Abstract
To capture the spatial distribution of phenanthrene in an urban setting we used vegetation biomonitoring with Jeffrey pine trees (Pinus jeffreyi). The major challenge in characterizing spatial variation in polycyclic aromatic hydrocarbon (PAH) concentrations within a metropolitan area has been sampling at a fine enough resolution to observe the underlying spatial pattern. However, field and chamber studies show that the primary pathway through which PAHs enter plants is from air into leaves, making vegetation biomonitoring a feasible way to examine the spatial distribution of these compounds. Previous research has shown that phenanthrene has adverse health effects and that it is one of the most abundant PAHs in urban air. We collected 99 pine needle samples from 91 locations in Fresno in the morning on a winter day, and analyzed them for PAHs in the inner needle. All 99 pine needle samples had detectable levels of phenanthrene, with mean concentration of 41.0 ng g−1, median 36.9 ng g−1, and standard deviation of 28.5 ng g−1 fresh weight. The ratio of the 90th:10th percentile concentrations by location was 3.3. The phenanthrene distribution had a statistically significant Moran’s I of 0.035, indicating a high degree of spatial clustering. We implemented land use regression to fit a model to our data. Our model was able to explain a moderate amount of the variability in the data (R2 = 0.56), likely reflecting the major sources of phenanthrene in Fresno. The spatial distribution of modeled airborne phenanthrene shows the influences of highways, railroads, and industrial and commercial zones.
Keywords: Polycyclic aromatic hydrocarbons, Phenanthrene, Biomonitoring, Spatial analysis, Air pollution, Epidemiology, Fresno, California
1. Introduction
In order to capture the short-term spatial distribution of airborne polycyclic aromatic hydrocarbons (PAHs) in an urban setting we employed a vegetation biomonitoring approach using Jeffrey pine trees (Pinus jeffreyi) in Fresno, California. Other researchers have used vegetation biomonitoring for examining local source–receptor relationships (Hwang and Wade, 2008; Lehndorff and Schwark, 2004; Meharg et al., 1998), whereas our primary interest is to establish the magnitude and shape of the PAH spatial distribution to inform future air monitoring studies and exposure assessment in epidemiology studies. Because PAH concentrations vary widely within most urban areas and because air sampling for PAHs is a labor-, equipment-, and time-intensive task, urban air monitoring has been limited to relatively few simultaneous sampling locations (Guo et al., 2003; Manoli et al., 2004; Noth et al., 2011; Thornhill et al., 2008). We elected to use a vegetation biomonitor because it potentially offers a convenient, available, and reliable passive monitor for characterizing PAHs.
We have focused our research on vapor-phase phenanthrene, the second most abundant airborne PAH in the environment and the PAH implicated in multiple adverse health outcomes (Gale et al., 2012; Miller et al., 2004; Nadeau et al., 2010; Tsien et al., 1997). PAHs are ubiquitous toxic air pollutants with complex spatial distributions. Major sources for ambient PAHs are open biomass burning, residential heating, power generation, trains, ships, motor vehicles, and industrial processes (EPA, 1998; Jenkins et al., 1996). Multiple studies in urban and suburban settings have shown phenanthrene to have the highest airborne concentrations of the 16 EPA PAHs, excepting naphthalene (Rogge et al., 2011; Zhu and Jia, 2012). Additionally, in personal exposures to PAHs the concentration of phenanthrene is the highest for PAH with three or more rings when compared either for total (vapor- and particle-phase), or vapor-phase (Li et al., 2010; Nethery et al., 2012).
PAHs are well-known to be carcinogenic individually and in mixtures (International Agency for Research on Cancer, 1987), but there is an extensive literature developing that implicates phenanthrene as a toxic agent in adverse health outcomes from subclinical immunological changes through asthma and wheeze. Phenanthrene exposure in ex vivo human studies caused the conversion of regulatory T-cells to pro-allergic Th-2 effector T-cell phenotype, which is associated with allergic asthma (Liu et al., 2013). In vitro studies by Schober et al. (2006, 2007) demonstrated that phenanthrene enhanced the allergic reaction to birch pollen by strongly inducing basophils taken from birch pollen allergic patients, significantly enhancing cytokine secretion (IL-4 and IL-8), and significantly enhancing histamine release (Schober et al., 2006, 2007). Furthermore, phenanthrene exerts these same pro-allergic effects on sensitized basophils from allergic individuals even in absence of the allergen itself (Lubitz et al., 2010). Phenanthrene has also been shown to enhance the allergic response to ragweed in ragweed-sensitive subjects by increasing IgE synthesis following in vivo human nasal challenge (Saxon and Diaz-Sanchez, 2000). Nadeau et al. (2010) have demonstrated an association between airborne PAH exposure and decreased FEV1, increased asthma severity, and suppression of regulatory T-cell function through methylation of the FoxP3 gene (Nadeau et al., 2010). Gale et al. (2012) found significant associations with ambient phenanthrene exposure and wheeze in a cohort of 315 asthmatic children (Gale et al., 2012). In conclusion, evidence is accumulating that respiratory health can be seriously impacted by phenanthrene exposure.
