Abstract
The term organophosphate (OP) refers to a diverse group of chemicals that are found in hundreds of products worldwide. As pesticides, their most common use, OPs are clearly beneficial for agricultural productivity and the control of deadly vector-borne illnesses. However, as a consequence of their widespread use, OPs are now among the most common synthetic chemicals detected in the environment as well as in animal and human tissues. This is an increasing environmental concern because many OPs are highly toxic and both accidental and intentional exposures to OPs resulting in deleterious health effects have been documented for decades. Some of these deleterious health effects include a variety of long-term neurological and psychiatric disturbances including impairments in attention, memory, and other domains of cognition. Moreover, some chronic illnesses that manifest these symptoms such as Gulf War Illness and Aerotoxic Syndrome have (at least in part) been attributed to OP exposure. In addition to acute acetylcholinesterase inhibition, OPs may affect a number of additional targets that lead to oxidative stress, axonal transport deficits, neuroinflammation, and autoimmunity. Some of these targets could be exploited for therapeutic purposes. The purpose of this review is thus to: 1) describe the important uses of organophosphate (OP)-based compounds worldwide, 2) provide an overview of the various risks and toxicology associated with OP exposure, particularly long-term neurologic and psychiatric symptoms, 3) discuss mechanisms of OP toxicity beyond cholinesterase inhibition, 4) review potential therapeutic strategies to reverse the acute toxicity and long term deleterious effects of OPs.
Keywords: Pesticide, Cholinesterase inhibitor, Agriculture, Gulf War Illness, Aerotoxic Syndrome, memory
1. Introduction
The term organophosphate (OP) refers to a diverse group of chemicals that are typically derived from phosphoric, phosphonic and phosphinic acids. Prototypic OPs were first synthesized in the nineteenth century and related compounds are now found in hundreds of products that are used worldwide including pesticides, defoliants, fire retardants, industrial solvents, lubricants, plasticizers, fuel additives, prescription drugs, and nerve agents (see Table 1 for representative examples and reviews, Soltaninejad and Shadnia, 2014; Costa 2018). OPs are particularly common among the group of compounds generically known as pesticides, which includes insecticides, anthelmintics, fungicides, and herbicides. These compounds have contributed to improvements in agricultural productivity across the world for decades through higher crop yields and improved product quality by controlling, insects, nematodes, and plant pathogens. Moreover, they also reduce the amount of labor, machinery, and fuel used for mechanical weed control (reviewed, Fernandez-Cornejo et al., 2014). While the extensive use of OP-based pesticides is often criticized due to the risks associated with their toxicity to humans and other non-target species, an important role of OPs in agriculture is likely to continue in the near future. This is due to their high effectiveness and broad spectrum against numerous types of pests, the availability of generic OP-based products of low cost, and the decades of experience with their use (reviewed, Casida and Durkin, 2013; see also Costa, 2018). Some commonly used OPs (e.g., chlorpyrifos) have additional advantages such as a relatively short persistence in the environment after application and chemical characteristics that provide flexibility for use in multiple delivery systems (Dow AgroSciences, 2017; reviewed, Solomon et al., 2014).
Table 1.
Commonly used Organophosphates in the United States and Worldwide and Nerve Agents (representative examples)
| Application | Representative Examples | Representative Chemical Structure |
|---|---|---|
| Agricultural Insecticides |
chlorpyrifos, malathion, diazinon | 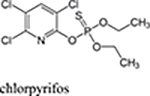 |
| Vector-Born Illness Prevention |
malathion, fenitrothrion, pirimiphos-methyl, naled | 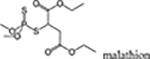 |
| Herbicides | glyphosate, glufosinate | |
| Fungicides | iprobenfos, edifenphos | 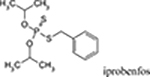 |
| Pharmaceutical Agents |
echothiophate, fosinopril, fotemustine | 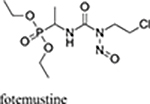 |
| Flame Retardants | tris (1, 3 dichloro-2-propyl) phosphate - TDCPP, tris (2chloroethyl) phosphate – TCEP tricresyl phosphate (TCP) |
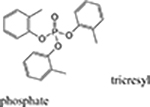 |
| Highly Toxic Nerve Agents |
sarin, soman, tabun, VX | 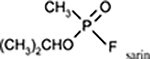 |
References: EPA 2017, CDC 2017; Costa 2018; Killeen et al., 2017
In addition to optimizing agricultural productivity, the value of OPs in the control of deadly vector-borne illnesses is also clear (reviewed, Cooper and Dobson, 2007). For example, along with pyrethroids, organochlorines, and carbamates, OPs continue to play a major role in the prevention regimen against one of the world’s most deadly diseases, malaria (World malaria report 2017). In at least some regions of the world OPs may offer distinct advantages over the other aforementioned insecticides. For example, in standard insecticide susceptibility testing across western Kenya it was determined that the Anopheles gambiae mosquito had acquired high resistance to pyrethroids and the organochlorine DDT, patchy resistance to carbamates (bendiocarb), but no resistance to the OP malathion (Wanjala et al., 2015). Notably, Anopheles gambiae is one of the most important vectors of malaria in sub-Saharan Africa. OP insecticides (e.g., malathion and naled) are also commonly used successfully by state and local agencies in the United States for adult mosquito control to prevent the spread of Zika, West Nile Virus, dengue, and chikungunya (Petersen et al., 2013; CDC 2017; EPA 2017). While the number of OP-based commercial products available to the public has decreased to some extent in the last several years, they continue to be available in a variety of products to combat home and garden pests (e.g., flies, roaches, ants, mosquitoes, etc.).
2. Risks associated with OP exposure
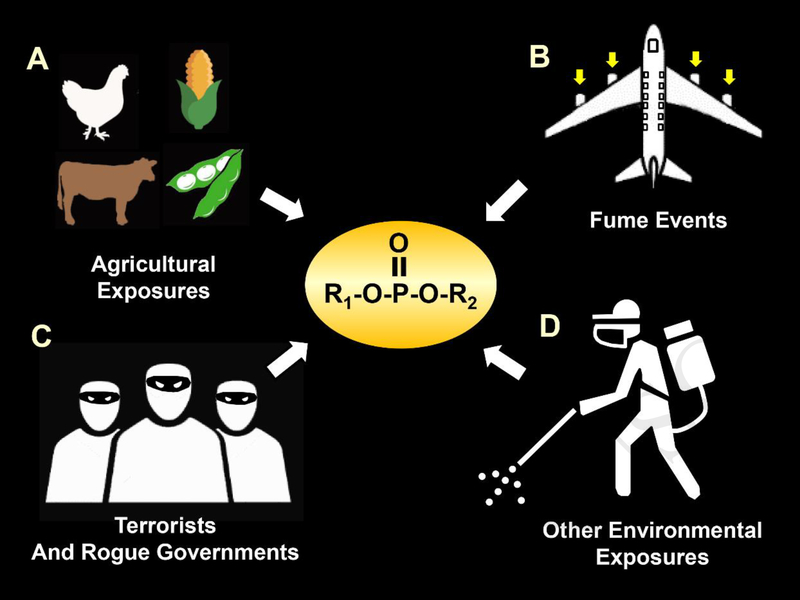
Please see Fig 1 for an illustration of several representative sources whereby humans and other non-target species may be exposed to toxic levels of OPs. Systemic absorption of OPs can occur by inhalation and after mucous membrane, dermal, conjunctival, and gastrointestinal exposure (Burillo-Putze and Xarau, 2016).
Fig 1.
Illustration of several representative sources of toxic exposures to OPs by humans and other non-target species. A. OP exposures in the agricultural setting, which may come from insecticides, anthelmintics, fungicides, and herbicides. B. OP exposures from terrorist attacks and chemical warfare assaults by rogue governments. C. Exposures as a result of “fume events” where the cabin air of an airplane can be contaminated with heated engine oil fumes that contain OPs. D. Exposures from insecticides used by public health officials to combat vector borne illnesses.
2.1. OP-Pesticides
As a consequence of their widespread use as pesticides, OPs (and their residues) are now among the most common synthetic chemicals detected in rivers, groundwater, soil, air, and plants, as well as in animal and human tissues worldwide (Schnoor, 1992; Davisson et al., 2005; Barr et al., 2011; Clune et al., 2012; Voorhees et al., 2017). This fact has become an increasing environmental concern because of the deleterious health effects of OPs that have been documented in both adults and children for decades (reviewed, Rohlman et al., 2011; Reiss et al., 2015; Worek et al., 2016; Masson and Nachon, 2017, see also section 3 below). In many places in the world, governments have had some success at reducing toxic exposures to OPs by imposing stricter regulations on their use. However, in some Eastern Mediterranean, Asiatic, and Western Pacific countries (especially those that are heavily reliant on agriculture), accidental OP exposures and suicidal ingestions remain as a persistent problem that may actually be worsening (Tsatsakis et al., 1996; Tsatsakis et al., 2008; Jin et al., 2010; Kumar et al., 2010; Costa, 2018; Abhimanyu and Madhan, 2018; reviewed, Pope, 1999; Iyer et al., 2015,Gunnell et al., 2007.
2.2. OP Nerve Agents as Components of Chemical Weapons
While the risk of toxicity from pesticide exposures is considerably higher for most people in the world, the threat from intentional poisonings with OPs by rogue governments and terrorists is also an ongoing concern (reviewed, Eisenkraft and Falk, 2016). Over the last 35 years, there have been multiple (well-documented) cases where OP-nerve agents were used against military soldiers and/or civilians. For example, in the 1980s the Iraqi military attacked Iranian military soldiers (Majnoon Island) and Kurdish civilians (Halabja) with OP-based nerve agents producing casualties estimated to be as high as “tens of thousands” (Barnaby, 1988; Macilwain, 1993; O’Leary, 2002; Hawrami et al., 2004). The Tokyo Sarin attack in March of 1995 by the domestic terrorist group Aum Shinrikyo resulted in the deaths of 12 people and the emergency medical evaluation and/or treatment of more than 5,000 other individuals (Suzuki et al., 1995; Nagao et al., 1997). The Organization for the Prohibition of Chemical Weapons (OPCW) and the United Nations have now concluded that Sarin has been used against civilians in Syria on multiple occasions (Sellström et al., 2013; Guterres, 2017), and the fatal sarin poisoning in 2013 has also been verified by a network of international laboratories (John et al., 2018). The recent news reports of the use of the OP VX in the assassination of the North Korean dictator’s half-brother in Malaysia (Latiff and Chow, 2017) and the attempted assassination of a former Russian military intelligence officer and his daughter with the OP Novichok in Britain (Smout and Holden, 2018) demonstrates the continued risks of intentional OP attacks on a global scale.
