Abstract
Objective:
Alterations in the serum metabolome may be detectable in at-risk individuals prior to the onset of coronary heart disease (CHD). Identifying metabolomic signatures associated with CHD may provide insight into disease etiology and prevention.
Approach and Results:
Metabolomic profiling (chromatography-mass spectrometry) was performed in 2,232 African Americans and 1,366 European Americans from the Atherosclerosis Risk in Communities (ARIC) study. We applied Cox regression with least absolute shrinkage and selection operator (LASSO) to select metabolites associated with incident CHD. A metabolite risk score (MRS) was constructed to evaluate whether the MRS predicted CHD risk beyond traditional risk factors (TRFs). After 30 years of follow-up, we observed 633 incident CHD cases. Thirty-two metabolites were selected by LASSO to be associated with CHD, and 19 of the 32 showed significant individual associations with CHD, including a sugar substitute, erythritol. Theophylline (HR [95% CI] = 1.16 [1.09- 1.25]) and gamma linolenic acid (HR [95% CI] = 0.89 [0.81- 0.97]) showed the greatest positive and negative associations with CHD, respectively. A 1 SD greater standardized MRS was associated with a 1.37 fold higher risk of CHD (HR [95% CI] = 1.37 [1.27- 1.47]). Adding the MRS to the TRFs significantly improved model predictive performance (30-year risk prediction: Δ C-statistic [95% CI] = 0.016 [0.008- 0.024], continuous net reclassification index [95% CI] = 0.522 [0.480- 0.556], integrated discrimination index [95%CI] = 0.038 [0.019- 0.065]).
Conclusion:
We identified 19 metabolites from known and novel metabolic pathways that collectively improved CHD risk prediction.
Keywords: metabolome, biomarker, coronary heart disease, risk prediction, Cardiovascular Disease, Biomarkers, Epidemiology, Risk Factors, Primary Prevention
Graphical Abstract
Introduction
Despite a decline in coronary heart disease (CHD) incidence in most countries, CHD remains the leading cause of death globally 1, 2. Identifying individuals at increased risk of CHD can improve prospects for delayed onset and improved treatment. Over the last decades, a number of traditional risk factors (TRFs) have been discovered for CHD 3–6, and those risk factors act synergistically. Multiple studies have demonstrated that integration of novel biomarkers, such as a genetic risk score, cardiac troponin-T or coronary artery calcium, can enhance CHD risk assessment 7. However, such studies have identified few novel and tractable biological pathways. Other technologies, such as metabolomics, may help to identify novel biomarkers and pathways involved in CHD onset and progression.
The metabolomic approach systematically evaluates small-molecule metabolites in biologic samples that reflect the state of the system or whole organism provide additional insights into disease pathology 8–10. Studies have successfully identified novel signatures of CHD risk using metabolomic approaches, and the potential value of such signatures beyond the TRFs has been demonstrated 11–18. However, these studies were carried out predominantly in individuals of European-ancestry and the spectrum of metabolites was limited.
We hypothesized that a collection of metabolites is associated with CHD risk independent of established risk factors, and adding a combination of CHD related metabolites to TRFs provides improved risk prediction. Hence, we conducted a metabolome-wide analysis of CHD risk in 3,598 African and European Americans from the Atherosclerosis Risk in Communities (ARIC) study to identify metabolomic signatures that better identify individuals at-risk.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) (https://biolincc.nhlbi.nih.gov) and the database of Genotypes and Phenotypes (dbGaP) (Study Accession: phs000090.v1.p1).
Study Population
The ARIC study is a prospective cohort study of 15,792 individuals aged 45-64 years from four U.S. communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD) originally sampled between 1987 and 1989. A detailed description of the ARIC study design was published elsewhere 19. Metabolomic profiles of baseline stored serum samples were measured in 1,997 African Americans in 2010 (phase 1), and 2,152 African and European Americans in 2014 (phase 2). Included in this analysis were 3,598 participants who did not have prevalent CHD or impaired renal function (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2) at the baseline examination, with CHD follow-up and complete covariate information. The ARIC study was approved by the institutional review boards at each site, and written informed consent was obtained from all study participants.
Assessment of incident CHD and Covariates
In ARIC, CHD incidence was ascertained by reviewing death certificate and hospital discharge records, and contacting participants annually, identifying hospitalizations and deaths during the prior year 20. Incident CHD was defined as definite fatal CHD, definite or probable myocardial infarction, silent myocardial infarction between examinations as determined by electrocardiography, or coronary revascularization 19 that occurred on or before December 31st 2016. Covariates used in the analyses were measured at the baseline examination or home interview. Blood pressure was measured by trained technicians following a standard protocol 21. Plasma total cholesterol levels were measured by enzymatic methods 22, 23. High-density lipoprotein (HDL) cholesterol was measured after precipitation of the plasma with MgCl2 and dextran sulfate by the method of Warnick et al 24. Self-reported smoking status (categorized as current smoker, former smoker and never smoker) and antihypertensive medication use during the past 2 weeks were obtained by questionnaire. Prevalent CHD was defined as a self-report of previous myocardial infarction or coronary reperfusion procedure, or electrocardiography (ECG) evidence of a previous myocardial infarction. Diabetes was defined as a fasting serum glucose level ≥126 mg/dL or non-fasting serum glucose level ≥200 mg/dL, a self-reported physician diagnosis of diabetes, or the use of hypoglycemic medication. eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation 25. Carotid intima-media thickness (C-IMT) was measured using the ultrasound procedure that has been previously described 26–28. Briefly, the C-IMT was assessed in three 1cm segments in the distal common carotid, the carotid artery bifurcation and the proximal internal carotid arteries. We used the mean of the mean measurements for both right and left sides of these segments at the baseline examination for analyses. Plaque presence or absence was adjudicated by trained readers; plaque was adjudicated to be present if two of three of the following criteria were abnormal: wall thickness, wall shape, and wall texture 27, 29.
