Abstract
Introduction
Despite guidelines for prevention of recurrent renal calculi, routine dietary modification and metabolic evaluation are often not performed.
Objective
To determine feasibility of a multicenter, pharmacist-staffed program to enroll patients at high risk of recurrent kidney stones and provide dietary instruction, metabolic evaluation, and medical therapy via telemedicine.
Methods
A total of 536 consecutive adult patients were referred from 3 Northern California Kaiser Permanente facilities. We determined the proportion of patients who enrolled, received dietary counseling, and completed metabolic evaluation at 12 months. The program was staffed by a clinical pharmacist and supervised by urologists following a protocol based on the American Urological Association guidelines. Patients were contacted entirely via telemedicine. Cystine or struvite kidney stones, renal tubular acidosis, and primary hyperoxaluria were exclusion criteria.
Results
Of the 536 patients, 500 agreed to enrollment. Among patients enrolled for 3 months, 99% self-reported compliance with at least 3 of 5 aspects of dietary advice. A complete metabolic evaluation including 24-hour urine collection was performed in 80% of patients by 12 months. A significant improvement in all urinary parameters occurred in 52 patients with calcium stones who repeated 24-hour urine testing. The 12-month dropout rate was 12.4%.
Conclusion
A telemedicine-administered, pharmacist-staffed, protocol-driven program can provide dietary advice and obtain compliance with metabolic testing for patients at high risk of recurrent kidney stones. Rates of metabolic testing and dropout compare favorably with previously reported rates. This report represents, to our knowledge, the first telemedicine-administered, pharmacist-staffed, kidney stone prevention program published in the literature.
Keywords: compliance, diet, kidney, pharmacist, practice guidelines, prevention, technology
INTRODUCTION
Kidney stone disease is extremely common, recurrent, and costly. The lifetime prevalence of kidney stones is about 10%.1–4 Kidney stones are likely to recur, with a recurrence rate of at least 50% within 10 years in first-time stone-forming patients.4–6 Recurrent stone formers have a much higher rate of subsequent recurrence, reported to be 50% at 1 to 5 years.7,8 The cost to care for patients with renal calculi was estimated at $5 billion annually in the US in 2013.9
Dietary modifications, metabolic evaluation, and medical treatment have been shown to dramatically lower recurrence rates. The American Urological Association4 and the European Association of Urology5 both currently recommend dietary modification in all kidney stone formers and metabolic evaluation and medical treatment for high-risk or recurrent stone formers.
Despite these recommendations, routine dietary modification and metabolic evaluation are often not performed. Several studies have shown that compliance with current best practice guidelines is less than ideal.10 Patients are often unaware of the correct dietary interventions, suggesting that dietary instruction is inadequate. Often, 24-hour urine testing is not performed, with completion rates as low as 7%.11,12 Even in those patients receiving care to prevent stones, compliance can be difficult to maintain despite the proven benefits, and the dropout rate is substantial.13,14 Compliance with metabolic testing and dietary modification can be challenging and time consuming, and community-based urologists may not have the time or interest to deal with this problem.
Patient compliance with diet and medical regimens is challenging for many other chronic medical conditions, and care models have been developed to improve compliance and clinical outcomes. Several studies have shown that clinical teams using standardized evidence-based protocols aided by the addition of nonphysicians, such as medical assistants, registered nurses, and clinical pharmacists, can result in improved compliance and improved clinical outcomes.15–17 Clinical pharmacists, who are well trained in dietary modification and medication compliance, have proved beneficial in achieving guideline-based care in many chronic medical conditions.18–21 Clinical pharmacist-administered dietary education, lifestyle modification, and management of drug therapy have been shown to be effective at improving diabetic control, hypertension, and other clinical outcomes. There is even a report of the successful use of telepharmacy consultations in a chronic care management program.22
In our organization, we have the additional challenge of trying to follow a single best practice guideline in 21 separate facilities with more than 100 urologists. A single program following 1 best practice guideline administered via telemedicine across multiple facilities could possibly address this challenge. For these reasons, we piloted a telemedicine-administered, pharmacist-staffed, protocol-driven program for metabolic evaluation, dietary instruction, and medical management of high-risk kidney stone formers at 3 of our medical centers.