2.3. OPs and Gulf War Illness
There is now a large body of published evidence indicating that approximately 25–32% of the United States (US) veterans who served in the 1990–1991 Persian Gulf War (Operation Desert Shield and Operation Desert Storm) are affected by a constellation of chronic health symptoms now known collectively as Gulf War Illness (GWI). These symptoms have not been commonly observed in US veterans of the same era who did not deploy to the Gulf region or veterans who were deployed in other areas of the world (e.g., Bosnia, Germany). However, they have been reported in veterans from other countries who also participated in the Gulf War (United Kingdom, Canada, Australia, Denmark, and France, reviewed, Iverson et al., 2007; White et al., 2016). The symptoms of GWI are diverse and may include unexplained fatigue, headaches, respiratory problems, musculoskeletal pain, gastrointestinal distress, skin rashes, and a variety of neurological and neuropsychiatric problems including cognitive impairment (Sullivan et al., 2018).
While a variety of possible contributing factors to GWI symptoms have been discussed (heat, stress, vaccinations, smoke from oil well fires, infectious organisms), a particularly plausible explanation for the neurological-based symptoms is exposure to one or more acetylcholinesterase inhibitors (AChEIs) (see Golomb, 2008, reviewed, White et al., 2016). It has been estimated that at least 41,000 military personnel were over-exposed to insecticides that contained either carbamate or OP-based AChEIs (Winkenwerder, 2003). For general military personnel, sources of exposures included fly baits, pest strips, sprayed liquids and powders and for military pesticide applicators, pesticide fogs and prisoner delousing compounds were additional sources of exposure. In addition to OP-pesticides, exposures to OP-nerve agents may have also been a contributing factor to GWI. It is now well documented that as many as 100,000 soldiers may have been exposed to low (i.e., non-acutely toxic) levels of sarin/cyclosarin following the destruction of an Iraqi munitions storage complex at Khamisiyah, Iraq, in March 1991 (Berardocco, 1997).
It is important to note, however, that more than two decades after the Gulf War, GWI remains a controversial topic (reviewed, Nettleman, 2015; Reardon, 2015). Members of the United States Institute of Medicine (IOM) and other scientists continue to argue that there is not enough evidence implicating either the prophylactic agent pryidostigimne or pesticide use as conclusive enough to assign causality. In particular, they cite the lack of objective exposure data, the need to rely on self-reports, and a lack of consistency of symptoms across patients as barriers to establishing causality.
2.4. OPs and Aerotoxic Syndrome
For more than 20 years, there have been reports from around the world from both airline crewmembers and some passengers of sickness following exposure to toxic fumes in airplane cabins. The symptoms (now collectively referred to as “Aerotoxic Syndrome”, reviewed Winder and Balouet, 2000) are diverse and include both short and long-term effects. Examples include ear/nose/throat irritation, skin conditions, nausea and vomiting, respiratory problems, headaches, dizziness, weakness and fatigue, sensory changes and nerve pain, tremors, chemical sensitivity and cognitive impairment (see reviews, Harrison and Mackenzie Ross, 2016; Michaelis et al., 2017). Aerotoxic Syndrome is thought to occur after the cabin air of an airplane (which is “bled” in from the engines to pressurize it), is compromised due to mechanical failures, faulty seals, the overfilling of oil or hydraulic reservoirs etc. This bleed air during the so-called “fume event” can be contaminated with heated engine oil fumes that contain a variety of hazardous chemicals (e.g., phenyl-naphthylamine, toluene, xylenes and the OPs, tri-butyl phosphate and tricresyl phosphate-TCP). Of these chemicals, the tri-ortho-cresyl phosphate (ToCP) isomer of TCP (an anti-wear additive to jet engine oil), has been the most commonly implicated and studied since it is a known neurotoxin associated with chronic OP-induced delayed polyneuropathy (OPIDP) (see Costa, 2017, see also section 3 below). It should be noted, however, that the published evidence collected thus far to support a causal connection between TCP or TOCP and the symptoms associated with aerotoxic syndrome is a subject of debate (see reviews, de Boer et al., 2015; Costa, 2017). The concentrations of these compounds in flight deck and cabin air detected thus far are very low (de Ree et al., 2014). Likewise, the levels of urine metabolites and butyrylcholinesterase adducts of these compounds in aircrews and airplane passengers, respectively, was very low (Schindler et al., 2013; Liyasova et al., 2011). Moreover, no significant inhibition of lymphocytic neurotoxic esterase (NTE) or erythrocyte AChE activity was observed in symptomatic flight crewmembers after fume events (Heutelbeck et al., 2016). In primary rat cortical neurons in culture, biochemical, morphological and electrophysiological data demonstrated that TCP isomers and mixtures induced no or only limited cytotoxicity at concentrations up to 100 μM, concentrations well above estimated systemic levels based on recent measurements of cabin air concentrations of TCPs (Duarte et al., 2017).
2.5. OPs and Incidence of Neurodegenerative Diseases
A potential role of pesticides of different classes (including OPs) in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis has long been suspected since they share common features such as the ability to induce oxidative stress, mitochondrial dysfunction, and neuronal cell loss (reviewed, Zaganas et al., 2013; Sanchez-Santed et al., 2016; Yan et al., 2016). Moreover, the notion that gene x environment interactions (e.g., epigenetic risk factors where the environmental factor is pesticide exposures) contribute to the etiology of neurodegenerative diseases is a particularly attractive hypothesis. Interestingly, some epidemiological studies have specifically linked OP exposures to the risk of Alzheimer’s disease (Hayden et al., 2010). However, the epidemiological studies are unable to provide a causal connection between OP exposure and neurodegenerative illnesses and reviews of the literature point to inconsistencies and lack of strong mechanistic arguments (see Baltazar et al., 2014; reviewed, Sanchez-Santed et al., 2016). Similar limitations appear to exist in the animal literature (at least as it applies to AD). For example, Peris-Sampedro and colleagues conducted several studies with chlorpyrfos using transgenic mice that express the human abeta protein or the apoE2, apoE3 and apoE4 isoforms. In each study, chlorpyrifos was associated with cognitive dysfunction (spatial learning and memory deficits, Peris-Sampedro et al., 2014; 2015, deficits in sustained attention, Peris-Sampedro et al., 2016). However, there was no clear connection between the cognitive deficits and specific risk factors for human AD (abeta protein, apoE4 allele).
3. Long Term Neuropsychiatric Effects of Acute and Repeated Exposures to OPs
The acute toxicity of OPs in humans has been associated with a host of central nervous system, cardiovascular, respiratory, gastrointestinal, sensory, and motor manifestations (see reviews, Bardin, 1994; Collombet, 2011; Pereira et al., 2014). Depending on the exposure level, some of the acute effects of OPs including excessive secretions, cardiorespiratory depression, and seizures can be life threatening. However, acute exposures to OPs can also lead to a variety of long-term neurological and psychiatric consequences in survivors. These consequences can include motor impairments, psychotic episodes, depressed mood, as well as deficits in signal detection and information processing, sustained attention, memory, sequencing and problem solving, abstraction, and cognitive flexibility (Savage et al., 1988; Rosenstock et al., 1991; Steenland et al., 1994; Dassanayake et al., 2007, Pereira et al., 2014). Chronic exposure to Ops can also be associated with neurologic and psychiatric abnormalities including motor dysfunction (extrapyramidal symptoms) deficits in eye-hand coordination and reaction time, anxiety, depression, psychotic symptoms, deficits in attention, information processing, and learning and memory (Amr et al., 1997; Salvi et al., 2003; Stephens et al., 1995; and reviewed Singh and Sharma, 2000). These symptoms are collectively known as chronic OP-induced neuropsychiatric disorders (COPIND).
In addition to the consequences of acute and chronic OP poisoning described above, a variety of long-term effects of repeated exposures to levels of OPs below the threshold for acute “cholinergic” toxicity have been documented. This type of exposure is relatively common in agricultural workers and pesticide sprayers and it has been associated with persistent alterations in psychomotor speed, executive function, visuospatial ability, working and visual memory (see Ross et al., 2013). Many of the symptoms described above for GWI and Aerotoxic Syndrome may also be result of OP exposures that were below the threshold for symptoms of acute toxicity.
Prospective animal studies support the clinical and epidemiological observations described above and indicate that chronic (or repeated) exposures to OPs at levels that are not associated with acute toxicity can result in a variety of neurobehavioral symptoms, particularly cognitive deficits (reviewed, Terry, 2012).
Several epidemiological studies have also found associations between prenatal or early postnatal exposure to OPs and behavioral abnormalities, particularly deficits in attention, learning and memory (see reviews, Eskenazi and Castorina, 1999; Eaton et al., 2008; Muñoz-Quezada et al., 2013; Gonzalez-Alzaga et al., 2014; Reiss et al., 2015). Here it is important to note that exposure to OPs, as revealed in biological monitoring studies in children, was elevated in inner cities and farming communities, but at levels below those causing significant AChE inhibition (reviewed in Costa, 2017). There is also considerable evidence from animal studies to suggest that both prenatal and early postnatal exposure to OPs can result in a variety of protracted cognitive impairments (Levin et al., 2001; reviewed, Slotkin 2004; Slotkin 2005; Timofeeva et al., 2008). Moreover, these impairments can become more pronounced as the exposed subject ages (Levin et al., 2010). These adverse developmental effects of OPs, however, with few exceptions, were only observed at levels causing significant AChE inhibition (reviewed Eaton et al., 2008; Timofeeva and Levin, 2010).
4. Mechanisms of the Long Term Neuropsychiatric Effects of Acute and Repeated Exposures to OPs
4.1. Acetylcholinesterase Inhibition
In the case of acute OP poisoning, the mechanism of both the acute symptoms of OP toxicity and the long-term neurological consequences has been attributed to the inhibition of acetylcholinesterase (AChE). This inhibition leads to marked elevations in synaptic acetylcholine levels which in turn lead to excessive stimulation of both muscarinic and nicotinic acetylcholine receptors (reviewed Ecobichon, 2001; Pereira et al., 2014). However, it is important to note that COPIND can occur without antecedent cholinergic symptoms and it does not necessarily appear to be dependent on acetylcholinesterase inhibition (Brown and Brix, 1998; reviewed, Ray and Richards, 2001 and Singh and Sharma, 2000).