Assessment of the Serum Metabolome
Metabolomic profiling was completed in two phases, 2010 and 2014, using serum samples which had been stored at −80 °C since collection at the baseline examination in 1987-1989. A total of 384 metabolites were detected and semi-quantified by Metabolon Inc. (Durham, USA) using untargeted gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry (GC-MS and LC-MS) 30, 31. 139 metabolites were excluded because: 1) their values were missing/below-the-detection-limit in more than 25% of the samples in either phase; or 2) their Pearson correlation coefficients were < 0.3 between 97 pairs of duplicate samples measured during both phases. After this assessment, 245 metabolites, including amino acids, lipids, nucleotides, peptides, carbohydrates, cofactors and vitamins, xenobiotics, and energy related metabolites were included in the present study.
Statistical Analysis
Within each phase, metabolite levels were winsorized at the 1st and 99th percentile, and missing/below-the-detection-limit values were imputed with the lowest detected value of that metabolite. Prior to the analyses, metabolites were standardized (mean = 0 and SD = 1) within each phase.
To select a subset of the most informative metabolites that were associated with incident CHD, Cox proportional hazard regression models with least absolute shrinkage and selection operator (LASSO) procedures were applied in the combined phase 1 and 2 samples. We fit LASSO with 10-fold cross validation incorporating 245 metabolites and CHD TRFs defined according to ARIC CHD risk score, including age, sex, race, smoking status, systolic blood pressure (SBP), anti-hypertensive medication use, HDL and total cholesterol levels, and diabetes status 4, as well as phase and study center. The penalty parameter was chosen as theλthat gave the minimum mean error as determined by 10-fold cross validation. To explore the individual effect of each LASSO selected metabolite on incident CHD, we performed analyses using a Cox model adjusting for TRFs, phase and study centers. The false discovery rate of 5% or less was used to define significance for associations between individual metabolites and CHD.
Metabolites selected by the LASSO procedure were further reduced to those that had significant individual associations with incident CHD, and this subset of metabolites were used to construct a metabolomics risk score (MRS). A continuous MRS was constructed using the sum of selected metabolite levels taking into account the direction of effect. The continuous MRS was standardized (mean = 0 and SD = 1) for further analysis. Quartiles of the continuous MRS were analyzed to examine any potential non-linear associations. Cox regression models were used to calculate whether the continuous MRS or MRS quartiles were associated with incident CHD adjusting for the same covariates described above. The proportional hazards assumption was tested in each Cox model and no violation of the assumption was observed. To explore potential sex- and race- specific effects, we performed secondary analyses testing the association between continuous MRS and incident CHD stratified by sex and race separately. Additionally, we tested the association between the MRS and baseline C-IMT and plaque measurement. The significance threshold was defined as p < 0.05 for the MRS analysis.
To investigate whether the MRS improved risk reclassification and prediction, we computed statistical measures of discrimination, including area under the receiver operating characteristic curve (AUC), the continuous net reclassification index (NRI), and the integrated discrimination index (IDI) 32, 33. The C-statistic and differences in C-statistic were calculated using the method for censored survival data proposed by Uno et al. 34. Additionally, to confirm the internal validity of the MRS and ensure a nearly unbiased estimate of our MRS model prediction performance, we performed 10-fold cross-validations with 100 replicates and reported the average cross-validation C-statistics 34, 35. All statistical analyses were carried out using R version 3.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
During 30 years of follow-up, we identified 633 (17.6%) incident CHD cases among 3,598 African and European American ARIC participants (Table 1). The CHD incidence rate was lower in European Americans as compared to African Americans. In general, ARIC European Americans showed a more favorable baseline CHD risk profile, including less current smokers, lower SBP levels, and less prevalent diabetes, except for lipid profiles, which were similar between African and European Americans. The C-IMT and plaque presence measurements did not differ between the two groups. Baseline characteristics by incidence CHD cases and non-cases were summarized in Supplemental Table I.
Table 1.
