Abstract
Many studies use the global clinical dementia rating (CDR) of 0.5 as a criterion for mild cognitive impairment, but past studies have not fully discussed its validity. The authors developed the ABC Dementia Scale (ABC-DS) to accurately monitor the changes in activities for daily living, behavioral and psychological symptoms of dementia, and cognitive function. When we carried out a cluster analysis of ABC-DS scores of 110 individuals for whom global CDR was 0.5, there were three groups with different levels of activities for daily living and cognitive function. O’Bryant et al. proposed a new guideline to stage dementia using the CDR sum of boxes scores (CDR-SOB). We used their proposal and ABC-DS scores to evaluate the validity of CDR 0.5 as a definition of mild cognitive impairment (MCI). We concluded that the CDR-SOB scores and ABC-DS score are more accurate than global CDR of 0.5 for specifying individuals with MCI.
Keywords: ABC dementia scale, clinical dementia rating, cluster analysis, mild cognitive impairment, three-dimensional distance
INTRODUCTION
There is no reliable diagnostic scale to confirm the presence of mild cognitive impairment (MCI) in individuals. Based on the medical history provided by patients and the results of various tests, doctors decide whether MCI can most reasonably explain the cause of any symptoms individuals are experiencing. Many doctors diagnose MCI based on the following criteria: Petersen criteria [1]; Diagnostic and Statistical Manual of Mental Disorders V [2]; International Statistical Classification of Diseases-10 [3]; Clinical Dementia Rating (CDR) [4]; National Institute on Aging-Alzheimer’s Association workgroups [5]; and the International Working Group-2 [6]. Despite the different criteria available to diagnose MCI, many previous studies have used global CDR of 0.5 as a diagnostic criterion [7–10]. Duara et al. reported that the Clinical Dementia Rating Sum of Boxes (CDR-SOB) was informative for diagnosing MCI [11]. Past studies, however, have not fully discussed the statistical and clinical validities for the definition. Instead of global CDR, O’Bryant et al. proposed a new interpretive guideline to stage dementia using the CDR-SOB scores: if the range of SOB is 0.5–2.0, 2.5–4.0 or 4.5–9.0 the stage is questionable impairment, very mild dementia or mild dementia, respectively [12, 13].
The authors established a new, brief assessment scale called the ABC Dementia Scale (ABC-DS) in a clinical trial (TRIAD1412) [14, 15]. As a component of this new scale, we developed three-dimensional distance (TDD) as a novel approach for estimating the overall severity of Alzheimer’s disease (AD), considering that the scale consists of three domains: Activity for Daily Living (ADL), Behavioral and Psychological Symptoms of Dementia (BPSD), and Cognitive Function (CF) (See Supplementary Figure 1). When we plotted the three domain scores on a three-dimensional axis for individuals whose global CDR was 0.5, we recognized that the distribution of dots constituted several groups (data not shown). This finding motivated us to investigate the validity of global CDR 0.5 as a criterion of MCI.
In this study, by using ABC-DS and O’Bryant’s guidelines, we assessed the validity of the global CDR score of 0.5 as a definition of MCI. This study used the data obtained in TRIAD1412.
MATERIALS AND METHODS
Data
For the TRIAD1412 study, we recruited 312 patients who had been diagnosed with 1) AD, based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria; 2) probable AD, based on the criteria of the National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroups, or the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; or 3) MCI, based on the International Working group on MCI criteria [16] or NIA-AA diagnostic criteria [17].
In TRIAD1412, doctors diagnosed the severities of dementia by subjective diagnosis following the diagnosis criteria [16, 17], although these criteria do not define the severities. Accordingly, doctors rated the severities based on their clinical experience. The doctors’ diagnoses consisted of four ordinal categories of AD: probable MCI, and mild, moderate, and severe dementia. On the other hand, trained clinical psychologists used global CDRs 0.5, 1, 2, and 3 to rate the severities of AD. The clinical psychologists used the Japanese version of the CDR and participated in a training seminar for the standardization of the evaluation prior to the clinical trial. There were 22 doctors and 38 clinical psychologists. In this study, the psychologists did not disclose the CDR rating scores to the doctors until the completion of the study. We obtained data from 110 individuals whose global CDR was 0.5, as assessed by the psychologists.