While the inhibition of acetylcholinesterase undoubtedly plays a key role in both the acute and long-term toxicology of OPs, there is nearly twenty years of experimental evidence to support the argument that this mechanism cannot alone account for the wide range of symptoms that have been reported (reviewed, Pope, 1999; Duysen, et al., 2001; Terry, 2012; Burke et al., 2017). OPs are known to affect literally hundreds of enzymes, receptors, and other proteins and it is becoming increasingly evident that some of these targets may be especially relevant to the long-term CNS effects of chronic OP exposure and developmental neurotoxicity. Consequently, interactions with these targets should be considered as part of a cumulative risk assessment of OPs (reviewed, Costa, 2018). In Table 2, we have provided a referenced (representative) list of potential non-acetylcholinesterase targets of OPs and their known or theoretical functions. In Fig 2, we have illustrated potential consequences of OP interactions with some of these targets (i.e., axonal transport deficits, oxidative stress, neuroinflammation, and autoimmunity). In the text below, we have provided an overview of the effects of OPs beyond acetylcholinesterase inhibition, which includes interactions with particular targets as well as physiological processes that these targets are known to influence. It is also important to note that acetylcholinesterase inhibition can occur in the absence of acute OP toxicity, but not vice versa; therefore the non-acetylcholinesterase targets discussed could be affected in both the case of acute-high level exposure as well as repeated lower level exposure, however, in the acute setting the non-acetylcholinesterase related physiologic effects might be difficult to distinguish. While each of these putative (noncholinergic) mechanisms of toxicity are discussed separately, it is unlikely that they are mutually exclusive and they may converge to induce deleterious effects.
Table 2.
Non-Cholinesterase Targets of OPs and Downstream Effects
| Target | Description/Function | Reference(s) |
|---|---|---|
| Neuroinflammation | Cytokines mediate a variety of inflammatory processes (IL-6, IL-1β, CCL2). |
Koo et al., 2018, Mohammadzadeh et al., 2018 |
| Oxidative Stress | Process by which reactive oxygen species are generated in excess or improperly cleared to cause widespread cell damage. | Eftekhari et al., 2018, Abolaji et al., 2017 |
| Gene Expression | Changes in gene expression can occur throughout all biological systems in virtually all cell types. Pathological changes in gene expression have been associated with a wide array of disorders. | Moyano et al., 2017, Savy et al., 2018, Lee et al., 2016 |
| Autoimmunity | Formation of autoantibodies that target proteins, especially those known to play important roles in the structure and function of neurons including myelination and axonal transport (e.g., MBP, NFP, Tau, MAP-2) |
Abou-Donia et al., 2013; AbouDonia et al., 2017; El-Rahman et al., 2018 |
| Mitochondria | Organelle responsible for ATP production. Changes in transport and morphology can affect function. Also associated with the release of apoptotic factors. | Middlemore-Risher et al 2011, Eftekhari et al., 2018 |
| Gut Microbiome | Large group of bacteria found in the gut existing in symbiosis with the host. Implicated in a wide range of functions and downstream effects ranging from immune function to cognitive development. | Gao et al., 2017 |
| DNA | DNA Damage (i.e. fragmentation, single and double strand breaks) is associated with carcinogenic and reproductive toxicity | Li et al., 2015 |
| Dynactin | Modulates the binding of cargoes to molecular motor proteins (dynein and kinesin-2) | Song et al., 2012 |
| Dynein | Motor protein responsible for retrograde axonal transport. | Song et al., 2012 |
| Tubulin | Globular proteins that polymerize into microtubules (i.e., α- and β-tubulin assemblies which serve structural roles in the cytoskeleton, support intracellular transport, mitosis, etc.). | Jiang et al., 2010 |
| Transferrin | Plasma glycoprotein that regulates iron levels. | Grigoryan et al., 2009 |
| Kinesin | Motor protein responsible for anterograde axonal transport in neurons as well as other cellular functions such as mitosis, meiosis, etc. | Grigoryan et al., 2009 |
| ATP Synthase | Enzyme responsible for ATP synthesis in mitochondria. | Grigoryan et al., 2009 |
| Nerve Growth Factor (NGF) and its receptors TrkA and P-75NTR |
NGF-Neurotrophin associated with survival, growth, and proliferation in neurons. TrkA-high affinity NGF receptor responsible for mediating neurotrophic effects of NGF. P-75NTR-low affinity neurotrophin receptor, which mediates apoptosis and cell death. | Terry et al., 2011 |
| Choline acetyltransferase (ChAt) |
Enzyme important for the synthesis of acetylcholine. | Terry et al., 2007, 2011 |
| α7-nicotinic acetylcholine receptors (nAChRs) | Acetylcholine receptor with known role in learning and memory. | Terry et al., 2007, 2011 |
| Butyrylcholinesterase | Serine hydrolase which nonspecifically hydrolyzes choline-based esters. | Li et al., 2007 |
| Monoacylglycerol lipase | Responsible for 2-AG hydrolysis in endocannabinoid inactivation. | Quistad et al, 2006, Reviewed Casida et al.,2008 |
| Serine Hydrolase KIAA 1363 |
Regulates lipid metabolism, involved with the invasiveness of tumors. | Nomura et al., 2006, Reviewed Casida et al.,2008 |
| Albumin | Globular protein responsible for transport | Peeples et al., 2005 |
| Cannabinoid CB1 receptors | G protein-coupled receptors, which mediate endocannabinoid signaling. | Quistad et al., 2002 |
| Fatty acid amide hydrolase | Serine hydrolase enzyme that catabolizes a class of bioactive lipids called the fatty acid amides, traditionally associated with endocannabinoid catabolism. | Quistad et al., 2001 |
| M2 muscarinic acetylcholine receptors | Second messenger coupled autoreceptor, modulates acetylcholine signaling. | Bomser and Casida 2001* |
| Acylpeptide hydrolase | Serine protease enzyme that catalyzes the removal of N-acylated amino acids from acetylated peptides, this plays a role in the coordinated protein-degradation | Richards et al., 2000 |
| Adenylyl cyclase | Enzyme that is required for the conversion of ATP to cyclic AMP/Important in the G protein signaling cascade |
Huff et al., 1994; Song et al., 1997; Auman et al., 2000 |
| Neuropathy target esterase | Phospholipase enzyme with known role in phospholipid metabolism, neurite outgrowth and process elongation during neuronal differentiation. | Lush et al., 1998 |
| Papain | Lysozomal cysteine protease. | Chaiken and Smith, 1969 |
OP-phosphorylation of M2 receptors was initially reported by Bomser and Casida 2001 (using chlorpyrifos oxon), but was not observed in experiments where paraoxon or a biotinlabeled fluorophosphonate were evaluated (Proskocil et al., 2010)
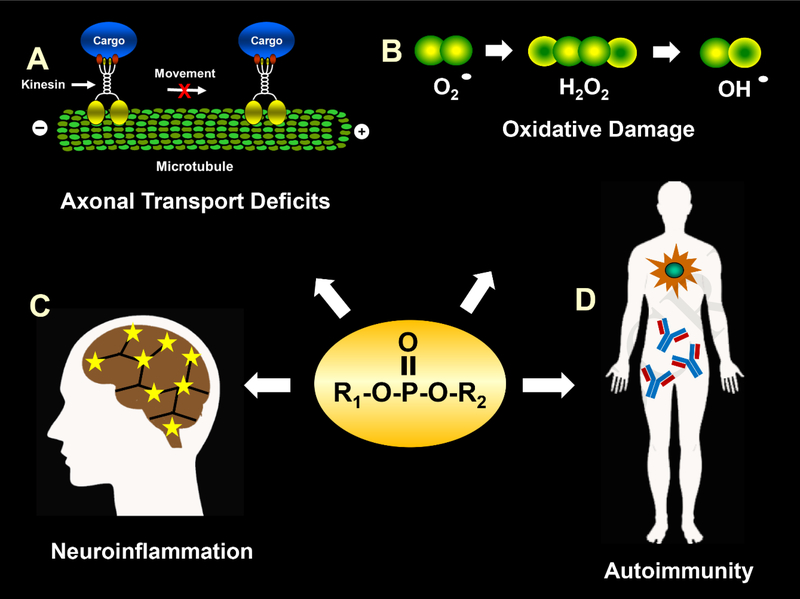
Fig 2.
Illustration of several representative physiological processes that may be affected by OPs to result in long-term deleterious health effects. A. OPs may impair axonal transport by altering the function of motor proteins such as kinesin and/or components of the neuronal cytoskeleton (e.g., microtubules). B. OPs can increase free radical formation and oxidative stress, which can lead to mitochondrial dysfunction and DNA damage to cells. C. OPs can lead to microglia activation and an increase in proinflammatory cytokines, which in turn may lead to neuroinflammation. D. OP exposure may lead to the generation of autoantibodies that target multiple proteins known to play important roles in both the structure and function of neurons including myelination and axonal transport.
4.2. Axonal Transport Deficits
Axonal transport is a fundamental process within neurons, whereby various organelles, membrane bound vesicles, and additional cargoes are transported along microtubules between the cell body and distal tip of the axon. The process is essential to neuronal development and axonal outgrowth as well as the maintenance and function of fully developed neurons (reviewed, Maday et al., 2014). Early indicators that OPs might impair axonal transport were evident in a report where relatively high doses of OPs (i.e., phenylphosphonothioate esters and TOCP) known to be associated with OPIDN impaired fast anterograde axonal transport in a rat optic nerve preparation (Reichert and Abou-Donia, 1980). Later studies in our laboratories (Terry et al., 2003; Terry et al., 2007) indicated that both anterograde and retrograde transport of vesicles in the sciatic nerves (ex vivo) were impaired in rats repeatedly exposed to chlorpyrifos, an OP not associated with OPIDN except at doses well above the LD50 (reviewed, Richardson, 1995). Importantly, the chlorpyrifos doses in these studies were below the threshold for acute toxicity and the deficits in axonal transport were persistent (detected for up to 14 days after the last chlorpyrifos injection). In more recent studies in our laboratory, using time-lapse imaging techniques in primary neuronal culture, we have observed impairments in the axonal transport of mitochondria (Middlemore-Risher et al., 2011) as well as membrane bound organelles (MBOs) (Gao et al., 2016) in axons associated with both chlorpyrifos and its metabolite CPF-oxon. Similar impairments in vitro were observed with the nerve agent diisopropylfluorophosphate (DFP) (Gao et al., 2016). In all of these in vitro studies, the impairments in axonal transport could be detected at concentrations that did not inhibit acetylcholinesterase activity and they were not blocked by cholinergic receptor antagonists. These data indicate that OPs with considerably different chemical structures (alkylphosphate versus the oxon metabolite of a phosphorothioate) can exhibit quite similar profiles of fast, anterograde axonal transport impairments at very low concentrations. Importantly, we have also observed persistent deficits in axonal transport in vivo in living rats exposed to chlorpyrifos or DFP using manganese-enhanced magnetic resonance imaging (MEMRI) (Hernandez et al., 2015; Naughton et al., 2018).
Other indications of OP-induced dysfunction in axons are evident in studies in vitro demonstrating decreased axonal outgrowth after exposure to chlorpyrifos or chlorpyrifos-oxon (CPO) (Howard et al., 2005; Yang et al., 2008). Chlorpyrifos-oxon has also been shown to disrupt axonal growth as well as motor behavior in zebrafish (Yang et al., 2011).