METHODS
Adult patients at high risk of recurrent kidney stone disease (defined as recurrent stone formers or first-time urate stone formers) were eligible for referral to the program. Patients with cystine, xanthine, or struvite stones were excluded. Patients with known renal tubular acidosis (RTA) or primary hyperoxaluria were also excluded. Referrals were accepted from 3 Kaiser Permanente (KP) Northern California facilities (South San Francisco, Oakland, and Richmond). Referral was at the discretion of the primary urologist after an initial evaluation. Referred patients received a telephone consultation with a pharmacist who introduced the program, provided dietary education, and proceeded with metabolic evaluation and medical treatment according to our stone prevention protocol.
The protocol was based on the American Urological Association and European Association of Urology guidelines and was reviewed and approved by a group of urologists, nephrologists, endocrinologists, and rheumatologists.4,5 The protocol and the clinical pharmacist’s scope of practice were reviewed and approved by the KP South San Francisco Pharmacy and Therapeutics Committee. Our clinical pharmacist had received 1 year of postgraduate training in the areas of telemedicine and diet and medication compliance in addition to the normal areas of practice. The pharmacist was supervised by a urologist and an endourologist.
Telephone follow-up occurred at a minimum of 6-week, 3-month, 6-month, and 12-month intervals the first year; more frequent follow-up occurred if laboratory, medication, or compliance issues arose. After the first year, telephone follow-up occurred annually, or sooner if the patient experienced a new stone event. All patients were requested to repeat a 24-hour urine test yearly. Patients who experienced a new stone event were requested to repeat serum testing as well as a 24-hour urine collection.
Dietary recommendations followed the clinical practice guidelines and were approved by clinical experts as well as dietitians. These diet recommendations were then scripted and delivered at every telephone encounter and included a detailed discussion on 1) fluid intake, 2) salt intake, 3) protein intake, 4) calcium consumption, and 5) oxalate consumption. Written materials were provided by secure email messaging or postal mail. Results of the initial 24-hour urine collection guided future more detailed and specific dietary instructions. Compliance with the diet was defined as the self-reported adherence to at least 3 of 5 aspects of dietary advice listed earlier and was obtained at every follow-up interval.
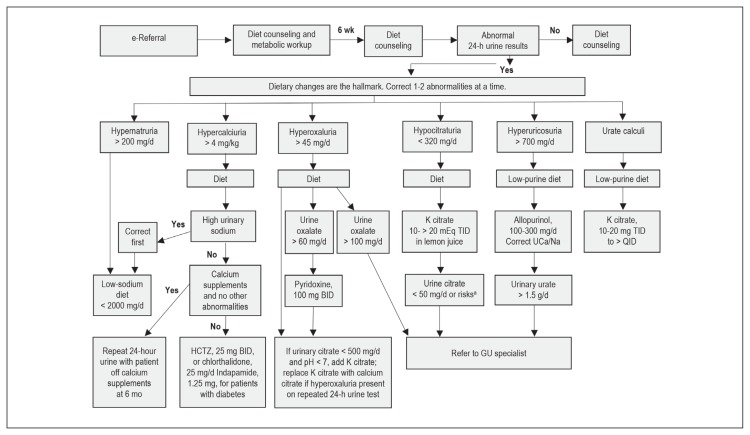
Metabolic evaluation included a baseline basic metabolic panel (CHEM-7), calcium, and uric acid measurements as well as a 24-hour urine collection. The 24-hour urine collection included assessment of volume, creatinine, calcium, oxalate, uric acid, citrate, sodium, potassium, phosphate, and pH. Urine abnormalities were corrected 1 to 2 abnormalities at a time. Patients with abnormal initial results of the 24-hour urine test were asked to repeat a second urine test 6 months later.
Dietary changes were emphasized for all stone formers. Medications were limited to specific indications only, and correction was made on only 1 to 2 urinary abnormalities at a time. A repeated 24-hour urine collection was recommended at 6-month follow-up for all patients with a nonvolume abnormality on initial collection; abnormal was defined as any deviation from the normal laboratory range for that constituent. Mixed stones were treated as calcium stones unless urate was 80% or higher. Patients who were noted to have certain extreme values in the initial workup were referred to a specialist (endourologist) as noted in Figure 1. Patients with high or high-normal serum calcium levels obtained a repeated serum calcium test along with a serum parathyroid hormone measurement and if the test results were abnormal, they were referred to an endocrinologist. Patients with major risk factors for RTA were referred to a nephrologist. Risk factors for RTA were defined as urinary citrate excretion below 50 mg/d alone, or urinary citrate excretion of 50 mg/d to 150 mg/d with low serum potassium, high chloride, or low CO2 level, more than 50% brushite or hydroxyapatite stones, or nephrocalcinosis (on the basis of the 2016 consensus statement23). Patients who noted a recurrent stone were referred to their primary urologist for reevaluation. Patients deemed at risk of RTA on the basis of the inability to correct urinary citrate levels to above 150 mg/d despite compliance with the protocol also were referred to a nephrologist.