We conducted the TRIAD1412 research following the World Medical Association Declaration of Helsinki (1964) and its amendments and subsequent clarifications. The institutional review board approved the study protocol, and all caregivers and participants provided written informed consent. We registered the clinical trial with the University Hospital Medical Information Network (http://www.umin.ac.jp/ No.: UMIN000021134).
ABC Dementia Scale
The ABC-DS consists of 13 items (Q1–Q13) and measures the current stage of three domains of dementia: ADL, BPSD, and CF. Evaluators interview care-givers and rate the scores by 9 levels from 9 to 1 or the best to the worst. We designed the scale so that we can time-sequentially monitor changes in ADL, BPSD, and CF, following the item response theory [18]. We statistically validated the reliability, construct validity, concurrent validity, responsiveness, and the item response characteristics of ABC-DS. We calculated three domain scores of ADL, BPSD, and CF that were defined by the following sums of item scores; “Q1 + Q2 + Q3 + Q4 + Q11 + Q12”, “Q7 + Q8 + Q9”, and“Q5 + Q6 + Q10 + Q13”, respectively. (See the details of the items in Supplementary Table 1) We estimated the overall severity by the sum of the 13 item scores (total score) or the TDD calculated using
ABC-DS in English, French, Chinese, and Korean languages can be downloaded from the following site under the terms and conditions specified: https://eprovide.mapi-trust.org/instruments/abc-dementia-scale. It should be noted that TDD is a patent-protected technique and requires a contract with the owner (please contact: abc_scale@tri-kobe.org).
Statistical methods
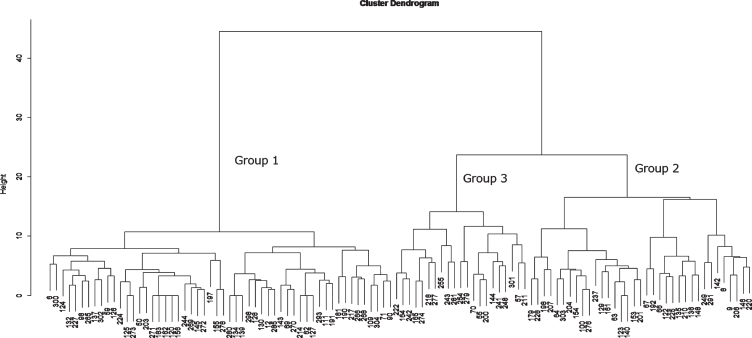
Using the 13 items of the ABC-DS, we performed a hierarchical cluster analysis that classified individuals with similar score patterns in the same group, referred to as a cluster [19]. This analysis provides a cluster dendrogram in which each cluster has a branch of a tree, each branch is distinct from the other branches, and the individuals (often called “leaves”) on each branch have broadly similar patterns of the ABC-DS scores to each other. This statistical technique is commonly used for genetic analysis in medicine. Accordingly, if some individuals have a similar pattern of the ABC-DS scores as for ADL, BPSD, and CF, they will be shown as leaves on the same branch in the cluster dendrogram. We classified the individuals whose global CDR was 0.5 by using the Ward method that connects the branches by optimizing the variance between clusters. We cut the tree at the first generation (branch) and obtained three main clusters (Fig. 1). We calculated the basic statistics of ABC-DS including minimum, first quarter, median, mean, third quarter, and maximum. These analyses were performed using the data analysis software R i386 3.5.1 [20].
Fig.1.
Cluster Dendrogram.
RESULTS
There were many discrepancies between the doctors’ empirical judgments of the severities and the global CDR scores by the clinical psychologists (Table 1). Of the 110 individuals with a global MCI of 0.5, the doctors diagnosed probable MCI in 55 (50%) patients and diagnosed 33, 21, and one patient with mild, moderate, and severe dementia, respectively. In Table 2, we provide the profiles of ABC-DS scores for the 55 individuals who were diagnosed with probable MCI by doctors. The median of the three domain scores for ADL, BPSD, and CF were 52.0, 26.0, and 30.0, respectively. The medians of the total score, TDD score, and CDR-SOB were 108.0, 65.7, and 2.0, respectively. According to O’Bryant’s guidelines, the stage of the individuals was questionable impairment.
Table 1.
