1. Introduction
By the end of 2018, approximately 22, 240 women in the U.S. are estimated to receive an ovarian cancer (OC) diagnosis.1 Considered the most fatal of the gynecological cancers,2 this malignancy kills more than 14,000 U.S. women each year.1 Fortunately, substantial advances in treatment in the last four decades have led to gradual but consistent improvements in survival.3 Stage-specific guidelines have been established by the National Comprehensive Cancer Network (NCCN)4 for best care practices in treating OC and adherence to these recommendations has been validated as a significant predictor of disease-specific survival.5 Despite these evidence-based guidelines, inequities in access to and delivery of appropriate care still exist.
Although most efforts to understand the drivers of OC disparities have largely focused on factors such as race and socioeconomic status,6–13 there has been growing consideration of the role that geographic location may play.6,8,14–17 A study of national data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program explored spatial variations in the delivery of OC treatment for Medicare recipients and found discrepancies existed by Hospital Referral Region.14 In British Columbia, despite theoretically having equal health care access under a single-payer system, differences were found in treatment practices by health authority region.15 Even with rising consensus that receiving specialized care is critical for OC outcomes,6,8,14–24 a U.S. nationwide study emphasized the disparities in access to gynecological oncologists, highlighting their concentration in metropolitan-centers.16 The vast geographic areas without specialists represent a geographic barrier for those who must cover greater distances to reach them.
The objective of the current study is to examine how geocoded residence at diagnosis contributes to receiving care that is compliant with OC NCCN treatment guidelines in California (CA) among women of all stages, while exploring differences by race/ethnicity, SES, and insurance. CA accounts for 11% of all ovarian cancer cases diagnosed nationally and is highly relevant as a standalone study setting because of the large number of cases and its racial/ethnic diversity.
2. Methods
2.1. Study Population
We used a retrospective study design to examine the relationship between residential address at diagnosis and adherence to NCCN treatment guidelines. All cases of invasive epithelial OC diagnosed in CA between January 1, 1996 and December 31, 2014 were ascertained from the California Cancer Registry (CCR), with follow up data obtained through December 31, 2016. Reporting to the CCR within 6 months of diagnosis is close to 99%, with follow up nearly as high (95%).25,26 CCR data was linked with California’s Office of Statewide Health Planning and Development (OSHPD) patient discharge data.
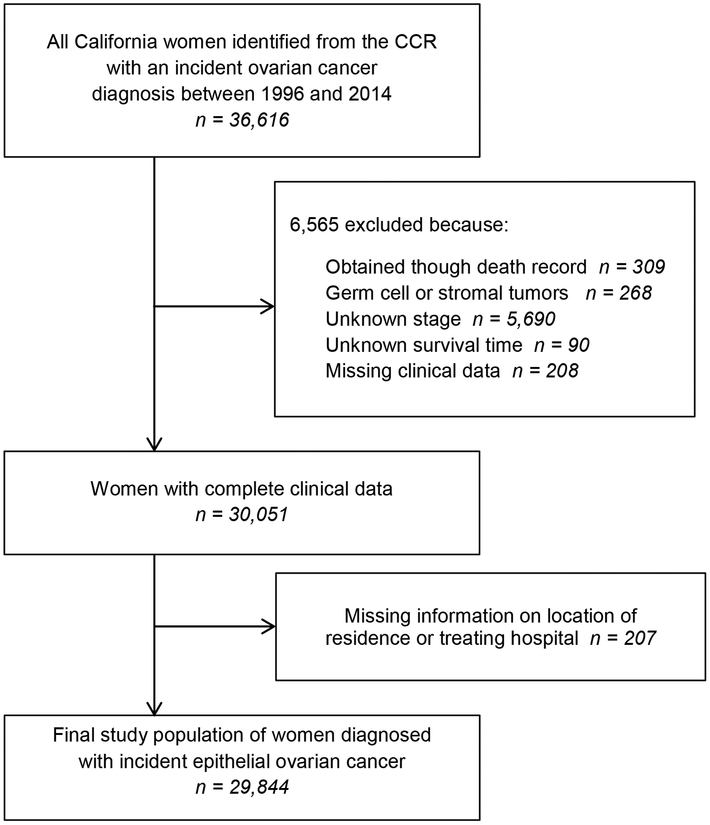
Women of all OC stages (International Federation of Gynecology and Obstetrics (FIGO) - Stage I-IV) were eligible for inclusion. Cases 18 years of age or older at time of diagnosis were identified from the CCR using the International Classification of Disease Codes for Oncology (ICD-O-3 C56.9). The analysis was restricted to women with complete clinical information and no prior history of OC. After exclusions, presented in Figure 1, a total of 29,844 women diagnosed with incident invasive epithelial OC were included in the analyses. The study was approved by the Institutional Review Board of the University of California, Irvine (UCI 14–66/HS# 2014–1476).
Figure 1: Study Population Exclusions.
This diagram details how patients diagnosed between 1996 to 2014 were included in the study. CCR stands for the California Cancer Registry.
2.2. Study Data
The primary outcome was non-adherence to stage-specific NCCN treatment guidelines, examined as a binary variable (adherent vs. non-adherent). Non-adherence required either surgical or chemotherapy treatment be non-adherent to the NCCN guidelines of women’s respective diagnosis period.19,27–32 Surgical guideline adherence for stages I-IIIB was a minimum of oophorectomy (± hysterectomy), pelvic and/or para-aortic lymph node biopsy, and omentectomy. Adherence for stages IIIC-IV was a minimum of oophorectomy (± hysterectomy) and omentectomy. For chemotherapy, receiving no adjuvant treatment was only appropriate for early stage and grade (stages IA-IB, grades 1–2). For all other stages (stage IC-IV) and grade 3 disease, multi-agent chemotherapy was guideline adherent. Chemotherapy must have been delivered subsequent to surgery, with the exception of stages IIIC-IV, in which it could have been received before or after surgery.19,27–32
Several important patient characteristics were included as predictors: age at diagnosis, race/ethnicity, marital status, SES, insurance type, diagnosis year, tumor characteristics, and the Deyo-adapted Charlson Comorbidity Score.33,34 Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander, and American Indian/Other. Insurance type was categorized as managed care, Medicare, Medicaid, other insurance, not insured, and unknown. SES was stratified into quintiles using the Yost score35 for patients diagnosed prior to 2006 and the Yang index36 for those with a diagnosis after 2006. Tumor characteristics included tumor grade, size, histology type, and stage at diagnosis. The observed-to-expected (O/E) adherence ratio of women’s initial reporting hospital was included as a measure of hospital quality.19 This metric was calculated as the number of OC cases that received NCCN adherent care divided by the amount expected to receive standard care for that hospital, distributed into quartiles, and classified by annual hospital case volume.19 High quality hospitals were in the highest O/E quartile and had ≥5 cases/year.
To assess the role of geographic location on accessibility and potential barriers to treatment, we calculated the distance from each woman’s geocoded residential address at the time of diagnosis to the geocoded location of their initial reporting hospital. We also calculated how far each woman lived from the nearest high-quality hospital. Each variable was categorized into quintiles, with distances for both measures calculated with a geographic information system (GIS) using the Streetmaps routing dataset in the network analysis extension (ArcGIS version 10.4.1, ESRI; Redlands, CA).