The literature on vegetation biomonitoring for PAHs is well-developed. Chamber studies and field studies, controlled and observational, have been used to examine distributions of PAHs and other persistent organic pollutants (POPs) in vegetation and dependencies of vegetation concentrations on local sources. Controlled field and physico-chemical studies show that the primary pathway through which phenanthrene enters plants is from air into the leaves, and uptake from soil is negligible (Barber et al., 2004; Kipopoulou et al., 1999; Ryan et al., 1988; Simonich and Hites, 1995; Welsch-Pausch et al., 1995). For the purposes of using pine needles as passive samplers for vapor-phase phenanthrene, the inner needle concentrations provide the most stable and least variable concentrations. Minimizing the non-spatial variability is especially important because we wanted to focus on spatial variability. Vapor-phase phenanthrene can penetrate into the inner needle directly through the stomata or by diffusing through the outer waxy layer (Paterson et al., 1991; Schönherr and Riederer, 1989). Photolysis and photodegredation of PAHs contribute to the higher variability of the outer wax layer, relative to the inner needle (Niu et al., 2003; Simonich and Hites, 1995; Wang et al., 2005; Wild et al., 2005). Controlled experiments found that the half-life of PAHs in the outer needle surface of conifers was shorter by at least 50% than the half-life in the inner needle (Wild et al., 2005). The half-life for phenanthrene in the whole needle was found by Wang et al. (2005) to be 34.5 h Wenzel et al. (1998) show that analysis of the inner needle results in good precision, with relative standard deviations under 20% (Wenzel et al., 1998), and that most of the total phenanthrene measured in the needle is present in the inner needle compartment. Experiments show that the majority of phenanthrene is present in the inner needle portion of 2-year old Pinus sylvestris L. needles collected in urban environments in Argentina and Germany (mean = 73% of total needle phenanthrene concentration is from the inner needle) (Wenzel et al., 1998) and in Germany and Russia (mean = 73% of total needle phenanthrene concentration is from the inner needle) (Wenzel et al., 1998).
Field studies show that accumulation of PAHs in leaves is sensitive to variations and changes in air concentration (Alfani et al., 2001, 2005; Hwang et al., 2003; Hwang and Wade, 2008; Lehndorff and Schwark, 2004; Wagrowski and Hites, 1997). Wagrowski and Hites (1997), Hwang et al. (2003), Hwang and Wade (2008), Alfani et al. (2001, 2005) and Lehndorff and Schwark (2004) each collected and analyzed vegetation for PAH concentrations and found an increasing concentration gradient along the rural-urban gradient. Additionally, Hwang and Wade (2008) and Meharg et al. (1998) show that point sources can be detected in vegetation concentrations (Hwang and Wade, 2008; Meharg et al., 1998). Hwang and Wade (2008) found that two sites located in Houston, Texas, USA near the “largest petrochemical complex in the United States,” had very high concentrations of PAH in pine needles (Pinus taeda) when compared to samples collected in other parts of the Houston metropolis. Meharg et al. (1998) showed that phenanthrene concentrations in grasses downwind from a large chemical fire were up to 67 times the concentrations in grasses upwind.
The goal of this research was to use Jeffrey pines (P. jeffreyi) to characterize the spatial distribution of one of the more volatile PAHs, phenanthrene, in Fresno, California, USA. We selected phenanthrene because of the research showing a relationship between exposure and health outcomes and because it is one of the most abundant of the PAHs in ambient air. The general approach was to obtain a cross-sectional dataset of PAHs at approximately 100 locations and use regression modeling with land use, traffic data, and other neighborhood characteristics to build a spatial model of phenanthrene concentrations for Fresno, CA.
2. Methods
2.1. Field collection
Two methods informed the choice of locations for pine needle sampling. In the first method, systematic sampling of the grid of 1-square mile United States Public Land Survey System (PLSS) blocks was used to capture the spatial range of ambient PAH concentrations throughout Fresno. Fresno contains approximately 150 PLSS blocks. This number was reduced to 42 blocks by selection of alternating blocks within each row of blocks. Each selected 1-square mile block was visited and the locations of Jeffrey pine trees were recorded on a paper map and electronically with a Garmin eTrex GPS device (Olathe, KS, USA).
In the second method, a “demand” surface was created that indicated areas of Fresno with high density of participants in the Fresno Asthmatic Children’s Environment Study (FACES) and where PAH concentrations were likely to be very high or very low (Noth et al., 2011). FACES is an epidemiology study that examines the relationship between asthma attacks and air pollutant concentrations. Our results will inform future collection of air monitoring data for epidemiology studies such as FACES, therefore the population density of participants is of particular interest. The demand surface used traffic density as a proxy for PAH concentrations. The traffic count data used roadway locations from the TeleAtlas MultiNet™ USA (TAMN) roadway database and the annual average daily traffic (AADT) count from the California Department of Transportation for vehicle activity data (Margolis et al., 2009). The range for Fresno was from 51 to 113,600 vehicles day−1. In ArcMap 9.2 (ESRI, Redlands, CA), the two input surfaces (traffic count and FACES population density) were combined and reclassified. For collection, we identified locations with higher FACES population densities and equally distributed by traffic density. Each of these areas was surveyed for locations of Jeffrey pine trees, and recorded on paper maps and with a GPS device.
There were a total of 158 possible sampling locations for Jeffrey pine trees identified when both surveys were combined. To reduce this number to approximately 100, pairs or groups of trees that were located closer together than approximately 10 m were reduced to a single representative tree, selected randomly. Next, trees that were located in the same PLSS block and representing the same “demand” level were reduced throughout the sampling area, again based on random numbers, until the total sample locations reached 100 samples.