Concordance between the diagnosis of doctors and the global CDR assessed by clinical psychologists with respect to the severities of dementia
| Global CDR rated by psychologists | |||||
| CDR3 | CDR2 | CDR1 | CDR0.5 | ||
| Doctors’ diagnosis | Severe | 31 | 18 | 5 | 1 |
| Moderate | 1 | 44 | 40 | 21 | |
| Mild | 0 | 3 | 51 | 33 | |
| Probable MCI | 1 | 0 | 3 | 55 | |
Table 2.
ABC-DS score profiles of individuals whom doctors diagnosed as probable MCI (N = 55)
| ADL | BPSD | Cognitive | Total | TDD | SOB | |
| Min. | 37.0 | 19.0 | 16.0 | 84.0 | 49.8 | 0.0 |
| 1st Qu. | 50.0 | 25.0 | 26.0 | 102.0 | 62.3 | 1.0 |
| Median | 52.0 | 26.0 | 30.0 | 108.0 | 65.7 | 2.0 |
| Mean | 51.2 | 25.6 | 29.2 | 106.0 | 64.4 | 2.3 |
| 3rd Qu. | 54.0 | 27.0 | 34.0 | 112.0 | 67.6 | 3.0 |
| Max. | 54.0 | 27.0 | 36.0 | 116.0 | 69.8 | 15.0 |
N, number of patients; Total, total score; TDD, three-dimensional distance score; Min., minimum; 1st Qu., first quarter; 3rd Qu., third quarter; Max., maximum; SOB, CDR sum of boxes.
When we performed a cluster analysis to classify the pattern of scores on the 13 items of ABC-DS for the 110 individuals with a CDR of 0.5 assessed by the clinical psychologists, we found a hierarchical relationship among individuals, as shown in Figure 1. We also identified three major groups in the cluster dendrogram: Group 1 (n = 54 : 49.1%), Group 2 (n = 35 : 31.8%), and Group 3. (n = 21 : 19.1%). Table 3 presents the basic statistics of ABC-DS and CDR-SOB for each group. The median of ADL decreased from Group 1 to Group 2 only and ceased the decrease in Group 3. There was no major difference in BPSD score among the groups. The median of CF score gradually decreased from Group 1 to Group 3, and the median in Group 3 became half of Group 1. The medians of TDD score in Group 1, Group 2, and Group 3 were 67.4, 61.8, and 58.2, respectively. The medians of CDR-SOB in Group 1, Group 2, and Group 3 were 2.0, 2.5 and 3.5, respectively; Group 1 corresponded to “questionable impairment”; Group 2 and Group 3 were categorized as “very mild dementia,” according to O’Bryant et al.
Table 3.
ABC-DS score profiles of the clustered groups for individuals with CDR 0.5
| Group | Statistics | ADL | BPSD | Cognitive | Total | TDD | SOB |
| 1 | Min. | 49.0 | 21.0 | 28.0 | 103.0 | 62.5 | 0.5 |
| (N = 54) | 1st Qu. | 52.0 | 25.0 | 30.0 | 109.0 | 65.8 | 1.0 |
| Median | 54.0 | 26.5 | 32.0 | 111.0 | 67.4 | 2.0 | |
| MCI *: 35 | Mean | 52.9 | 25.9 | 32.2 | 111.1 | 67.2 | 2.0 |
| (64.8%) | 3rd Qu. | 54.0 | 27.0 | 34.0 | 114.8 | 69.2 | 3.0 |
| Max. | 54.0 | 27.0 | 36.0 | 117.0 | 70.3 | 4.0 | |
| 2 | Min. | 37.0 | 19.0 | 18.0 | 85.0 | 50.7 | 1.0 |
| (N = 35) | 1st Qu. | 45.5 | 24.5 | 23.5 | 95.5 | 58.2 | 2.0 |
| Median | 50.0 | 25.0 | 24.0 | 101.0 | 61.8 | 2.5 | |
| MCI: 19 | Mean | 49.2 | 25.0 | 24.5 | 98.7 | 60.5 | 2.6 |
| (54.2%) | 3rd Qu. | 52.5 | 27.0 | 26.0 | 103.5 | 63.5 | 3.5 |
| Max. | 54.0 | 27.0 | 28.0 | 107.0 | 65.7 | 4.0 | |
| 3 | Min. | 39.0 | 19.0 | 10.0 | 76.0 | 48.5 | 3.0 |
| (N = 21) | 1st Qu. | 44.0 | 23.0 | 15.0 | 85.0 | 54.4 | 3.5 |
| Median | 50.0 | 26.0 | 16.0 | 91.0 | 58.2 | 3.5 | |
| MCI: 1 | Mean | 48.4 | 25.0 | 16.1 | 89.4 | 56.9 | 3.7 |
| (4.8%) | 3rd Qu. | 52.0 | 27.0 | 19.0 | 95.0 | 59.9 | 4.0 |
| Max. | 54.0 | 27.0 | 20.0 | 99.0 | 63.0 | 4.5 |
The median values are indicated in bold. *The number of individuals who were diagnosed as probable MCI by doctors and their proportion in the group.