The mechanisms of the OP-related deficits in axonal transport noted above are unclear, but several hypotheses have been developed. There is now a significant amount of evidence that OPs may alter the function of motor proteins such as kinesin and/or components of the neuronal cytoskeleton (e.g., microtubules) that are important for axonal transport. In vitro evidence that OPs might interact with kinesin to negatively affect kinesin-driven axonal transport is evident in microtubule motility experiments where an increase in the number of locomoting microtubules that detached from kinesin-coated glass when kinesin was preincubated with the OPs chlorpyrifos, chlorpyrifos-oxon, or DFP was observed (Gearhart et al., 2007). These data suggested that OPs modify kinesin and weaken the kinesin-microtubule interactions that facilitate anterograde axonal transport. This hypothesis was supported by a later mass spectrometry study where (using the biotin-tagged OP agent, FP-biotin) OP binding to tyrosine in the human kinesin 3C motor domain was demonstrated (Grigoryan et al., 2009)
There is also evidence that OPs impair tubulin polymerization and other factors that can affect microtubule assembly and stability, which in turn leads to impairments of axonal transport. For example in spectrophotometric studies, Prendergast et al., 2007 demonstrated that chlorpyrifos-oxon inhibited the polymerization of tubulin, and, moreover, utilizing organotypic slice cultures of rodent brain and histological methods that chlorpyrifos-oxon caused a marked decrease in the concentration of microtubule associated protein-2 (MAP-2). Interestingly, multiple OPs (soman, sarin, DFP, CPO, FP-Biotin) have been shown to covalently bind to tyrosine residues on tubulin, an effect that may explain the disruptions in tubulin polymerization (Grigoryan et al., 2008; Jiang et al., 2010).
Other studies suggest that OPs can trigger changes in the post-translational modifications of molecules that are important for axonal transport. As an example, DFP was recently shown to decrease tubulin acetylation to impair the axonal movements of mitochondria in both cultured rodent and human derived neurons. The effect of DFP was exacerbated by corticosterone or cortisol, in rat and human neurons, respectively (to mimic stress), and these negative effects were attenuated by tubacin, a drug that inhibits HDAC6, the tubulin deacetylase (Rao et al., 2017). Moreover, our recent study indicated that chronic exposure to chlorpyrifos leads to a significant and persistent inhibition of tubulin acetylation in the brain of rats (Yang et al., 2018).
Finally, decreases in retrograde axonal transport related proteins dynein and dynactin have been observed in the spinal cord and cerebral cortex of hens after exposure to acutely toxic doses of TOCP (Song et al., 2012).
4.3. Oxidative Stress and Neuroinflammation
It is well accepted that OPs, particularly at high concentrations, can increase oxidative stress and DNA damage to cells, (reviewed, Soltaninejad and Abdollahi, 2009; Pearson and Patel, 2016; Vanova et al., 2018). In addition, chlorpyrifos, can induce lipid peroxidation in the developing rat brain at concentrations that only cause mild signs of systemic toxicity (Slotkin, 2005). Chronic low-level exposure to dichlorvos in adult rats has been shown to induce apoptotic neurodegeneration by raising mitochondrial Ca++ levels, impairing the activity of mitochondrial complexes I, III and IV, and increasing oxidative stress (Kaur et al., 2007). The OP-exposure regimen in this study was also associated with an increase in lipid peroxidation and decreases in the mitochondrial antioxidants glutathione and superoxide dismutase. Moreover, long-term exposure by rabbits to the OPs, diazinon or propoxur (at doses not associated with overt signs of acute toxicity) resulted in oxidative stress, histopathological lesions, and genotoxic effects in the liver and kidneys (Tsitsimpikou et al., 2013). Low-level repeated exposure to chlorpyrifos has also been shown to induce inflammatory responses in cultured astrocytes (i.e., increases in IL-6, GFAP, and p-ERK1/2) (Mense et al., 2006). Acute and chronic exposures to multiple OPs have also been shown to trigger neuroinflammation via microglia activation and increases in proinflammatory cytokines in mice (reviewed, Banks and Lein, 2012; Viviani et al., 2014), as well as in 3-dimensional brain cell cultures (Monnet-Tschudi et al., 2007). In a mouse model developed to mimic Gulf War Illness (GWI), acute exposure to DFP was found to produce a strong inflammatory response across multiple brain regions. Increased levels of mRNA for the inflammatory markers TNF-α, IL6, CCL2, IL-1β, LIF and OSM were reported and interestingly, these effects were potentiated by pretreatment with the stress related hormone corticosterone (O’Callaghan et al., 2015). A follow-up (GWI-related) study in rats later demonstrated that acute DFP exposure increased the expression of a number of cytokine-related genes associated with inflammation (IL-6, IL1β, CCL2 etc.) in a manner that was exacerbated by corticosterone pre-treatment. Using a novel high-order diffusion magnetic resonance imaging (MRI) technique, alterations in micro-scale diffusivity in the frontal cortex and hippocampus were found to accompany the alterations in cytokine mRNA expression (Koo et al., 2018). The authors noted that the association of these diffusion changes with neuroinflammatory cytokine expression indicates the potential for GWI-relevant exposures to result in connectivity changes in the brain.
Some studies suggest that the increases in inflammation observed after OP exposure may be directly related to changes in oxidative stress. For example, chlorpyrifos exposure can lead to the induction of the NLRP3 inflammasome in a manner that is reversible by the blockade of mitochondrial reactive oxygen species (mROS). Stimulation of mROS by chlorpyrifos is thought to be first step in a process by which mROS-induced inflammation can lead to pyrotosis and apoptosis in human keratinocyte HaCat cell lines (Jang et al., 2015). More recent experiments in rats demonstrated that increased levels of oxidative stress markers (GSH, GSGG, 3-NT/Tyrosine) in the brain after acute DFP exposure were accompanied by increases in inflammatory cytokines (TNF-α, IL1-β, IL-6, KC/GRO) (Liang et al, 2018). Interestingly, DFP induced changes in both oxidative stress and inflammation could be attenuated by treatment with a catalytic antioxidant. Similar findings in which antioxidant treatment was able to attenuate an increase in inflammatory markers elicited by exposure to the OP malathion have also been reported (Mohammadzade et al., 2018). Furthermore, the co-occurrence of oxidative stress and inflammation has been reported in rats exposed to chlorpyrifos, an effect that could be reduced by a drug with known antioxidant properties (Abolaji et al., 2017). Please see further discussion to support the relationship between neuroinflammation and oxidative stress in the “Potential Therapeutic Strategies” section below.
4.4. Autoimmunity
There is a small, but evolving body of literature to suggest that OP exposures may lead to autoimmune responses in some individuals, effects that might offer one explanation for why OP exposures can lead to chronic (in some cases lifelong) symptoms. Examples come from studies designed to identify peripheral biomarkers of neurotoxicity in veterans with GWI and farmers exposed to pesticides who developed neurological symptoms or evidence of neurological damage (Abou-Donia et al., 2017; El Rahman et al., 2018). In the Abou-Donia et al. study, the serum of 20 veterans with GWI and 10 non-veteran symptomatic (low back pain) controls were screened for the presence of autoantibodies to central nervous system-specific proteins. In the El Rahman et al. 2018 study, serum samples from 50 farmers chronically exposed to different mixtures of pesticides (including a variety of OPs) and 25 non-exposed controls were analyzed. In each study, higher levels of autoantibody reactivity against the following proteins were detected in both the veterans with GWI and the pesticide exposed farmers (compared to controls): neurofilament triplet proteins (NFP), tubulin, microtubule associated tau proteins (Tau), microtubule associated protein-2 (MAP-2), myelin basic protein (MBP), myelin associated glycoprotein (MAG), glial fibrillary acidic protein (GFAP), and calcium-calmodulin kinase II (CaMKII). The findings of these two studies were similar to a previous study (Abou-Donia et al., 2013) of flight crewmembers who experienced symptoms now referred to as “Aerotoxic Syndrome” and who reported being exposed to cabin air emissions which may have contained OPs. Collectively, while the three human studies described here were relatively small and retrospective, they support the argument that OP exposure may lead to the generation of autoantibodies that target proteins, especially those known to play important roles in both the structure and function of neurons including myelination and axonal transport.
4.5. Gene Expression
Recent advances in gene sequencing have made it possible to simultaneously evaluate the expression of hundreds of different genes in a single study (reviewed, Wang et al., 2009). Due to the ability of OPs to modify a number of different targets, such studies may be of particular utility in understanding the vast array of OP-induced toxic effects. Alterations in the expression of hundreds of different genes in the rat hippocampus have been recently reported via microarray and RNA-seq analysis after repeated chlorpyrifos exposure (Lee et al., 2016). In particular, alterations in six mRNAs were found to be common among multiple transcriptomics analyses (Bdnf, Cort, Crhbp, Nptx2, Npy and Pnoc). The genes affected by chlorpyrifos exposure are involved in the expression of neurotrophins and secreted neuropeptides. Alterations in the expression and function of the aforementioned neurotrophins and neuropeptides have been associated with a range of cognitive and psychiatric disorders (Lee et al., 2016) and thus could offer one explanation for the cognitive and psychiatric disturbances in OP-exposed individuals. Furthermore, a recent study demonstrated that chronic, low level exposure to the OP diazinon in adult rats induces changes in the expression of genes associated with psychological disorders and cell-to-cell signaling specifically involving glutamatergic and monoaminergic neurotransmission (Savy et al., 2018).
In vitro changes in gene expression in response to both acute and long-term exposure to OPs have also been reported. For example, multiple changes in the expression profiles of genes related to apoptosis and necrosis were observed after 24hrs of acute, or 14 days of repeated, exposures to chlorpyrifos in SN56 cells (Moyano et al., 2017). Given that the SN56 cell line is derived from cholinergic septal neurons, it is believed that the reported changes in cell death related pathways may underlie cholinergic neurodegeneration and may be responsible for previously reported deficits in learning and memory after chlorpyrifos exposure.
5. Potential Therapeutic Strategies
5.1. Antioxidants
Given the aforementioned perturbations in mitochondrial function and related oxidative stress, the use of antioxidants to treat OP related dysfunction has become a growing field of study. In support of this premise, the mitochondria-targeted antioxidant MitoQ attenuated dichlorvos-induced ROS production, it increased MnSOD activity and glutathione levels, and it decreased lipid peroxidation, protein and DNA oxidation in rats. MitoQ also suppressed dichlorvos-related DNA fragmentation, cytochrome c release and caspase-3 activity and it attenuated mitochondrial swelling, loss of cristae and chromatin condensation (Wani et al., 2011). More recently, the antioxidant carotenoid compound crocin was shown to be protective against parkinsonian-like motor deficits resulting from chronic exposure to the OP malathion in rats (Mohammadzadeh et al., 2018). In addition, the catalytic antioxidant AEOL10150 was shown to reduce markers for oxidative stress, inflammation, and cell death after administration of an acute, seizure-inducing, dose of DFP in rats. The neuroprotective nature and blood-brain-barrier permeability of AEOL10150 makes it a particularly strong candidate as a potential therapeutic (Liang et al., 2018). Further evidence for the benefit of antioxidants in treating OP toxicity has been provided by studies in rats using nanoparticles to deliver coenzyme Q10 (CoQ10). Eftekhari and colleagues demonstrated that nanoparticle based delivery of CoQ10 is effective in protecting against dichlorvos induced hepatotoxicity. Here, the authors demonstrated protection against mitochondrial dysfunction and the generation of reactive oxygen species with increased bioavailability over traditional CoQ10 (Eftekhari et al., 2018).