Baseline characteristics and number of incident coronary heart disease events among participants, Atherosclerosis Risk in Communities study.
| African Americans | European Americans | P-values | Overall | |
|---|---|---|---|---|
| Participants, n | 2232 | 1366 | 3598 | |
| Incident CHD, n (%) | 359 (16.1%) | 274 (20.1%) | 0.003 | 633 (17.6%) |
| Age (years) | 52.8 ± 5.6 | 54.2 ± 5.7 | <0.001 | 53.3 ± 5.7 |
| Male, n (%) | 801 (35.9%) | 602 (44.1%) | <0.001 | 1403 (40.0%) |
| SBP (mm Hg) | 127.9 ± 21.0 | 119.1 ± 18.2 | <0.001 | 124.5 ± 20.4 |
| Anti-hypertensive medication use, n (%) | 828 (37.1%) | 276 (20.2%) | <0.001 | 1104 (30.7%) |
| HDL-c (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.4 | <0.001 | 1.4 ± 0.4 |
| TC (mmol/L) | 5.5 ± 1.1 | 5.6 ± 1.0 | 0.34 | 5.5 ± 1.1 |
| Prevalent Diabetes, n (%) | 367 (16.4%) | 90 (6.6%) | <0.001 | 457 (12.7%) |
| Smoking status | <0.001 | |||
| Current, n (%) | 1070 (47.9%) | 556 (40.7%) | 1626 (45.3%) | |
| Former, n (%) | 526 (23.6%) | 463 (33.9%) | 989 (27.6%) | |
| Never, n (%) | 636 (28.5%) | 347 (25.4%) | 983 (27.3%) | |
| C-IMT | 0.7 (0.1) | 0.7 (0.2) | 0.9 | 0.7 (0.1) |
| Plaque presence (%) | 592 (28.1%) | 425 (31.6%) | 0.03 | 1017 (29.4%) |
Abbreviations: CHD, coronary heart disease; SBP, systolic blood pressure; HDL-c, high-density lipoprotein cholesterol, TC, serum total cholesterol, and C-IMT, carotid intima-media thickness.
Data are expressed as mean ± standard errors for quantitative traits.
Thirty-two metabolites were identified by LASSO as the best subset predictive of incident CHD. We examined each of the selected metabolites for its association with CHD risk, and 19 out of 32 metabolites had significant individual associations, including 8 amino acids, 3 lipids, 2 nucleotides, 1 peptide, 1 carbohydrates, and 4 xenobiotics (Supplemental Table II). The median hazard ratio (HR) per SD was 1.11 across the 19 metabolites; the 25th percentile and 75th percentile HR per SD was 1.08 and 1.14. Theophylline (HR [95% confidence interval (CI)] = 1.16 [1.09- 1.25]) and linolenate [alpha or gamma; (18:3n3 or 6)] (HR [95% CI] = 0.89 [0.81- 0.97]) had the highest and lowest HRs for CHD, respectively (Figure 1). Fourteen out of 19 selected metabolites were not correlated (|r| < 0.3), while five other metabolites showed moderate inter-correlations (Supplemental Figure I).
Figure 1.
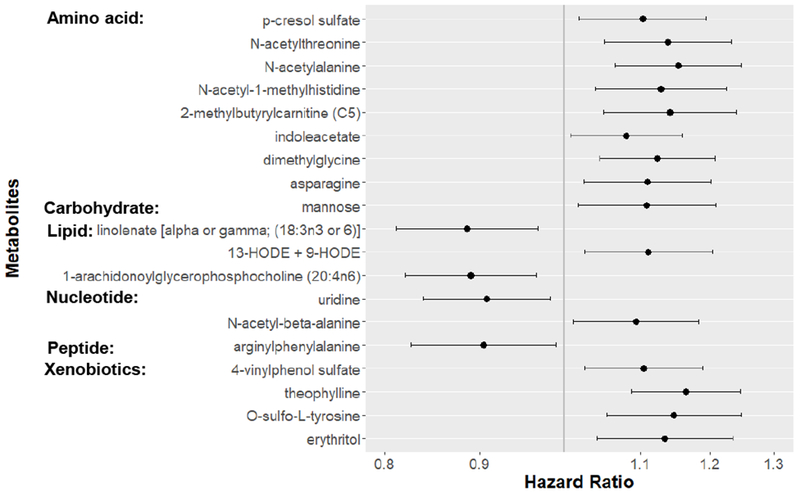
Individual metabolite hazard ratios (95% confidence intervals) per SD increment for 19 LASSO metabolites with significant individual associations with coronary heart disease risk.
The MRS constructed from the 19 metabolites was approximately normally distributed (Supplemental Figure II). Baseline participants’ characteristics by deciles of the MRS were summarized in Supplemental Table III. A 1 SD increase in the standardized MRS was associated with a 1.37 fold greater risk of incident CHD adjusting for TRFs (HR [95% CI]) = 1.37 [1.27-1.47], Table 2). There was a graded association between the MRS quartiles and risk of incident CHD events (p for linear trend = 1.82×10−13) (Table 2), where participants with the highest quartile of MRS had more than 2 fold higher risk of developing CHD compared to participants in the lowest quartile (Q4 vs. Q1 HR [95% CI] = 2.21 [1.72-2.84]). Additional analyses adjusting for BMI and total energy intake did not change the association between MRS and CHD risk. Sex-stratified and race-stratified analyses did not suggest different continuous MRS associations with CHD among males and females, or among African- and European- Americans (Supplemental Table IV). The MRS we created for CHD risk was significantly associated with greater intima-media thickness (log transformed C-IMT measurement, coefficient = 0.007, p = 0.03). But the association between the MRS and plaque presence was modest (Odds Ratio = 0.07, p = 0.07).