2.2. Collection method
Prior to collection, experiments were conducted to determine if collecting needles from all four “sides” of an individual tree (two directions parallel to the street, and two directions perpendicular) was necessary to avoid bias from branch-to-branch variations. The goal of the sampling is to obtain an unbiased average for the tree. Pine needle samples were collected from four branches. The samples were aliquoted into three samples each, to examine the precision of the laboratory analysis [Online Supplement Tables 1 and 2]. The variability among the branches resulted in an observable difference among samples collected on different branches on the same tree. Therefore, in order to measure the average value on a tree, the protocol was to collect needles from a minimum of two branches on each tree, located as far apart on the tree as possible, and at breast height (approximately 1.4 m). Additionally, needles were collected from the second bunch back to ensure that the needles collected were of a uniform age, and hence minimize aging effects, either from decay or from different biological functioning (Piccardo et al., 2005). Samples were wrapped in solvent-washed aluminum foil, labeled, sealed with tape, and stored in coolers on dry ice during the collection period. After returning to the laboratory, pine needle samples were stored in the freezer at −20 °C.
2.3. Laboratory method
We used a published method for the extraction, clean-up, and analysis of pine needles for phenanthrene (Hubert et al., 2001, 2003; Wenzel et al., 1997, 1998). Before analysis, samples were removed from the freezer and stripped of the outer wax layer by following published methods of 10 min sonication in dichloromethane (Hubert et al., 2001; Wenzel et al., 1997, 1998). After wax stripping, the solvent was allowed to evaporate from the needles before they were weighed. The needles were then chopped into 1/8-inch pieces using a new solvent-washed razor blade for each sample. The mean mass for the chopped samples was 9.8 g. The chopped needles were mixed with 10 mg of diatomaceous earth, and placed in a stainless steel extraction cell. Any empty space left in the cell was filled with solvent-washed Ottawa sand.
Samples were extracted using the Dionex Accelerated Solvent Extractor 200 (Dionex Corp, Sunnyvale, CA), or ASE. Each cell was extracted with heptane for four 10-min cycles at 120 °C, under 10 MPa (1500 psi). The flush volume for the cycles was 30% of the cell volume and the nitrogen purge was 120 s at 1 Mpa (150 psi). Following extraction the sample was evaporated to near dryness under nitrogen with a Zymark TurboVapII (Caliper Life Sciences, Hopkinton, MA) and then redissolved with 1.5 ml or 2.0 ml of tetrahydrofuran depending on a low or high wax volume in the sample. Following transfer to tetrahydrofuran, 200ul or 400ul of the sample was injected into the HPLC (Waters626 Pump/600controller, Waters717 + autosampler, Waters Corporation, Milford, MA) for fractionation using a gel permeation chromatograph column (Nucleogel GPS 50–100, Macherey-Nagel, Bethlehem, PA). Both injection volumes were tested for adequate recoveries with standard solution and spiked pine needle samples for the full extraction and clean-up procedure. The flow rate was 0.5 mL min−1 of tetrahydrofuran. The 11–17 min fraction was retained, evaporated to 0.5 mL, and quantitatively transferred to heptane for analysis by GC/MS. Analyses were performed on a Hewlett Packard model 6890 Gas Chromatograph (Hewlett Packard, Palo Alto, CA) equipped with a 30 m (50%-Phenyl)- methylpolysiloxane fused silica capillary column and a 5972 Mass Selective detector operating in the selected ion-monitoring mode for enhanced sensitivity. The 11–17 min fraction contained 100% of the PAHs in the injected extract. Triplicate analyses showed the method had good precision, with relative standard deviations under 20%.
Concentrations of phenanthrene found in the inner needle sample were normalized by total mass in grams of the pine needle sample as weighed after the needle outer layer was removed (ng phenanthrene g−1 fresh weight); phenanthrene had a limit of quantitation of 0.90 ng g−1 fresh weight.
We used standard solutions and spiked pine needle samples to determine recovery levels for the method on the GC/MS. Standard solution samples were run by adding the standard solution to sand and performing the full method of extraction and analysis. The standard solution recovery for phenanthrene was 100% and the spiked pine needle recovery was 120%.
A storage study to test and quantify any significant losses to PAHs in the pine needle samples during storage in the freezer found that the average phenanthrene concentration in replicate samples tested at the beginning and end of the 8-week duration of the analysis period were not statistically different, as found by a two-tailed t-test.
2.4. Spatial distribution
To characterize the spatial distribution of phenanthrene, we calculated the Moran’s I statistic and examined the bivariate models of phenanthrene with local environmental characteristics (see below). We also plotted the sample variogram to look for an overall covariance structure in the data. Following these preliminary steps, phenanthrene concentrations for all of the City of Fresno were modeled using linear regression. The model was fit manually in SAS 9.3 (Cary, NC). Covariates in the model were required to be statistically significant at an alpha level of 0.10. Residual analysis on the model included an examination of the quantile–quantile and histogram plots for normality, of the residual versus predicted scatterplot for absence of structure, and of the Moran’s I statistic for spatial autocorrelation. Additionally, correlations between the covariates were checked for collinearity.
2.4.1. Quantification of local environmental characteristics
Geographic information was collected, compiled and processed in ArcGIS 9.2 (ESRI, Redlands, CA) and, when necessary, further data calculations were completed in SAS 9.3 (SAS, Cary, NC).