Many compounds extracted from plants of the Zingiberaceae (ginger) family are also well known for their medicinal value. Curcumin is one such compound, and is an extract from Curcuma longa (Turmeric). Curcumin pretreatment in SH-SY5Y and IMR-32 cells in vitro was shown to reduce oxidative stress caused by a synergistic interaction between amyloid beta and the commonly used OPs monocrotophos or chlorpyrifos. The positive effects of curcumin in this study were reported to depend on activation the Nrf2-antioxidant response element-signaling pathway (Sarkar et al., 2017). Due to its activity in both antioxidant and DNA repair settings, curcumin may thus make an effective prophylactic treatment against OP exposure (Sarkar et al., 2017). Other compounds of the Zingiberaceae family may also be effective therapeutics against OP toxicity by acting in a multifunctional manner. Due to the wide variety of targets affected by OPs, compounds with the ability to target both multiple molecular pathways as well as multiple organs may be of particular utility. 6-Gingerol-rich fraction (6-GRF) from Zingiber officinale(Garden Ginger) was recently shown to be protective against chlorpyrifos-induced oxidative stress (H2O2 and MDA) and disruption of antioxidant related proteins (catalase, GST, GSH, SOD, GPx) in rats. Moreover, 6-GRF reduced CPF-related increases in inflammatory (NO, MPO and TNF-α) and apoptotic (caspase-3) markers (Abolaji et al., 2017).
5.2. Axonal Transport: Microtubule and Signaling Kinase Targeted Therapies
Increasing microtubule stability may serve as another potential strategy for reversing OP induced deficits in axonal transport and cognition. While no data are currently available regarding OP exposures and microtubule-target therapeutic agents, studies in other disease contexts may be relevant. For example, the microtubule stabilizing compound epitholone D has previously shown efficacy in reducing axonal dysfunction as well as improving axonal transport and cognitive performance in a transgenic mouse model for Alzheimer’s disease (Zhang et al., 2012; Brunden et al., 2010). Tubulin acetylation may be another potential target for reversing OP induced deficits in axonal transport and microtubule dysfunction. It was recently shown that chronic exposure to CPF can lead to decreased acetylation of α-tubulin at Lys-40 (Yang et al., 2018) in rats. Similarly, Rao et al demonstrated reductions in tubulin acetylation after DFP exposure in both cultured rat and human (stem cell derived) neurons. Moreover, the effect of DFP was reversed by treatment with the HDAC6 inhibitor tubacin (Rao et al., 2017).
In addition to microtubule-associated proteins and post-translational modifications, a number of different kinases can also act as regulators of axonal transport. A recent review identified 10 different kinases that could regulate various aspects of axonal transport (JNK, Cdk5, GSKIIIβ, ERK1/2, PKA etc.), including the phosphorylation of motors, adapter proteins, and cargoes (reviewed, Gibbs et al., 2015). Accordingly, some of these kinases could be targeted by new therapeutic agents to improve axonal transport.
5.3. Additional Strategies against Acute OP Toxicity
The current standard of care for treating acute poisoning with OPs includes airway support (intubation), the muscarinic antagonist atropine, the AChE reactivating agent pralidoxime (2-PAM), and benzodiazepines (e.g., diazepam) to control seizures (reviewed, Kats and Brooks 2017). Unfortunately, the mortality rate with this regimen is relatively high and the efficacy of some components of this regimen (especially 2-PAM) has been challenged (reviewed, Eddleston & Chowdhury, 2016). Accordingly, there remains a need for more effective therapeutic regimens and several relatively recent studies have identified new (potential) approaches. For example, blockade of intracellular Ca2+ release following OP induced status epilepticus has shown to be an effective strategy for neuroprotection against OP induced toxicity. As an example, the compounds dantrolene and carisbamate reduced hippocampal cell death resulting from a seizure inducing exposure to the OP paraoxon in rats via the inhibition of a post injury Ca2+ plateau (Deshpande et al., 2016). N-acetylcysteine (NAC) is an antioxidant compound used in the treatment of acetaminophen overdose, which may also be a useful adjunct to the traditional standard of care after acute OP exposure. In a randomized, controlled, parallel-group clinical trial, intravenous NAC was shown to reduce the amount of atropine required for symptomatic relief after acute organophosphate poisoning (El-Ebiary et al., 2016). Recently it has also been shown that the essential omega-3 polyunsaturated fatty acid alpha-linolenic acid can rescue soman induced behavior deficits in rats and to reduce rates of corresponding cell death in multiple brain regions (Pan et al., 2015). Catalytic bioscavengers represent another potential route for protecting against OP toxicity, especially in a prophylactic context. Bacterial derived enzymes with phosphotriesterase (PTE) are highly efficient in the degradation of OPs, but present certain immunogenic concerns. Similar, human derived, enzymes such as paraoxonase-1(PON1) represent additional possibilities with less immunogenic concerns. In particular, the PON1 mutant IIG1 may offer prophylactic protection against a broad range of OPs (reviewed, Iyer et al., 2015). The use of butyrylcholinesterase as a bioscavenger to remove OPs from circulation, thus preventing their toxic effects is another potential avenue for therapeutic intervention (see review, Pope and Brimijoin, 2018). Equine serum derived BChE delivered via a gel coated nanocapsule has been shown to bind the OP paraoxon in a non-immunogenic fashion in rats (Zhang et al., 2016).
Supplemental antidotes to acute OP toxicity, such as magnesium, clonidine and sodium bicarbonate, have also been evaluated in small clinical trials; however, the results to date have been equivocal. Other, potential antidotes such as nicotinic receptor antagonists, beta‐adrenergic agonists, and lipid emulsions (to bind and sequester lipophilic OPs, see review Eisenkraft and Falk, 2016) are currently being evaluated in animal models and in small pilot clinical trials (reviewed, Eddleston & Chowdhury, 2016).
6. Conclusion
With the widespread use of OPs worldwide to control pests as well as to prevent vector borne illnesses comes the environmental risks to humans and other non-target organisms. This risk is exacerbated by the tendency of rogue governments and terrorists to employ OPs against individuals they consider enemies which can include both military personnel and civilians. It is essential that novel treatments be developed to combat both the acute toxicity of OPs as well as their long-term deleterious health effects, since there are currently no ideal therapeutic approaches available. In order for these new treatments to be developed it is imperative that a better understanding of the mechanisms that contribute to the toxicity be of OPs be elucidated. In addition to the well-known inhibitory effects of OPs on acetylcholinesterase, there is an evolving literature to suggest that OPs affect a number of additional targets that lead to oxidative stress, axonal transport deficits, neuroinflammation, and autoimmunity. There is significant (and growing) evidence that some of these targets could exploited for therapeutic purposes.
Acknowledgments
The authors would like to thank Ms. Ashley Davis for her administrative assistance in preparing this article. The work of the corresponding author’s laboratory cited in this review was supported by the National Institute of Environmental Health Sciences (ES012241) and the Congressionally Directed Medical Research Programs (CDMRP), specifically, the Gulf War Illness Research Program (GWIRP), grant number W81XWH-12–1-0536.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abhimanyu P, Madhan R. (2018). Assessment of Socio-demographic Profile, Pattern, and Outcomes of Poisoning Cases in Emergency Department of A Tertiary Care Teaching Hospital– A Prospective Study, Toxicology Reports (2018), 10.1016/j.toxrep.2018.05.013. [DOI] [Google Scholar]
- Abolaji AO, Ojo M, Afolabi TT, Arowoogun MD, Nwawolor D, Farombi EO. (2017) Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem Biol Interact. May 25;270:15–23. doi: 10.1016/j.cbi.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Abou-Donia MM, ElMasry EM, Monro JA, Mulder MF. (2013). Autoantibodies to nervous system-specific proteins are elevated in sera of flight crew members: biomarkers for nervous system injury. J Toxicol Environ Health A. 76(6):363–80. doi: 10.1080/15287394.2013.765369. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Conboy LA, Kokkotou E, Jacobson E, Elmasry EM, Elkafrawy P, Neely M, Bass CR’, Sullivan K. (2017) Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicol Teratol. May;61:36–46. doi: 10.1016/j.ntt.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Amr MM, Halim ZS, Moussa SS. (1997). Psychiatric disorders among Egyptian pesticide applicators and formulators. Environ Res 73(1–2):193–9. doi: 10.1006/enrs.1997.3744 [DOI] [PubMed] [Google Scholar]
- Auman JT, Seidler FJ, Slotkin TA. (2000) Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: implications for neurotoxicity. Brain Res Dev Brain Res. May 11;121(1):19–27. [DOI] [PubMed] [Google Scholar]
- Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. (2014) Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases--a mechanistic approach. Toxicol Lett October 15;230(2):85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Banks CN, Lein PJ. (2012) A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology June;33(3):575–84. doi: 10.1016/j.neuro.2012.02.002. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin PG, van Eeden SF, Moolman JA, Foden AP, Joubert JR. (1994) Organophosphate and carbamate poisoning. Arch Intern Med. July 11;154(13):1433–41. Review. [PubMed] [Google Scholar]
- Barnaby F (1988). Iran-Iraq War: the use of chemical weapons against the Kurds. Ambio 17: 407–8. [Google Scholar]
- Barr DB, Wong LY, Bravo R, Weerasekera G, Odetokun M, Restrepo P, Kim DG, Fernandez C, Whitehead RD Jr, Perez J, Gallegos M, Williams BL, Needham LL. (2011). Urinary concentrations of dialkylphosphate metabolites of organophosphorus pesticides: national health and nutrition examination survey 1999–2004. Int. J. Environ. Res. Public Health 8, 3063–3098. doi: 10.3390/ijerph8083063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardocco D (1997). DoD, CIA release Khamisiyah modeling data. GulfNEWS; 1:3. [Google Scholar]
- Bomser JA, Casida JE. (2001) Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro. Toxicol Lett February 3;119(1):21–6. [DOI] [PubMed] [Google Scholar]
- Brown MA, Brix KA. (1998) Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol. Nov-Dec;18(6):393–408. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, Iba M, James MJ, Xie SX, Ballatore C, Smith AB 3rd, Lee VM, Trojanowski JQ. (2010) Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci October 13;30(41):13861–6. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burillo-Putze G, Xarau S. Pesticides In: Tintinalli JE, Stapczynski J, Ma O, Yealy DM, Meckler GD, Cline DM. eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 8e New York, NY: McGraw-Hill; 2016. http://accessmedicine.mhmedical.com/content.aspx?bookid=1658§ionid=154158300. Accessed August 02, 2018. [Google Scholar]
- Burke RD, Todd SW, Lumsden E, Mullins RJ, Mamczarz J, Fawcett WP, Gullapalli RP, Randall WR, Pereira EFR, Albuquerque EX. (2017) Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J Neurochem August;142 Suppl 2:162–177. doi: 10.1111/jnc.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Nomura DK, Vose SC, Fujioka K. (2008) Organophosphate-sensitive lipases modulate brain lysophospholipids, ether lipids and endocannabinoids. Chem Biol Interact. September 25;175(1–3):355–64. doi: 10.1016/j.cbi.2008.04.008 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Durkin KA. (2013) Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu Rev Entomol. 58:99–117. doi: 10.1146/annurev-ento-120811-153645. Review. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) [Accessed 02/22/18];2017 https://www.cdc.gov/zika/vector/aerial-spraying.