2.3. Statistical Analysis
The main predictor variable of interest was geographic location as a smooth function of women’s geocoded residential location at diagnosis. We used generalized additive models (GAMs) to estimate the effect of a locally-weighted loess smoother of longitude and latitude on the log odds of not receiving adherent treatment while also adjusting for covariates.37,38 Details of the methods used are described elsewhere.38 Briefly, log odds of adherence was computed at thousands of locations points throughout California holding all other covariables constant, using the average odds for all of California as the referent group. Odds were not computed for areas with very few or no cases.8 The amount of smoothing depends on the span size, which represents the proportion of cases used locally to calculate the log odds at each point. The span size of 0.3 was chosen because it minimized the Akaike’s Information Criterion (AIC) for the majority of the models.37,38
Our base model examined the effect of residential location, while adjusting only for age and cancer characteristics at diagnosis. A fully-adjusted model additionally adjusted for demographic and treatment factors: SES, race/ethnicity, insurance type, marital status, quality of reporting hospital, comorbidities, and the two distance variables. Any differences in geographic areas of increased or decreased risk between the base and fully-adjusted models indicate that the additional demographic and treatment factors were affecting geographic variation in adherent care. We also conducted stage-stratified analyses. Permutation tests were used to calculate a global p-value for the importance of location. We produced color maps for each model displaying the odds ratios for treatment non-adherence throughout California, with contour lines delineating geographic areas that excluded odds ratios of 1. We also conducted Chi Square tests for differences between racial/ethnic, SES, and insurance groups across the distribution of distance variables. Statistical modeling and mapping were done in R version 3.4.0 using the MapGAM package.
3. Results
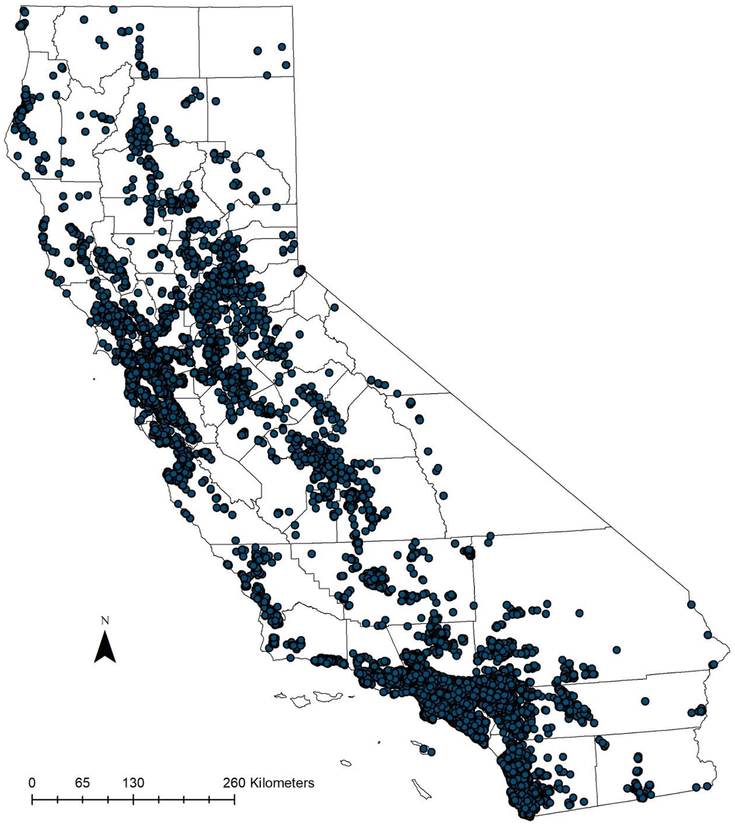
Patient characteristics are detailed in Table 1, with the case distribution shown in Figure 2. Of the 29,844 cases included in the analysis, 9,734 (32.6%) women were diagnosed at early stages (Stage1 and Stage2). The majority of the population was non-Hispanic White (63.4%) and the median age at time of diagnosis was 60 years old. Only 11,419 (38.3%) of all patients received care adherent to the National Comprehensive Care Network (NCCN) treatment guidelines. Women with Stage 3 disease were more likely to receive adherent care as compared to those diagnosed in early stages or Stage 4 (52.8% versus 25.2% and 34.2%, respectively).
Table 1:
Patient Characteristics by NCCN Treatment Adherence (n=29,844)
| Characteristic | Treatment Adherent | Treatment Non-Adherent | ||
|---|---|---|---|---|
| N | % | N | % | |
| Total | 11419 | 38.3 | 18425 | 61.7 |
| Age Group | ||||
| 18–44 | 1511 | 35.9 | 2699 | 64.1 |
| 45–54 | 2806 | 43.7 | 3617 | 56.3 |
| 55–64 | 3359 | 46.5 | 3862 | 53.5 |
| 65+ | 3743 | 31.2 | 8247 | 68.8 |
| Race/Ethnicity | ||||
| Non-Hispanic White | 7533 | 39.8 | 11387 | 60.2 |
| Non-Hispanic Black | 424 | 29.9 | 992 | 70.1 |
| Hispanic | 2020 | 35.1 | 3729 | 64.9 |
| Asian/PI | 1378 | 38.7 | 2186 | 61.3 |
| American Indian/Other | 64 | 32.8 | 131 | 67.2 |
| Socioeconomic Status | ||||
| Lowest SES | 1222 | 30.3 | 2815 | 69.7 |
| Lower-Middle SES | 1878 | 34.6 | 3557 | 65.4 |
| Middle SES | 2374 | 37.5 | 3950 | 62.5 |
| Higher-Middle SES | 2769 | 40.4 | 4091 | 59.6 |
| Highest SES | 3176 | 44.2 | 4012 | 55.8 |
| Insurance Type | ||||
| Managed Care | 5830 | 41.2 | 8320 | 58.8 |
| Medicare | 2438 | 31.9 | 5215 | 68.1 |
| Medicaid | 1001 | 36.7 | 1724 | 63.3 |
| Other Insurance | 1636 | 42.8 | 2189 | 57.2 |
| Not insured | 275 | 30.9 | 614 | 69.1 |
| Unknown | 239 | 39.7 | 363 | 60.3 |
| Marital Status | ||||
| Not Married | 5029 | 34.2 | 9659 | 65.8 |
| Married | 6390 | 42.2 | 8766 | 57.8 |
| Charlson Comorbidity Score | ||||
| CCS 0 | 5931 | 41.7 | 8288 | 58.3 |
| CCS 1 | 2743 | 40.3 | 4064 | 59.7 |
| CCS 2+ | 2078 | 30.9 | 4648 | 69.1 |
| CCS Unknown | 667 | 31.9 | 1425 | 68.1 |
| Stage | ||||
| Stage 1 | 1720 | 23.8 | 5518 | 76.2 |
| Stage 2 | 731 | 29.3 | 1765 | 70.7 |
| Stage 3 | 5943 | 52.8 | 5320 | 47.2 |
| Stage 4 | 3025 | 34.2 | 5822 | 65.8 |
| Hospital Quality Measure | ||||
| Low | 1912 | 27.4 | 5078 | 72.6 |