2.4.1.1. Traffic.
Two datasets were used to define traffic-related spatial variables: roadway locations from the TeleAtlas MultiNet™ USA (TAMN) roadway database and vehicle activity data from the California Department of Transportation (Caltrans). The vehicle activity data were GIS-based and contained estimates of annual average daily traffic (AADT) volumes traveling both directions on select road segments, and truck traffic-volumes for freeways and state freeways (Margolis et al., 2009).
Five categories of traffic variables were defined: roadway proximity, roadway density, traffic intensity, and home location in relation to school bus emissions. Roadway proximity measured the distance from the sampling site to the nearest of each of five major road types (freeway, major arterial, minor arterial, major collector, and minor collector). Roadway density was defined as the sum of total roadway length of each of the five major road types within each of 6 circular buffers (50 m, 100 m, 200 m, 300 m, 400 m, and 500 m) around the sampling site. Traffic intensity at each site was calculated based on AADT counts from Caltrans, assuming Gaussian decay of exhaust emissions with distance to the roadway (Wilhelm and Ritz, 2003). Roadway proximity and density were also calculated separately for the major freeways in Fresno because of the difference in predominant vehicle type by freeway. US Route 99, on the western edge of the city, is a heavily used regional trucking corridor whereas centrally-located State Route 41 restricts truck traffic and is primarily local, light-duty vehicles. Last, we accounted for city and school buses. City bus routes and bus stops were each available from the City of Fresno. The intensity and proximity of bus routes and bus stops was calculated using the 6 buffers described above. The impact of diesel school buses was represented using the distance to the nearest elementary school as a proxy for exposure to bus exhaust. Elementary school proximity was used to capture this source in Fresno, because elementary schools are used as primary bus stops for the school district, not children’s homes. School buses do not have established routes, but vary their route depending on the driver and traffic conditions (Fresno Unified School District, personal communication, 2005).
2.4.1.2. Land use.
Land use data were obtained from the California Department of Water Resources county-wide California Land & Water Use surveys from Fresno County (2000) and Madera County (2001). Land use types included were urban, vacant urban (including parking lots), landscaped urban, residential urban, commercial, industrial, agricultural, semi-agricultural, native vegetation, and native riparian. To describe the neighborhood, each sampling site was assigned three sets of values: land use type on which the tree was located; land use types within 6 circular buffers (radii 50 m, 100 m, 200 m, 300 m, 400 m, and 500 m) near the home; and total land use area, by type, within the same 6 buffers around the home.
2.4.1.3. Neighborhood variables.
Data from the United States 2000 Census SF3 dataset was selected for transportation, home fuel use, or socioeconomic characteristics and assigned to each sample by location.
3. Results
3.1. Samples collected
On February 20, 2008, four teams of researchers collected 99 samples from 91 locations in Fresno between 8:10am and 12:50pm. The samples were collected by four teams, each of which started in a different quadrant of the city. This was done so that the time of collection for the samples was not spatially autocorrelated.
3.2. Phenanthrene distribution in samples
All 99 pine needle samples had detectable levels of phenanthrene, with mean concentration of 41.0 ng g−1 fresh weight, median 36.9 ng g−1 fresh weight, and standard deviation of 28.5 ng g−1 fresh weight. The ratio of the 90th:10th percentile concentrations was 3.3 and the ratio of the maximum:minimum was 21.6. There were eight locations at which pine needle samples were taken at the start of sampling in the morning and again from the same eight trees at the end of sampling midday. We saw no significant difference, as tested by paired t-test (mean absolute difference was less than 4 ng g−1 between morning and afternoon samples), between 8 pairs of these samples, indicating that our pine needle samples are virtually contemporaneous.
3.3. Spatial modeling
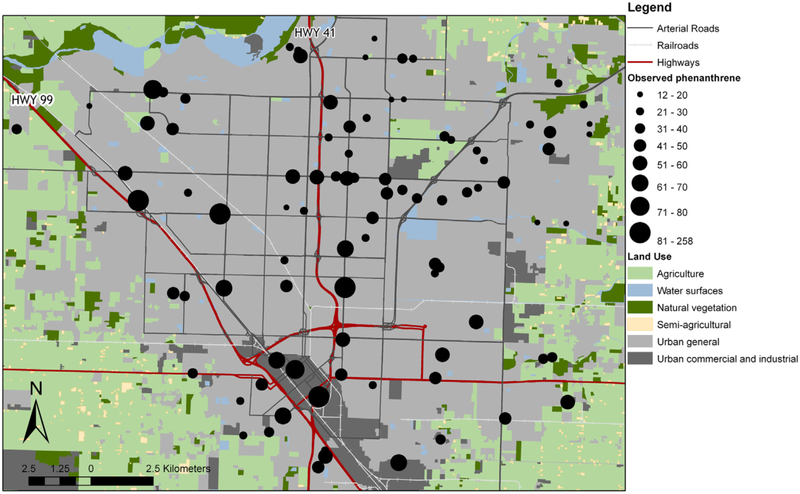
The phenanthrene distribution had a statistically significant Moran’s I of 0.035, indicating a high degree of spatial clustering (Fig. 1). The sample variogram did not show a systematic covariance structure. We log-transformed the data prior to modeling, to achieve a normal distribution. Additionally, we adjusted a single extreme high value (greater than mean plus five standard deviations), from 257.7 ng g−1 fresh weight to 102.0 ng g−1 fresh weight (mean plus two standard deviations), to avoid biasing the results of our regression model toward this one point. This is still the highest phenanthrene value in the dataset, the next highest value is 85.6 ng g−1 fresh weight.
Fig. 1.
