Abstract
Lactose is the main source of calories in milk, an essential nutriedigestion, patients with visceral hypersensitivity nt in infancy and a key part of the diet in populations that maintain the ability to digest this disaccharide in adulthood. Lactase deficiency (LD) is the failure to express the enzyme that hydrolyses lactose into galactose and glucose in the small intestine. The genetic mechanism of lactase persistence in adult Caucasians is mediated by a single C→T nucleotide polymorphism at the LCTbo −13’910 locus on chromosome-2. Lactose malabsorption (LM) refers to any cause of failure to digest and/or absorb lactose in the small intestine. This includes primary genetic and also secondary LD due to infection or other conditions that affect the mucosal integrity of the small bowel. Lactose intolerance (LI) is defined as the onset of abdominal symptoms such as abdominal pain, bloating and diarrhoea after lactose ingestion by an individual with LM. The likelihood of LI depends on the lactose dose, lactase expression and the intestinal microbiome. Independent of lactose digestion, patients with visceral hypersensitivity associated with anxiety or the Irritable Bowel Syndrome (IBS) are at increased risk of the condition. Diagnostic investigations available to diagnose LM and LI include genetic, endoscopic and physiological tests. The association between self-reported LI, objective findings and clinical outcome of dietary intervention is variable. Treatment of LI can include low-lactose diet, lactase supplementation and, potentially, colonic adaptation by prebiotics. The clinical outcome of these treatments is modest, because lactose is just one of a number of poorly absorbed carbohydrates which can cause symptoms by similar mechanisms.
Keywords: lactase, malabsorption, functional bowel disorder, diet, hydrogen breath tests
Introduction
Lactose metabolism continuous to fascinate anthropologists, geneticists, physiologists and clinicians.1–3 Studying the mechanisms of lactose digestion and intolerance has provided insights not only into dietary causes of functional intestinal symptoms but also into human evolution and nutrition, culture and lifestyle (box 1). Recent evidence has demonstrated the impact of lactose digestion on the human microbiota and general health. Considering these issues has raised possible concerns of a dairy-free diet. This review will emphasise recent developments in the clinical diagnosis and management of this condition (box 2).
Box 1. Pathophysiology of lactose malabsorption.
Lactose malabsorption is typically caused by lactase downregulation after infancy due to lactase non-persistence which in Caucasians is mediated by the LCT −13’910:C/C genotype.
Lactase non-persistence is the genetic wildtype and not a disease. Both lactase persistence and non-persistence are common phenotypes in healthy humans.
The lactase genetic region is among the genetic regions strongest shaped by human evolution within the last 10 000 years, with lactase persistence providing a selective advantage of up to 4%–5% per generation.
The LCT −13’910 is the region within the human genome with the strongest interaction with the intestinal microbiota. The LCT −13’910:C/C genotype is associated with higher Bifidobacteria levels on lactose consumption (bifidogenic effect).
Genetic and physiological studies suggest higher bone mineral density and larger height in individuals with lactase persistence.
Box 2. Clinical relevance of lactose intolerance.
Lactose intolerance is defined as symptoms on lactose exposure in individuals with lactose malabsorption.
Most individuals with lactose malabsorption tolerate a dose of at least 12 g lactose (corresponding to 250 mL of milk) without problems. Larger doses may be tolerated if consumed with food or spread over a whole day.
Symptoms of lactose intolerance depend on the strength of the stimulus (ie, lactose dose) and the presence of visceral hypersensitivity, as observed in many patients with IBS.
Treatment options for lactose intolerance include a low-lactose diet, oral lactase enzyme replacement, prebiotics that produce bacterial lactase in the colon and, potentially, prebiotics that adapt the colonic microbiota.
Intolerance of low–moderate lactose doses often indicates the presence of IBS. Such individuals are sensitive to a range of poorly absorbed, fermentable foods (‘FODMAPs’). Effective dietary treatment in this group requires not a low-lactose but a low-FODMAP diet.
FODMAP, fermentable oligosaccharide, disaccharide and monosaccharide and polyols.
Lactose is the main sugar in milk
Milk production by the mammary gland is a defining feature of mammals and lactose (‘milk sugar’; β-galactosyl-1,4 glucose) is the main source of carbohydrate in human milk and that of other mammals, except for sea lions and walruses which produce low volume, viscous and fatty lactose-free milk.4
Infants are uniquely adapted to lactose-based nutrition. In a randomised controlled study, infants fed with breast milk or lactose-based formula had higher levels of glucose and other nutrients (eg, amino acids) in the blood compared with infants with lactose-free formula.5 Lactose also seems to be the only monosaccharide or disaccharide that does not increase the risk of dental caries.6 In adults, dairy products account for approximately 14% of energy intake in Europe and North America. In recent years, the amount of milk consumed has slightly decreased in these regions. By contrast, in China and many developing countries, milk intake contributes only 4% to energy intake; however, consumption is increasing rapidly.3
Cow’s milk contains approximately 5 g lactose per 100 mL, equating to 12.5 g lactose in a typical serving size of 250 mL. Lactose is also present in cultured milk products such as yoghurt and cheese (the second-largest fermentation industry after alcohol).3 Yoghurt contains ≈50% of the lactose of unprocessed milk; whereas, cheese has low lactose content, especially if long-ripened products are consumed.3 Additionally, lactose powder is also a common additive in typical processed foods, enhancing the texture and flavour of sausages, gravy, margarines, bread, sauces, and many prepared meals (table 1).
Table 1.
Lactose content in dairy products and foods (representative values are provided)
| Food | Lactose content (g) per 100 g | Lactose content per typical serving (g) |
| Milk (full) | 4.7 | 15 |
| Milk (skimmed) | 4.8 | 15 |
| Lactose-free milk | <0.1 | <0.1 |
| Goat’s milk | 4.5 | 13 |
| Buttermilk | 3.0 | 9.0 |
| Butter | 0.5 | 0.1 |
| Yoghurt (fresh) | 3.0 | 9.3 |
| Yoghurt (biological) | 4.0 | 9.5 |
| Cream cheese | 3.0 | 0.9 |
| Soft cheese (eg, camembert) | 0.3 | 0.1 |
| Hard cheese (eg, cheddar and gruyere) | 0.1 | <0.1 |
| Cream | 3.6 | 3.2 |
| Soft ice cream | 6.4 | 5.7 |
| Latte Macchiato | 4.3 | 8.6 |
| Lasagne | 1.1 | 2.6 |
| Cheeseburger | 0.9 | 1.1 |
| Ready sauces | 3.6 | 4.5 |
| Pudding/custard | 3.6 | 4.5 |
| Rice, nut, soy or oat beverages | 0.0 | 0.0 |
| Meat and alternatives contain very little lactose. Products that may include lactose are those prepared with milk or milk products such as some processed meat, sausage, breaded or battered meat or fish, commercial egg substitutes, scrambled eggs, soufflés. | ||
| Fats and oils contain very little lactose. Products that may include lactose are those prepared with milk or milk products such as butter or margarine made with milk or whey powder and salad dressings (eg, ranch style or buttermilk). | ||
| Prepared foods may include lactose when made with milk or milk products. These include store bought gravy or sauce mixes, vegetable or chip dips, soups, chips or snack crackers (eg, cheese flavoured), artificial whipped toppings, powdered meal replacement supplements and cream-based liqueurs. | ||
Lactose digestion and absorption
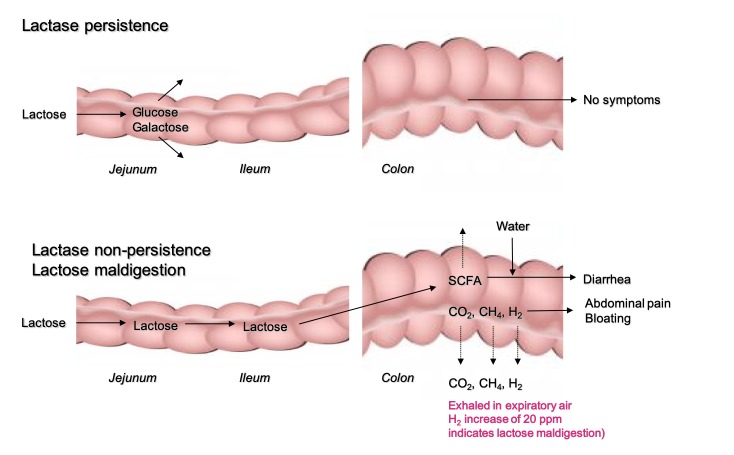
Digestion and absorption of lactose takes place in the small intestine (figure 1).7 8 Lactose is the main substrate of lactase-phlorizin hydrolase expressed on the brush border of villi with its highest expression in the mid-jejunum. The enzyme spans the apical membrane of mature enterocytes and is made up of two identical extracellular 160 kDa polypeptide chains, as well as a short intracytoplasmic part. The alpha-glucosidase activity of this enzyme cleaves the milk sugar disaccharide into the monosaccharides glucose and galactose which are then actively transported into epithelial cells (enterocytes) by the sodium(+)/glucose (galactose) co-transporter (SGLT1). At higher concentrations, a second facilitative transporter (GLUT2) becomes involved.9 From the enterocytes, glucose moves into the surrounding capillaries by facilitated diffusion.