| Intermediate | 6533 | 37.8 | 10742 | 62.2 |
| High | 2974 | 53.3 | 2605 | 46.7 |
| Distance Traveled to Care | ||||
| <6 km | 1911 | 32.0 | 4058 | 68.0 |
| 6–9 km | 2133 | 35.7 | 3836 | 64.3 |
| 10–16 km | 2262 | 37.9 | 3706 | 62.1 |
| 17–32 km | 2358 | 39.5 | 3611 | 60.5 |
| >32 km | 2755 | 46.2 | 3214 | 53.8 |
| Closest High Quality Hospital | ||||
| <9 km | 2501 | 41.9 | 3468 | 58.1 |
| 9–14 km | 2247 | 37.6 | 3722 | 62.4 |
| 15–24 km | 2228 | 37.3 | 3740 | 62.7 |
| 25–48 km | 2289 | 38.3 | 3680 | 61.7 |
| >48 km | 2154 | 36.1 | 3815 | 63.9 |
CCS, Charlson Comorbidity Score; km, kilometers; NCCN, National Comprehensive Cancer Network; PI, Pacific Islander; SES, socioeconomic status
Figure 2:
Epithelial Ovarian Cancer Case Distribution in California between 1996 – 2014
3.1. Spatial Analysis of Treatment Adherence
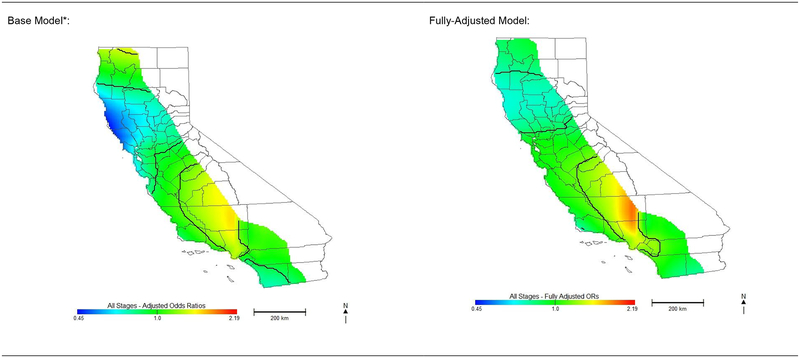
Residential location was significantly associated with non-adherence to NCCN treatment guidelines among California women diagnosed with OC. All analyses, including stage-stratified models, resulted in a highly significant global test for location (<0.001). Compared to base model odds ratios (ORs) adjusted for only age and cancer characteristics for all stages (OR range: 0.46–1.57), fully-adjusted ORs were attenuated in some locations, but increased in other areas (OR range: 0.70–1.89). Figure 3 shows the protective effects observed in northern California were attenuated and no longer present in the San Francisco Bay area after full adjustment. Although the reduced risk observed in the southern-most portion of California was no longer present after full adjustment, risk in northern Los Angeles County and western Kern County increased. Spatial variability in risk of non-adherent treatment with the fully-adjusted models indicates geographic disparities in adherent treatment that are not explained by distance to initial reporting hospital, distance from the nearest high-quality hospital, or the other demographic and treatment variables included in our analyses.
Figure 3: Odds of Receiving Non-Adherent Care for Ovarian Cancer in California.
Effect of geographic location on risk of receiving non-adherent National Comprehensive Cancer Network guideline treatment for invasive epithelial ovarian cancer.
*Base model is adjusted for age and cancer characteristics only
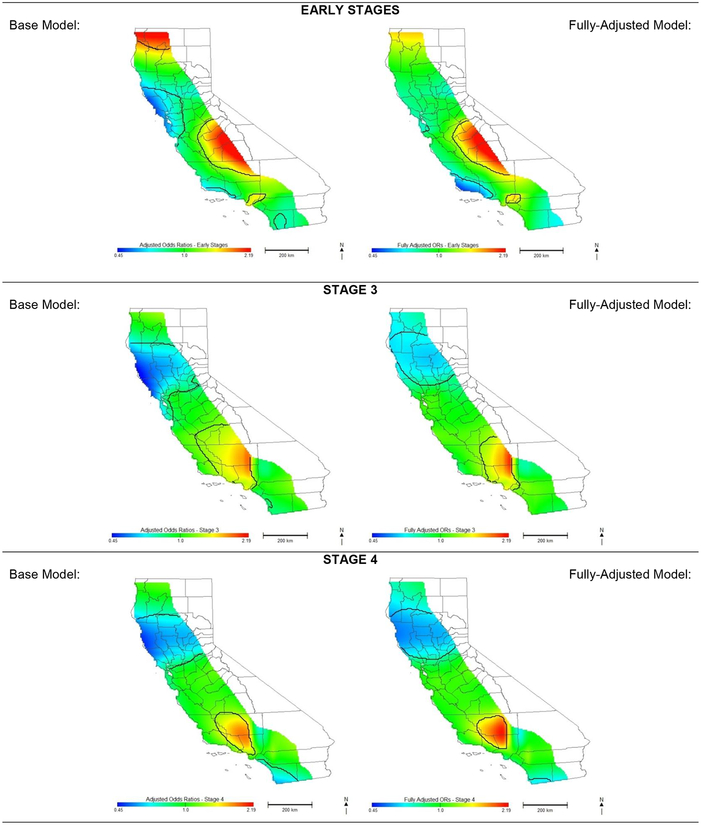
Patterns of geographic risk for NCCN non-adherence varied across the different stage-stratified analyses. Regions of increased and decreased risk in the early stage analyses differed greatly from the other stages (comparison of Figure 3 to Figure 4). When controlling for age and cancer characteristics alone, we identified areas of increased risk of non-adherent treatment for early stage OC in mid-Central Valley and decreased risk in Northern California (OR range: 0.49–2.90). After full adjustment of the early stage model, there is no longer an association in northern California and San Diego County; however, Ventura and Santa Barbara Counties in the Central Coast become largely protective (OR range: 0.49–2.90). Models for Stages 3 and 4 display similar patterns to those of all stages combined although areas of higher and lower risk are smaller (Figure 4) and the magnitude of ORs are attenuated (OR ranges 0.61–2.13 and 0.47–1.86 for Stages 3 and 4 respectively).
Figure 4: Odds of Receiving Non-Adherent Care for Ovarian Cancer in California.
Effect of geographic location on risk of receiving non-adherent National Comprehensive Cancer Network guideline treatment for invasive epithelial ovarian cancer, stratified by stage. Early stages includes stage 1 and stage 2.
*Base model is adjusted for age and cancer characteristics only.
Associations between all-stage non-adherent care and additional variables included in the spatial model are presented in Table 2. Increasing distance traveled to receive care decreased non-adherence ORs. Compared to patients living <6km of their initial reporting facility, those traveling >32km had decreased odds (OR=0.76, 95%CI=0.70–0.84) of receiving care that deviated from the NCCN guidelines. Increasing distance traveled to receive care was significantly protective against receiving non-adherent care for those in early stages (Table 3). Compared to women in closest proximity to a high-quality hospital (within 9km), living >48km was a significant deterrent to receiving adherent care for women diagnosed in early stages. Patterns with distance were similar for Stages 3 and 4 but generally not significant (not shown).