Table 2.
Hazard ratios relating metabolite risk score with incident coronary heart disease.
| Contrast | HR | 95% CI | P-value | |
|---|---|---|---|---|
| Continuous MRS (per SD change) | 1.37 | 1.27- 1.47 | <2×10−16 | |
| MRS Quartiles | Q2 vs. Q1 | 1.08 | 0.82- 1.43 | 0.57 |
| Q3 vs. Q1 | 1.36 | 1.04- 1.77 | 0.02 | |
| Q4 vs. Q1 | 2.21 | 1.72- 2.84 | 6.51×10−10 | |
| Trend | 4.63×10−13 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; MRS, metabolite risk score; SD, standard deviation. Each model adjusted for age, sex, race, study center, phase, smoking status, systolic blood pressure, anti-hypertensive medication use, diabetes status, total cholesterol, and high-density lipoprotein cholesterol levels.
Adding the continuous MRS to the TRFs model significantly improved model discrimination performance. The C-statistic [95% CI] increased from 0.724 [0.704-0.744] to 0.740 [0.720-0.759] for 30-year CHD risk prediction (Δ C-statistic [95% CI] = 0.016 [0.008-0.024], Figure 2). Addition of the MRS also showed significant incremental benefit in the analysis of the continuous NRI and IDI (continuous NRI [95% CI] = 0.522 [0.480-0.556], p < 0.001, and IDI [95% CI] = 0.038 [0.019-0.065], p = 0.002). Adding MRS quartiles to the TRFs model similarly improved model performance. The Δ C-statistic [95% CI] for 30-year CHD risk prediction was 0.014 [0.005- 0.022]), and the continuous NRI [95% CI] = 0.475 [0.271-0.634] and IDI [95% CI] = 0.048 [0.014- 0.085].
Figure 2.
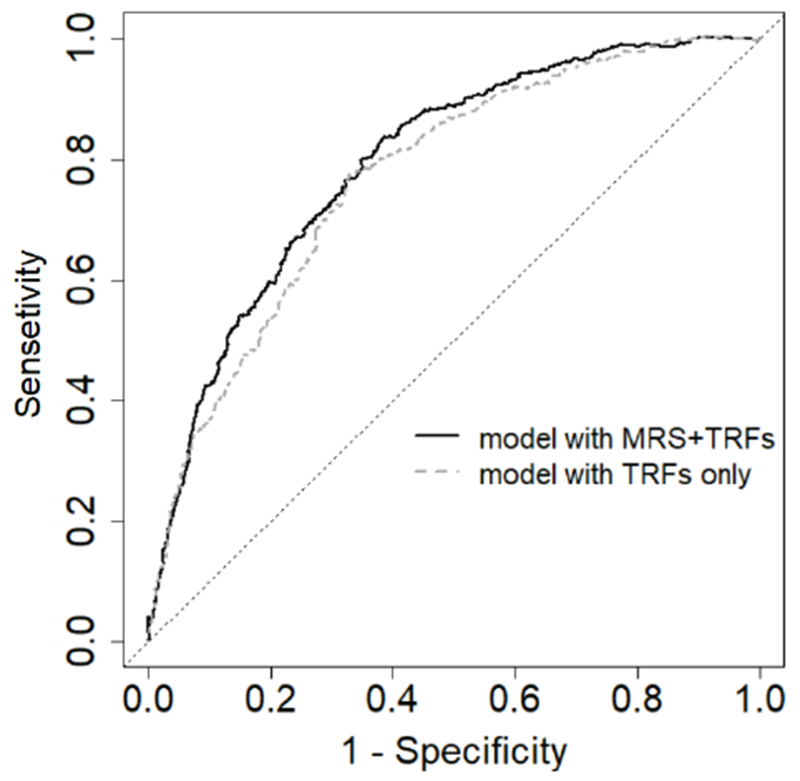
Receive operating characteristic curves for coronary heart disease 30-year risk prediction, comparing a model with traditional risk factors (TRF) only and a model with TRF plus continuous metabolite risk score (MRS).
Using 10-fold cross validation with 100 replicates, we computed cross-validated C-statistics for the TRFs only model (corrected C-statistic: 0.718) and the model with continuous MRS and the TRFs (corrected C-statistic: 0.735). Cross validation did not change the conclusion that adding the continuous MRS to the TRFs model improved discrimination (cross validation corrected Δ C-statistic vs. one time estimated Δ C-statistic: 0.017 vs. 0.016).