Phenanthrene concentrations in pine needles, ng g−1 fresh weight, at 91 locations in Fresno, CA on 20 February 2008.
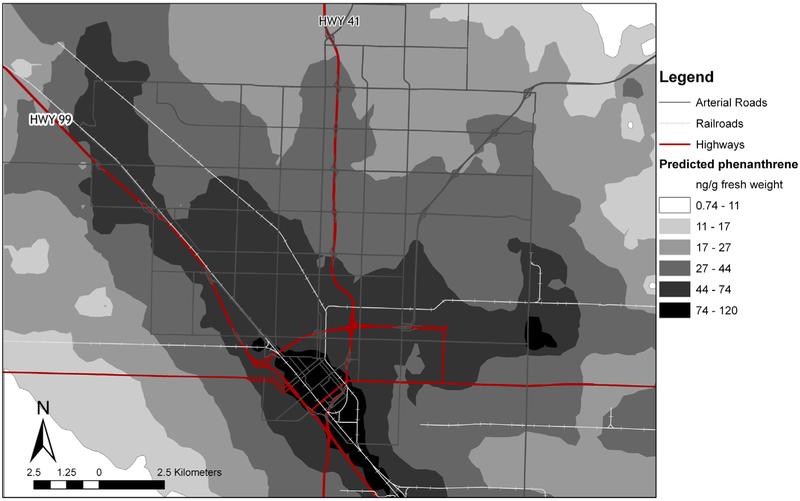
The model for phenanthrene accounted for a moderate amount of variability in the data (R2 = 0.56, Table 1). The residual analyses did not show any bias. The Moran’s I for the residuals was not statistically significant, indicating that there was no remaining spatial autocorrelation. In a sensitivity analysis, we found that the model fit with the full dataset resulted in similar beta coefficients and coefficient of determination (R2 = 0.55, Online Supplement Table 3). Table 1 shows that each covariate has a fairly large range, making the primary predictive variable(s) likely to be different for different locations. However, the partial R2 value shows that the distance from the sampling site to the nearest railroad tracks has the largest explanatory power in the model. Fig. 2 shows the results of the model through an inverse distance weighting interpolation of modeled concentrations at 1096 locations. The range in the modeled data is from 1.0 to 125 ng g−1 fresh weight, with a 90th:10th percentile ratio of 6.2. The range in the modeled data is larger than that in the observed data because there is a wider range of microenvironments in the modeled locations than in the observed locations. In particular, our modeled locations included sites that were closer to highways and railroads than in the observed data.
Table 1.
Final model for the spatial distribution of phenanthrene in pine needles (ng g−1 fresh weight).
| Variable description | Parameter estimate | Pr > |t| | Partial R2 | Range of parameter |
|---|---|---|---|---|
| Model intercept | 3.55 | <0.0001 | ||
| Distance from sampling site to nearest railroad tracks | −8.94E–05 | <0.0001 | 0.21 | 47–8706 m |
| Area (m2) of natural vegetation land type within 500 m radius | −3.29E–06 | 0.0004 | 0.10 | 0–219,370 m2 |
| Number of blockgroup residents with a longer commute (45–59 min) | 0.0058 | 0.0002 | 0.08 | 0–107 people |
| Total length (m) of any roadway within 100 m | 5.69E–04 | 0.001 | 0.06 | 54–1247 m |
| Distance from sampling site to nearest highway | −1.36E–04 | 0.0001 | 0.04 | 7–1462 m |
| Area (m2) of urban industrial or commercial land type within 50 m radius | 7.25E–05 | 0.004 | 0.04 | 0–7833 m2 |
| Number of households with children at home | 4.47E–04 | 0.038 | 0.02 | 2–939 households |
| Number of blockgroup residents with a short commute (less than 5 min) | −6.57E-03 | 0.009 | 0.004 | 0–62 people |
Fig. 2.
Predicted phenanthrene concentrations in ng g−1 fresh weight, based on land use regression modeling at 1000 locations. Surface generated through inverse distance weighting for purposes of presentation.
4. Discussion and conclusion
The use of 91 sites for simultaneous phenanthrene measurement in a single urban area is unprecedented for vegetation biomonitoring or air monitoring of PAHs. In general, air concentrations of PAHS have been measured simultaneously at less than 15 locations when using air sampling techniques. As expected from previous air concentration measurement data in Fresno, phenanthrene was the most abundant PAH in the pine needle samples (Noth et al., 2011). Applying vegetation biomonitoring, we were able to observe that the short-term (approximately daily) spatial variability of phenanthrene in Fresno is significant, with significant clustering of concentrations. Our model explains a moderate amount of the variability in the data, likely reflecting the major sources of phenanthrene in Fresno. In particular, the spatial distribution of modeled phenanthrene clearly shows the influences of highways, railroads, and industrial and commercial zones within the urban environment. We also saw that sampling sites in blockgroups with a higher number of residents who commuted less than 5 min to work had lower phenanthrene concentrations. Because Fresno commuters almost exclusively drive in private cars, the shorter commuters may be the few residents who walk, bike, carpool or work at home; such a decrease in local car activity would explain the lower phenanthrene concentrations in those blockgroups. In contrast, block-groups with higher numbers of residents with longer commutes (45–60 min) had higher phenanthrene concentrations. This may be an indicator of more intense private vehicle use, which would increase neighborhood phenanthrene concentrations. What our model appears to not capture is the very local, small-scale variability for which we have insufficient range of sites. For example, we suspect from our data that proximity to certain types of industries (e.g. welding) or minor sources may be associated with increased phenanthrene concentrations, but we do not have enough sampling sites near each minor source to adequately account for them in the regression model.