Table 2:
Multivariate Analysis of NCCN Treatment Non-adherence for All Stages
| Characteristic | OR | 95% Confidence Interval | ||
|---|---|---|---|---|
| Age | 1.02 | 1.02 | - | 1.02 |
| Size Category | ||||
| <50mm | 1.00 | Referent | ||
| 50–99mm | 0.93 | 0.85 | - | 1.02 |
| 100+mm | 0.91 | 0.83 | - | 0.99 |
| Size Unknown | 1.12 | 1.03 | - | 1.22 |
| Grade | ||||
| Grade I | 1.00 | Referent | ||
| Grade II | 1.00 | 0.89 | - | 1.13 |
| Grade III | 0.85 | 0.76 | - | 0.95 |
| Grade IV | 0.73 | 0.65 | - | 0.83 |
| Grade Unknown | 2.25 | 2.00 | - | 2.54 |
| Stage | ||||
| Stage 1 | 1.00 | Referent | ||
| Stage 2 | 0.75 | 0.68 | - | 0.84 |
| Stage 3 | 0.25 | 0.23 | - | 0.27 |
| Stage 4 | 0.33 | 0.30 | - | 0.36 |
| Histology | ||||
| Serous | 1.00 | Referent | ||
| Mucinous | 1.40 | 1.24 | - | 1.58 |
| Endometrioid | 1.22 | 1.11 | - | 1.34 |
| Clear cell | 0.91 | 0.81 | - | 1.03 |
| Adenocarcinoma, NOS | 2.89 | 2.59 | - | 3.22 |
| Others | 1.78 | 1.66 | - | 1.91 |
| Race/Ethnicity | ||||
| Non-Hispanic White | 1.00 | Referent | ||
| Non-Hispanic Black | 1.21 | 1.06 | - | 1.39 |
| Hispanic | 1.01 | 0.93 | - | 1.09 |
| Asian/Pacific Islander | 1.02 | 0.93 | - | 1.11 |
| American Indian/Other | 1.47 | 1.05 | - | 2.05 |
| Socioeconomic Status | ||||
| Lowest SES | 1.28 | 1.16 | - | 1.42 |
| Lower-middle SES | 1.15 | 1.06 | - | 1.26 |
| Middle SES | 1.09 | 1.01 | - | 1.19 |
| Higher-middle SES | 1.06 | 0.98 | - | 1.14 |
| Highest SES | 1.00 | Referent | ||
| Insurance | ||||
| Managed Care | 1.00 | Referent | ||
| Medicare | 1.10 | 1.03 | - | 1.19 |
| Medicaid | 1.04 | 0.94 | - | 1.15 |
| Other Insurance | 1.01 | 0.93 | - | 1.10 |
| Not insured | 1.34 | 1.14 | - | 1.58 |
| Unknown | 0.99 | 0.82 | - | 1.20 |
| Marital Status | ||||
| Not Married | 1.00 | Referent | ||
| Married | 0.85 | 0.81 | - | 0.90 |
| Charlson Comorbidity Score | ||||
| CCS 0 | 1.00 | Referent | ||
| CCS 1 | 0.99 | 0.92 | - | 1.05 |
| CCS 2+ | 1.19 | 1.10 | - | 1.28 |
| CCS Unknown | 1.26 | 1.13 | - | 1.41 |
| Year of Diagnosis | 1.01 | 1.00 | - | 1.01 |
| Hospital Quality Measure | ||||
| Low | 2.57 | 2.35 | - | 2.81 |
| Intermediate | 1.76 | 1.64 | - | 1.89 |
| High | 1.00 | Referent | ||
| Distance Traveled to Care | ||||
| <6 km | 1.00 | Referent | ||
| 6–9 km | 0.92 | 0.85 | - | 1.00 |
| 10–16 km | 0.89 | 0.82 | - | 0.97 |
| 17–32 km | 0.91 | 0.84 | - | 1.00 |
| >32 km | 0.76 | 0.70 | - | 0.84 |
| Closest High Quality Hospital | ||||
| <9 km | 1.00 | Referent | ||
| 9–14 km | 1.06 | 0.97 | - | 1.15 |
| 15–24 km | 1.05 | 0.97 | - | 1.15 |
| 25–48 km | 1.13 | 1.04 | - | 1.23 |
| >48 km | 1.18 | 1.07 | - | 1.29 |
CCS, Charlson Comorbidity Score; km, kilometers; NCCN, National Comprehensive Cancer Network; NOS, Not otherwise specified; OR, Odds Ratio
Table 3:
Multivariate Analysis of NCCN Treatment Non-adherence for Early Stages (Stage 1 and Stage 2)
| Variable | OR* | 95% Confidence Interval | ||
|---|---|---|---|---|
| Distance Traveled to Care | ||||
| <6 km | 1.00 | Referent | ||
| 6–9 km | 0.82 | 0.69 | - | 0.96 |
| 10–16 km | 0.83 | 0.70 | - | 0.98 |
| 17–32 km | 0.77 | 0.66 | - | 0.91 |
| >32 km | 0.57 | 0.49 | - | 0.68 |
| Closest High Quality Hospital | ||||
| <9 km | 1.00 | Referent | ||
| 9–14 km | 0.96 | 0.82 | - | 1.12 |
| 15–24 km | 1.02 | 0.87 | - | 1.20 |
| 25–48 km | 1.14 | 0.97 | - | 1.34 |
| >48 km | 1.25 | 1.05 | - | 1.49 |
km, kilometers; NCCN, National Comprehensive Cancer Network; OR, Odds Ratio
OR adjusted for geographic location, age, race/ethnicity, socioeconomic status, insurance, marital status, Charlson comorbidity score, stage, hospital quality
3.3. Geographic Disparities
The distribution of distance traveled to the reporting hospital by patient characteristics are shown in Table 4. Greater proportions of women treated at high-quality facilities traveled further for care. Among women reported by a high-quality hospital, more than a third (38.1%) lived within 9km, whereas only 9.3% lived >48km (Table 5). In contrast, women reported by low quality hospitals tended to live further from high-quality hospitals (31.4% in furthest category vs. 11.8% in closest).