The ABC-DS score profile of Group 1 was very similar to that of individuals who were diagnosed with probable MCI by the doctors. The doctors diagnosed 35 of 52 (64.8%), 19 of 35 (54.2%), and 1 of 21 (4.8%) individuals with probable MCI in Group 1, Group 2, and Group 3, respectively. The ABC-DS score profiles of 35 individuals with probable MCI in Group 1 were almost identical to those of Group 1 as a whole (data not shown). The lower and upper values of the 99% confidence interval of the mean TDD score for Group 1 were 66.5 and 67.9, respectively.
DISCUSSION
In this study, we found a discrepancy in the severities of dementia diagnosed by the doctors and the global CDR scores rated by the clinical psychologists. We also found that individuals with CDR 0.5 constituted three heterogeneous groups of individuals with varying severity of ADL and CF when we assessed them by using the ABC-DS. We recognized that the individuals whom doctors diagnosed with probable MCI almost corresponded to Group 1 in our cluster analysis. When we select individuals with MCI by CDR 0.5 for clinical research, we should carefully interpret the study results. To identify individuals with MCI, CDR-SOB and ABC-DS might be more accurate than global CDR. The ABC-DS score profile in Group 1 may be relevant to define MCI.
We found several articles discussing that there are some additional problems for the diagnosis of MCI relying on a global CDR score of 0.5. First, the global scores produced from the six component scores were pointed out to be occasionally inconsistent [21]. In the Memory Impairment Study, Grundman et al. analyzed 769 patients with MCI diagnosed according to their operational criteria, 107 cognitively normal elderly controls, 122 patients with very mild AD rated as having a CDR of 0.5, and 183 patients with mild AD rated as having a CDR of 1.0 [22]. The patients met operational criteria for amnestic MCI, and controls were individuals who had a CDR of 0. They reported that the mean of the CDR-SOB of the CDR 0.5 AD group (n = 122) was 1.7 times higher than that for individuals in the MCI group (n = 769) whom the doctor diagnosed without using CDR. This result indicated that the group of individuals with a CDR of 0.5 contained not only MCI individuals but also the group of individuals whose severity was worse than MCI. Our analysis with ABC-DS supported their observations that individuals with a CDR of 0.5 are heterogeneous.
There are also other limitations of the global CDR scores on MCI diagnosis when we consider disease progression. When the MCI participants with global CDR scores of 0.5 were divided into groups with relatively intact or impaired ratings on the instrumental activities of daily living (IADL) subscale of the CDR, the MCI participants with impaired IADL were more likely than those with intact IADL to progress to a clinical diagnosis of probable AD within the next two years [23]. These findings indicate that individuals with global CDR scores of 0.5 manifest heterogeneous states of cognitive impairment ranging from healthy elders to mild AD, and we needed a more exact scale to define MCI.
O’Bryant et al. proposed new CDR-SOB interpretive guidelines and defined questionable cognitive impairment if CDR-SOB ranges from 0.5 to 4.0. Our cluster analysis showed that CDR-SOB for the individuals of three groups fell within this range. The median of CDR-SOB for individuals whom doctors diagnosed probable MCI corresponded to questionable cognitive impairment, according to their guideline. We consider that O’Bryant’s guidelines will be informative to define MCI precisely.