Figure 1.
Physiology of lactose malabsorption. SCFA, short chain fatty acids.
Lactase deficiency and lactose malabsorption
The terms relating to lactose metabolism are often mixed-up which may cause confusion (table 2). Lactase deficiency (LD) is the failure to express lactase at the brush border of the small intestine. Lactose malabsorption (LM) refers to any cause of failure to digest and/or absorb lactose in the small intestine. Lactose intolerance (LI) is the occurrence of symptoms such as abdominal pain, bloating or diarrhoea in LM patients after ingestion of lactose.
Table 2.
Glossary with definitions related to lactase deficiency, lactose malabsorption and lactose intolerance
| Concept | Definition | |
| Congenital lactase deficiency | CLD | Very rare genetic disorder (typically frameshift mutations) leading to lack of expression of lactase and severe symptoms immediately after birth |
| Lactase non-persistence | LNP | Decrease of intestinal lactase expression in the first two decades of life. Phenotype in most individuals worldwide (biological wildtype) |
| Lactase persistence | LP | Continued expression of intestinal lactase expression beyond infancy; dominant phenotype in Western countries. |
| Lactase deficiency | LD | Inability to digest large amounts of lactose due to low lactase expression in the small intestine |
| Lactose malabsorption | LM | Passage of lactose into the large intestine as a consequence of LD or other pathology (eg, rapid transit) |
| Primary lactose malabsorption | Lactose malabsorption due to lactase non-persistence (dominant phenotype worldwide). | |
| Secondary lactose malabsorption | Lactose malabsorption due to lower lactase expression, typically in the setting of intestinal inflammation (may be reversible). | |
| Lactose intolerance | LI | Appearance of typical intestinal symptoms such as abdominal pain, bloating, diarrhoea in individuals with LM after lactose ingestion determined by appropriate testing (ideally blinded testing). |
| Functional lactose intolerance | Symptoms of LI on lactose challenge in individuals without lactose malabsorption. | |
| Self-reported lactose intolerance | SLI | History of LI symptoms without formal testing of either LM or LI. |
Congenital lactase deficiency is a very rare paediatric condition that causes severe symptoms and failure to thrive in infants.10 The most common cause of LM in adolescents and adults is primary (genetic) lactase non-persistence (LNP). The activity of lactase in the small intestine reaches a peak at the time of birth but is reduced in most populations during childhood, a process which is thought to facilitate weaning. However, in some individuals, high activity of lactase persists, enabling consumption of large amounts of lactose also in adulthood. It should be emphasised that, worldwide, most individuals have LNP with phenotypic LD and LM (figure 2). Thus, LNP, LD and LM are not diseases but normal variants of human metabolism.11 Other causes of LM include secondary (acquired) LD, rapid small intestinal transit and small bowel bacterial overgrowth.
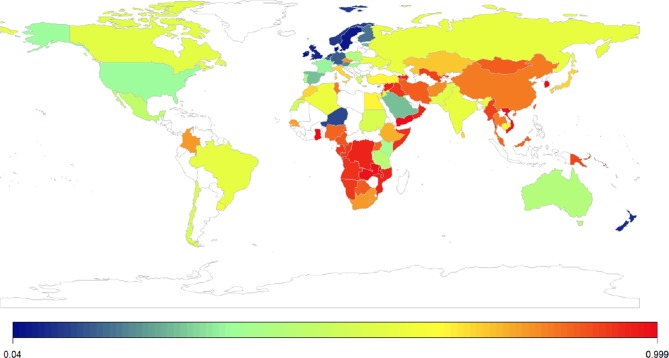
Figure 2.
Worldwide prevalence of lactose malabsorption. Online supplement 1 breaks down the the evidence base in terms of the investigative modality used to acquire the epidemiological information (eg, genetic test and breath test). Online supplement 2 provides the complete reference list.
gutjnl-2019-318404supp001.pdf (151.9KB, pdf)
gutjnl-2019-318404supp002.pdf (491.9KB, pdf)
In case of LM (primary or secondary), undigested lactose comes into contact with the intestinal microbiota. Bacterial fermentation of lactose results in production of gasses including hydrogen (H2), carbon dioxide (CO2), methane (CH4) and short chain fatty acids (SCFA) that have effects on GI function (figure 1).
Lactose intolerance
Lactose malabsorption (LM) is a necessary precondition for lactose intolerance (LI). However, the two must not be confused and the causes of symptoms must be considered separately. Many individuals with LM have no symptoms after ingestion of a standard serving of dairy products (table 1) whereas others develop symptoms (‘intolerance’) such as abdominal pain, borborygmi (rumbling tummy) and bloating after lactose intake (figure 1). The onset of these symptoms is strongly correlated to the appearance of hydrogen gas during breath tests12. Further, undigested lactose in the small intestine lead to osmotic trapping of water and the osmotic load in the colon is increased about eightfold by fermentation of lactose to SCFA.13 14 Diarrhoea will result if the respective load of lactose exceeds the capacity of the colonic microbiota for fermentation or the SCFA load exceeds the colon capacity for resorption.15
The likelihood of developing symptoms after lactose ingestion is multifactorial (figure 3). Extrinsic factors include the amount of lactose ingested and whether dairy products are ingested with other foods that affect intestinal transit and the rate of lactose delivery to the colon. Intrinsic factors include expression of lactase at the brush border of the small intestine, history of GI disorders or abdominal surgery and the composition of the intestinal microbiome. When incubated in vitro with lactose, faecal samples from lactose-intolerant subjects mediated faster and higher production of SCFA than samples from lactose-tolerant subjects.16 17 However, an impact of SCFA on symptoms has not been directly demonstrated in humans. Further, in the anaerobic environment of the intestinal tract, generation of reducing equivalents result in rapid hydrogen production, and in several clinical studies, the amount of gas production correlated with the presence and severity of intestinal symptoms.18 19 Other patient factors not directly related to lactose digestion are also associated with LI. These include the presence of anxiety disorders, high levels of psychosocial stress and the presence of functional GI disorders such as IBS (figures 3 and 4).
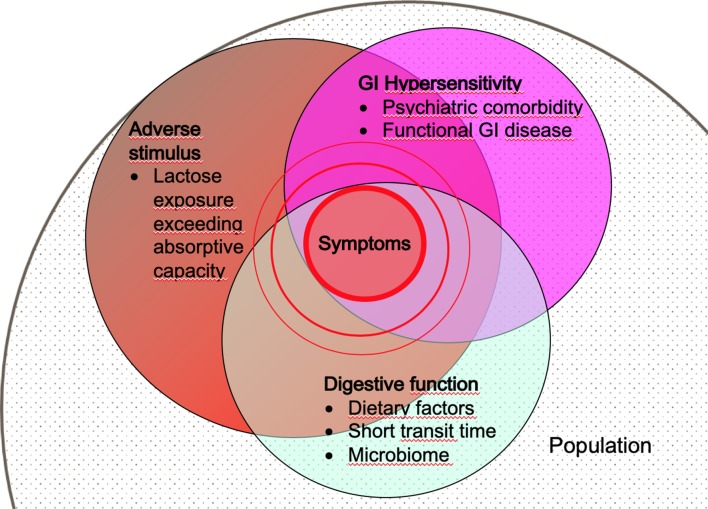
Figure 3.
Schematic model of the pathogenesis of lactose intolerance symptoms. In a given population, a fraction of individuals will have digestive dysfunction resulting in lactose malabsorption. Within this population, individuals with anxiety disorders or GI disease that increase visceral sensitivity are more susceptible to the lactose challenge. In this model, the risk of developing symptoms increases with lactose dose, severity of digestive dysfunction (lactose malabsorption) and visceral sensitivity. This model of disease is not restricted to lactose but is likely to be shared by other FODMAPs, fermentable oligosaccharides, disaccharides and monosaccharides and polyols.
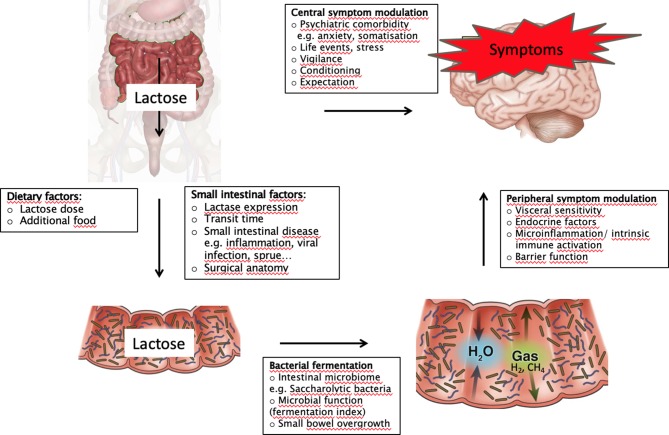
Figure 4.