Figure 1.
Schematic diagram representing an overview of the protocol.
a Presence of risk factors for renal tubular acidosis; see Methods section.
BID = twice a day; GU = genitourinary; HCTZ = hydrochlorothiazide; K = potassium; QID = 4 times a day; TID = 3 times a day; UCa/Na = urinary calcium/urinary sodium.
Patients who were referred to the program but declined to participate were defined as those who verbally declined on initial contact or who did not respond to the 6-week follow-up phone call. The dropout rate was defined as the number of patients who were unable to be reached after 5 attempts, who declined to continue in the program, or who died of unrelated issues divided by the total number enrolled for that time period.
Statistical analysis involved use of the Student 2-tailed t-test. Results were expressed as the mean and standard deviation.
RESULTS
A total of 536 patients were referred to the kidney stone prevention program during a 17-month period. Five hundred patients were enrolled, as defined by agreeing to receive the initial consultation as well as completing the 6-week follow-up phone call; 36 patients declined enrollment.
Patient demographics are listed in Table 1. Of the 500 patients enrolled, 100% completed dietary counseling and 266 of the 268 patients (99%) enrolled for at least 6 months in the program self-reported compliance with at least 3 of 5 aspects of dietary advice.
Table 1.
Demographic Characteristics of Patients Enrolled in Program (N = 500)
| Characteristic | Value |
|---|---|
| Age, mean (median); range, y | 55 (57); 23–88 |
| Sex, % women/men | 34/66 |
| Previous stone composition,a no. of patients | |
| Calcium oxalate | 315 |
| Unknown composition | 102 |
| Urate | 54 |
| Calcium phosphate | 25 |
| Pure apatite | 2 |
| Pure brushite | 2 |
| Average no. of previous stone events | 2.8 |
| Enrollment period, mo | 17 |
| Mean follow-up, d | 267 |
Stone analysis data obtained from Quest Diagnostics (San Juan Capistrano, CA).
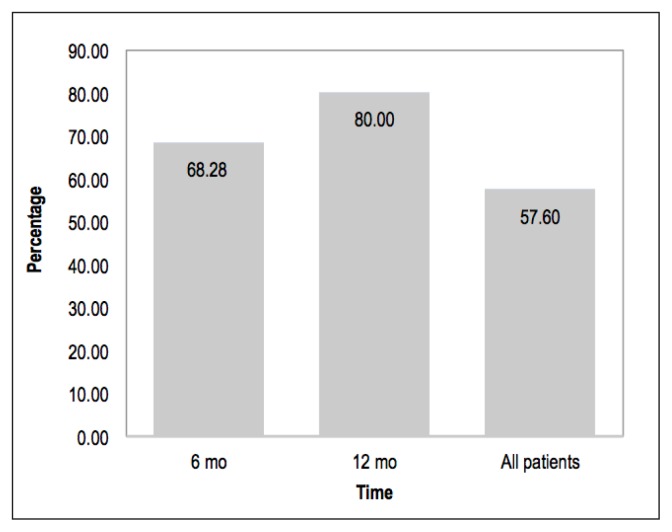
Overall, 288 of the 500 patients completed a 24-hour urine test. Of the 268 patients in the program at least 6 months, 183 (68%) completed both serum and 24-hour urine testing. Of the 120 patients in the program at least 12 months, 96 (80%) completed both serum and 24-hour urine testing (Figure 2).
Figure 2.
Completion rate of 24-hour urine test.
Abnormal results of the 24-hour urine test were found in 251 of the 288 patients (87%). Of these 251 patients, 92 (37%) had solitary abnormalities, whereas 159 patients (63%) had multiple abnormalities; 56 of 251 patients (22%) had 3 or more abnormalities. Hyperoxaluria was the most common abnormality at 24%, followed by hyperuricosuria at 21% and low volume at 17% (Table 2).
