles in signaling though other plasma membrane receptors, including FSHR and INSR, which have also been implicated in GWAS and other genetic studies to be involved in the pathophysiology of PCOS. Additional information is available in an online repository [53–59].
Acknowledgments
The authors thank Dr. Deborah Segaloff for the gift of pGFP2-N2-myc-hLHCGR, and Jamaia Marks for assistance with the signaling schematic.
Financial Support: This work was primarily funded by National Institutes of Health (NIH) Grants U54HD3449 (to J.F.S. and J.M.M.), R01HD033852 (to J.M.M.), R01HD058300 (to J.M.M.), and R01HD083323 (to J.M.M. and J.F.S.). Microscopy was performed at the Virginia Commonwealth University Department of Anatomy and Neurobiology Microscopy Facility, supported in part by funding from the NIH/National Institute of Neurological Disorders and Stroke Center Core Grant 5 P30 NS047463 and in part by funding from the NIH/National Cancer Institute Cancer Center Support Grant P30 CA016059.
Glossary
Abbreviations:
- CHO
Chinese hamster ovary
- DENN
differentially expressed in normal and neoplastic cells
- GWAS
genome-wide association study
- hCG
human chorionic gonadotropin
- IF
immunofluorescence
- PCOS
polycystic ovary syndrome
Additional Information
Disclosure Summary: J.M.M. and J.F.S. jointly hold patents dealing with the diagnosis and treatment of PCOS based on DENND1A.V2. Some of these patents are licensed by Prescient Medicine. J.F.S. is a scientific advisory board member to Prescient Medicine, a diagnostic company. The remaining authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Rodgers R, Avery J, Moore V, Davies M, Azziz R, Stener-Victorin E, Moran L, Robertson S, Stepto N, Norman R, Teede HJ. Complex diseases and co-morbidities: polycystic ovary syndrome and type 2 diabetes mellitus. Endocr Connect. 2019;8(3):R71–R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110(5):794–809. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):16057. [DOI] [PubMed] [Google Scholar]
- 4. Legro RS, Driscoll D, Strauss JF III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95(25):14956–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. [DOI] [PubMed] [Google Scholar]
- 6. Liu H, Zhao H, Chen ZJ. Genome-wide association studies for polycystic ovary syndrome. Semin Reprod Med. 2016;34(4):224–229. [DOI] [PubMed] [Google Scholar]
- 7. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–59. [DOI] [PubMed] [Google Scholar]
- 8. Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Zhao J, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li W, Li B, Wan C, Qin Y, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang Y, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–1025. [DOI] [PubMed] [Google Scholar]
- 9. Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations [published correction appears in Nat Commun. 2016;7:10762]. Nat Commun. 2015;6(1):7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Hu LM, Wang YF, Yang HY, Huang XY, Zhou W, Sun HX. Genome-wide association study for SNPs associated with PCOS in human patients. Exp Ther Med. 2017;14(5):4896–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, Krauss RM, Rotter JI, Ankener W, Legro RS, Azziz R, Strauss JF III, Dunaif A, Urbanek M. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012;49(2):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louwers YV, Stolk L, Uitterlinden AG, Laven JSE. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(12):E2006–E2012. [DOI] [PubMed] [Google Scholar]
- 13. Marat AL, McPherson PS. The connecdenn family, Rab35 guanine nucleotide exchange factors interfacing with the clathrin machinery. J Biol Chem. 2010;285(14):10627–10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kulasekaran G, Nossova N, Marat AL, Lund I, Cremer C, Ioannou MS, McPherson PS. Phosphorylation-dependent regulation of connecdenn/DENND1 guanine nucleotide exchange factors. J Biol Chem. 2015;290(29):17999–18008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye B, Duan B, Deng W, Wang Y, Chen Y, Cui J, Sun S, Zhang Y, Du J, Gu L, Lin L, Tang Y. EGF stimulates Rab35 activation and gastric cancer cell migration by regulating DENND1A-Grb2 complex formation. Front Pharmacol. 2018;9:1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF III. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA. 2014;111(15):E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tee MK, Speek M, Legeza B, Modi B, Teves ME, McAllister JM, Strauss JF III, Miller WL. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol Cell Endocrinol. 2016;434:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McAllister JM, Han AX, Modi BP, Teves ME, Mavodza GR, Anderson ZL, Shen T, Christenson LK, Archer KJ, Strauss JF. miRNA profiling reveals miRNA-130b-3p mediates DENND1A variant 2 expression and androgen biosynthesis. Endocrinology. 2019;160(8):1964–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson DB, Wilson MP. Identification and subcellular localization of human rab5b, a new member of the ras-related superfamily of GTPases. J Clin Invest. 1992;89(3):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McAllister JM, Legro RS, Modi BP, Strauss JF III. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26(3):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang M, Guan R, Segaloff DL. Revisiting and questioning functional rescue between dimerized LH receptor mutants. Mol Endocrinol. 2012;26(4):655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickenheisser JK, Biegler JM, Nelson-Degrave VL, Legro RS, Strauss JF III, McAllister JM. Cholesterol side-chain cleavage gene expression in theca cells: augmented transcriptional regulation and mRNA stability in polycystic ovary syndrome. PLoS One. 2012;7(11):e48963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF III, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19(2):379–390. [DOI] [PubMed] [Google Scholar]