Mechanistic model of lactose digestion in patients with lactase persistence and lactase deficiency illustrating the relationship between lactose malabsorption, visceral sensitivity and symptoms.
Products of lactose fermentation may also trigger extra-intestinal symptoms. A recent review of results from >2000 patients with a clinical diagnosis of functional GI disorders, reported a high frequency of neurological symptoms such as tiredness and headache after lactose or fructose ingestion.20 However, it is uncertain whether the occurrence of neurological symptoms was caused by LM, because these patients have a high prevalence of nonspecific somatic complaints21, there was no placebo control, no statistical relationship between H2 production and symptoms was present and no mechanistic explanation was provided.20
Epidemiology
A recent meta-analysis estimated the prevalence of LM worldwide at 68% with higher rates reported for genetic tests than hydrogen breath tests (HBTs).22 LM is lowest in Nordic countries (<5% in Denmark) and highest in Korean and Han Chinese populations (approaches 100%). Large variations in LM are seen on a regional level (figure 2),22 reflecting the underlying genetic heritage and prevalence of primary LD in these populations. Testing for LI is more complex and would require standardised hydrogen breath testing in large, carefully selected populations and, for this reason, the prevalence of LI is unknown.
Genetics
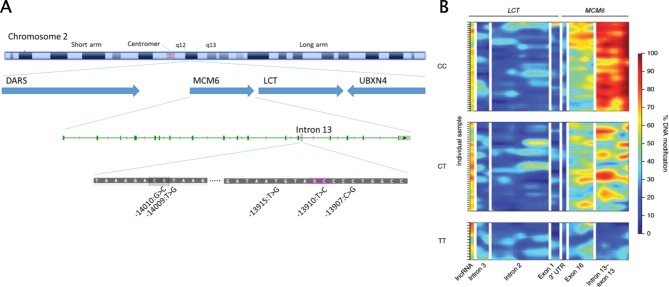
In the Caucasian population, lactase persistence (LP) is due to a gain-of-function mutation 13.9 kb upstream of the lactase gene (LCT-13’910:C→T, ‘‘T’ for tolerance’) on chromosome 2. This single nucleotide polymorphism (SNP) is far upstream of the protein forming unit within the intron of an unrelated gene (figure 5A).23 This mutation creates a new binding site for the transcription factor that promotes persistent lactase expression after infancy.24
Figure 5.
Genetics of lactose malabsorption. (A) Organisation of the lactase genetic locus on chromosome 2. The positions of the lactase gene (LCT) and the neighbouring genes aspartyl-tRNA synthetase (DARS), minichromosome maintenance complex component 6 (MCM6) and UBX domain-containing protein 4 (UBXN4) are indicated. Polymorphisms relevant for lactose malabsorption are located within intron 13 of the MCM6 gene, upstream of the lactase gene. (B) Differential levels of methylation of intron 13 of MCM6 and the LCT gene in individuals with genetic lactose malabsorption (LCT −13910:C/C), lactose tolerance (LCT −13910:T/T) and the clinically silent, physiologically intermediate genotype LCT −13910:C/T. Hypermethylation (red colour) results in genetic silencing of the respective gene. (Source: From Labrie et al 26).
Genetic LP is considered a dominant genotype, and only individuals with two LCT-13’910:C alleles should be considered to have LNP. However, heterozygotes with LCT-13’910:CT genotype may have higher H2 levels in the HBT than LCT-13’910:TT individuals.25 This intermediate phenotype might be relevant during nutritional challenges or intestinal diseases. By contrast, epigenetic regulation of the lactase gene appears to be critical. Methylation patterns in the region of the LCT-13’910:C/T polymorphism in small intestinal enterocytes strongly differ dependent on the genotype, from >80% modification with the LNP genotype to 20% with the LP genotype (figure 5B). It has also been shown that LCT promotor methylation is low after birth but increases in childhood in the presence of LCT-13’910:C but not LCT-13’910:T.26 Thus, LNP is a good example of a condition in which DNA sequence variations set the stage for age-dependent methylation which later results in a clinical phenotype, a mechanism that might be applicable also to complex diseases.27
The LCT-13’910:T SNP associated with LP in Europe and many near Asian regions resides on the same haplotype, indicating rapid spread of a single mutation.28 The mutation appears in prehistoric skeletons for the first time approximately 10 500 years ago in Anatolia with spread to Europe and Northern Africa over time in line with domestication of animals. In Africa and the Middle East, different mutations in the same genetic region are responsible for LP1 29 (table 3), indicating convergent evolution.
Table 3.
Genetic variations affecting lactase persistence and LM1
| Mutation associated with lactase persistence | Geographic region | SNP |
| LCT −13’910:T* | Northern Europe | rs4988235 |
| LCT −13’915:G | Middle East | rs41380347 |
| LCT −13’907:G | Ethiopia and Sudan | rs41525747 |
| LCT −14’009:G | Ethiopia and Sudan | rs820486563 |
| LCT −14’010:C | Kenya, Tanzania and South Africa | rs145946881 |
*This mutation is in strong linkage disequilibrium with the LCT −22’018-A mutation. Mechanistic evidence indicates that the −13’910 mutation is responsible for lactase persistence.
LM, lactose malabsorption; SNP, single nucleotide polymorphism.
Source: Adapted from Segurel and Bon 1
There is convincing genetic evidence for a strong selection pressure for LP. In a whole genome analysis of skeletons originating between 6500 and 300 BC, the LCT-13’910:T allele showed increasing prevalence over time.30 LP provided an advantage of up to 4%–5% per generation, which is one of the largest selection pressures observed for any gene in recent human evolution and is in the same order of magnitude as resistance genes for malaria (4%–9%), skin pigmentation in Europeans (3%) and genes associated with hypoxia response in Tibetan populations at high altitude.1
The ability to digest lactose after infancy made milk a source of nutrition (calories, protein) and clean water accessible to adults. This is likely to have been critical in periods of famine. However, why LP increased fitness to such a high degree is unclear since many individuals with LM can consume 250 mL of milk without developing symptoms, and processing of milk to yoghurt, cheese or butter decreases the advantage of LP further.31 Further, the ‘cost’ of LNP with generally mild abdominal symptoms seems modest and individuals with LNP may even benefit from milk consumption due to prebiotic activity of lactose on the colonic microbiota.1 Increased intake of vitamin D from milk could also provide a selective advantage, especially in Northern Europe with a high risk of vitamin D deficiency due to low ultraviolet exposure.3 32
Taken together, impressive selection pressure took place at the lactase genetic locus after the uptake of pastoralist farming, favouring LP in many regions worldwide; however, the specific advantage of milk consumption that increased survival and whether these are present only during times of dietary or health stress or continuously remain unclear.
Secondary lactose malabsorption
Secondary LM refers to the development of LM in individuals who are potentially able to digest lactose (ie, LP individuals).33 34 Lactase is situated at the tip of intestinal villi and thus vulnerable to intestinal injury, especially since new immature enterocytes are lactase deficient.6 As a consequence, secondary LM can complicate GI conditions including infectious gastroenteritis, IBD, coeliac disease and systemic sclerosis (SSc).
The incidence of secondary LM, which is often transitory, caused by infectious gastroenteritis is increased and can be clinically relevant,35 especially in infants for whom milk is the staple food. In a paediatric study (mean age 12 months) with 126 patients with rotavirus infection and 62 controls with rotavirus negative diarrhoea, LM was more frequent in the former group (60% vs 49%, p=0.002).36 Similarly, in adult patients with chronic diarrhoea after kidney transplantation, those with norovirus colonisation had a much higher risk for LM than a control group (100% vs 12.5%).37 A systematic review concluded that exclusion of lactose would reduce the duration of acute diarrhoea in children by up to a day and reduce ‘treatment failure’ (RR: 0.5, 95% CI: 0.4 to 0.7), variously described in studies as requirement for unscheduled intravenous fluid injection or persistent stool weight >30 g/ kg after 3 days.38
Similar but more persistent results are seen in IBD. In a meta-analysis, the overall OR for LM in patients with IBD was 1.6 (95% CI: 1.0 to 2.6, p=0.048), being highest in Crohn’s disease (CD) affecting the small bowel.39 In line with this observation, a paediatric study showed reduced lactase expression in CD patients with the risk of LD correlated with villous atrophy.39 40 High prevalence of secondary LM has also been reported in other conditions that affect the mucosal integrity or function of the small bowel. Patients with a new diagnosis of coeliac disease often have a positive lactose HBT; however, many recover the ability to digest lactose after 6–12 months on a gluten-free diet.41Patients with SSc also have a high prevalence of LM on breath testing, a finding that is associated with more advanced disease.42 Secondary LD may complicate environmental enteric dysfunction (EED), a condition that affects mainly children in an environment with low resources, poor hygiene and poor nutrition. EED is characterised by intestinal atrophy and dysbiosis associated with enzyme deficiencies, malabsorption and malnutrition.43
Lactose malabsorption and the microbiota
The human body harbours approximately 40 trillion bacteria with approximately 99% of the microbiome contained within in the human colon. Fermentation of lactose by saccharolytic (‘sugar digesting’) bacteria in individuals with LM can cause abdominal symptoms (figures 1 and 4). However, this process also has benefits. SCFA and other products of fermentation are required for colonic health and liberate additional calories from otherwise indigestible carbohydrates. Moreover, the intestinal microbiota adapts to facilitate intake of dairy products. As a result, although lactase expression is not upregulated by lactose ingestion, regular consumption of lactose appears to reduce breath hydrogen excretion and reduced lactose intolerant symptoms.44 Both in vitro and in vivo studies demonstrate increases in Bifidobacteria and/or Lactobacilli that are considered to be healthy components of the microbiome.45 46 In a large study of healthy Japanese, the abundance of Bifidobacteria correlated positively with dietary dairy intake.47 As this population is 90%–100% LNP, this could reflect the effect of regular lactose intake on the microbiota; however, the reverse cannot be excluded.