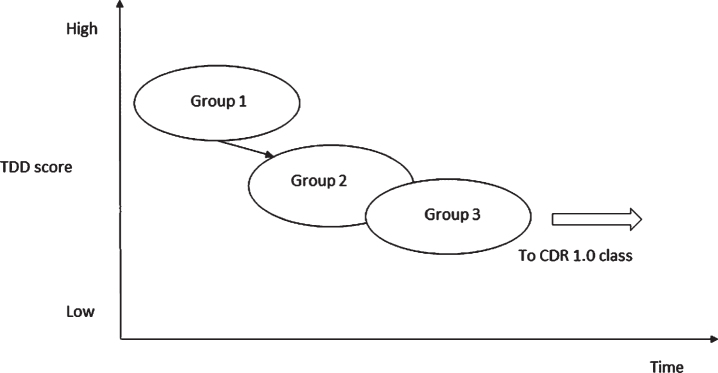
We used ABC-DS scores for our cluster analysis and found that there were three types of MCI individuals, Groups 1, 2, and 3. We may consider that Groups 1, 2, and 3 should be stages 1, 2, and 3 indicating the peculiar progressions of symptoms in MCI: the median of ADL decreased from Group 1 to Group 2 but stopped the decrease in Group 3: the median of BPSD was almost unchanged from Group 1 to Group 3: however, CF rapidly decreased from Group 1 to Group 3. As cognitive functions deteriorate, ADL usually worsen, but the changes do not always occur parallelly [24]. Preserved implicit memory maintains its functional state of daily living in AD patients [25]. Attribution of cognitive dysfunction to ADL may differ according to the cognitive domains. The executive function is more strongly associated with ADL than with memory [26]. Taken together, we suggest that if the level of cognitive function decreases, but the compensation mechanism works, the decrease in ADL may not be apparent for a certain period of time, and Group 3 might be that period. Individuals in Group 3 will move to global CDR 1 class when the cognitive function further deteriorates, and the compensation will not work anymore. Based on these findings, we present a hypothetical progression pathway from MCI to mild dementia (Fig. 2).
Fig.2.
A hypothetical schema for the progression from MCI to mild dementia. In Group 3, implicit memory compensatory strategies such as ADL may work to compensate for explicit memory deficits. It is likely that if explicit memory further deteriorates and the compensation mechanism fails, Group 3 will move to a subgroup in CDR1.0 class.
If we know a TDD score of a new patient and the patient is probably MCI, we can define the patient’s group by calculating a distance to the nearest the median of the TDD among the three groups for MCI. We can also monitor the progression of the symptoms or transferring between groups at MCI using ABC-DS scores.
Conclusion
Many clinicians and researchers routinely use a global CDR of 0.5 as a definition of MCI. In the present study, we showed that the profiles of ABC-DS scores for individuals with a CDR of 0.5 were heterogeneous, and there were three subgroups among the individuals with CDR 0.5. This result suggested that a global CDR of 0.5 might not be an accurate criterion to specify individuals with MCI. For this purpose, we should use CDR-SOB or ABC-DS score, and consider that ABC-DS is useful to monitor the changes of the AD symptoms.
If we measure biomarker values such as amyloid beta and tau protein in cerebrospinal fluids and the z-scores of Voxel-based Specific Regional analysis system for Alzheimer’s disease for the patients (VSRAD) belonging to Groups 1, 2, and 3, we can characterize the three groups and investigate the relationships between the groups and the responsiveness to interventions.
However, a limitation of the ABC-DS is that we do not know the threshold score between healthy older individuals and individuals in Group 1 in Table 2. To identify this threshold, we need to conduct another clinical trial.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mr. Tukada, Mr. Sugihara, and Mrs. Nakagawa for data analysis. We also offer our appreciation to Mr. Sakamine for his contribution to this study as a study manager.
Daiichi Sankyo Co., Ltd. funded TRIAD1714 research; this company, however, did not have any role in the design of the study, the data collection, statistical analyses, nor writing of this paper. The authors did not disclose this manuscript to the company before submission to this journal.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/ADR-190126.