- Chaiken IM, Smith EL. (1969) Reaction of a specific tyrosine residue of papain with diisopropylfluorophosphate. J Biol Chem. August 10;244(15):4247–50. [PubMed] [Google Scholar]
- Clune AL, Ryan PB, Barr DB. (2012). Have regulatory efforts to reduce organophosphorus insecticide exposures been effective? Environ. Health Perspect 120, 521–525. doi: 10.1289/ehp.1104323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombet JM. (2011) Nerve agent intoxication: recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicol Appl Pharmacol. September 15;255(3):229–41. doi: 10.1016/j.taap.2011.07.003 Review. [DOI] [PubMed] [Google Scholar]
- Cooper Jand Dobson Hans. (2007). The benefits of pesticides to mankind and the environment. Crop Protection 26; 1337–1348. [Google Scholar]
- Costa LG. (2018) Organophosphorus Compounds at 80: Some Old and New Issues. Toxicol Sci. Mar 1;162(1):24–35. doi: 10.1093/toxsci/kfx266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassanayake T, Weerasinghe V, Dangahadeniya U, Kularatne K, Dawson A, Karalliedde L, Senanayake N. (2007) Cognitive processing of visual stimuli in patients with organophosphate insecticide poisoning. Neurology 68:2027–30. doi: 10.1212/01.wnl.0000264423.12123.f0 [DOI] [PubMed] [Google Scholar]
- Davisson ML, Love AH, Vance A, Reynolds JG. (2005). Environmental Fate of Organophosphorus Compounds Related to Chemical Weapons Livermore, CA: Lawrence Livermore National Laboratory with the U.S. Department of Energy at the University of California; (Contract W-7405-Eng-48) [Google Scholar]
- de Boer J, Antelo A, van der Veen I, Brandsma S, Lammertse N. (2015) Tricresyl phosphate and the aerotoxic syndrome of flight crew members--current gaps in knowledge. Chemosphere January;119 Suppl:S58–61. doi: 10.1016/j.chemosphere.2014.05.015. [DOI] [PubMed] [Google Scholar]
- de Ree H, van den Berg M, Brand T, Mulder GJ, Simons R, Veldhuijzen van Zanten B, Westerink RH. (2014) Health risk assessment of exposure to TriCresyl Phosphates (TCPs) in aircraft: a commentary. Neurotoxicology December;45:209–15. doi: 10.1016/j.neuro.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Huang BA, Phillips KF, DeLorenzo RJ. (2016) Pharmacological blockade of the calcium plateau provides neuroprotection following organophosphate paraoxon induced status epilepticus in rats. Neurotoxicol Teratol Jul-Aug;56:81–86. doi: 10.1016/j.ntt.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow AgroSciences; http://www.dowagro.com/chlorp/na/science/prop.htm. Accessed March 25, 2018.
- Duarte DJ, Rutten JMM, van den Berg M, Westerink RHS. (2017) In vitro neurotoxic hazard characterization of different tricresyl phosphate (TCP) isomers and mixtures. Neurotoxicology March;59:222–230. doi: 10.1016/j.neuro.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, Lockridge O. (2001) Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther. November;299(2):528–35. [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. (2008) Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol.38 Suppl 2:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ. (2001). Pesticide use in developing countries. Toxicology. 7;160(1–3):27–33. Review. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Chowdhury FR. (2016) Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br J Clin Pharmacol. March;81(3):462–70. doi: 10.1111/bcp.12784. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Ahmadian E, Azami A, Johari-Ahar M, Eghbal MA. (2018) Protective effects of coenzyme Q10 nanoparticles on dichlorvos-induced hepatotoxicity and mitochondrial/lysosomal injury. Environ Toxicol February;33(2):167–177. doi: 10.1002/tox.22505. [DOI] [PubMed] [Google Scholar]
- Eisenkraft A, Falk A. (2016) Possible role for anisodamine in organophosphate poisoning. Br J Pharmacol. June;173(11):1719–27. doi: 10.1111/bph.13486. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ebiary AA, Elsharkawy RE, Soliman NA, Soliman MA, Hashem AA. (2016) N-acetylcysteine in Acute Organophosphorus Pesticide Poisoning: A Randomized, Clinical Trial. Basic Clin Pharmacol Toxicol August;119(2):222–7. doi: 10.1111/bcpt.12554. [DOI] [PubMed] [Google Scholar]
- El Rahman HAA, Salama M, Gad El-Hak SA, El-Harouny MA, ElKafrawy P, Abou-Donia MB. (2018). A Panel of Autoantibodies Against Neural Proteins as Peripheral Biomarker for Pesticide-Induced Neurotoxicity. Neurotox Res February;33(2):316–336. doi: 10.1007/s12640-017-9793-y. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) [Accessed 02/22/2018];2017 www.epa.gov/mosquitocontrol/controlling-adult-mosquitoes.
- Eskenazi B, Castorina R. (1999) Association of prenatal maternal or postnatal child environmental tobacco smoke exposure and neurodevelopmental and behavioral problems in children. Environ Health Perspect. December;107(12):991–1000. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cornejo Jorge, Nehring Richard, Osteen Craig, Wechsler Seth, Martin Andrew, and Vialou Alex. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960–2008, EIB-124, U.S. Department of Agriculture, Economic Research Service, May 2014. [Google Scholar]
- Gao J, Naughton SX, Wulff H, Singh V, Beck WD, Magrane J, Thomas B, Kaidery NA, Hernandez CM, Terry AV Jr. (2016) Diisopropylfluorophosphate Impairs the Transport of Membrane-Bound Organelles in Rat Cortical Axons. J Pharmacol Exp Ther. March;356(3):645–55. doi: 10.1124/jpet.115.230839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Naughton SX, Beck WD, Hernandez CM, Wu G, Wei Z, Yang X, Bartlett MG, Terry AV Jr. (2017) Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. Neurotoxicology September;62:111–123. doi: 10.1016/j.neuro.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV Jr. (2007) Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol. January 1;218(1):20–9. [DOI] [PubMed] [Google Scholar]
- Gibbs KL, Greensmith L, Schiavo G. (2015) Regulation of Axonal Transport by Protein Kinases. Trends Biochem Sci. October;40(10):597–610. doi: 10.1016/j.tibs.2015.08.003. Review [DOI] [PubMed] [Google Scholar]
- Golomb BA. (2008) Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A. March 18;105(11):4295–300. doi: 10.1073/pnas.0711986105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alzaga B, Lacasaña M, Aguilar-Garduño C, Rodríguez-Barranco M, Ballester F, Rebagliato M, Hernández AF. (2014) A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett October 15;230(2):104–21. doi: 10.1016/j.toxlet.2013.11.019. Review. [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. (2008) Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents. Chem Biol Interact. 2008. September 25;175(1–3):180–6. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Li B, Anderson EK, Xue W, Nachon F, Lockridge O, Schopfer LM. (2009) Covalent binding of the organophosphorus agent FP-biotin to tyrosine in eight proteins that have no active site serine. Chem Biol Interact. 2009. August 14;180(3):492–8. doi: 10.1016/j.cbi.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D, Eddleston M, Phillips MR, Konradsen F. (2007) The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health December 21;7:357. doi: 10.1186/1471-2458-7-357. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterres A (2017). Seventh report of the Organisation for the Prohibition of Chemical Weapons-United Nations Joint Investigative Mechanism (S/2017/904).