Recent data point to interactions between human genes and the microbiota. In association studies between human genetic variations and the microbiota, to date, the association most consistently described is that between the LCT-13’910:C/T SNP and the abundance of Bifidobacterium.48–50 Such interactions might have practical implications because SCFAs produced by microbial fermentation of lactose are involved in immune regulation,51 glucose and lipid homeostasis,52 colonocyte differentiation53 with implications for homeostasis and gut-brain modulation.54 Taken together, some experts suggest that LNP subjects may have ‘more to gain than to lose’ by consumption of small amounts of lactose-containing foods.55
Lactose intolerance and IBS
The relationship of LI and IBS has been extensively studied in a South Chinese population with near 100% LNP on genetic testing. A double-blinded, randomised, cross-over comparison of lactose tolerance at 10, 20 and 40 g lactose was performed in IBS patients with diarrhoea (IBS-D) and healthy controls.56 There was a very strong correlation between the appearance of hydrogen gas in the breath and reports of bloating, pain and other symptoms in patients with lactose intolerance. However, the correlation between the amount of hydrogen gas in the breath and the severity of symptoms was much weaker. Consistent with preliminary findings in a European population,57 a key observation was that the risk of symptoms and the severity of symptoms were greatly increased in IBS patients, especially at the low to moderate doses found in the normal diet. It is well known that many patients with functional GI disorders have psychological comorbidity and are hypersensitive to dietary and physical stimuli that affect the digestive tract. 21 58 Further work demonstrated that anxiety, visceral hypersensitivity (defined by rectal barostat) and high levels of gas production on breath tests all increased the severity of abdominal symptoms after ingestion of 20 g lactose.19 Moreover, mucosal biopsies from the ileum and colon showed increased numbers of mast cells and intraepithelial lymphocytes in lactose-sensitive patients and showed that the release of inflammatory cytokines (eg, tumour necrosis factor) after lactose intake was higher in this group than controls.59
These observations are similar to those in post-infective IBS58 and provide insight into the pathophysiological basis not only of food intolerance but, more generally, functional GI symptoms. IBS is a heterogenous condition; however, symptoms related to intake of food items with poorly absorbed, fermentable carbohydrates such as lactose are reported by up to 70% of patients with this diagnosis.60–62 Patients with LI and IBS complain of similar symptoms, have high rates of psychological comorbidity and markers of an activated innate mucosal immune system. Moreover, both respond to similar dietary interventions (see below). Together, this evidence suggests a common pathological basis in which a susceptible individual with a sensitive (‘irritable’) bowel develops symptoms when exposed even to a modest stimulus, such as low–moderate doses of lactose (figure 3).33
Lactose intolerance and quality of life
Like other functional GI disorders, LI is not a trivial condition but has a negative impact on quality of life and nutrition. Anxiety increases the risk of symptoms (‘intolerance’) after lactose ingestion, but the fear that food will trigger bloating, pain and diarrhoea is also a cause of anxiety. Indeed, in studies, not only patients with LI but also those with self-diagnosed LI who do not have the condition describe a lower quality of life than individuals without concerns about food intolerance.61 62 This anxiety generalises to other foods, and patients with LI often describe intolerance to a range of products, especially those known to cause bloating (eg, legumes and dried fruit).62 As a result, individuals might adopt a restrictive diet that could impact on health in a variety of ways.60 62 63 In severe cases, this form of behaviour is termed avoidant/restrictive food intake disorder by DSM-5, a form of eating disorder that is associated with weight loss but not with body dysmorphia.64
Testing for lactose malabsorption and intolerance
Five tests of lactose digestion are available, each of which investigates different aspects of the process and has specific advantages and disadvantages (table 4).
Table 4.
Brief characterisation of the diagnostic tests available for lactose malabsorption
| Hydrogen breath test | Lactose tolerance test | Duodenal lactase activity | Serum gaxilose or urine galactose test | Genetic test | |
| Lactose challenge | Yes | Yes | No | Yes (gaxilose) | No |
| Assessment of symptoms and LI | Yes/possible | Yes/possible | No/not possible | No/not possible | No/not possible |
| Test principle | Detection of H2 in expiratory air | Increase in plasma glucose after lactose challenge | Lactase enzymatic activity in duodenal biopsy | Detection of D-xylose in serum or Galactose in urine after cleavage of orally administered 4-galactosylxylose (gaxilose) by lactase | Detection of −13910 C/T polymorphism |
| Detection of secondary LM | Yes | Yes | Yes | Yes | No |
| Costs | Low | Lowest | High (if costs for endoscopy are included) | Intermediate | High |
| Limitations | False-negative tests by H2-non- producer. False-positive tests with SIBO, rapid transit, altered bowel anatomy | Disorders of glucose metabolism, altered bowel anatomy | Patchy expression of lactase | Variable test performance in literature. False-positive tests with SIBO, rapid transit and other conditions | False negative in the presence of atypical mutations (relevant in non-Caucasian populations) |
| Best use | Test of choice to assess LM and symptoms (LI) | Low resource setting, LM epidemiology | If gastroscopy is performed for other reasons | To be determined | LD/LNP epidemiology |
LD, lactase deficiency; LI, lactose intolerance; LM, lactose malabsorption; LNP, lactase non-persistence; SIBO, small intestinal bacterial overgrowth.
Genetic tests apply real-time PCR or sequencing on DNA extracted from a venous blood or buccal swab sample and are most appropriate in epidemiological studies. In Caucasians, LP is nearly uniformly mediated by the LCT-13910:C/T polymorphism, and genetic testing can detect genetic LNP. However, the genetic situation is more complex in patients with African or Asian heritage (table 3) and genetic tests are currently not advocated in these populations for clinical purposes. Importantly, secondary LM will not be detected by genetic tests.
Tests for lactase enzymatic activities on intestinal biopsies will detect primary and secondary LM. While an endoscopy with sedation is not indicated for this purpose, this test can be performed if endoscopy is indicated for other reasons. It should be noted that lactase activity is patchy and more than a single biopsy may be required for optimal test accuracy.65
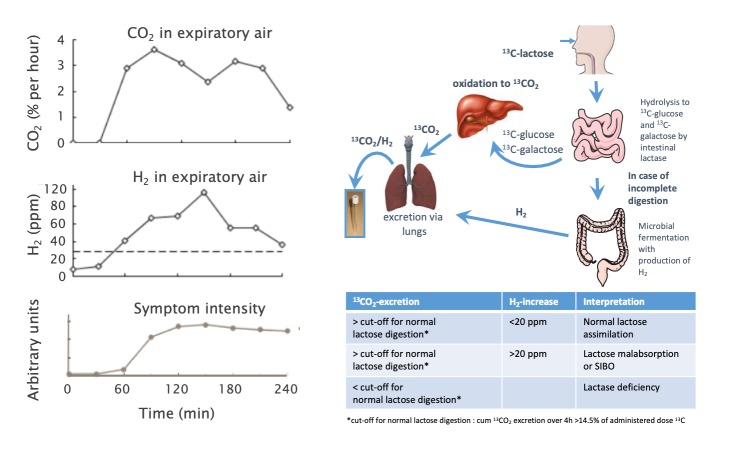
The lactose HBT measures the excretion of hydrogen in expiratory air after an oral challenge with a standard dose of lactose. As hydrogen is not produced by mammalian enzymes, its presence indicates contact of the sugar with bacteria indicating LM, although small intestinal bacterial overgrowth cannot be excluded. In clinical practice, an intermediate lactose dosage of 20–25 g may be optimal.56 66 Smaller amounts of lactose lack sensitivity for LM. Larger amounts used in epidemiological studies (eg, 40–50 g, figure 6) induce symptoms even in healthy individuals with LM that tolerate the amount of lactose present in normal diets (see below).56 A baseline H2 value <20 ppm is a requirement for a reliable test and an increase ≥20 ppm within 3 hours is diagnostic of LM.66 Reducing observation time impairs sensitivity; however, only four measurements (0, 90, 120, 180 min) are required for valid results.67 An H2-non-producing microbiota can lead to false-negative HBT. In some of these individuals, methanogenic bacteria (eg, Methanobrevibacter smithii) convert hydrogen to methane (CH4) in a 4:1 ratio resulting in lower H2 excretion and a lower fraction of positive tests.66 Simultaneous assessment of methane can partially overcome this limitation. Therefore, even though the increase in CH4 is often low (<20 ppm) and not correlated to symptoms, combined H2/CH4-measurements are recommended by some authors.66 68 A more reliable approach involves the use of 13C-lactose with simultaneous breath measurements of 13CO2 as a marker of lactose digestion and H2 as a marker of LM (figure 7); however, this technique is not available outside specialist centers.69
Figure 6.