- 24. Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 25. Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925–5933. [DOI] [PubMed] [Google Scholar]
- 26. Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF III, McAllister JM. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304–2311. [DOI] [PubMed] [Google Scholar]
- 27. Strauss JF., III Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome. Ann N Y Acad Sci. 2003;997:42–48. [DOI] [PubMed] [Google Scholar]
- 28. Wickenheisser JK, Nelson-DeGrave VL, Quinn PG, McAllister JM. Increased cytochrome P450 17α-hydroxylase promoter function in theca cells isolated from patients with polycystic ovary syndrome involves nuclear factor-1. Mol Endocrinol. 2004;18(3):588–605. [DOI] [PubMed] [Google Scholar]
- 29. Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM, Strauss JF III. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol. 2004;63(1):51–60. [DOI] [PubMed] [Google Scholar]
- 30. Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss JF III. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278(29):26380–26390. [DOI] [PubMed] [Google Scholar]
- 31. Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, Wood JR, Legro RS, Strauss JF III, McAllister JM. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology. 2004;145(2):799–808. [DOI] [PubMed] [Google Scholar]
- 32. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bani Mohammad M, Majdi Seghinsara A. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pac J Cancer Prev. 2017;18(1):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. RRID:AB_2801335, https://scicrunch.org/resolver/AB_2801335.
- 35. RRID:AB_2783818, https://scicrunch.org/resolver/AB_2783818.
- 36. RRID:AB_2783817, https://scicrunch.org/resolver/AB_2783817.
- 37. RRID:AB_2340851, https://scicrunch.org/resolver/AB_2340851.
- 38. RRID:AB_2338006, https://scicrunch.org/resolver/AB_2338006.
- 39. RRID:AB_2340619, https://scicrunch.org/resolver/AB_2340619.
- 40. RRID:AB_2336933, https://scicrunch.org/resolver/AB_2336933.
- 41. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DiRienzo JA, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat versión 2018. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Córdoba, Argentina. Available at: http://www.infostat.com.ar.
- 44. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/671d296ef60e01c221fb. [DOI] [PMC free article] [PubMed]
- 45. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/83b0ef5f014a108e0d65. [DOI] [PMC free article] [PubMed]
- 46. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/5f82ff51e80c2c91bdfa. [DOI] [PMC free article] [PubMed]
- 47. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/0b8c228b464bbacf5cc5. [DOI] [PMC free article] [PubMed]
- 48. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/1200283a08944d49114d. [DOI] [PMC free article] [PubMed]
- 49. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/c4048b357d2b59083313. [DOI] [PMC free article] [PubMed]
- 50. Gulappa T, Clouser CL, Menon KMJ. The role of Rab5a GTPase in endocytosis and post-endocytic trafficking of the hCG-human luteinizing hormone receptor complex. Cell Mol Life Sci. 2011;68(16):2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jean-Alphonse F, Bowersox S, Chen S, Beard G, Puthenveedu MA, Hanyaloglu AC. Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments. J Biol Chem. 2014;289(7):3960–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rashid S, Pilecka I, Torun A, Olchowik M, Bielinska B, Miaczynska M. Endosomal adaptor proteins APPL1 and APPL2 are novel activators of beta-catenin/TCF-mediated transcription. J Biol Chem. 2009;284(27):18115–18128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/378d3e8a69a7b51cdae4. [DOI] [PMC free article] [PubMed]
- 54. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/1f8230630042c9934b30. [DOI] [PMC free article] [PubMed]
- 55. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/538875005aa443a972d6. [DOI] [PMC free article] [PubMed]
- 56. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/b7dcbd3df7142426ca13. [DOI] [PMC free article] [PubMed]
- 57. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/e003a0385afa4df3b890. [DOI] [PMC free article] [PubMed]
- 58. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/092e4bd25d9a88e65ccf. [DOI] [PMC free article] [PubMed]
- 59. Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JM III. Data from: Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. figshare 2019. Deposited 21 August 2019. https://figshare.com/s/b196b93ae52b1c3c5050. [DOI] [PMC free article] [PubMed]