Table 4:
Comparison of Patient Characteristics by Distance Traveled to Receive Care (n=29,844)*
| <6 km | 6 – 9 km | 10 – 16 km | 17 – 32 km | > 32 km | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | N | % | N | % | N | % | N | % | N | % | N |
| 18–44 | 737 | 17.5 | 815 | 19.4 | 887 | 21.1 | 930 | 22.1 | 841 | 20.0 | 4210 |
| 45–54 | 1148 | 17.9 | 1130 | 17.6 | 1303 | 20.3 | 1421 | 22.1 | 1421 | 22.1 | 6423 |
| 55–64 | 1252 | 17.3 | 1320 | 18.3 | 1432 | 19.8 | 1535 | 21.3 | 1682 | 23.3 | 7221 |
| 65+ | 2832 | 23.6 | 2704 | 22.6 | 2346 | 19.6 | 2083 | 17.4 | 2025 | 16.9 | 11990 |
| Race/Ethnicity | |||||||||||
| Non-Hispanic White | 3831 | 20.2 | 3716 | 19.6 | 3545 | 18.7 | 3657 | 19.3 | 4171 | 22.0 | 18920 |
| Non-Hispanic Black | 291 | 20.6 | 293 | 20.7 | 345 | 24.4 | 300 | 21.2 | 187 | 13.2 | 1416 |
| Hispanic | 1099 | 19.1 | 1205 | 21.0 | 1241 | 21.6 | 1226 | 21.3 | 978 | 17.0 | 5749 |
| Asian/PI | 715 | 20.1 | 718 | 20.1 | 804 | 22.6 | 750 | 21.0 | 577 | 16.2 | 3564 |
| American Indian/Other | 33 | 16.9 | 37 | 19.0 | 33 | 16.9 | 36 | 18.5 | 56 | 28.7 | 195 |
| Socioeconomic Status | |||||||||||
| Lowest SES | 924 | 22.9 | 828 | 20.5 | 858 | 21.3 | 672 | 16.6 | 755 | 18.7 | 4037 |
| Lower-Middle SES | 1127 | 20.7 | 1027 | 18.9 | 1070 | 19.7 | 1039 | 19.1 | 1172 | 21.6 | 5435 |
| Middle SES | 1239 | 19.6 | 1212 | 19.2 | 1178 | 18.6 | 1257 | 19.9 | 1438 | 22.7 | 6324 |
| Higher-Middle SES | 1357 | 19.8 | 1303 | 19.0 | 1382 | 20.1 | 1420 | 20.7 | 1398 | 20.4 | 6860 |
| Highest SES | 1322 | 18.4 | 1599 | 22.2 | 1480 | 20.6 | 1581 | 22.0 | 1206 | 16.8 | 7188 |
| Insurance Type | |||||||||||
| Managed Care | 2528 | 17.9 | 2763 | 19.5 | 2926 | 20.7 | 3111 | 22.0 | 2822 | 19.9 | 14150 |
| Medicare | 1913 | 25.0 | 1687 | 22.0 | 1423 | 18.6 | 1194 | 15.6 | 1436 | 18.8 | 7653 |
| Medicaid | 604 | 22.2 | 532 | 19.5 | 534 | 19.6 | 563 | 20.7 | 492 | 18.1 | 2725 |
| Other Insurance | 675 | 17.6 | 717 | 18.7 | 730 | 19.1 | 764 | 20.0 | 939 | 24.5 | 3825 |
| Not insured | 145 | 16.3 | 155 | 17.4 | 202 | 22.7 | 223 | 25.1 | 164 | 18.4 | 889 |
| Unknown | 104 | 17.3 | 115 | 19.1 | 153 | 25.4 | 114 | 18.9 | 116 | 19.3 | 602 |
| Marital Status | |||||||||||
| Not Married | 3300 | 22.5 | 3051 | 20.8 | 2905 | 19.8 | 2821 | 19.2 | 2611 | 17.8 | 14688 |
| Married | 2669 | 17.6 | 2918 | 19.3 | 3063 | 20.2 | 3148 | 20.8 | 3358 | 22.2 | 15156 |
| Charlson Comorbidity Score | |||||||||||
| CCS 0 | 2664 | 18.7 | 2699 | 19.0 | 2844 | 20.0 | 2967 | 20.9 | 3045 | 21.4 | 14219 |
| CCS 1 | 1360 | 20.0 | 1394 | 20.5 | 1328 | 19.5 | 1321 | 19.4 | 1404 | 20.6 | 6807 |
| CCS 2+ | 1597 | 23.7 | 1468 | 21.8 | 1338 | 19.9 | 1204 | 17.9 | 1119 | 16.6 | 6726 |
| CCS Unknown | 348 | 16.6 | 408 | 19.5 | 458 | 21.9 | 477 | 22.8 | 401 | 19.2 | 2092 |
| Stage | |||||||||||
| Stage 1 | 1345 | 18.6 | 1391 | 19.2 | 1485 | 20.5 | 1542 | 21.3 | 1475 | 20.4 | 7238 |
| Stage 2 | 473 | 19.0 | 480 | 19.2 | 461 | 18.5 | 548 | 22.0 | 534 | 21.4 | 2496 |
| Stage 3 | 2148 | 19.1 | 2161 | 19.2 | 2203 | 19.6 | 2238 | 19.9 | 2513 | 22.3 | 11263 |
| Stage 4 | 2003 | 22.6 | 1937 | 21.9 | 1819 | 20.6 | 1641 | 18.5 | 1447 | 16.4 | 8847 |
| NCCN Treatment Adherence | |||||||||||
| Adherent | 1911 | 16.7 | 2133 | 18.7 | 2262 | 19.8 | 2358 | 20.6 | 2755 | 24.1 | 11419 |
| Non-Adherent | 4058 | 22.0 | 3836 | 20.8 | 3706 | 20.1 | 3611 | 19.6 | 3214 | 17.4 | 18425 |
| Hospital Quality Measure | |||||||||||
| Low | 2157 | 30.9 | 1594 | 22.8 | 1315 | 18.8 | 1121 | 16.0 | 803 | 11.5 | 6990 |
| Intermediate | 3036 | 17.6 | 3488 | 20.2 | 3590 | 20.8 | 3597 | 20.8 | 3564 | 20.6 | 17275 |
| High | 776 | 13.9 | 887 | 15.9 | 1063 | 19.1 | 1251 | 22.4 | 1602 | 28.7 | 5579 |
| Closest High Quality Hospital | |||||||||||
| <9 km | 1843 | 30.9 | 1850 | 31.0 | 975 | 16.3 | 809 | 13.6 | 492 | 8.2 | 5969 |
| 9–14 km | 1100 | 18.4 | 1240 | 20.8 | 2003 | 33.6 | 1109 | 18.6 | 517 | 8.7 | 5969 |
| 15–24 km | 860 | 14.4 | 1082 | 18.1 | 1404 | 23.5 | 1879 | 31.5 | 743 | 12.4 | 5968 |
| 25–48 km | 1023 | 17.1 | 1052 | 17.6 | 862 | 14.4 | 1375 | 23.0 | 1657 | 27.8 | 5969 |
| >48 km | 1143 | 19.1 | 745 | 12.5 | 724 | 12.1 | 797 | 13.4 | 2560 | 42.9 | 5969 |
Statistical significance of differences between groups were calculated using chi-square tests. P-values were <0.001 for all categories
CCS, Charlson Comorbidity Score; km, kilometers; NCCN, National Comprehensive Cancer Network; PI, Pacific Islander; SES, socioeconomic status
Table 5:
Patient Characteristics by Distance to Closest High Quality Hospital (n=29,844)*
| < 9 km | 9 – 14 km | 15 – 24 km | 25 – 48 km | > 48 km | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | N | % | N | % | N | % | N | % | N | % | N |
| 18–44 | 877 | 20.8 | 893 | 21.2 | 895 | 21.3 | 817 | 19.4 | 728 | 17.3 | 4210 |