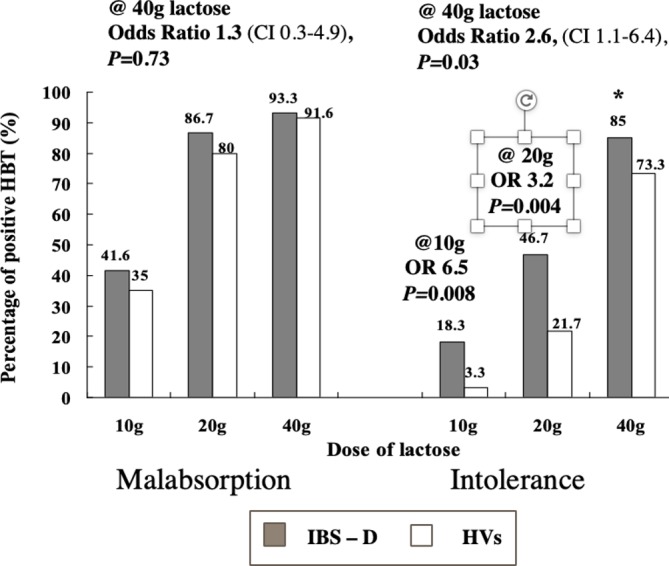
Symptoms in individuals with lactose malabsorption depend on lactose dose and visceral hypersensitivity. A population of Chinese individuals (100% primary lactase non-persistence) including HV with no history of abdominal symptoms and individuals with IBS-D was tested three times with different lactose dosages (10, 20 and 40 g) in a blinded fashion. The likelihood of a clinically positive HBT was higher in individuals with IBS-D for the low and the intermediate lactose dose. HBT, hydrogen breath test; HV, healthy volunteers; IBS-D, diarrhoea-predominant irritable bowel syndrome. (Source: Adapted from Yang et al.56)
Figure 7.
Results of a hydrogen breath test of an individual with lactose intolerance with simultaneous assessment symptoms and H2 levels, indicating lactose fermentation by the microbiota. An H2 increase by ≥20 ppm over baseline indicates lactose malabsorption. When 13C-labelled lactose is administered, 13CO2 levels indicate absorption and metabolisation of 13C-labelled lactose by the subject. Patient reports of abdominal symptoms subsequent to increases in these markers is diagnostic of lactose intolerance.
The lactose tolerance test measures glucose in plasma at different times (e.g. 0, 30, 60, 120 min) after ingestion of 50 g lactose. Although the test does not require complex or expensive equipment, its invasive nature (multiple blood samples) limits its utility. Use of capillary blood measurements with portable glucose metres makes the test less invasive but does not offer the same diagnostic accuracy as measurements in venous blood.70
The gaxilose test involves the administration of the lactase substrate gaxilose (4-galactosylxylose) with measurement of D-xylose in urine or blood. Conceptually, gaxilose measurements are ideal for assessment of intestinal lactase since activity over the entire small intestine is measured.71 In a manufacturer-sponsored trial, the diagnostic accuracy of gaxilose tests (0.93) was higher than HBT (0.85) or lactose tolerance tests (0.79) in comparison to duodenal biopsies.71 However, this was not confirmed in an independent study when the genetic test (LCT-13’910:C/T) was used as reference.72
Testing for lactose intolerance
The major limitation of the genetic, enzymatic and gaxilose tests is that LM is common in healthy individuals, and a positive test does not confirm that symptoms are caused by this condition. For this reason, in our practice, HBT is the method of choice because reasonably reliable information about digestive function and patient symptoms are obtained.
The diagnosis of LI requires appropriate testing of symptoms using validated questionnaires designed for the purpose.73 A National Institute of Health consensus conference defined LI as ‘the onset of GI symptoms following a blinded, single-dose challenge of ingested lactose by an individual with LM, which are not observed when the person ingests an indistinguishable placebo’,74 thus supporting the case for blinded testing of symptoms. Although rarely performed outside clinical studies, blinded testing might be useful since in clinical practice, the correlation between self-reported symptoms of LI and objective findings on tests for lactose digestion is low.62 Indeed, among individuals referred for HBT, about half of those with normal lactose digestion report abdominal discomfort after an unblinded lactose challenge.69 Further, intolerance to dairy products is reported by 20% of all individuals75 and up to 70% of IBS patients in European populations with low rates of genetic LNP.60
A ‘blinded multiple dose challenge’ would provide clarity not only regarding lactose digestion but also identify the amount of lactose that individuals could ‘safely’ consume (figure 6).56 Moreover, in subjects with known LNP, these could be performed at patients’ homes with a negative control, low and intermediate lactose challenge (eg, 12.5 and 25 g, corresponding to 250 and 500 mL milk, respectively).34 This could help educate patients because, in real life, it is self-reported intolerance and not the objective results of testing that best predicts food choices.62 However, to the best of our knowledge, blinded home-based testing has not been tested in routine clinical practice. The need for a well-accepted, practical and cost-effective investigation of food intolerance that predicts the outcome of dietary therapy is a key clinical challenge in functional GI disorders. The ability to predict the outcome of dietary therapy would be the measure for an appropriate symptom assessment.
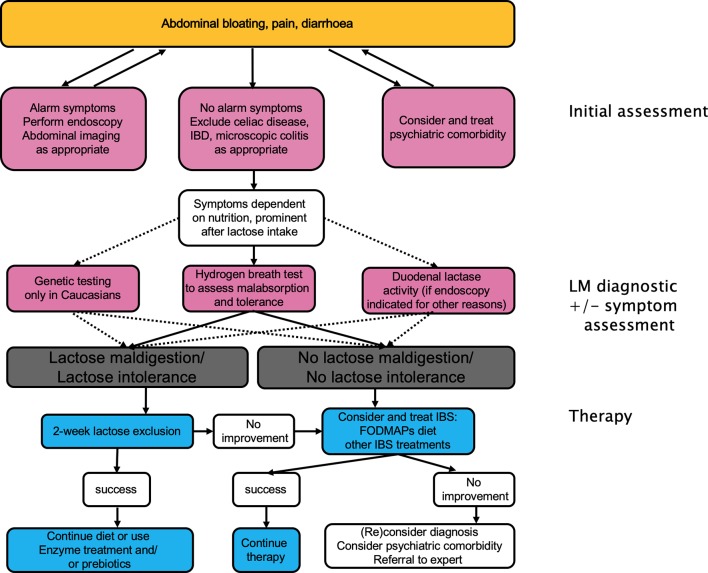
Therapeutic options
Therapy of lactose intolerance aims to improve patient symptoms and to avoid risk for undernutrition or malnutrition in the long term (figure 8). A diet low in lactose is typically recommended and this is supported by common sense and clinical evidence.56 However, in contrast to the management of sprue or food allergies, a strict lactose-free diet is not required since patients with LI often tolerate up to 250 mL milk (12 g lactose) without symptoms and more when consumed with food.31
Figure 8.
Management of lactose malabsorption and lactose intolerance. FODMAPs, fermentable oligosaccharides, disaccharides and monosaccharides and polyols.