Discussion
In this population based prospective study of African and European Americans, we identified a set of 19 metabolites, including amino acids, lipids, peptides, carbohydrates, nucleotide and xenobiotics, that were collectively associated with CHD risk during thirty years of follow-up. A MRS constructed from the 19 metabolites were positively association with C-IMT, a well-described surrogate marker for cardiovascular disease 36, and showed a graded association with CHD risk that were consistent across race and sex. Adding the MRS to the TRFs model significantly improved 30-year incident CHD risk prediction and discrimination. The predictive ability of the MRS was internally validated. Our findings indicate that a metabolite panel can be used to improve CHD risk prediction, compared with TRFs alone.
Previous metabolomic studies have identified multiple metabolites that are associated with CHD risk 11–18, 37–41, some of which were corroborated in our study. For example, metabolites involved in amino acid metabolism, including asparagine, dimethylglycine, and short chain acylcarnitines such as 2-methylbutyrylcarnitine (C5), were associated positively with CHD occurrence in this and other studies 37, 38, 40, 41. We observed that α-linoleic acid was negatively associated with CHD, which is consistent with previous metabolomic study findings, and in line with previous observations that polyunsaturated fatty acids were inversely associated with cardiovascular disease in contrast to saturated and monounsaturated fatty acids 42, 43. Other metabolites such as 13-HODE + 9-HODE (oxidized derivatives of linoleic acid), uridines, and mannose also have been previously reported to be associated with CHD 14, 16, 38, 39, 44, 45. Some metabolites reported previously were not included in our MRS, such as TMAO (14, 15) which was not in our analyzed metabolomic panel. Other CHD metabolites, for examples, polyunsaturated fatty acids, were not selected probably because of high correlations with other LASSO selected metabolites, as LASSO tends to select only one variable from a group of highly correlated variables 46.
Twelve of our 19 selected metabolites have not been previously reported to be associated with CHD (Supplemental Table II). Among these, four metabolites have been shown previously to be related to one or more CHD risk factors. For example, higher urinary excretion levels of 4-vinylphenol sulfate, a styrene metabolite, has been reported to associate with smoking 47, which can be explained as styrene is one of many chemicals found in cigarettes. DNA methylation studies have previously reported several CpG loci that associate with serum 4-vinylphenol sulfate levels 48, which are the same CpG loci associated with tobacco smoking 49. Greater N-acetylthreonine levels was identified to be a biomarker for the progression of renal dysfunction 50, and N-acetyl-1-methylhistidine, a metabolite involved in histidine metabolism, has been associated positively with incident chronic kidney disease 51. Three metabolites have been associated with heart failure risk: higher levels of N-acetylalanine and p-cresol sulfate 52, and lower levels of 1-arachidonoylglycerophosphocholine (20:4n6), also known as lysoPC (20:4) 53. In addition, p-cresol sulfate levels have been reported to be higher among those elderlies as compared to younger age group 54, and we observed the same pattern at baseline (data not shown). Such observations suggest that some of the metabolites we selected might partially reflect aging process, a major contributor to CHD risk. Theophylline, which we found associated with the highest CHD HR, is a drug used to treat asthma and chronic obstructive pulmonary disease (COPD), and is well known for having cardiotoxic side effects 55, 56. We conducted sensitivity analyses by excluding COPD cases, defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria 57, or individuals with self-reported ever asthma. The association between theophylline and CHD risk in the fully adjusted model remained unchanged (data not shown), although the association still may reflect residual confounding effects of smoking.
An interesting metabolite associated positively with CHD in our study was erythritol. Erythritol is a sugar substitute widely used in processed foods, especially artificial sweetened beverages (ASB). Like conventional sugar sweetened beverages, greater ASB intake has been associated with negative health outcomes, including increased risk for CHD 58, 59 and hypertension 60. Studies of ASB often fail to distinguish the sugar replacement sweetener used in these beverages; therefore, the etiologic connection of sugar substitutes with CHD remains poorly understood. Hootman et al. showed that erythritol can be synthesized from glucose via the pentose-phosphate pathway and associated with adiposity gain 61, which is in contrast to previously evidence that erythritol cannot be metabolized in humans 62. The association between erythritol and CHD we observed may be explained by weight gain 63; however, additional adjustment for body mass index did not change the association we observed between erythritol and CHD risk (data not shown).
The metabolite, 1-arachidonoylglycerophosphocholine (20:4n6) [LysoPC (20:4)], showed a novel negative association with CHD. LysoPC is mainly formed by the enzyme, lecithin:cholesterol acyltransferase (LCAT), which converts cholesterol and phosphatidylcholine to cholesterol esters and lysophosphatidylcholine 64, 65. It has been reported that lysoPC (20:4) levels are lower in heart failure patients with reduced ejection fraction as compared to controls 53. lysoPC (20:4) has been suggested to be a potential biomarkers for discriminating CHD patients from controls (lower levels in CHD patients) 66. Fan et al. (54) provided evidence supporting multiple lysoPCs, for example, lysoPC (16:0), lysoPC (18:1), and lysoPC (20:3), as metabolites with relatively lower concentrations in CHD patients or in more severe patients vs. controls. Our results further document an association of lysoPC with CHD risk.