Table 2.
Type of abnormalities on initial 24-hour urine test (N = 438)a
| Abnormality | Number (%) |
|---|---|
| Hypercalciuria | 49 (11.19) |
| Hyperuricosuria | 91 (20.78) |
| Hyperoxaluria | 107 (24.43) |
| Hypocitraturia | 64 (14.61) |
| Low volume | 74 (16.89) |
| Hypernatruria | 53 (12.10) |
N = total number of abnormalities. Please note 159 of 251 patients had multiple abnormalities
When we separated out the 446 calcium oxalate stone formers, 248 completed an initial 24-hour urine test, of which 212 had abnormal results. Of these 212 abnormalities, 178 were nonvolume abnormalities. Fifty-two of these 178 patients (29%) were in the program long enough to repeat a second collection and were found to have a statistically significant improvement in the averages of all listed urinary parameters (Table 3).
Table 3.
Urine abnormalities (N = 99) in 52 patients with pure or mixed calcium oxalate stones who repeated 24-hour urine test
| Urine abnormality | No. | Mean value (standard deviation) | Percentage difference | p value | Percentage normalized | |
|---|---|---|---|---|---|---|
| Baseline | After intervention | |||||
| Hypercalciuria (250 mg/d) | 17 | 388.4 (83.6) | 292.2 (71.9) | 25 | 0.001 | 24 |
| Hypernatriuria (> 200 mg/d) | 17 | 230.4 (63.7) | 166.6 (60.6) | 28 | 0.005 | 82 |
| Hyperuricosuria (> 700 mg/d) | 24 | 874.3 (227.5) | 694.8 (258.9) | 21 | 0.014 | 54 |
| Hyperoxaluria (> 45 mg/d) | 25 | 60.6 (14.4) | 49.3 (19.1) | 19 | 0.022 | 48 |
| Hypocitraturia (< 320 mg/d) | 16 | 156.7 (68.2) | 297.1 (177.7) | 90 | 0.006 | 31 |
Seventeen hypernatruric patients repeated a 24-hour urine test by 17 months, and 14 (82%) of the test results normalized. Additionally, 25 hyperoxaluric patients repeated a 24-hour urine test and 12 (48%) had results that normalized. For the 16 hypocitraturic patients who repeated a 24-hour urine test, 5 (31%) results normalized. Among hypercalciuric patients who repeated a 24-hour urine test, 4 (24%) of 17 results normalized. Finally, 24 hyperuricosuric patients repeated a 24-hour urine test and 13 test results (54%) normalized.
A total of 114 patients (23%) were receiving medical therapy on the basis of the protocol. Three patients were found to have primary hyperparathyroidism. Five patients were found to be receiving the carbonic anhydrase inhibitor acetazolamide or topiramate.
Of the 500 patients who enrolled in the program, the overall dropout rate was 11.8% (59/500); 57 patients could not be contacted for follow-up, and 2 patients died of unrelated causes. The dropout rate was 12.4% (17/137) for those in the program longer than 12 months.
DISCUSSION
Kidney stone disease is extremely common, recurrent, and costly. Diet and lifestyle changes as well as medical treatment of underlying metabolic abnormalities can greatly affect a patient’s risk of recurrence, and this provides a compelling reason to counsel, evaluate, and treat these individuals. The American Urological Association4 and European Association of Urology5 both currently recommend dietary modification in all stone formers with metabolic evaluation and medical treatment for high-risk or recurrent stone formers or those who are interested.
Despite these recommendations, routine dietary modification and metabolic evaluation are often not being performed with compliance; 24-hour urine testing has been reported to be as low as 7.4% in high-risk patients and 16.8% in recurrent stone formers.11,12 Although patients and urologists are often extremely motivated to treat the initial painful stone episode, interest in long-term dietary changes or onerous metabolic evaluations is often very low. Even in those patients getting dietary instruction, metabolic evaluation, and medical treatment, compliance can be difficult to maintain despite the proven benefits.13 Dauw et al24 reported dismally low compliance rates with pharmacologic therapy for patients with kidney stones, including an adherence rate of only 13.4% for citrate therapy.