Improved lactose tolerance by manipulating the colonic microbiota could also be achieved by ingestion of prebiotics.44 A randomised placebo-controlled study in 85 LI patients reported that regular ingestion of short-chain galacto-oligosaccharides (GOS, RP-G28) tended to reduce H2 production and improve abdominal pain during lactose HBT. After 1 month, 30% of GOS-treated patients versus 6% of placebo-treated patients considered themselves lactose tolerant.76 Microbiological workup revealed a transient increase in lactose fermenting Bifidobacterium spp. on GOS treatment and a negative correlation between Bifidobacterium levels and abdominal pain, and re-introduction of milk prompted a further shift in bacterial composition, including an increase in the genus Roseburia.77
Lactose-free dairy products in which lactase is added to milk are widely available and considered safe, although allergic reactions have been reported.78 Lactase treatment of milk products also reduces crystallisation of lactose, increasing sweetness and fermentation for production of yoghurt.79 However, residual side proteolytic activity of lactase can degenerate casein and impair taste, especially after long storage.80
Lactase supplementation by tablets improves both lactose digestion (reduced H2 production) and symptoms81 82 although the effects are modest (eg, 18% with overall reduction of symptoms82). An alternative approach is to ingest probiotics such as Lactobacillus spp., Bifidobacterium longum or Bifidobacterium animalis that produce lactase in the gut. A recent systematic review of this treatment option confirmed an overall positive effect; however, the effect size was not consistently better than lactase supplementation and study quality was poor.83
In many clinical studies, only a minority of patients with LI on HBT report satisfactory improvement in symptoms after treatment to reduce intake of dairy products or supplement lactase. Moreover, it remains unclear, to what extent the therapy itself and conditioning of patient expectations contribute to outcome. Lack of improvement can also be due to the presence of functional bowel disorders, which are present in many patients referred for investigation. These patients are sensitive to various nutrients, mechanical and chemical stimuli and, therefore, rarely respond to restriction of dairy products alone.84
IBS patients develop symptoms after ingestion of a range of poorly absorbed, fermentable carbohydrates (fermentable oligosaccharides, disaccharides and monosaccharides and polyols (FODMAPs)) that includes but is not restricted to lactose even in LM patients.85 A low-FODMAP diet improves abdominal symptoms in 50%–80% of IBS patients.86 87 This dietary therapy requires commitment from the patient and is best delivered by professional dietician. Identification of factors predicting dietary outcome would improve compliance and cost-effectiveness of this intervention; however, in a large clinical study neither clinical presentation nor HBT results (high dose lactose 50 g or fructose 35 g) predicted response to the low-FODMAP diet.87 The response to an intermediate dose of a representative, non-absorbable FODMAP (eg, lactulose 20 g) that rarely c symptoms in health, but often induces bloating, abdominal pain and diarrhea in FGID patients may improve the ability of HBT to identify individuals that respond to this dietary intervention. Alternatively, bioassays to identify saccharolytic bacteria and/or fermentation capacity in faecal samples might be developed that predict outcome of lactose (or FODMAP) restriction in patients.88
Long-term complications of lactose intolerance
Considering the objective effects of genetic LD on intestinal microbiota and recent human evolution (see above), LM and LI are likely to have a relevant impact on nutrition. Dairy products are valuable sources of protein, calcium and vitamin D.89 However, these nutrients can also be acquired from other food sources.
The relationship between lactose tolerance and height has been demonstrated, although some of this effect could be explained by population stratification.90 Daily milk consumption of 245 mL is associated with increased body height (0.39 cm, 95% CI: 0.29 to 0.48).90 Similarly, milk intake and LP have been linked with higher body mass index (BMI) in some studies.91
Effects of nutrition on health are difficult to address in interventional studies due to need for long-term follow-up, costs and limited compliance in patients. However, since LM in Caucasians is a monogenic condition (LCT −13’910C genotype), this question can be addressed by applying a Mendelian randomisation approach that limits confounding by social, environmental or behavioural factors. A recent study using this methodology confirmed higher milk consumption in individuals with genetic LP and this was associated with vitamin D levels which were 2.3-fold (OR: 1.6–3.4) lower in individuals with LCT-13’910:CC and 1.5-fold lower in LCT-13’910:CT compared with LCT-13’910:TT.92 Vitamin D is important for bone mineralisation and a separate meta-analysis showed a higher bone mineral density and a lower risk of fractures for TT versus CT/CC (OR: 0.81, 95% CI: 0.7 to 0.94, p=0.005).93 However, this finding was not confirmed in a European study that applied a similar study design.91
Results regarding other effects of milk consumption such as cardiovascular health and cancer are controversial.94 In a large Swedish study, individuals with high consumption of non-fermented milk and other dairy products had a higher all-cause mortality (HR: 1.32, 95% CI: 1.18 to 1.48); however, these results were not robust in the subgroup for which a Mendelian randomisation study could be performed.95 Some of these findings might be explained by an effect of LP on BMI (see above). In any case, these conflicting results are not surprising considering the complexity of the diet with regards to availability of lactose-free milk, intake of calcium, vitamin D, saturated fats, cholesterol, proteins and calories. Moreover, other genetic markers for lipid metabolism and polymorphisms of the vitamin D receptor might also impact on health.94 Additional studies with new approaches accounting for multiple nutrients and multiple genetic markers are needed to clarify the relationship of milk consumption, LM and long-term outcomes.
Outlook
Primary genetic LP and non-persistence are common in healthy humans; however, ingestion of milk by individuals with LD leads to LM and, in susceptible patients, to symptoms of lactose intolerance. Diagnosis is based on detection either of the genetic mutation, loss of lactase activity in the enteric mucosa or evidence of malabsorption by breath tests. However, the association between self-reported LI, objective findings of tests and clinical outcome of dietary intervention is variable. Recent studies have provided important new insight into the complex relationship between LD, LM and symptom generation. This work has shed light on the important issue of food intolerance as a cause of symptoms in IBS and other functional GI disorders.
The development of a well-accepted, practical and cost-effective investigation of food intolerance that predicts the outcome of dietary therapy is one of the biggest clinical challenges in the field of functional GI disorders. Understanding the biological mechanism for food intolerance will help clinicians make a definitive diagnosis and guide rational dietary and medical management. Ongoing studies will provide high-quality evidence to document the clinical outcome, cost-effectiveness and long-term effects of these strategies.
Acknowledgments
The authors would like to thank Professor Ning Dai, President of the school of medicine, Zhejiang University City College, and her team at the Sir Run Run Shaw Hospital in Hangzhou for their excellent work on the Sino–Swiss trials into the mechanism of lactose intolerance that inform key sections of this review referred to in this article. We thank also Dr Lars Fadnes of the Department of Global Public Health and Primary Care, University of Bergen, attached to Haukeland University Hospital. The figures and supplemental file were prepared by Lars Fadnes for this publication. His work collating epidemiological studies of lactose malabsorption and intolerance is presented in figure 2 and supplemental files.
Footnotes
Contributors: MB and BM performed the literature search, collated the information and produced the first draft of the manuscript. KV and MRF contributed additional material and edited the publication. All authors discussed and revised the draft and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MF has received research funding from Nestlé International for studies of lactose digestion and tolerance.
Provenance and peer review: Commissioned; externally peer reviewed.
Collaborators: Professor Ning Dai, Department of Gastroenterology, Zhejiang University School of Medicine, Sir Run Run Shaw Hospital, Hangzhou, Zhejiang, China, 310016; E-mail: 2267454962@qq.com. Dr Lars Fadnes, Department of Global Public Health and Primary Care, University of Bergen and Bergen Addiction Research Group, Department of Addiction Medicine, Haukeland University Hospital, Post box 7804, 5020 Bergen, Norway (Web: http://www.uib.no/en/persons/Lars.Thore.Fadnes) E-mail: Lars.Fadnes@uib.no
Patient consent for publication: Not required.