| 45–54 | 1328 | 20.7 | 1309 | 20.4 | 1273 | 19.8 | 1353 | 21.1 | 1160 | 18.1 | 6423 |
| 55–64 | 1444 | 20.0 | 1452 | 20.1 | 1455 | 20.1 | 1401 | 19.4 | 1469 | 20.3 | 7221 |
| 65+ | 2320 | 19.3 | 2315 | 19.3 | 2345 | 19.6 | 2398 | 20.0 | 2612 | 21.8 | 11990 |
| Race/Ethnicity | |||||||||||
| Non-Hispanic White | 3418 | 18.1 | 3399 | 18.0 | 3547 | 18.7 | 4034 | 21.3 | 4522 | 23.9 | 18920 |
| Non-Hispanic Black | 308 | 21.8 | 319 | 22.5 | 436 | 30.8 | 234 | 16.5 | 119 | 8.4 | 1416 |
| Hispanic | 1121 | 19.5 | 1275 | 22.2 | 1267 | 22.0 | 1050 | 18.3 | 1036 | 18.0 | 5749 |
| Asian/PI | 1083 | 30.4 | 939 | 26.3 | 692 | 19.4 | 619 | 17.4 | 231 | 6.5 | 3564 |
| American Indian/Other | 39 | 20.0 | 37 | 19.0 | 26 | 13.3 | 32 | 16.4 | 61 | 31.3 | 195 |
| Socioeconomic Status | |||||||||||
| Lowest SES | 814 | 20.2 | 902 | 22.3 | 691 | 17.1 | 553 | 13.7 | 1077 | 26.7 | 4037 |
| Lower-Middle SES | 851 | 15.7 | 1094 | 20.1 | 1006 | 18.5 | 955 | 17.6 | 1529 | 28.1 | 5435 |
| Middle SES | 1165 | 18.4 | 1163 | 18.4 | 1196 | 18.9 | 1185 | 18.7 | 1615 | 25.5 | 6324 |
| Higher-Middle SES | 1433 | 20.9 | 1264 | 18.4 | 1480 | 21.6 | 1529 | 22.3 | 1154 | 16.8 | 6860 |
| Highest SES | 1706 | 23.7 | 1546 | 21.5 | 1595 | 22.2 | 1747 | 24.3 | 594 | 8.3 | 7188 |
| Insurance Type | |||||||||||
| Managed Care | 3034 | 21.4 | 2867 | 20.3 | 3055 | 21.6 | 3166 | 22.4 | 2028 | 14.3 | 14150 |
| Medicare | 1412 | 18.5 | 1421 | 18.6 | 1385 | 18.1 | 1393 | 18.2 | 2042 | 26.7 | 7653 |
| Medicaid | 595 | 21.8 | 662 | 24.3 | 496 | 18.2 | 399 | 14.6 | 573 | 21.0 | 2725 |
| Other Insurance | 647 | 16.9 | 684 | 17.9 | 702 | 18.4 | 749 | 19.6 | 1043 | 27.3 | 3825 |
| Not insured | 166 | 18.7 | 187 | 21.0 | 203 | 22.8 | 167 | 18.8 | 166 | 18.7 | 889 |
| Unknown | 115 | 19.1 | 148 | 24.6 | 127 | 21.1 | 95 | 15.8 | 117 | 19.4 | 602 |
| Marital Status | |||||||||||
| Not Married | 3205 | 21.8 | 3049 | 20.8 | 2926 | 19.9 | 2815 | 19.2 | 2693 | 18.3 | 14688 |
| Married | 2764 | 18.2 | 2920 | 19.3 | 3042 | 20.1 | 3154 | 20.8 | 3276 | 21.6 | 15156 |
| Charlson Comorbidity Score | |||||||||||
| CCS 0 | 2844 | 20.0 | 2800 | 19.7 | 2789 | 19.6 | 2925 | 20.6 | 2861 | 20.1 | 14219 |
| CCS 1 | 1324 | 19.5 | 1319 | 19.4 | 1380 | 20.3 | 1360 | 20.0 | 1424 | 20.9 | 6807 |
| CCS 2+ | 1296 | 19.3 | 1363 | 20.3 | 1407 | 20.9 | 1250 | 18.6 | 1410 | 21.0 | 6726 |
| CCS Unknown | 505 | 24.1 | 487 | 23.3 | 392 | 18.7 | 434 | 20.7 | 274 | 13.1 | 2092 |
| Stage | |||||||||||
| Stage 1 | 1476 | 20.4 | 1548 | 21.4 | 1500 | 20.7 | 1396 | 19.3 | 1318 | 18.2 | 7238 |
| Stage 2 | 536 | 21.5 | 502 | 20.1 | 475 | 19.0 | 477 | 19.1 | 506 | 20.3 | 2496 |
| Stage 3 | 2246 | 19.9 | 2177 | 19.3 | 2261 | 20.1 | 2312 | 20.5 | 2267 | 20.1 | 11263 |
| Stage 4 | 1711 | 19.3 | 1742 | 19.7 | 1732 | 19.6 | 1784 | 20.2 | 1878 | 21.2 | 8847 |
| NCCN Treatment Adherence | |||||||||||
| Adherent | 2501 | 21.9 | 2247 | 19.7 | 2228 | 19.5 | 2289 | 20.0 | 2154 | 18.9 | 11419 |
| Non-Adherent | 3468 | 18.8 | 3722 | 20.2 | 3740 | 20.3 | 3680 | 20.0 | 3815 | 20.7 | 18425 |
| Hospital Quality Measure | |||||||||||
| Low | 827 | 11.8 | 1042 | 14.9 | 1448 | 20.7 | 1477 | 21.1 | 2196 | 31.4 | 6990 |
| Intermediate | 3017 | 17.5 | 3712 | 21.5 | 3604 | 20.9 | 3687 | 21.3 | 3255 | 18.8 | 17275 |
| High | 2125 | 38.1 | 1215 | 21.8 | 916 | 16.4 | 805 | 14.4 | 518 | 9.3 | 5579 |
| Distance to Receive Care | |||||||||||
| <6 km | 1843 | 31.5 | 1100 | 18.8 | 860 | 14.7 | 1023 | 17.5 | 1023 | 17.5 | 5849 |
| 6–9 km | 1850 | 29.5 | 1240 | 19.8 | 1082 | 17.2 | 1052 | 16.8 | 1052 | 16.8 | 6276 |
| 10–16 km | 975 | 16.0 | 2003 | 32.8 | 1404 | 23.0 | 862 | 14.1 | 862 | 14.1 | 6106 |
| 17–32 km | 809 | 12.4 | 1109 | 16.9 | 1879 | 28.7 | 1375 | 21.0 | 1375 | 21.0 | 6547 |
| >32 km | 492 | 9.7 | 517 | 10.2 | 743 | 14.7 | 1657 | 32.7 | 1657 | 32.7 | 5066 |
Statistical significance of differences between groups were calculated using chi-square tests. P-values were <0.001 for all categories
CCS, Charlson Comorbidity Score; km, kilometers; NCCN, National Comprehensive Cancer Network; PI, Pacific Islander; SES, socioeconomic status
Non-Hispanic White women and American Indian/Other race made up the largest proportions of women traveling >32km for care (Table 4). Non-Hispanic Black women were the least likely to travel greater distances for care across all analyses. Overall, women diagnosed in Stage 4 were the least likely to travel far, regardless of race, SES, or insurance (data not shown). Noteworthy, Asian/Pacific Islanders (30.4%) and Non-Hispanic Blacks (21.8%) made up the largest proportion of those living <9km of the closest high-quality hospital (Table 5). While less than 10% of women of the highest SES lived >48km from a high-quality hospital, more than 25% of the lower SES quintiles lived >48km.