Dropout rates of 20% per year have been reported by Parks and colleagues.13,14 There is also difficulty in obtaining and interpreting 24-hour urine results, and the benefit has been called into question by some authors.25
In addition, medical prevention must be cost effective. Cost-effectiveness depends on recurrence rate, intervention rate, and cost of care.26 Recurrence rates vary widely from less than 0.1 stones per year for first-time stone formers to greater than 1.0 stones per year for certain recurrent stone formers.8 The percentage of patients who require urologic intervention varies widely from 23% to 60%, as does the cost of care.14 Cost estimates from Europe in the late 1990s suggest that the recurrence rate must be more than 0.2 episodes per year to be cost effective and that metabolic evaluation in first-time stone formers is unlikely to be cost effective.27,28 For these reasons, we selected 2 high-risk groups for evaluation: Recurrent stone formers and urate stone formers.
Like hypertension, diabetes mellitus, and cardiovascular disease, kidney stone disease is dramatically affected by simple compliance with dietary and lifestyle changes. A team approach involving the addition of nurses and pharmacists to the delivery of standardized protocols can result in improved compliance and improved clinical outcomes compared with usual care.15–17 Clinical pharmacists are specifically trained in dietary modification, lifestyle modification, medication compliance, and telemedicine, and transferring many of these activities to a clinical pharmacist has proved beneficial in other chronic medical conditions.18–22,29
Hyperoxaluria was the most common nonvolume urinary abnormality identified in our study, which is consistent with the recent rising trend of hyperoxaluria in the US.30 We could show early postintervention improvement in both urinary oxalate and urinary sodium excretion, unlike in other studies.14
CONCLUSION
Our pilot study shows that it is feasible to enroll patients in a telemedicine-administered, pharmacist-staffed, protocol-driven, kidney stone prevention program and obtain good compliance with dietary counseling and metabolic testing. Ninety-three percent of patients agreed to enrollment, followed up with at least 2 phone consultations, and received at least 2 episodes of dietary counseling. Most enrolled patients (80%) completed a full metabolic workup, including a 24-hour urine test by 1 year. Encouraging early results were noted in the correction of hyperoxaluria and hypernatruria with dietary measures. The dropout rate of 12.4% for patients in the program over 12 months compares favorably with that reported in the literature.
This report represents, to our knowledge, the first pharmacist-staffed, telemedicine-administered, kidney stone prevention program published in the literature. Further studies will need to address more objective measures of success related to dietary instruction, ongoing patient compliance, the impact on future stone recurrence, and the cost-effectiveness of such a program.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012 Jul;62(1):160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States, 1976–1994. Kidney Int. 2003 May;63(5):1817–23. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri A. Epidemiology of urolithiasis. Arch Ital Urol Androl. 1996 Sep;68(4):203–49. [PubMed] [Google Scholar]
- 4.Pearle MS, Goldfarb DS, Assimos DG, et al. American Urological Association. Medical management of kidney stones: AUA guideline. J Urol. 2014 Aug;192(2):316–24. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Skolarikos A, Straub M, Knoll T, et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol. 2015 Apr;67(4):750–63. doi: 10.1016/j.eururo.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med. 1989 Dec 15;111(12):1006–9. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 7.Parks JH, Coe FL. Evidence for durable kidney stone prevention over several decades. BJU Int. 2009 May;103(9):1238–46. doi: 10.1111/j.1464-410X.2008.08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotan Y, Cadeddu JA, Roerhborn CG, Pak CY, Pearle MS. Cost-effectiveness of medical management strategies for nephrolithiasis. J Urol. 2004 Dec;172(6 Pt 1):2275–81. doi: 10.1097/01.ju.0000141498.11720.20. [DOI] [PubMed] [Google Scholar]