References
- 1. Ségurel L, Bon C. On the evolution of lactase persistence in humans. Annu Rev Genomics Hum Genet 2017;18:297–319. 10.1146/annurev-genom-091416-035340 [DOI] [PubMed] [Google Scholar]
- 2. Bayless TM, Brown E, Paige DM. Lactase non-persistence and lactose intolerance. Curr Gastroenterol Rep 2017;19:23 10.1007/s11894-017-0558-9 [DOI] [PubMed] [Google Scholar]
- 3. Silanikove N, Leitner G, Merin U. The Interrelationships between lactose intolerance and the modern dairy industry: global perspectives in evolutional and historical backgrounds. Nutrients 2015;7:7312–31. 10.3390/nu7095340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reich CM, Arnould JPY. Evolution of Pinnipedia lactation strategies: a potential role for α-lactalbumin? Biol Lett 2007;3:546–9. 10.1098/rsbl.2007.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slupsky CM, He X, Hernell O, et al. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs regular infant formula: A randomized controlled trial. Sci Rep 2017;7:3640 10.1038/s41598-017-03975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grenov B, Briend A, Sangild PT, et al. Undernourished children and milk lactose. Food Nutr Bull 2016;37:85–99. 10.1177/0379572116629024 [DOI] [PubMed] [Google Scholar]
- 7. Skovbjerg H, Norén O, Sjöström H, et al. Further characterization of intestinal lactase/phlorizin hydrolase. Biochim Biophys Acta 1982;707:89–97. 10.1016/0167-4838(82)90400-9 [DOI] [PubMed] [Google Scholar]
- 8. Amiri M, Diekmann L, von Köckritz-Blickwede M, et al. The diverse forms of lactose intolerance and the putative linkage to several cancers. Nutrients 2015;7:7209–30. 10.3390/nu7095332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, Tuo B, Dong H. Regulation of intestinal glucose absorption by ion channels and transporters. Nutrients 2016;8:43 10.3390/nu8010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enattah N, Pekkarinen T, Välimäki MJ, et al. Genetically defined adult-type hypolactasia and self-reported lactose intolerance as risk factors of osteoporosis in Finnish postmenopausal women. Eur J Clin Nutr 2005;59:1105–11. 10.1038/sj.ejcn.1602219 [DOI] [PubMed] [Google Scholar]
- 11. Misselwitz B, Fox M. What is normal and abnormal in lactose digestion? Lancet Gastroenterol Hepatol 2017;2:696–7. 10.1016/S2468-1253(17)30180-2 [DOI] [PubMed] [Google Scholar]
- 12. Zhao J, et al. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2010;31:892–900. [DOI] [PubMed] [Google Scholar]
- 13. Suarez F, Levitt M. Lactose malabsorption and diarrhea. Nutrition 1997;13:53–4. 10.1016/S0899-9007(96)00294-8 [DOI] [PubMed] [Google Scholar]
- 14. Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of fodmaps (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol 2014;109:110–9. 10.1038/ajg.2013.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 2010;72:297–313. 10.1146/annurev-physiol-021909-135817 [DOI] [PubMed] [Google Scholar]
- 16. He T, Venema K, Priebe MG, et al. The role of colonic metabolism in lactose intolerance. Eur J Clin Invest 2008;38:541–7. 10.1111/j.1365-2362.2008.01966.x [DOI] [PubMed] [Google Scholar]
- 17. Windey K, Houben E, Deroover L, et al. Contribution of colonic fermentation and fecal water toxicity to the pathophysiology of lactose-intolerance. Nutrients 2015;7:7505–22. 10.3390/nu7095349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wotzka SY, Kreuzer M, Maier L, et al. Microbiota stability in healthy individuals after single-dose lactulose challenge—A randomized controlled study. PLoS One 2018;13:e0206214 10.1371/journal.pone.0206214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Y, Zheng X, Cong Y, et al. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol 2013;108:1516–25. 10.1038/ajg.2013.198 [DOI] [PubMed] [Google Scholar]
- 20. Wilder-Smith CH, Olesen SS, Materna A, et al. Fermentable sugar ingestion, gas production, and gastrointestinal and central nervous system symptoms in patients with functional disorders. Gastroenterology 2018;155:1034–44. 10.1053/j.gastro.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 21. Fox MR, Kahrilas PJ, Roman S, et al. International Working Group for Disorders of Gastrointestinal Motility and Function. Clinical measurement of gastrointestinal motility and function: who, when and which test? Nat Rev Gastroenterol Hepatol 2018;15:568–79. 10.1038/s41575-018-0030-9 [DOI] [PubMed] [Google Scholar]
- 22. Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:738–46. 10.1016/S2468-1253(17)30154-1 [DOI] [PubMed] [Google Scholar]
- 23. Enattah NS, Sahi T, Savilahti E, et al. Identification of a variant associated with adult-type hypolactasia. Nat Genet 2002;30:233–7. 10.1038/ng826 [DOI] [PubMed] [Google Scholar]
- 24. Lewinsky RH, Jensen TG, Møller J, et al. T-13910 DNA variant associated with lactase persistence interacts with Oct-1 and stimulates lactase promoter activity in vitro. Hum Mol Genet 2005;14:3945–53. 10.1093/hmg/ddi418 [DOI] [PubMed] [Google Scholar]
- 25. Dzialanski Z, Barany M, Engfeldt P, et al. Lactase persistence versus lactose intolerance: Is there an intermediate phenotype? Clin Biochem 2016;49:248–52. 10.1016/j.clinbiochem.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 26. Labrie V, Buske OJ, Oh E, et al. Lactase nonpersistence is directed by DNA-variation-dependent epigenetic aging. Nat Struct Mol Biol 2016;23:566–73. 10.1038/nsmb.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swallow DM, Troelsen JT. Escape from epigenetic silencing of lactase expression is triggered by a single-nucleotide change. Nat Struct Mol Biol 2016;23:505–7. 10.1038/nsmb.3238 [DOI] [PubMed] [Google Scholar]
- 28. Enattah NS, Trudeau A, Pimenoff V, et al. Evidence of still-ongoing convergence evolution of the lactase persistence T-13910 alleles in humans. Am J Hum Genet 2007;81:615–25. 10.1086/520705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ranciaro A, Campbell MC, Hirbo JB, et al. Genetic origins of lactase persistence and the spread of pastoralism in Africa. Am J Hum Genet 2014;94:496–510. 10.1016/j.ajhg.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 2015;528:499–503. 10.1038/nature16152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 2010;152:797–803. 10.7326/0003-4819-152-12-201006150-00241 [DOI] [PubMed] [Google Scholar]
- 32. Sverrisdóttir Oddný Ósk, Timpson A, Toombs J, et al. Direct estimates of natural selection in iberia indicate calcium absorption was not the only driver of lactase persistence in Europe. Mol Biol Evol 2014;31:975–83. 10.1093/molbev/msu049 [DOI] [PubMed] [Google Scholar]
- 33. Deng Y, Misselwitz B, Dai N, et al. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015;7:8020–35. 10.3390/nu7095380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Misselwitz B, Pohl D, Frühauf H, et al. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J 2013;1:151–9. 10.1177/2050640613484463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davidson GP, Goodwin D, Robb TA. Incidence and duration of lactose malabsorption in children hospitalized with acute enteritis: Study in a well-nourished urban population. J Pediatr 1984;105:587–90. 10.1016/S0022-3476(84)80425-4 [DOI] [PubMed] [Google Scholar]
- 36. Hu Y, Gui L, Chang J, et al. The incidence of infants with rotavirus enteritis combined with lactose intolerance. Pak J Pharm Sci 2016;29(1 Suppl):321–3. [PubMed] [Google Scholar]
- 37. Bonani M, Pereira RM, Misselwitz B, et al. Chronic norovirus infection as a risk factor for secondary lactose maldigestion in renal transplant recipients: a prospective parallel cohort pilot study. Transplantation 2017;101:1455–60. 10.1097/TP.0000000000001376 [DOI] [PubMed] [Google Scholar]
- 38. Gaffey MF, Wazny K, Bassani DG, et al. Dietary management of childhood diarrhea in low- and middle-income countries: a systematic review. BMC Public Health 2013;13(Suppl 3):S17 10.1186/1471-2458-13-S3-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szilagyi A, Galiatsatos P, Xue X. Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr J 2015;15:67 10.1186/s12937-016-0183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiecek S, Wos H, Radziewicz Winnicki I, et al. Disaccharidase activity in children with inflammatory bowel disease. Turk J Gastroenterol 2014;25:185–91. 10.5152/tjg.2014.3994 [DOI] [PubMed] [Google Scholar]
- 41. Ojetti V, Gabrielli M, Migneco A, et al. Regression of lactose malabsorption in coeliac patients after receiving a gluten-free diet. Scand J Gastroenterol 2008;43:174–7. 10.1080/00365520701676138 [DOI] [PubMed] [Google Scholar]
- 42. Marie I, Leroi A-M, Gourcerol G, et al. Lactose malabsorption in systemic sclerosis. Aliment Pharmacol Ther 2016;44:1123–33. 10.1111/apt.13810 [DOI] [PubMed] [Google Scholar]
- 43. Kvissberg MA, Dalvi PS, Kerac M, et al. Carbohydrate malabsorption in acutely malnourished children and infants: a systematic review. Nutr Rev 2016;74:48–58. 10.1093/nutrit/nuv058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr 1996;64:232–6. 10.1093/ajcn/64.2.232 [DOI] [PubMed] [Google Scholar]
- 45. Szilagyi A, Shrier I, Heilpern D, et al. Differential impact of lactose/lactase phenotype on colonic microflora. Can J Gastroenterol 2010;24:373–9. 10.1155/2010/649312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mäkivuokko HA, Saarinen MT, Ouwehand AC, et al. Effects of lactose on colon microbial community structure and function in a four-stage semi-continuous culture system. Biosci Biotechnol Biochem 2006;70:2056–63. 10.1271/bbb.60022 [DOI] [PubMed] [Google Scholar]