Arginylphenylalanine, a dipeptide composed of arginine and phenylalanine, was negatively associated with CHD risk. Although no previous study has identified the dipeptide arginylphenylalanine as associated with CHD, its two components, arginine and phenylalanine, have been recognized for their roles in cardiovascular diseases 16, 67–69. Arginine has substantial cardiovascular benefits, including lowering blood pressure, peripheral vascular resistance and plasma homocysteine, as well as improving endothelial function 68, 69. Previous metabolomic studies associated higher serum phenylalanine levels with increased cardiovascular risk 16, 67. The possible pathophysiology of arginylphenylalanine in CHD development is unknown, but we speculate it may be via these two amino acids since it is a short-lived intermediate on the way to amino acids degradation pathways.
To our knowledge, this study is the first to evaluate prospectively whether a comprehensive set of metabolites predicts CHD in a biracial population. Our study has some limitations that are worth noting. We did not replicate our findings in other independent population-based studies. Yet, our use of LASSO and a false discovery rate of 5% strengthened the selection of metabolites. Our MRS included 19 metabolites with significant individual associations with CHD risk. Further investigation of LASSO selected metabolites not included in the MRS is warranted. In addition, LASSO picks the statistically most significant metabolites for CHD prediction, not necessarily the most biologically relevant ones. We assessed the metabolome on samples drawn in 1987-89 to predict CHD over 30 years. ARIC participants’ metabolomes and confounding variables may have changed significantly over the 30 years of follow-up. The lack of longitudinal metabolomic data did not allow us to examine the change of these metabolites in relation to CHD risk. Furthermore, the matabolomic panel we used focused on small molecules, which did not contain complex lipids. Information on complex lipids, such as lipidomic profiles, may provide additional insights for CHD risk 42, 43.
We selected metabolites based on their associations with incidence CHD beyond traditional risk factors, including conventional lipid variables. Our data demonstrated that adding MRS to current conventional risk scores led to improved CHD prediction. Although the strength of association of MRS was similar to conventional lipid parameters used in CHD risk prediction, it is premature to suggest routine assessment of metabolites in clinical practice. Future investigation is warranted to examine long-term metabolic changes in relation to CHD risk prediction, costs and availability of the measurement assays, appropriate therapeutic cutpoints, and clinical evidence of benefits for achieving these cutpoints based on randomized clinical trials.
In conclusion, a MRS constructed from 19 metabolites was associated with the risk of developing CHD and improved CHD 30 years risk prediction beyond TRFs. Our results suggest several new pathways in the etiology of CHD, and highlight the use of metabolomics in CHD risk prediction. Further work is warranted to replicate our novel findings and shed light on potential mechanisms in CHD etiology.
Supplementary Material
Highlights.
Identifying metabolomic signatures may provide insight into disease etiology and prevention.
Using least absolute shrinkage and selection operator and a false discovery rate of 5%, we selected 19 metabolites that were associated with the risk of developing coronary heart disease, including a sugar substitute, erythritol.
A metabolite risk score constructed from the 19 selected metabolites improved coronary heart disease 30 years risk prediction beyond traditional risk factors.
Our results suggest several new pathways in the etiology of coronary heart disease, and highlight the use of metabolomics in CHD risk prediction.
Acknowledgement
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The metabolomics measurements were supported by the National Genome Research Institute (HG004402). This project also was supported by the American Heart Association (Yu, 17SDG33661228).
Abbreviations:
- TRFs
Traditional risk factors
- MRS
Metabolomics risk score
- LASSO
Least absolute shrinkage and selection operator
- ARIC study
Atherosclerosis Risk in Communities study
- CHD
Coronary heart disease
Footnotes
Disclosure
None
References
- 1.Organization WH. World health statistics 2017: Monitoring health for the sdgs Sustainable Development Goals. Geneva: WHO; 2017 [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362 [DOI] [PubMed] [Google Scholar]
- 4.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the atherosclerosis risk in communities (aric) study. J Clin Epidemiol 2003;56:880–890 [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase a2, high-sensitivity c-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the atherosclerosis risk in communities (aric) study. Circulation. 2004;109:837–842 [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the atherosclerosis risk in communities (aric) study. Am Heart J 2002;144:233–238 [DOI] [PubMed] [Google Scholar]
- 7.Vasan R Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335. [DOI] [PubMed] [Google Scholar]
- 8.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical chemistry. 2009;81:6656–6667 [DOI] [PubMed] [Google Scholar]
- 9.Madsen R, Lundstedt T, Trygg J. Chemometrics in metabolomics—a review in human disease diagnosis. Analytica chimica acta. 2010;659:23–33 [DOI] [PubMed] [Google Scholar]
- 10.Villas-Bôas SG, Mas S, Åkesson M, Smedsgaard J, Nielsen J. Mass spectrometry in metabolome analysis. Mass spectrometry reviews. 2005;24:613–646 [DOI] [PubMed] [Google Scholar]