- Harrison V, Mackenzie Ross SJ. (2016) An emerging concern: Toxic fumes in airplane cabins. Cortex. January;74:297–302. doi: 10.1016/j.cortex.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JC, Welsh-Bohmer KA; Cache County Study Investigators. (2010) Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology. May 11;74(19):1524–30. doi: 10.1212/WNL.0b013e3181dd4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrami SA, Ibrahim N. (2004) Experiencing chemical warfare: two physicians tell their story of Halabja in Northern Iraq. Can J Rural Med. Summer;9(3):178–81 [PubMed] [Google Scholar]
- Hernandez CM, Beck WD, Naughton SX, Poddar I, Adam BL, Yanasak N, Middleton C, Terry AV Jr. (2015) Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology. March;47:17–26. doi: 10.1016/j.neuro.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Heutelbeck AR, Bornemann C, Lange M, Seeckts A, Müller MM. (2016) Acetylcholinesterase and neuropathy target esterase activities in 11 cases of symptomatic flight crew members after fume events. J Toxicol Environ Health A. 79(22–23):1050–1056. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. (2005) Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. September 1;207(2):112–24. [DOI] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. (1994) Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther. April;269(1):329–35. [PubMed] [Google Scholar]
- Iversen A, Chalder T, Wessely S. (2007). Gulf War Illness: lessons from medically unexplained symptoms. Clin Psychol Rev. 27(7):842–54 doi: 10.1016/j.cpr.2007.07.006. Review. [DOI] [PubMed] [Google Scholar]
- Iyer R, Iken B, Leon A. (2015) Developments in alternative treatments for organophosphate poisoning. Toxicol Lett. March 4;233(2):200–6. doi: 10.1016/j.toxlet.2015.01.007. Review. [DOI] [PubMed] [Google Scholar]
- Jang Y, Lee AY, Jeong SH, Park KH, Paik MK, Cho NJ, Kim JE, Cho MH. (2015) Chlorpyrifos induces NLRP3 inflammasome and pyroptosis/apoptosis via mitochondrial oxidative stress in human keratinocyte HaCaT cells.Toxicology. December 2;338:37–46. doi: 10.1016/j.tox.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. (2010) Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. May;115(1):183–93. doi: 10.1093/toxsci/kfq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Ravella R, Ketchum B, Paul R, Bierman R, Heaney P, White TS, Brantley SL. (2010) Mineral weathering and elemental transport during hillslope evolution at the susquehanna/shale hills critical zone observatory. Geochimica et. Cosmochimica. Acta 74: 3669–3691. [Google Scholar]
- John H, van der Schans MJ, Koller M, Spruit HET, Worek F, Thiermann H, Noort D. (2018) Fatal sarin poisoning in Syria 2013: forensic verification within an international laboratory network. Forensic Toxicol 36(1): 61–71. 10.1007/s11419-017-0376-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz KD, Brooks DE. (2017) Organophosphate Toxicity, September 5, 2017. In: Medscape from WebMD, Medscape, LLC, New York, New York: Available: https://emedicine.medscape.com/article/167726 (accessed April 4, 2018). [Google Scholar]
- Kaur P, Radotra B, Minz RW, Gill KD. (2007) Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicology. November;28(6):1208–19. doi: 10.1016/j.neuro.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Killeen GF, Masalu JP, Chinula D, Fotakis EA, Kavishe DR, Malone D, Okumu F. (2017) Control of Malaria Vector Mosquitoes by Insecticide-Treated Combinations of Window Screens and Eave Baffles. Emerg Infect Dis. May;23(5):782–789. doi: 10.3201/eid2305.160662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BB, Michalovicz LT, Calderazzo S, Kelly KA, Sullivan K, Killiany RJ, O’Callaghan JP. (2018) Corticosterone potentiates DFP-induced neuroinflammation and affects high-order diffusion imaging in a rat model of Gulf War Illness. Brain Behav Immun January;67:42–46. doi: 10.1016/j.bbi.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Fareedullah MD, Sudhakar Y, Venkateswarlu B, Kumar EA. (2010) Current review on organophosphorus poisoning. Arch Appl Sci Res.2:199–215. [Google Scholar]
- Latiff R, Chow E. (2017). Chemical weapon VX nerve agent killed North Korean leader’s half-brother: Malaysian police. Reuters February 23, 2017 https://www.reuters.com/article/us-northkorea-malaysia-kim/chemical-weapon-vx-nerve-agent-killed-north-korean-leaders-half-brother-malaysian-police-idUSKBN16303Z. Accessed March 22, 2018. [Google Scholar]
- Lee YS, Lewis JA, Ippolito DL, Hussainzada N, Lein PJ, Jackson DA, Stallings JD. (2016) Repeated exposure to neurotoxic levels of chlorpyrifos alters hippocampal expression of neurotrophins and neuropeptides. Toxicology. January 18;340:53–62. doi: 10.1016/j.tox.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher ND, Seidler FJ, Slotkin TA. (2001). Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res, 130, pp. 83–89. [DOI] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, Yang L, Petro A, Ryde IT, Wrench N, Seidler FJ, Slotkin TA. (2010). Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res April 2;208(2):319–27. doi: 10.1016/j.bbr.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Huang Q, Lu M, Zhang L, Yang Z, Zong M, Tao L. (2015) The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere September;135:387–93. doi: 10.1016/j.chemosphere.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Li H, Schopfer LM, Nachon F, Froment MT, Masson P, Lockridge O. (2007) Aging pathways for organophosphate-inhibited human butyrylcholinesterase, including novel pathways for isomalathion, resolved by mass spectrometry. Toxicol Sci. 2007. November;100(1):136–45 doi: 10.1093/toxsci/kfm215 [DOI] [PubMed] [Google Scholar]
- Liang LP, Pearson-Smith JN, Huang J, McElroy P, Day BJ, Patel M. (2018) Neuroprotective Effects of AEOL10150 in a Rat Organophosphate Model. Toxicol Sci April 1;162(2):611–621. doi: 10.1093/toxsci/kfx283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyasova M, Li B, Schopfer LM, Nachon F, Masson P, Furlong CE, Lockridge O. (2011) Exposure to tri-o-cresyl phosphate detected in jet airplane passengers. Toxicol Appl Pharmacol. November 1;256(3):337–47. doi: 10.1016/j.taap.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush MJ, Li Y, Read DJ, Willis AC, Glynn P. (1998) Neuropathy target esterase and a homologous Drosophila neurodegeneration-associated mutant protein contain a novel domain conserved from bacteria to man. Biochem J. May 15;332 ( Pt 1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macilwain C (1993) Study proves Iraq used nerve gas, Nature 363, p. 3. [DOI] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. (2014) Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. October 22;84(2):292–309. doi: 10.1016/j.neuron.2014.10.019. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P, Nachon F. (2017) Cholinesterase reactivators and bioscavengers for pre- and post-exposure treatments of organophosphorus poisoning. J Neurochem. August;142 Suppl 2:26–40. doi: 10.1111/jnc.14026. Review. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Lan C, Zhou M, Bentsman G, Volsky DJ, Whyatt RM, Perera FP, Zhang L. (2006) The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol Sci September;93(1):125–35. doi: 10.1093/toxsci/kfl046 [DOI] [PubMed] [Google Scholar]
- Michaelis S, Burdon J, Vyvyan Howard C. Aerotoxic syndrome: a new occupational disease? Public Health Panorama. s June 2017 [Google Scholar]
- Middlemore-Risher ML, Adam BL, Lambert NA, Terry AV Jr. (2011) Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J Pharmacol Exp Ther. 2011. November;339(2):341–9. doi: 10.1124/jpet.111.184762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh L, Hosseinzadeh H, Abnous K, Razavi BM. (2018) Neuroprotective potential of crocin against malathion-induced motor deficit and neurochemical alterations in rats. Environ Sci Pollut Res Int. February;25(5):4904–4914. doi: 10.1007/s11356-017-0842-0. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich M-G, Honegger P. (2007) Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Human & Experimental Toxicology Vol 26, Issue 4, pp. 339 – 346 doi: 10.1177/0960327107074589 [DOI] [PubMed] [Google Scholar]
- Moyano P, Del Pino J, Anadon MJ, Díaz MJ, Gómez G, Frejo MT. (2017) Toxicogenomic profile of apoptotic and necrotic SN56 basal forebrain cholinergic neuronal loss after acute and long-term chlorpyrifos exposure. Neurotoxicol Teratol. Jan-Feb;59:68–73. doi: 10.1016/j.ntt.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, Iglesias V, Alvarado S, Concha C, Rojas E, Vega C. (2013) Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotoxicology. December;39:158–68. doi: 10.1016/j.neuro.2013.09.003. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Takatori T, Matsuda Y, Nakajima M, Iwase H, Iwadate K (1997) Definitive evidence for the acute sarin poisoning diagnosis in the Toyko subway. Toxicol Appl Pharmacol 144 (1):198–203. doi: 10.1006/taap.1997.8110 [DOI] [PubMed] [Google Scholar]
- Naughton SX, Hernandez CM, Beck WD, Poddar I, Yanasak N, Lin PC, Terry AV Jr. (2018). Repeated exposures to diisopropylfluorophosphate result in structural disruptions of myelinated axons and persistent impairments of axonal transport in the brains of rats. Toxicology. June 9;406–407:92–103. doi: 10.1016/j.tox.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Nettleman M (2015). Gulf War Illness: Challenges Persist. Transactions of the American Clinical and Climatological Association, vol. 126. [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Durkin KA, Chiang KP, Quistad GB, Cravatt BF, Casida JE. (2006) Serine hydrolase KIAA1363: toxicological and structural features with emphasis on organophosphate interactions. Chem Res Toxicol. September;19(9):1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM. (2015) Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J Neurochem. June;133(5):708–21. doi: 10.1111/jnc.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary CA. (2002) The Kurds of Iraq: recent history, future prospects. Middle East Rev Int Affairs (MERIA) J. 6(4). [Google Scholar]
- Organization for the Prohibition of Chemical Weapons (OPCW) website www.opcw.org accessed March 30, 2018.