Understanding the spatial distribution of PAH has been critical for the epidemiology studies conducted in Fresno, CA (Gale et al., 2012; Nadeau et al., 2010; Noth et al., 2011). Spatial variability in epidemiology studies is often overlooked as being far less important than temporal variability (Brauer et al., 2003; Hoek et al., 2008; Wilson et al., 2005). However, the distribution of phenanthrene in these data, representing the full space of the city of Fresno on one day, show a 3-fold difference between the 90th and 10th percentile observed concentrations (6 fold in modeled concentrations) and more than a 20-fold difference between the maximum and minimum observed concentrations (over 100 fold in modeled). In comparison, the outdoor airborne PAH concentrations measured at the Fresno EPA Supersite in February 2008 show a 5-fold difference in the 90th:10th percentile daily concentrations and a 9-fold difference between maximum and minimum daily concentrations (Hammond et al., 2010). This shows clearly that for short-term exposure assessment the inclusion of spatial variability is equally important to correct quantification of temporal variability.
Bearing in mind the differences between species, extraction methods and clean-up procedures, the vast majority of published research on PAHs in pine needles reports phenanthrene concentrations to be the highest of the 16 EPA Priority PAHs measured (Hwang and Wade, 2008; Lehndorff and Schwark, 2004; Piccardo et al., 2005). Many studies have examined the distribution of phenanthrene in pine needles collected over a large geographic region (Amigo et al., 2011; Lehndorff and Schwark, 2009a,b; Ratola et al., 2010); relatively few have looked at intra-urban variability (Hwang and Wade, 2008; Lehndorff and Schwark, 2004). Similar to our data, Lehndorff and Schwark (2004) reported that of the 46 locations sampled in Cologne PAHs were highest near railroad operations as compared to other sampling locations. Hwang and Wade (2008) found that within their 18 sites of pine needle sampling those in more dense residential areas and with higher traffic volumes were higher in total PAH, most of which was phenanthrene.
In summary, our data and model show that there is a high degree of variability in phenanthrene concentrations across a moderate-sized urban environment. Traffic-generated pollution is important as well as rail pollution. When planning urban air monitoring campaigns to observe or model the spatial distribution of urban PAH concentrations, it is important to evaluate the microenvironments present and select those locations that will maximize variability in both road and rail traffic.
Supplementary Material
HIGHLIGHTS.
We examine the spatial distribution of phenanthrene using vegetation biomonitoring.
Phenanthrene concentrations in Fresno show significant spatial clustering.
The major sources of phenanthrene in our data in Fresno are highways and railroads.
Acknowledgments
We wish to thank Charles Perrino, Cynthia Turpin, Masahiko Sugihara, Daniel Burley, Quan Gan, and Sa Lui. We also wish to thank the Mickey Leland National Urban Air Toxics Research Program (RFA 2005–01). The research described in this article was funded by the Mickey Leland National Urban Air Toxics Research Center (NUATRC), an organization jointly funded by the United States Environmental Protection Agency and private industry sponsors. The contents of this article do not necessarily reflect the views of NUATRC, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or any of the private industry sponsors. We also wish to acknowledge additional support from the University of California, Berkeley/Stanford Children’s Environmental Health Center, funded by NIEHS 1P20ES018173 and EPA RD-83459601–0.
Footnotes
Appendix A. Supplementary material
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.atmosenv.2013.05.056.
References
- Alfani A, Maisto G, Prati MV, Baldantoni D, 2001. Leaves of Quercus ilex L. as biomonitors of PAHs in the air of Naples (Italy). Atmospheric Environment 35, 3553–3559. [Google Scholar]
- Alfani A, De Nicola F, Maisto G, Prati MV, 2005. Long-term PAH accumulation after bud break in Quercus ilex L. leaves in a polluted environment. Atmospheric Environment 39, 307–314. [Google Scholar]
- Amigo JM, Ratola N, Alves A, 2011. Study of geographical trends of polycyclic aromatic hydrocarbons using pine needles. Atmospheric Environment 45, 5988–5996. [Google Scholar]
- Barber JL, Thomas GO, Kerstiens G, Jones KC, 2004. Current issues and uncertainties in the measurement and modelling of air–vegetation exchange and within-plant processing of POPs. Environmental Pollution 128, 99–138. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hoek G, van Vliet P, Meliefste K, Fischer P, Gehring U, Heinrich J, Cyrys J, Bellander T, Lewne M, Brunekreef B, 2003. Estimating long-term average particulate air pollution concentrations: application of traffic indicators and geographic information systems. Epidemiology 14, 228–239. [DOI] [PubMed] [Google Scholar]
- EPA, 1998. Locating and Estimating Air Emissions from Sources of Polycyclic Organic Matter U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Research Triangle Park, NC. [Google Scholar]
- Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB, 2012. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. Journal of Exposure Science and Environmental Epidemiology 22, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lee SC, Ho KF, Wang XM, Zou SC, 2003. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmospheric Environment 37, 5307–5317. [Google Scholar]
- Hammond SK, Noth EM, Tager IB, Biging GS, Gale S, Mann JK, 2010. Short-and Long-term Respiratory Effects of Exposure to Traffic PAHs in a Cohort of Children with Asthma. NUATRC Research Report. Mickey Leland Urban Air Toxics Research Center. [Google Scholar]
- Hoek G, Beelen R, de Hoogh K, Vienneau D, Gulliver J, Fischer P, Briggs D, 2008. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmospheric Environment 42, 7561–7578. [Google Scholar]
- Hubert A, Wenzel K-D, Engelwald W, Schuurmann G, 2001. Accelerated solvent extraction – more efficient extraction of POPs and PAHs from real contaminated plant and soil samples. Reviews in Analytical Chemistry 20, 101–144. [DOI] [PubMed] [Google Scholar]
- Hubert A, Popp P, Wenzel K, Engewald W, 2003. One-step cleanup for PAH residue analysis in plant matrices using size-exclusion chromatography. Analytical and Bioanalytical Chemistry 376, 53–60. [DOI] [PubMed] [Google Scholar]
- Hwang H, Wade T, Sericano J, 2003. Concentrations and source characterization of polycyclic aromatic hydrocarbons in pine needles from Korea, Mexico, and United States. Atmospheric Environment 37, 2259–2267. [Google Scholar]
- Hwang H, Wade T, 2008. Aerial distribution, temperature-dependent seasonal variation, and sources of polycyclic aromatic hydrocarbons in pine needles from the Houston metropolitan area, Texas, USA. Journal of Environmental Science and Health, Part A 43, 1243–1251. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 1987. Overall evaluations of carcinogenicity: an updating of IARC monographs. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vols. 1 to 42, p. 38. [PubMed] [Google Scholar]
- Jenkins BM, Jones AD, Turn SQ, Williams RB, 1996. Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environmental Science & Technology 30, 2462–2469. [Google Scholar]
- Kipopoulou A, Manoli E, Samara C, 1999. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environmental Pollution 106, 369–380. [DOI] [PubMed] [Google Scholar]
- Lehndorff E, Schwark L, 2004. Biomonitoring of air quality in the Cologne Conurbation using pine needles as a passive sampler – Part II: polycyclic aromatic hydrocarbons (PAH). Atmospheric Environment 38, 3793–3808. [Google Scholar]
- Lehndorff E, Schwark L, 2009a. Biomonitoring airborne parent and alkylated three-ring PAHs in the Greater Cologne Conurbation I: temporal accumulation patterns. Environmental Pollution 157, 1323–1331. [DOI] [PubMed] [Google Scholar]
- Lehndorff E, Schwark L, 2009b. Biomonitoring airborne parent and alkylated three-ring PAHs in the Greater Cologne Conurbation II: regional distribution patterns. Environmental Pollution 157, 1706–1713. [DOI] [PubMed] [Google Scholar]
- Li Z, Mulholland JA, Romanoff LC, Pittman EN, Trinidad DA, Lewin MD, Sjodin A, 2010. Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. Journal of Environmental Monitoring 12, 1110–1118. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang L, Winterroth LC, Garcia M, Weisman S, Wong J, Sunwoo J, Nadeau KC, 2013. Epigenetically-mediated pathogenic effects of phenanthrene on regulatory T cells. Journal of Toxicology, 13 10.1155/2013/967029. Article ID 967029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz S, Schober W, Pusch G, Effner R, Klopp N, Behrendt H, Buters JTM, 2010. Polycyclic aromatic hydrocarbons from diesel emissions exert proallergic effects in birch pollen allergic individuals through enhanced mediator release from basophils. Environmental Toxicology 25, 188–197. [DOI] [PubMed] [Google Scholar]
- Manoli E, Kouras A, Samara C, 2004. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 56, 867–878. [DOI] [PubMed] [Google Scholar]
- Margolis HG, Mann JK, Lurmann FW, Mortimer KM, Balmes JR, Hammond SK, Tager IB, 2009. Altered pulmonary function in children with asthma associated with highway traffic near residence. International Journal of Environmental Health Research 19, 139–155. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Wright J, Dyke H, Osborn D, 1998. Polycyclic aromatic hydrocarbon (PAH) dispersion and deposition to vegetation and soil following a large scale chemical fire. Environmental Pollution 99, 29–36. [DOI] [PubMed] [Google Scholar]
- Miller RI, Garfield R, Horton M, Camann D, Perera FP, 2004. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 126, 1071–078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau KC, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes JR, Tager IB, 2010. Ambient air pollution impairs T-cell function in asthma. Journal of Allergy and Clinical Immunology 126, 845–852. [DOI] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, Sjodin A, Li Z, Romanoff LC, Foster W, Arbuckle TE, 2012. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. Journal of Exposure Science and Environmental Epidemiology 22, 70–81. [DOI] [PubMed] [Google Scholar]
- Niu J, Chen J, Martens D, Quan X, Yang F, Kettrup A, Schramm K-W, 2003. Photolysis of polycyclic aromatic hydrocarbons adsorbed on spruce [Picea abies(L.) Karst.] needles under sunlight irradiation. Environmental Pollution 123, 39–45. [DOI] [PubMed] [Google Scholar]