- 11.Shah SH, Sun J-L, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, Newgard CB, Califf RM, Newby LK. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. American Heart Journal. 2012;163:844–850.e841 [DOI] [PubMed] [Google Scholar]
- 12.Rizza S, Copetti M, Rossi C, Cianfarani MA, Zucchelli M, Luzi A, Pecchioli C, Porzio O, Di Cola G, Urbani A, Pellegrini F, Federici M. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis.232:260–264 [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaarhorst AA, Verhoeven A, Weller CM, et al. A metabolomic profile is associated with the risk of incident coronary heart disease. Am Heart J. 2014;168:45–52.e47 [DOI] [PubMed] [Google Scholar]
- 15.Kume S, Araki S-i, Ono N, et al. Predictive properties of plasma amino acid profile for cardiovascular disease in patients with type 2 diabetes. PLOS ONE. 2014;9:e101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M. Metabolite profiling and cardiovascular event risk: A prospective study of three population-based cohorts. Circulation. 2015;131:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137:841–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganna A, Salihovic S, Sundstrom J, et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet 2014;10:e1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ARIC Investigators. The atherosclerosis risk in communities (aric) study: Design and objectives. American journal of epidemiology. 1989;129:687–702 [PubMed] [Google Scholar]
- 20.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA, The AI. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (aric) study: Methods and initial two years’ experience. Journal of Clinical Epidemiology. 1996;49:223–233 [DOI] [PubMed] [Google Scholar]
- 21.Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, Pinsky JL, Klein R. Retinal arteriolar diameters and elevated blood pressure: The atherosclerosis risk in communities study. Am J Epidemiol 1999;150:263–270 [DOI] [PubMed] [Google Scholar]
- 22.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clinical Chemistry. 1983;29:1075–1080 [PubMed] [Google Scholar]
- 23.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. Journal of clinical chemistry and clinical biochemistry. Zeitschrift fur klinische Chemie und klinische Biochemie. 1984;22:165–174 [DOI] [PubMed] [Google Scholar]
- 24.Russell Warnick G, Mayfield C, Benderson J, Chen J-S, Albers JJ. Hdl cholesterol quantitation by phosphotungstate-mg2+ and by dextran sulfate-mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. American journal of clinical pathology. 1982;78:718–723 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Cai J, Tegeler C, Sorlie P, Metcalf PA, Heiss G. Reproducibility of extracranial carotid atherosclerotic lesions assessed by b-mode ultrasound: The atherosclerosis risk in communities study. Ultrasound in medicine & biology. 1996;22:791–799 [DOI] [PubMed] [Google Scholar]
- 27.Li R, Duncan BB, Metcalf PA, Crouse JR 3rd, Sharrett AR, Tyroler HA, Barnes R, Heiss G. B-mode-detected carotid artery plaque in a general population. Atherosclerosis risk in communities (aric) study investigators. Stroke. 1994;25:2377–2383 [DOI] [PubMed] [Google Scholar]
- 28.High-resolution b-mode ultrasound scanning methods in the atherosclerosis risk in communities study (aric). The aric study group. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 1991;1:68–73 [PubMed] [Google Scholar]
- 29.Hunt KJ, Sharrett AR, Chambless LE, Folsom AR, Evans GW, Heiss G. Acoustic shadowing on b-mode ultrasound of the carotid artery predicts chd. Ultrasound in medicine & biology. 2001;27:357–365 [DOI] [PubMed] [Google Scholar]
- 30.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–6667 [DOI] [PubMed] [Google Scholar]
- 31.Tetsuya O, Naoya M, Naohisa T, Tetsuya S, Matthew M, Michael VM, John AR, Kirk DB, Lining G. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in fischer 344 male rats. Toxicologic Pathology. 2009;37:521–535 [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, Agostino RBD, Agostino RBD, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Statistics in Medicine. 2008;27:157–172 [DOI] [PubMed] [Google Scholar]
- 33.Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the c-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011;30:1105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JMG, Ankerst DP, Andridge RR. Validation of biomarker-based risk prediction models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:5977–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The aric (atherosclerosis risk in communities) study. Journal of the American College of Cardiology. 2010;55:1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake KJ, Sidorov VY, McGuinness OP, Wasserman DH, Wikswo JP. Amino acids as metabolic substrates during cardiac ischemia. Experimental biology and medicine (Maywood, N.J.). 2012;237: 10.1258/ebm.2012.012025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y, Yu B, Alexander D, Steffen LM, Nettleton JA, Boerwinkle E. Metabolomic patterns and alcohol consumption in african americans in the atherosclerosis risk in communities study. The American journal of clinical nutrition. 2014;99:1470–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vangaveti V, Baune BT, Kennedy RL. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Therapeutic Advances in Endocrinology and Metabolism. 2010;1:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svingen GF, Ueland PM, Pedersen EK, Schartum-Hansen H, Seifert R, Ebbing M, Loland KH, Tell GS, Nygard O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2013;33:2041–2048 [DOI] [PubMed] [Google Scholar]