4. Discussion
Overall, just over one third of women received NCCN guideline-adherent care. This is possibly a result of comorbidities, disease progression, access to specialized facilities, and lower SES.5–7,12,13,39 Studies examining other cancers have shown similar low rates (<50%) of adherence to NCCN guidelines.40,41 The current study found residential location to be significantly associated with the likelihood of receiving NCCN adherent treatment for women diagnosed with OC. Due to a growing awareness of the impact of residential location on health and the development of more sophisticated analysis tools, the value of geospatial research in cancer is increasing.42 With the availability of geocoded addresses and the use of GAMs, we identified disparities within the state of California where women were more or less at risk of non-adherent care, despite adjusting for numerous important factors and further showed that the impact of location depended on stage at diagnosis. This methodology was previously used to examine late-stage OC survival disparities across California census tracts.8
Differences in spatial patterns of care are increasingly being recognized in the OC literature. One population-based study exploring geographic patterns in treatment delivery and epithelial OC mortality by Health Referral Regions found hospital region to be associated with regional discrepancies in cancer-specific surgery, with women in more remote areas less likely to receive it.14 Our results also show that women living in remote areas of central California, especially those diagnosed at early stages, are more vulnerable to receiving substandard care. Although there is a low density of high-quality centers in nonmetropolitan areas, the risk of treatment non-adherence in California differed depending on residential location. While patients living in rural areas of Northern California had favorable odds, those residing in counties encompassing greater Los Angeles received nonstandard care despite the availability of high-quality centers.
It is well documented that the location of initial treatment for OC is important, in particular high-volume and high-quality centers showing superior outcomes.19,20,22,43 A comprehensive cancer center examining its own patients’ travel distance found that those residing farthest from the hospital had worse cervical cancer outcomes,44 yet a similar analyses of gynecological malignancies treated at a National Cancer Institute-designated center found women living less than 10 miles were less likely to be treatment compliant although those making the longest journey had greater mortality before treatment completion.45 We found women were more likely to access a high-quality hospital if they lived close to one, an association similarly observed by Tracey and colleagues.17 They found more than half of women living within 5km of high-quality hospitals utilized these facilities compared to 16% of women in the farthest quintile.17
The considerable financial challenges already faced coupled with the additional burden that travel poses for women diagnosed with OC must be acknowledged.45 Travel is a geographic barrier to treatment and may disproportionately affect those of lower SES,46 a point illustrated by their overall remoteness from high-quality centers. The implications of geographic access and travel are worth noting, given that women of lower SES and with safety-net insurance or uninsured were less likely to travel for care, obtain care at quality centers, or receive NCCN guideline treatment. Furthermore, women may choose to stay local for care. One study found that approximately 20% of women indicated that they would not travel over 50 miles for care, despite the potential survival advantages.47 This may be particularly true for older women, those with comorbidities, or with limited social support. Greater distances may be less viable for women who may be managing multiple conditions.48 We found women with two or more comorbidities and over 65 years were less likely to travel farther, which is consistent with prior work that older age is associated with shorter journeys.24,48
The present study has several noteworthy features. Among them is the large sample size, with almost 20 years of data available from the CCR, a registry with demonstrated reliability. Additionally, the investigation of geocoded residential location and its differing effects on OC treatment adherence by stage and social demographics is novel. Unlike previous studies that used zip code and census block variables as spatial proxies, utilizing an individual-level measure of patient location allows for a more accurate assessment of the effect of geographic location. Furthermore, the network analyst tool in GIS provided a more precise calculation of travel distance. We were also able to adjust for several important covariates including comorbidity which has been shown to be a main reason for failure to complete chemotherapy.39 Lastly, the GAM framework is particularly useful for investigating nonlinear geographic disparities while accounting for known risk factors.
The study is limited by the potential for reporting bias and the presence of unmeasured confounders given reliance on previously collected registry data. Furthermore, the collection and interpretation of CCR data may be limited by the possibility that reporting facilities are not the main treating hospital, satellite hospitals may report under one hospital, and chemotherapy treatment may be underreported. However, these are thought to be uncommon and unlikely to influence the results in this secondary analysis of large population-based data. Also, we cannot account for several potentially important access characteristics such as travel times and utilization or availability of public transportation. We assumed patients would drive the shortest route between their residence and hospitals to compute distances. However, when reliable transportation is unavailable, difficult, or expensive, travel may pose additional burdens to patients of lower SES. Another limitation is that the CCR does not collect information on the treating physician’s OC case volume or their medical specialty. These characteristics may vary geographically and have been previously found to be predictors of treatment adherence and survival.49–51
5. Conclusions
Quality care is vital to decreasing OC mortality, yet the majority of women do not receive it. Future research should examine how location differentially affects access to care and impacts survival. Non-Hispanic Black women, those of lower SES and non-married women were less likely to travel far for care and were more likely to receive non-adherent treatment. Spatial analyses of geographic barriers, using linear and nonlinear methods may provide an opportunity for targeted intervention to broaden access to care among vulnerable populations. Providing transportation, opening satellite clinics, employing patient navigators, and ensuring that those services are covered by all insurance carriers are potential avenues to facilitate access to high-quality care, ultimately improving OC survival overall.16,45
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(6):588–594. doi: 10.1001/jama.2017.21926 [DOI] [PubMed] [Google Scholar]
- 3.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol. 2012;125(1):19–24. doi: 10.1016/j.ygyno.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Jonasch E, Agarwal N, et al. Ovarian cancer, Version 2. 2014 featured updates to the NCCN guidelines. JNCCN - J Natl Compr Cancer Nerwork. 2014;12(2):175–181. [Google Scholar]
- 5.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121(6):1226–1234. doi: 10.1097/AOG.0b013e3182922a17 [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Chang J, Ziogas A, Anton-Culver H, Vieira VM. Spatial analysis of adherence to treatment guidelines for advanced-stage ovarian cancer and the impact of race and socioeconomic status. Gynecol Oncol. 2014;134(1):60–67. doi: 10.1016/j.ygyno.2014.03.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bristow RE, Chang J, Ziogas A, Chavez LR, Anton-culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. 2015;125(4):833–842. doi: 10.1097/AOG.0000000000000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bristow RE, Chang J, Ziogas A, Gillen DL, Bai L, Vieira VM. Spatial analysis of advanced-stage ovarian cancer mortality in California. Am J Obstet Gynecol. 2015;213(1):43.e1–8. doi: 10.1016/j.ajog.2015.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodeib M, Chang J, Liu F, et al. Socioeconomic status as a predictor of adherence to treatment guidelines for early-stage ovarian cancer. Gynecol Oncol. 2015;138(1):121–127. doi: 10.1016/j.ygyno.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: A report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133(2):353–361. doi: 10.1016/j.ygyno.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronin KA, Howlader N, Stevens JL, Trimble EL, Harlan LC, Warren JL. Racial disparities in the receipt of guideline care and cancer deaths for women with ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2018:cebp.0285.2018. doi: 10.1158/1055-9965.EPI-18-0285 [DOI] [PubMed] [Google Scholar]