- Noth EM, Hammond SK, Biging GS, Tager IB, 2011. A spatial–temporal regression model to predict daily outdoor residential PAH concentrations in an epidemiologic study in Fresno, CA. Atmospheric Environment 45, 2394–2403. [Google Scholar]
- Paterson S, Mackay D, Bacci E, Calamari D, 1991. Correlation of the equilibrium and kinetics of leaf-air exchange of hydrophobic organic chemicals. Environmental Science & Technology 25, 866–871. [Google Scholar]
- Piccardo M, Pala M, Bonaccurso B, Stella A, Redaelli A, Paola G, Valerio F, 2005. Pinus nigra and Pinus pinaster needles as passive samplers of polycyclic aromatic hydrocarbons. Environmental Pollution 133, 293–301. [DOI] [PubMed] [Google Scholar]
- Ratola N, Amigo JM, Alves A, 2010. Comprehensive assessment of pine needles as bioindicators of PAHs using multivariate analysis. The importance of temporal trends. Chemosphere 81, 1517–1525. [DOI] [PubMed] [Google Scholar]
- Rogge WF, Ondov JM, Bernardo-Bricker A, Sevimoglu O, 2011. Baltimore PM2.5 Supersite: highly time-resolved organic compounds-sampling duration and phase distribution – implications for health effects studies. Analytical and Bioanalytical Chemistry 401, 3069–3082. [DOI] [PubMed] [Google Scholar]
- Ryan JA, Bell RM, Davidson JM, O’Connor GA, 1988. Plant uptake of non-ionic organic chemicals from soils. Chemosphere 17, 2299–2323. [Google Scholar]
- Saxon A, Diaz-Sanchez D, 2000. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology 48, 325–327. [DOI] [PubMed] [Google Scholar]
- Schober W, Belloni B, Lubitz S, Eberlein-Konig B, Bohn P, Saritas Y, Lintelmann J, Matuschek G, Behrendt H, Buters J, 2006. Organic extracts of urban aerosol (<= PM2.5) enhance rBet v. 1-induced upregulation of CD63 in basophils from birch pollen-allergic individuals. Toxicological Sciences 90, 377–384. [DOI] [PubMed] [Google Scholar]
- Schober W, Lubitz S, Belloni B, Gebauer G, Lintelmann J, Matuschek G, Weichenmeier I, Eberlein-Konig B, Buters J, Behrendt H, 2007. Environmental polycyclic aromatic hydrocarbons (PAHs) enhance allergic inflammation by acting on human basophils. Inhalation Toxicology 19, 151–156. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Riederer M, 1989. Foliar penetration and accumulation of organic chemicals in plant cuticles. Review of Environmental Contamination and Toxicology 108, 1–70. [Google Scholar]
- Simonich SL, Hites RA, 1995. Organic pollutant accumulation in vegetation. Environmental Science & Technology 29, 2905e–2914. [DOI] [PubMed] [Google Scholar]
- Thornhill DA, de Foy B, Herndon SC, Onasch TB, Wood EC, Zavala M, Molina LT, Gaffney JS, Marley NA, Marr LC, 2008. Spatial and temporal variability of particulate polycyclic aromatic hydrocarbons in Mexico City. Atmospheric Chemistry and Physics 8, 3093–3105. [Google Scholar]
- Tsien A, DiazSanchez D, Ma J, Saxon A, 1997. The organic component of diesel exhaust particles and phenanthrene, a major polyaromatic hydrocarbon constituent, enhances IgE production by IgE-secreting EBV-transformed human B cells in vitro. Toxicology and Applied Pharmacology 142, 256–263. [DOI] [PubMed] [Google Scholar]
- Wagrowski DM, Hites RA, 1997. Polycyclic aromatic hydrocarbon accumulation in urban, suburban, and rural vegetation. Environmental Science & Technology 31, 279–282. [Google Scholar]
- Wang D, Chen J, Zua Z, Qioa X, Huang L, 2005. Disappearance of polycyclic aromatic hydrocarbons sorbed on surfaces of pine [Pinua thunbergii] needles under irradiation of sunlight: volatilization and photolysis. Atmospheric Environment 39, 4583–4591. [Google Scholar]
- Welsch-Pausch K, McLachlan MS, Umlauf G, 1995. Determination of the principal pathways of polychlorinated dibenzo-p-dioxins and dibenzofurans to Lolium multiflorum (Welsh Ray Grass). Environmental Science & Technology 29, 1090–1098. [DOI] [PubMed] [Google Scholar]
- Wenzel KD, Weissflog L, Paladini E, Gantuz M, Guerreiro P, Puliafito C, Schuurmann G, 1997. Immission patterns of airborne pollutants in Argentina and Germany. 2. Biomonitoring of organochlorine compounds and polycyclic aromatics. Chemosphere 34, 2505–2518. [Google Scholar]
- Wenzel K-D, Hubert A, Manz M, Weissflog L, Engewald W, Schuurmann G, 1998. Accelerated solvent extraction of semivolatile organic compounds from biomonitoring samples of pine needles and mosses. Analytical Chemistry 70, 4827–4835. [Google Scholar]
- Wild E, Dent J, Thomas GO, Jones KC, 2005. Real-time visualization and quantification of PAH photodegration on and within plant leaves. Environmental Science & Technology 39, 268–273. [PubMed] [Google Scholar]
- Wilhelm M, Ritz B, 2003. Residential proximity to traffic and adverse birth outcomes in Los Angeles County, California, 1994–1996. Environmental Health Perspectives 111, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Kingham S, Pearce J, Sturman AP, 2005. A review of intraurban variations in particulate air pollution: Implications for epidemiological research. Atmospheric Environment 39, 6444–6462. [Google Scholar]
- Zhu XL, Jia CR, 2012. Apportioning variability of polycyclic aromatic hydrocarbons (PAHs) and chlordanes in indoor and outdoor environments. Journal of Environmental Monitoring 14, 1926–1934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.