- 41.Guasch-Ferré M, Zheng Y, Ruiz-Canela M, et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of mediterranean diet interventions. The American journal of clinical nutrition. 2016;103:1408–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toledo E, Wang DD, Ruiz-Canela M, et al. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the mediterranean diet. The American journal of clinical nutrition. 2017;106:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, Spector TD, Willeit J, Kiechl S, Mayr M. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based bruneck study. Circulation. 2014;129:1821–1831 [DOI] [PubMed] [Google Scholar]
- 44.Fretts AM, Mozaffarian D, Siscovick DS, Sitlani C, Psaty BM, Rimm EB, Song X, McKnight B, Spiegelman D, King IB, Lemaitre RN. Plasma phospholipid and dietary α-linolenic acid, mortality, chd and stroke: The cardiovascular health study. The British journal of nutrition. 2014;112:1206–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mardinoglu A, Stancakova A, Lotta LA, Kuusisto J, Boren J, Bluher M, Wareham NJ, Ferrannini E, Groop PH, Laakso M, Langenberg C, Smith U. Plasma mannose levels are associated with incident type 2 diabetes and cardiovascular disease. Cell metabolism. 2017;26:281–283 [DOI] [PubMed] [Google Scholar]
- 46.Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2005;67:301–320 [Google Scholar]
- 47.Manini P, De Palma G, Andreoli R, Goldoni M, Poli D, Lasagni G, Mutti A. [urinary excretion of 4-vinyl phenol after experimental and occupational exposure to styrene]. Giornale italiano di medicina del lavoro ed ergonomia. 2003;25 Suppl:61–62 [PubMed] [Google Scholar]
- 48.Petersen A-K, Zeilinger S, Kastenmüller G, et al. Epigenetics meets metabolomics: An epigenome-wide association study with blood serum metabolic traits. Human molecular genetics. 2014;23:534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeilinger S, Kühnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A, Strauch K, Waldenberger M, Illig T. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PloS one. 2013;8:e63812–e63812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. The Journal of clinical endocrinology and metabolism. 2016;101:696–704 [DOI] [PubMed] [Google Scholar]
- 51.Yu B, Zheng Y, Alexander D, Morrison AC, Coresh J, Boerwinkle E. Genetic determinants influencing human serum metabolome among african americans. PLoS Genet 2014;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Y, Yu B, Alexander D, Manolio TA, Aguilar D, Coresh J, Heiss G, Boerwinkle E, Nettleton JA. Associations between metabolomic compounds and incident heart failure among african americans: The aric study. American journal of epidemiology. 2013:kwt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JRB, Alberta H. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLOS ONE. 2015;10:e0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Giebultowicz J, Wroczynski P, Paczek L. Age-dependent increase in serum levels of indoxyl sulphate and p-cresol sulphate is not related to their precursors: Tryptophan and tyrosine. Geriatrics & Gerontology International. 2017;17:1022–1026 [DOI] [PubMed] [Google Scholar]
- 55.Starakis I, Starakis I, Lekkou A, Blikas A, Labropoulou-Karatza C. Drug-induced cardiotoxicity due to aminophylline treatment: A case report(). Current Therapeutic Research, Clinical and Experimental. 2003;64:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. American journal of respiratory and critical care medicine. 2017;195:557–582 [DOI] [PubMed] [Google Scholar]
- 57.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Nhlbi/who global initiative for chronic obstructive lung disease (gold) workshop summary. American journal of respiratory and critical care medicine. 2001;163:1256–1276 [DOI] [PubMed] [Google Scholar]
- 58.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. The American journal of clinical nutrition. 2009;89:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741, s1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. Journal of general internal medicine. 2012;27:1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hootman KC, Trezzi JP, Kraemer L, Burwell LS, Dong X, Guertin KA, Jaeger C, Stover PJ, Hiller K, Cassano PA. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E4233–e4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G. Metabolism of erythritol in humans: Comparison with glucose and lactitol. The British journal of nutrition. 1993;69:169–176 [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, Willett WC, Hu FB. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318:255–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosek AB, Durbin D, Jonas A. Binding affinity and reactivity of lecithin cholesterol acyltransferase with native lipoproteins. Biochemical and biophysical research communications. 1999;258:548–551 [DOI] [PubMed] [Google Scholar]
- 65.Hirsch-Reinshagen V, Donkin J, Stukas S, Chan J, Wilkinson A, Fan J, Parks JS, Kuivenhoven JA, Lutjohann D, Pritchard H, Wellington CL. Lcat synthesized by primary astrocytes esterifies cholesterol on glia-derived lipoproteins. Journal of lipid research. 2009;50:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng Q, Liu Z, Zhong S, et al. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Scientific Reports. 2016;6:22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tenori L, Hu X, Pantaleo P, Alterini B, Castelli G, Olivotto I, Bertini I, Luchinat C, Gensini GF. Metabolomic fingerprint of heart failure in humans: A nuclear magnetic resonance spectroscopy analysis. International Journal of Cardiology. 2013;168:e113–e115 [DOI] [PubMed] [Google Scholar]
- 68.Bode-Böger SM, Böger RH, Galland A, Tsikas D, Frölich JC. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic–pharmacodynamic relationship. British journal of clinical pharmacology. 1998;46:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.West SG, Likos-Krick A, Brown P, Mariotti Fo. Oral l-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. The Journal of Nutrition. 2005;135:212–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