- 12.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823–832. doi: 10.1093/jnci/djt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long B, Chang J, Ziogas A, Tewari KS, Anton-Culver H, Bristow RE. Impact of race, socioeconomic status, and the health care system on the treatment of advanced-stage ovarian cancer in California. Am J Obstet Gynecol. 2015;212(4):468.e1–468.e9. doi: 10.1016/j.ajog.2014.10.1104 [DOI] [PubMed] [Google Scholar]
- 14.Fairfield KM, Lee Lucas F, Earle CC, Small L, Trimble EL, Warren JL. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the Medicare population. Cancer. 2010;116(20):4840–4848. doi: 10.1002/cncr.25242 [DOI] [PubMed] [Google Scholar]
- 15.Dehaeck U, McGahan CE, Santos JL, Carey MS, Swenerton KD, Kwon JS. The impact of geographic variations in treatment on outcomes in ovarian cancer. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2013;23(2):282–287. doi: 10.1097/IGC.0b013e31827b87b1 [DOI] [PubMed] [Google Scholar]
- 16.Stewart SL, Cooney D, Hirsch S. Effect of gynecologic oncologist availability on ovarian cancer mortality. World J Obstet Gynecol. 2014;3(2):71–77. doi: 10.5317/wjog.v3.i2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tracey E, Hacker NF, Young J, Armstrong BK. Effects of access to and treatment in specialist facilities on survival from epithelial ovarian cancer in Australian women: A data linkage study. Int J Gynecol Cancer. 2014;24(7):1232–1240. doi: 10.1097/IGC.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 18.Cowan RA, O’Cearbhaill RE, Gardner GJ, et al. Is it time to centralize ovarian cancer care in the United States? Ann Surg Oncol. 2016;23(3):989–993. doi: 10.1245/s10434-015-4938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galvan-Turner VB, Chang J, Ziogas A, Bristow RE. Observed-to-expected ratio for adherence to treatment guidelines as a quality of care indicator for ovarian cancer. Gynecol Oncol. 2015;139(3):495–499. doi: 10.1016/j.ygyno.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115(3):334–338. doi: 10.1016/j.ygyno.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 21.Polsky D, Armstrong KA, Randall TC, et al. Explaining variations in chemotherapy utilization in ovarian cancer : The relative contribution of geography. Heal Serv Res. 2006:2201–2218. doi: 10.1111/j.1475-6773.2006.00596.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: Survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132(2):403–410. doi: 10.1016/j.ygyno.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 23.Chase DM, Fedewa S, Chou TS, Chen A, Ward E, Brewster WR. Disparities in the allocation of treatment in advanced ovarian cancer: Are there certain patient characteristics associated with nonstandard therapy? Obstet Gynecol. 2012;119(1):68–77. doi: 10.1097/AOG.0b013e31823d4006 [DOI] [PubMed] [Google Scholar]
- 24.Wasif N, Chang YH, Pockaj BA, Gray RJ, Mathur A, Etzioni D. Association of distance traveled for surgery with short- and long-term cancer outcomes. Ann Surg Oncol. 2016;23(11):3444–3452. doi: 10.1245/s10434-016-5242-z [DOI] [PubMed] [Google Scholar]
- 25.Parikh-Patel A, Allen M, Wright WE, et al. Validation of self-reported cancers in the California Teachers Study. Am J Epidemiol. 2003;157(6):539–545. doi: 10.1093/aje/kwg006 [DOI] [PubMed] [Google Scholar]
- 26.California Cancer Registry. How complete are California Cancer Registry data? http://ccrcal.org/Inside_CCR/FAQ.shtml#how complete are ccr data. Accessed December 15, 2018.
- 27.Morgan R, Alvarez R, Gerhsenson D, Al E. Update of the NCCN ovarian cancer practice guidelines. Oncology. 1997;11:95–107. [PubMed] [Google Scholar]
- 28.Morgan R, Alvarez R, Armstrong D, Copeland L, Fiorica J, Fishman D. NCCN practice guidelines for ovarian cancer. Version 2000. J Natl Compr Cancer Netw. 2000. [Google Scholar]
- 29.Morgan R, Alvarez R, Armstrong D, Copeland L, Fiorica J, Fishman D. Ovarian cancer guideline In: National Comprehensive Cancer Network. Fort Washington, PA; 2002. [Google Scholar]
- 30.Morgan R, Alvarez R, Armstrong D. Ovarian cancer. Version 1.2005 In: National Comprehensive Cancer Network. ; 2005. [Google Scholar]
- 31.Morgan R, Alvarez R, Armstrong D, et al. Ovarian cancer, version 3.2012. J Natl Compr Netw. 2012;10(11):1339–1349. [DOI] [PubMed] [Google Scholar]
- 32.Morgan R, Alvarez R, Armstrong D, et al. Ovarian cancer, version 2.2013. J Natl Compr Cancer Netw. 2013;11(10):1199–1209. [DOI] [PubMed] [Google Scholar]
- 33.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 34.Lichtensztajn DY, Giddings BM, Morris CR, Parikh-Patel A, Kizer KW. Comorbidity index in central cancer registries : the value of hospital discharge data. Clin Epidemiol. 2017;9:601–609. doi: 10.2147/CLEP.S146395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–711. doi: 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Schupp CW, Harrati A, Clarke C, Keegan THM GS. Developing an area-based socioeconomic measure from American Community Survey data Cancer Prevention Institute of California, Fremont, California: 2014. 2014:1–17. [Google Scholar]
- 37.Hastie T, Tibshirani R. Generalized Additive Model. 1990:297–310. [Google Scholar]
- 38.Webster T, Vieira V, Weinberg J, Aschengrau A. Method for mapping population-based case-control studies: An application using generalized additive models. Int J Health Geogr. 2006;5:1–10. doi: 10.1186/1476-072X-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson BK, Martin JY, Shah MM, Straughn JM, Leath CA. Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014;133(2):142–146. doi: 10.1016/j.ygyno.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser BC, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. Hpb. 2012;14(8):539–547. doi: 10.1111/j.1477-2574.2012.00496.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaendler KS, Chang J, Penner KR, Bristow RE. Disparities in Adherence to National Comprehensive Cancer Network Treatment Guidelines and Survival for Stage IB – IIA Cervical Cancer in California. Obstet Gynecol. 2018;131(5):899–908. doi: 10.1097/AOG.0000000000002591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulos DNK, Ghali RR, Ibrahim EM, Boulos MNK, Abdelmalik P. An eight-year snapshot of geospatial cancer research (2002–2009): Clinico-epidemiological and methodological findings and trends. Med Oncol. 2011;28(4):1145–1162. doi: 10.1007/s12032-010-9607-z [DOI] [PubMed] [Google Scholar]
- 43.Reames B, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260(2):244–251. doi: 10.1097/SLA.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrington DA, Dilley SE, Landers EE, et al. Distance from a Comprehensive Cancer Center: A proxy for poor cervical cancer outcomes? Gynecol Oncol. 2016;143(3):617–621. doi: 10.1016/j.ygyno.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temkin SM, Fleming SA, Amrane S, Schluterman N, Terplan M. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol Oncol. 2015;137(3):497–502. doi: 10.1016/j.ygyno.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 46.Guagliardo MF. Spatial accessibility of primary care: concepts, methods and challenges. Int J Heal Geogr. 2004;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shalowitz DI, Nivasch E, Burger RA, Schapira MM. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148(1):42–48. doi: 10.1016/j.ygyno.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 48.Jindal M, Zheng C, Quadri HS, et al. Why do long-distance travelers have improved pancreatectomy outcomes? J Am Coll Surg. 2017;225(2):216–225. doi: 10.1016/j.jamcollsurg.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: Impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118(3):262–267. doi: 10.1016/j.ygyno.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 50.Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer. 2008;44(7):992–999. doi: 10.1016/j.ejca.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 51.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109(10):2031–2042. doi: 10.1002/cncr.22604 [DOI] [PubMed] [Google Scholar]