- 9.Hyams ES, Matlaga BR. Cost-effectiveness treatment strategies for stone disease for the practicing urologist. Urol Clin North Am. 2013 Feb;40(1):129–33. doi: 10.1016/j.ucl.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Bos D, Kim K, Hoogenes J, Lambe S, Shayegan B, Matsumoto ED. Compliance of the recurrent stone former with current best practice guidelines. Can Urol Assoc J. 2018 Mar;12(3):E112–20. doi: 10.5489/cuaj.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milose JC, Kaufman SR, Hollenback BK, Wolf JS, Jr, Hollinsworth JM. Prevalence of 24-hour urine collection in high risk stone formers. J Urol. 2014 Feb;191(2):376–80. doi: 10.1016/j.juro.2013.08.080. [DOI] [PubMed] [Google Scholar]
- 12.Dauw CA, Alruwaily AF, Bierlein MJ, et al. Provider variation in the quality of metabolic stone management. J Urol. 2014 Mar;193(3):885–90. doi: 10.1016/j.juro.2014.09.111. [DOI] [PubMed] [Google Scholar]
- 13.Parks JH, Asplin JR, Coe FL. Patient adherence to long-term medical treatment of kidney stones. J Urol. 2001 Dec;166(6):2057–60. doi: 10.1016/s0022-5347(05)65505-x. [DOI] [PubMed] [Google Scholar]
- 14.Parks JH, Coe FL. Evidence for durable kidney stone prevention over several decades. BJU Int. 2009 May;103(9):1238–46. doi: 10.1111/j.1464-410X.2008.08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters A, Davidson MB. Application of a diabetes managed care program. The feasibility of using nurses and a computer system to provide effective care. Diabetes Care. 1998 Jul;21(7):1037–43. doi: 10.2337/diacare.21.7.1037. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013 Aug;310(7):699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffe MG, Young JD. The Kaiser Permanente Northern California Story: Improving hypertension control from 44% to 90% in 13 years. J Clin Hypertens (Greenwich) 2016 Apr;18(4):260–1. doi: 10.1111/jch.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015 Nov 10;66(19):2129–39. doi: 10.1016/j.jacc.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoag clinic: Improving patient care. Open Med. 2009 Feb 2;3(1):e16–21. [PMC free article] [PubMed] [Google Scholar]
- 20.Lifer SM, Musser MR, Kier KL. Evaluation of a pharmacist-run antiarrhythmic clinic in an ambulatory practice. J Am Pharm Assoc. 2015 Sep-Oct;55(5):546–51. doi: 10.1331/JAPhA.2015.14260. [DOI] [PubMed] [Google Scholar]
- 21.Wallgren S, Berry-Caban CS, Bowers L. Impact of clinical pharmacist intervention on diabetes-related outcomes in a military treatment facility. Ann Pharmacother. 2012 Mar;46(3):353–7. doi: 10.1345/aph.1Q564. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AM, Bingham J, Schussel K, et al. Integrating innovative telehealth solutions into interprofessional team-delivered care management pilot program. J Manag Care Spec Pharm. 2018 Aug;24(8):813–8. doi: 10.18553/jmcp.2018.24.8.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gambaro G, Croppi E, Coe F, et al. Consensus Conference Group. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: A consensus statement. J Nephrol. 2016 Dec;29(6):715–34. doi: 10.1007/s40620-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dauw CA, Bierlein M, Bierlein MJ, et al. Factors associated with preventive pharmacological therapy adherence among patients with kidney stones. Urology. 2016 Jul;93:45–9. doi: 10.1016/j.urology.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Hsi RS, Sanford T, Goldfarb DS, Stoller ML. The role of the 24-hour urine collection in the prevention of kidney stone recurrence. J Urol. 2017 Apr;197(4):1084–9. doi: 10.1016/j.juro.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Chandhoke PS. When is medical prophylaxis cost-effective for recurrent calcium stones? J Urol. 2002 Sep;168(3):937–40. doi: 10.1097/00005392-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Trinchieri A, Ostini F, Nespoli R, Rovera F, Montanari E, Zanetti G. A prospective study of recurrence rate and risk factors for recurrence after a first renal stone. J Urol. 1999 Jul;162(1):27–30. doi: 10.1097/00005392-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Robertson WG. The economic case for the biochemical screening of stone patients. Urolithiasis 2000: Proceedings of the 9th International Symposium on Urolithiasis Claremont; Cape Town, South Africa: University of Cape Town Press; 2000. pp. 403–5. [Google Scholar]
- 29.Mehuys E, Van Bortel L, De Bolle L, et al. Effectiveness of a community pharmacist intervention in diabetes care: A randomized controlled trial. J Clin Pharm Ther. 2011 Oct;36(5):602–13. doi: 10.1111/j.1365-2710.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- 30.Spradling K, Vernez SL, Khoyliar C, et al. Prevalence of hyperoxaluria in urinary stone formers: Chronological and geographical trends and a literature review. J Endourol. 2016 Apr;30(4):469–75. doi: 10.1089/end.2015.0676. [DOI] [PubMed] [Google Scholar]