REFERENCES
- [1]. Petersen RC (2011) Mild cognitive impairment, N Engl J Med 364, 2227–2234. [DOI] [PubMed] [Google Scholar]
- [2].American Psychiatric Association (2013) American Psychiatric Association, Arlington VA, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-5. [Google Scholar]
- [3].World Health Organization (1993) 10th Revision. World Health Organization, Geneva, International Statistical Classification of Diseases and Related Health Problems.. [Google Scholar]
- [4]. Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules, Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [5]. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease, Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria, Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [7]. Meguro K, Ishii H, Yamaguchi S, Ishizaki J, Sato M, Hashimoto R, Meguro M, Lee E, Tanaka Y, Kasuya M, Sekita Y (2004) Prevalence and cognitive performances of clinical dementia rating 0.5 and mild cognitive impairment in Japan: the Tajiri project, Alzheimer Dis Assoc Dis 18, 3–10. [DOI] [PubMed] [Google Scholar]
- [8]. Yang YH, Lai CL, Lin RT, Tai CT, Liu CK (2006) Cut-off values of blessed dementia rating scale and its clinical application in elderly Taiwanese, Kaohsiung J Med Sci 22, 377–384. [DOI] [PubMed] [Google Scholar]
- [9]. Chang YL, Bondi MW, McEvoy LK, Fennema-Notestine C, Salmon DP, Galasko D, Hagler DJ, Dale AM, Alzheimer’s Disease Neuroimaging Initiative (2011) Global clinical dementia rating of 0.5 in MCI masks variability related to level of function, Neurology 76, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Aretouli E, Okonkwo OC, Samek J, Brandt J (2011) The fate of the 0.5s: predictors of 2-year outcome in mild cognitive impairment, J Int Neuropsychol Soc 17, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Duara R, Loewenstein DA, Greig-Custo MT, Raj A, Barker W, Potter E, Schofield E, Small B, Schinka J, Wu Y, Potter H (2010) Diagnosis and staging of mild cognitive impairment, using a modification of the clinical dementia rating scale: the mCDR, Int J Geriatr Psychiatry 25, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R (2008) Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study, Arch Neurol 65, 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. O’Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, Massman PJ, Hobson V, Cullum CM (2010) Validation of the new interpretive guidelines for the Clinical Dementia Rating Scale Sum of Boxes score in the national Alzheimer’s coordinating center database, Arch Neurol 67, 746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Mori T, Kikuchi T, Umeda-Kameyama Y, Wada-Isoe K, Kojima S, Kagimura T, Kudoh C, Uchikado H, Ueki A, Yamashita M, Watabe T (2018) ABC Dementia Scale: a quick assessment tool for determining Alzheimer’s Disease severity, Dement Geriatr Cogn Dis Extra 8, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Kikuchi T, Mori T, Wada-Isoe K, Umeda-Kameyama Y, Kagimura T (2018) A novel dementia scale for Alzheimer’s Disease, J Alzheimers Dis Parkinsonism 8, 2. [Google Scholar]
- [16]. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H (2004) Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment, J Intern Med 256, 240–246. [DOI] [PubMed] [Google Scholar]
- [17]. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association Workgroup, Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Streiner D.L, Norman G.R., Cairnery J (2015) Health Measurement Scales: A Practical Guide to Their Development and Use (fifth edition), Oxford University Press, Oxford. [Google Scholar]
- [19]. Everitt B (2005) An S and S-PLUS Companion to Multivariate Analysis, Springer, London. [Google Scholar]
- [20]. Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5 299–314. Available at http://www.R-project.org. [Google Scholar]
- [21]. Gelb DJ, St Laurent RT (1993) Alternative calculation of the global clinical dementia rating, Alzheimer Dis Assoc Dis 7, 202–211. [DOI] [PubMed] [Google Scholar]
- [22]. Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR Jr, Galasko DR, Doody R, Kaye J (2004) Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials, Arch Neurol 61, 59–66. [DOI] [PubMed] [Google Scholar]
- [23]. Chang YL, Bondi MW, McEvoy LK, Fennema-Notestine C, Salmon DP, Galasko D, Hagler DJ, Dale AM; Alzheimer’s Disease Neuroimaging Initiative (2011) Global clinical dementia rating of 0.5 in MCI masks variability related to level of function, Neurology 76, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Arrighi HM, Gélinas I, McLaughlin TP, Buchanan J, Gauthier S (2013) Longitudinal changes in functional disability in Alzheimer’s disease patients, Int Psychogeriatr 25, 929–937. [DOI] [PubMed] [Google Scholar]
- [25]. Machado S, Cunha M, Minc D, Portella CE, Velasques B, Basile LF, Cagy M, Piedade R, Ribeiro P (2009) Alzheimer’s disease and implicit memory, Arq Neuropsiquiatr 67, 3334–42. [DOI] [PubMed] [Google Scholar]
- [26]. Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ; Committee on Research of the American Neuropsychiatric Association (2007) The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association, J Neuropsychiatry Clin Neurosci 19, 249–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.