- 47. Kato K, Ishida S, Tanaka M, et al. Association between functional lactase variants and a high abundance of Bifidobacterium in the gut of healthy Japanese people. PLoS One 2018;13:e0206189 10.1371/journal.pone.0206189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goodrich JK, Davenport ER, Clark AG, et al. The relationship between the human genome and microbiome comes into view. Annu Rev Genet 2017;51:413–33. 10.1146/annurev-genet-110711-155532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goodrich JK, Davenport ER, Beaumont M, et al. Genetic determinants of the gut microbiome in UK Twins. Cell Host Microbe 2016;19:731–43. 10.1016/j.chom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonder MJ, Kurilshikov A, Tigchelaar EF, et al. The effect of host genetics on the gut microbiome. Nat Genet 2016;48:1407–12. 10.1038/ng.3663 [DOI] [PubMed] [Google Scholar]
- 51. Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. 10.1016/B978-0-12-800100-4.00003-9 [DOI] [PubMed] [Google Scholar]
- 52. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–91. 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- 53. Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab 2013;14:994–1008. 10.2174/1389200211314090006 [DOI] [PubMed] [Google Scholar]
- 54. Dalile B, Van Oudenhove L, Vervliet B, et al. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 2019;12 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 55. Lukito W, Malik SG, Surono IS, et al. From ’lactose intolerance' to ’lactose nutrition'. Asia Pac J Clin Nutr 2015;24(Suppl 1):S1–8. 10.6133/apjcn.2015.24.s1.01 [DOI] [PubMed] [Google Scholar]
- 56. Yang J, Deng Y, Chu H, et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:262–8. 10.1016/j.cgh.2012.11.034 [DOI] [PubMed] [Google Scholar]
- 57. Beyerlein L, Pohl D, Delco F, et al. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Aliment Pharmacol Ther 2008;27:659–65. 10.1111/j.1365-2036.2008.03623.x [DOI] [PubMed] [Google Scholar]
- 58. Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009;136:1979–88. 10.1053/j.gastro.2009.02.074 [DOI] [PubMed] [Google Scholar]
- 59. Yang J, Fox M, Cong Y, et al. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther 2014;39:302–11. 10.1111/apt.12582 [DOI] [PubMed] [Google Scholar]
- 60. Böhn L, Störsrud S, Törnblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013;108:634–41. 10.1038/ajg.2013.105 [DOI] [PubMed] [Google Scholar]
- 61. Casellas F, Aparici A, Pérez MJ, et al. Perception of lactose intolerance impairs health-related quality of life. Eur J Clin Nutr 2016;70:1068–72. 10.1038/ejcn.2016.80 [DOI] [PubMed] [Google Scholar]
- 62. Zheng X, Chu H, Cong Y, et al. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices. Neurogastroenterol Motil 2015;27:1138–46. 10.1111/nmo.12602 [DOI] [PubMed] [Google Scholar]
- 63. Böhn L, Störsrud S, Simrén M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol Motil 2013;25:23–e1. 10.1111/nmo.12001 [DOI] [PubMed] [Google Scholar]
- 64. Hay P, Mitchison D, Collado AEL, et al. Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. J Eat Disord 2017;5:21 10.1186/s40337-017-0149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maiuri L, Rossi M, Raia V, et al. Morphological method for the diagnosis of human adult type hypolactasia. Gut 1994;35:1042–6. 10.1136/gut.35.8.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American Consensus. Am J Gastroenterol 2017;112:775–84. 10.1038/ajg.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang JF, Fox M, Chu H, et al. Four-sample lactose hydrogen breath test for diagnosis of lactose malabsorption in irritable bowel syndrome patients with diarrhea. World J Gastroenterol 2015;21:7563–70. 10.3748/wjg.v21.i24.7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Lacy Costello BPJ, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: a review. J Breath Res 2013;7:024001 10.1088/1752-7155/7/2/024001 [DOI] [PubMed] [Google Scholar]
- 69. Houben E, De Preter V, Billen J, et al. Additional value of CH4 measurement in a combined 13C/H2 lactose malabsorption breath test: a retrospective analysis. Nutrients 2015;7:7469–85. 10.3390/nu7095348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Domínguez Jiménez JL, Fernández Suárez A. Correlation between capillary and venous blood glucose in the lactose tolerance test. Dig Dis Sci 2016;61:208–14. 10.1007/s10620-015-3851-1 [DOI] [PubMed] [Google Scholar]
- 71. Aragón JJ, Hermida C, Martínez-Costa OH, et al. Noninvasive diagnosis of hypolactasia with 4-Galactosylxylose (Gaxilose): a multicentre, open-label, phase IIB-III nonrandomized trial. J Clin Gastroenterol 2014;48:29–36. 10.1097/MCG.0b013e318297fb10 [DOI] [PubMed] [Google Scholar]
- 72. Domínguez Jiménez JL, Fernández Suárez A, Muñoz Colmenero AÚ, et al. Primary hypolactasia diagnosis: Comparison between the gaxilose test, shortened lactose tolerance test, and clinical parameters corresponding to the C/T-13910 polymorphism. Clin Nutr 2017;36:471–6. 10.1016/j.clnu.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 73. Hammer HF, Hammer J, Fox M. Mistakes in the management of carbohydrate intolerance and how to avoid them. UEG Education 2019;19:9–14. [Google Scholar]
- 74. Suchy FJ, Brannon PM, Carpenter TO, et al. NIH consensus development conference statement: Lactose intolerance and health. NIH Consens State Sci Statements 2010;27:1–27. [PubMed] [Google Scholar]
- 75. Lomer MCE. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther 2015;41:262–75. 10.1111/apt.13041 [DOI] [PubMed] [Google Scholar]
- 76. Savaiano DA, Ritter AJ, Klaenhammer TR, et al. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial. Nutr J 2013;12:160 10.1186/1475-2891-12-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Azcarate-Peril MA, Ritter AJ, Savaiano D, et al. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci U S A 2017;114:E367–75. 10.1073/pnas.1606722113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Voisin MR, Borici-Mazi R. Anaphylaxis to supplemental oral lactase enzyme. Allergy Asthma Clin Immunol 2016;12:66 10.1186/s13223-016-0171-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Saqib S, Akram A, Halim SA, et al. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017;7:79 10.1007/s13205-017-0645-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Troise AD, Bandini E, De Donno R, et al. The quality of low lactose milk is affected by the side proteolytic activity of the lactase used in the production process. Food Res Int 2016;89(Pt 1):514–25. 10.1016/j.foodres.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 81. Ianiro G, Pecere S, Giorgio V, et al. Digestive enzyme supplementation in gastrointestinal diseases. Curr Drug Metab 2016;17:187–93. 10.2174/138920021702160114150137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ibba I, Gilli A, Boi MF, et al. Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting lactose malabsorption and intolerance. Biomed Res Int 2014;2014:1–7. 10.1155/2014/680196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oak SJ, Jha R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 2019;59 10.1080/10408398.2018.1425977 [DOI] [PubMed] [Google Scholar]
- 84. Parker TJ, Woolner JT, Prevost AT, et al. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur J Gastroenterol Hepatol 2001;13:219–25. 10.1097/00042737-200103000-00001 [DOI] [PubMed] [Google Scholar]
- 85. Shepherd SJ, Parker FC, Muir JG, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008;6:765–71. 10.1016/j.cgh.2008.02.058 [DOI] [PubMed] [Google Scholar]
- 86. Afify SM, Pali-Schöll I. Adverse reactions to food: the female dominance – A secondary publication and update. World Allergy Organ J 2017;10:43 10.1186/s40413-017-0174-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilder-Smith CH, Olesen SS, Materna A, et al. Predictors of response to a low-FODMAP diet in patients with functional gastrointestinal disorders and lactose or fructose intolerance. Aliment Pharmacol Ther 2017;45:1094–106. 10.1111/apt.13978 [DOI] [PubMed] [Google Scholar]
- 88. Zhang Y. The low-FODMAP diet and traditional dietary advice reduce symptoms of diarrheapredominant irritable bowel syndrome in Chinese population: a randomized controlled trial with analysis into factors associated with efficacy. Neurogastroenterol Mot 2018;30(Suppl 1):e13423. [Google Scholar]
- 89. Rozenberg S, Body J-J, Bruyère O, et al. Effects of dairy products consumption on health: benefits and beliefs—a commentary from the belgian bone club and the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases. Calcif Tissue Int 2016;98:1–17. 10.1007/s00223-015-0062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Beer H. Dairy products and physical stature: a systematic review and meta-analysis of controlled trials. Econ Hum Biol 2012;10:299–309. 10.1016/j.ehb.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 91. Yang Q, Lin SL, Au Yeung SL, et al. Genetically predicted milk consumption and bone health, ischemic heart disease and type 2 diabetes: a Mendelian randomization study. Eur J Clin Nutr 2017;71:1008–12. 10.1038/ejcn.2017.8 [DOI] [PubMed] [Google Scholar]
- 92. Alharbi O, El-Sohemy A. Lactose Intolerance (LCT-13910C>T) Genotype Is Associated with Plasma 25-Hydroxyvitamin D Concentrations in Caucasians: A Mendelian Randomization Study. J Nutr 2017;147:1063–9. 10.3945/jn.116.246108 [DOI] [PubMed] [Google Scholar]
- 93. Wu Y, Li Y, Cui Y, et al. Association of lactase 13910 C/T polymorphism with bone mineral density and fracture risk: a meta-analysis. J Genet 2017;96:993–1003. 10.1007/s12041-017-0866-8 [DOI] [PubMed] [Google Scholar]
- 94. Comerford KB, Pasin G. Gene-dairy food interactions and health outcomes: a review of nutrigenetic studies. Nutrients 2017;9:710 10.3390/nu9070710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tognon G, Nilsson LM, Shungin D, et al. Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr 2017;105:1502–11. 10.3945/ajcn.116.140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2019-318404supp001.pdf (151.9KB, pdf)
gutjnl-2019-318404supp002.pdf (491.9KB, pdf)