To the Editor:
Case reports1 and the high tumor mutational burden2 of basal cell carcinomas (BCCs) compared with other tumor types suggest that programmed death-ligand 1 (PD-L1) inhibitors may be active against advanced BCCs. Many advanced BCCs are refractory to3 or are recurrent4 after hedgehog pathway inhibitors, and therefore PD-L1 inhibitors could be a useful therapeutic option. We present a proof-of-principle, nonrandomized, open-label study of pembrolizumab (200 mg intravenously every 3 weeks), with or without vismodegib (150 mg orally daily), for eligible subjects with advanced BCCs. The primary outcome was the overall response rate (ORR) for all evaluable subjects at 18 weeks.
Sixteen participants, 9 receiving pembrolizumab monotherapy and 7 receiving pembrolizumab plus vismodegib, were evaluable by the revised Response Evaluation Criteria In Solid Tumors5 (version 1.1) at data cutoff. The ORR for all evaluable subjects was 38% (6/16 patients; 95% confidence interval 15–65%; P = .003) at 18 weeks (Table I, Fig 1). The ORR at 18 weeks for pembrolizumab monotherapy group was 44% (4/9 patients; 95% confidence interval 14–79%; P = .008), and for the dual therapy group was 29% (2/7 patients; 95% confidence interval 4–71%; P = . 15).
Table I.
Primary and secondary outcomes
| Outcome | All evaluable participants (N = 16) | Pembrolizumab monotherapy (n = 9) | Pembrolizumab plus vismodegib (n = 7) |
|---|---|---|---|
| Overall response rate (range), n | 38% (15–65%), 6 | 44% (14–79%), 4 | 29% (4–71%), 2 |
| One-year PFS probability, % | 70 | 62 | 83 |
| One-year OS probability, % | 94 | 89 | 100 |
| Median treatment duration, weeks (range) | 18.5 (5.9–73.0) | 19.0 (10–73.0) | 18.0 (5.9–38.6) |
| Median time to response, weeks (range), n | 10.4 (8.4–17.4), 6 | 12.4 (8.4–17.4), 4 | 10.3 (8.7–11.9), 2 |
| Median duration of response, weeks (range), n | 67.3 (28.0–82.0), 6 | 67.6 (31.4–82.0), 4 | 52.8 (28.0–77.6), 2 |
| Median time from pembrolizumab start date to next treatment start date, weeks (range), n | 21.9 (6.1–43.3), 6 | 21.9 (15.4–43.3), 4 | 19.2 (6.1–32.3), 2 |
Overall response rates were calculated at the 18-week time point. One-year progression-free survival and overall survival probabilities were calculated using the KaplaneMeier method.
OS, Overall survival; PFS, progression-free survival.
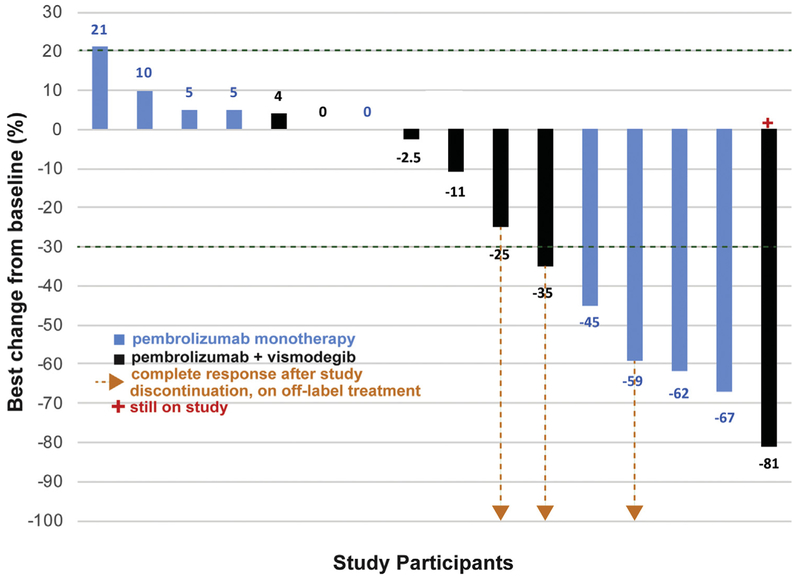
Fig 1.
Waterfall plot showing best percent change in the diameter of targeted basal cell carcinoma lesions from baseline for all evaluable subjects. The dotted arrows indicate 3 subjects who achieved complete response after study discontinuation, based on imaging and clinical documentation.
The median time to response for all responders (n = 6) was 10.4 weeks (range 8.4–17.4 weeks). The median duration of response for all responders (n = 6) was 67.3 weeks (range 28.0–82.0 weeks; Table I).
One-year progression-free survival probability was 70%, and the 1-year overall survival probability was 94% for all evaluable subjects (n = 16; Table I).
Before pembrolizumab, 29% (2/7 patients) expressed PD-L1 at ≥1% of tumor cells. There was no significant correlation between prepembrolizumab PD-L1 expression and best percentage change in BCC diameter.
There were no life-threatening adverse events (AEs) or deaths during the study. Three severe (grade 3) AEs occurred out of 98 AEs from 16 participants. Only 1 of the severe AEs, hyponatremia, was attributed to pembrolizumab. There were 23 immune-related AEs, with dermatitis and fatigue as the most common (all grade 1 or 2), and only 1 severe immune-related AE (the aforementioned hyponatremia).
As a proof-of-principle study, we conclude that pembrolizumab is active against BCCs. Although the 2 groups were not directly compared, the response rate of the pembrolizumab plus vismodegib group was not superior to the monotherapy group. The lack of life-threatening AEs or death suggests that pembrolizumab has a reasonable safety profile in patients with BCC.
This study is limited by its sample size, because advanced BCCs are an uncommon disease. Nevertheless, the efficacy and safety data presented here could be used in future meta-analyses and compared with forthcoming multi-institutional studies on PD-L1 inhibitors against advanced BCCs.
Acknowledgments
We thank Alex McMillan, PhD, for biostatistics consultation.
Merck, Sharp, and Dohme, Inc. provided funding for this study but were not involved in writing of the manuscript, decision to submit for publication, data collection, analysis, interpretation, trial design, patient recruitment or any other aspect pertinent to the study. The authors have not been paid to write this article by a pharmaceutical company or other agency. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Dr Chang was a consultant for Merck, Sharp and Dohme, Inc, is a clinical investigator for studies sponsored by Regeneron, Genentech-Roche, and Novartis. Ms Yost is funded by grants from the National Science Foundation, National Institutes of Health, and Parker Institute for Cancer Immunotherapy.
Footnotes
The other authors have no conflicts of interest to disclose.
This study is subject to Stanford Human Subjects Approval Protocol 34925 and is listed at clinicaltrials.gov ().
REFERENCES
- 1.Cannon JGD, Russell JS, Kim J, Chang ALS. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134:213–220. [DOI] [PubMed] [Google Scholar]
- 3.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang AL, Oro AE. Initial assessment of tumor regrowth after vismodegib in advanced basal cell carcinoma. Arch Dermatol. 2012;148:1324–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45: 228–247. [DOI] [PubMed] [Google Scholar]