- Pan H, Piermartiri TC, Chen J, McDonough J, Oppel C, Driwech W, Winter K, McFarland E, Black K, Figueiredo T, Grunberg N, Marini AM. (2015) Repeated systemic administration of the nutraceutical alpha-linolenic acid exerts neuroprotective efficacy, an antidepressant effect and improves cognitive performance when given after soman exposure. Neurotoxicology. December;51:38–50. doi: 10.1016/j.neuro.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Pearson JN, Patel M. (2016) The role of oxidative stress in organophosphate and nerve agent toxicity. Ann N Y Acad Sci. August;1378(1):17–24. doi: 10.1111/nyas.13115. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. (2005) Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005. February;83(2):303–12 doi: 10.1093/toxsci/kfi023 [DOI] [PubMed] [Google Scholar]
- Pereira EF, Aracava Y, DeTolla LJ Jr, Beecham EJ, Basinger GW Jr, Wakayama EJ, Albuquerque EX. (2014). Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J Pharmacol Exp Ther. 350(2):313–321. doi: 10.1124/jpet.114.214932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris-Sampedro F, Salazar JG, Cabré M, Reverte I, Domingo JL, Sánchez-Santed F, Colomina MT. (2014) Impaired retention in AβPP Swedish mice six months after oral exposure to chlorpyrifos. Food Chem Toxicol. October;72:289–94. doi: 10.1016/j.fct.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Peris-Sampedro F, Basaure P, Reverte I, Cabré M, Domingo JL, Colomina MT. (2015) Chronic exposure to chlorpyrifos triggered body weight increase and memory impairment depending on human apoE polymorphisms in a targeted replacement mouse model. Physiol Behav. May 15;144:37–45. doi: 10.1016/j.physbeh.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Peris-Sampedro F, Reverte I, Basaure P, Cabré M, Domingo JL, Colomina MT. (2016) Apolipoprotein E (APOE) genotype and the pesticide chlorpyrifos modulate attention, motivation and impulsivity in female mice in the 5-choice serial reaction time task. Food Chem Toxicol. June;92:224–35. doi: 10.1016/j.fct.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Petersen A, Jensen BH, Andersen JH, Poulsen ME, Christensen T, Nielsen E. (2013). Pesticide Residues, Results from the period 2004–2011. Søborg: Danmarks Tekniske Universitet, Fødevareinstituttet [Google Scholar]
- Pope CN. (1999) Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit Rev. 1999. Apr-Jun;2(2):161–81. doi: 10.1080/109374099281205. Review. [DOI] [PubMed] [Google Scholar]
- Pope CN, Brimijoin S. (2018) Cholinesterases and the fine line between poison and remedy. Biochem Pharmacol July;153:205–216. doi: 10.1016/j.bcp.2018.01.044. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV Jr. (2007) Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007. April 25;146(1):330–9. doi: 10.1016/j.neuroscience.2007.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskocil BJ, Bruun DA, Thompson CM, Fryer AD, Lein PJ. (2010) Organophosphorus pesticides decrease M2 muscarinic receptor function in guinea pig airway nerves via indirect mechanisms. PLoS One. May 10;5(5):e10562. doi: 10.1371/journal.pone.0010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. (2001) Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol Appl Pharmacol. May 15;173(1):48–55. doi: 10.1006/taap.2001.9175 [DOI] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. (2002) Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett. September 5;135(1–2):89–93. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. (2006) Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol Appl Pharmacol. February 15;211(1):78–83 doi: 10.1016/j.taap.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Rao AN, Patil A, Brodnik ZD, Qiang L, España RA, Sullivan KA, Black MM, Baas PW. (2017) Pharmacologically increasing microtubule acetylation corrects stress-exacerbated effects of organophosphates on neurons. Traffic July;18(7):433–441. doi: 10.1111/tra.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DE, Richards PG (2001) The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol Lett 120(1–3):343–51. doi: 10.1016/S0378-4274(01)00266-1. Review. [DOI] [PubMed] [Google Scholar]
- Reardon S (2015). The Insurmountable Gulf. Nature, Vol 517 pg. 132–134. [DOI] [PubMed] [Google Scholar]
- Reichert BL, Abou-Donia MB. (1980) Inhibition of fast axoplasmic transport by delayed neurotoxic organophosphorus esters: a possible mode of action. Mol Pharmacol January;17(1):56–60 [PubMed] [Google Scholar]
- Reiss R, Chang ET, Richardson RJ, Goodman M. (2015). A review of epidemiologic studies of low-level exposures to organophosphorus insecticides in non-occupational populations, Critical Reviews in Toxicology, 45:7, 531–641, doi: 10.3109/10408444.2015.1043976. Review. [DOI] [PubMed] [Google Scholar]
- Richards PG, Johnson MK, Ray DE. (2000) Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol Pharmacol. September;58(3):577–83. [DOI] [PubMed] [Google Scholar]
- Richardson RJ. (1995) Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. J Toxicol Environ Health. February;44(2):135–65. doi: 10.1080/15287399509531952. Review. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ. (2011). Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology 32, 268–276. doi: 10.1016/j.neuro.2010.12.008. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K. (1991) Chronic central nervous system effects of acute organophosphate pesticide intoxication. The Pesticide Health Effects Study Group. Lancet. July 27;338(8761):223–7. [DOI] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. (2013) Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. January;43(1):21–44. doi: 10.3109/10408444.2012.738645. Review. [DOI] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO (2003). Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci 72:267–271. doi: 10.1093/toxsci/kfg034 [DOI] [PubMed] [Google Scholar]
- Sánchez-Santed F, Colomina MT, Herrero Hernández E. (2016) Organophosphate pesticide exposure and neurodegeneration. Cortex. January;74:417–26. doi: 10.1016/j.cortex.2015.10.003. Review. [DOI] [PubMed] [Google Scholar]
- Sarkar B, Dhiman M, Mittal S, Mantha AK. (2017) Curcumin revitalizes Amyloid beta (25–35)-induced and organophosphate pesticides pestered neurotoxicity in SH-SY5Y and IMR-32 cells via activation of APE1 and Nrf2. Metab Brain Dis. 2017. December;32(6):2045–2061. doi: 10.1007/s11011-017-0093-2. [DOI] [PubMed] [Google Scholar]
- Savage EP, Keefe TJ, Mounce LM, Heaton RK, Lewis JA, Burcar PJ. (1988) Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Environ Health. Jan-Feb;43(1):38–45. doi: 10.1080/00039896.1988.9934372 [DOI] [PubMed] [Google Scholar]
- Savy CY, Fitchett AE, Blain PG, Morris CM, Judge SJ (2018) Gene expression analysis reveals chronic low level exposure to the pesticide diazinon affects psychological disorders gene sets in the adult rat. Toxicology. 2018. January 15;393:90–101. doi: 10.1016/j.tox.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Justinova Z, Lafleur D, Woods D, Roschke V, Hallak H, Sklair-Tavron L, Redhi GH, Yasar S, Bergman J, Goldberg SR. (2013) Modification of pharmacokinetic and abuse-related effects of cocaine by human-derived cocaine hydrolase in monkeys. Addict Biol. Jan;18(1):30–9. doi: 10.1111/j.1369-1600.2011.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor JL. (1992) Fate of Pesticides and Chemicals in the Environment John Wiley, New York. [Google Scholar]
- Sellström A, Cairns S and Barbeschi M (2013) United nations mission to investigate allegations of the use of chemical weapons in the Syrian Arab Republic. Final Report 12. [Google Scholar]
- Singh S, Sharma N. (2000) Neurological syndromes following organophosphate poisoning. Neurol India 48:308–313. Review [PubMed] [Google Scholar]
- Slotkin TA. (2004). Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol, 198, pp. 132–151. doi: 10.1016/j.taap.2003.06.001. Review. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. (2005) Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos In: Gupta RC , editor. Toxicity of organophosphate and carbamate pesticides. San Diego: Elsevier Academic Press; p. 293–314. [Google Scholar]
- Smout A, Holden M. (2018) UK’s May says ‘highly likely’ Russia behind nerve attack on spy. Reuters March 12, 2018. https://www.reuters.com/article/us-britain-russia/uks-may-says-highly-likely-russia-behind-nerve-attack-on-spy-idUSKCN1GO0MS Assessed March 22, 2018.
- Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM, Giesy JP. (2014) Properties and uses of chlorpyrifos in the United States. Rev Environ Contam Toxicol. 2014;231:13–34. doi: 10.1007/978-3-319-03865-0_2. Review. [DOI] [PubMed] [Google Scholar]
- Soltaninejad K, Abdollahi M. (2009) Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit. March;15(3):RA75–90. Review. [PubMed] [Google Scholar]
- Soltaninejad K, Shadnia S (2014) History of the Use and Epidemiology of Organophosphorus Poisoning In: Balali-Mood M, Abdollahi M. (eds) Basic and Clinical Toxicology of Organophosphorus Compounds. Springer, London [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. (1997) Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. July;145(1):158–74 doi: 10.1006/taap.1997.8171 [DOI] [PubMed] [Google Scholar]
- Song F, Zou C, Han X, Zeng T, Zhang C, Xie K. (2012) Reduction of retrograde axonal transport associated-proteins motor proteins, dynein and dynactin in the spinal cord and cerebral cortex of hens by tri-ortho-cresyl phosphate (TOCP). Neurochem Int. January;60(2):99–104. doi: 10.1016/j.neuint.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Steenland K, Jenkins B, Ames RG, O’Malley M, Chrislip D, Russo J. (1994) Chronic neurological sequelae to organophosphate pesticide poisoning. Am.J.Public Health 84; 731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. (1995) Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet 345:1135–1139. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Bradford W, Stone C, Thompson TA, Heeren T, White RF. (2018) Neuropsychological functioning in military pesticide applicators from the Gulf War: Effects on information processing speed, attention and visual memory. Neurotoxicol Teratol. Jan-Feb;65:1–13. doi: 10.1016/j.ntt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Morita H, Ono K, Maekawa K, Nagai R, Yazaki Y (1995) Sarin Poisoning in Tokyo subway. Lancet 345 (8955):980. [PubMed] [Google Scholar]
- Terry AV Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. (2003) Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther. April;305(1):375–84. doi: 10.1124/jpet.102.041897 [DOI] [PubMed] [Google Scholar]
- Terry AV Jr, Gearhart DA, Beck WD Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. (2007) Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther. September;322(3):1117–28. doi: 10.1124/jpet.107.125625 [DOI] [PubMed] [Google Scholar]
- Terry AV Jr, Buccafusco JJ, Gearhart DA, Beck WD, Middlemore-Risher ML, Truan JN, Schwarz GM, Xu M, Barlett MG, Kutiyanawala A, Pillai A. (2011) Repeated, intermittent exposures to diisopropylfluorophosphate in rats: protracted effects on cholinergic markers, nerve growth factor-related proteins, and cognitive function. Neuroscience 10; 176:237–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV Jr. (2012) Functional Consequences of Repeated Organophosphate Exposure: Potential Non-Cholinergic Mechanisms. Pharmacology & Therapeutics 134:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. (2008). Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide diazinon. Neurotoxicol Teratol, 30, pp. 38–45 doi: 10.1016/j.ntt.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O, Levin E. (2011). Glutamate and nicotinic receptor interactions in working memory: Importance for the cognitive impairment of schizophrenia. Neuroscience. 195 21–36. 10.1016/j.neuroscience.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Tsitsimpikou C, Tzatzarakis M, Fragkiadaki P, Kovatsi L, Stivaktakis P, Kalogeraki A, Kouretas D, Tsatsakis AM. (2013) Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology. May 10;307:109–14. doi: 10.1016/j.tox.2012.11.002 [DOI] [PubMed] [Google Scholar]
- Vanova N, Pejchal J, Herman D, Dlabkova A, Jun D. (2018) Oxidative stress in organophosphate poisoning: role of standard antidotal therapy. J Appl Toxicol. 2018. August;38(8):1058–1070. doi: 10.1002/jat.3605. Review. [DOI] [PubMed] [Google Scholar]
- Viviani B, Boraso M, Marchetti N, Marinovich M. (2014) Perspectives on neuroinflammation and excitotoxicity: a neurotoxic conspiracy? Neurotoxicology. July;43:10–20. doi: 10.1016/j.neuro.2014.03.004. Review. [DOI] [PubMed] [Google Scholar]
- Voorhees JR, Rohlman DS, Lein PJ, Pieper AA. (2017) Neurotoxicity in Preclinical Models of Occupational Exposure to Organophosphorus Compounds. Front Neurosci. January 18;10:590. doi: 10.3389/fnins.2016.00590. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. January;10(1):57–63. doi: 10.1038/nrg2484. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani WY, Gudup S, Sunkaria A, Bal A, Singh PP, Kandimalla RJ, Sharma DR, Gill KD. (2011) Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology. Dec;61(8):1193–201. doi: 10.1016/j.neuropharm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, Atieli HE, Zhou G, Githeko AK, Yan G. (2015) Pyrethroid and DDT Resistance and Organophosphate Susceptibility among Anopheles spp. Mosquitoes, Western Kenya. Emerg Infect Dis. 2015. December;21(12):2178–81. doi: 10.3201/eid2112.150814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R (2016) Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex January;74:449–75. doi: 10.1016/j.cortex.2015.08.022. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder C, Balouet JC. (2002) The toxicity of commercial jet oils. Environ Res. June;89(2):146–64. Review. [DOI] [PubMed] [Google Scholar]
- Winkenwerder W (2003). Environmental Exposure Report: Pesticides Final Report. Washington, DC: U.S. Department of Defense, Office of the Special Assistant to the Undersecretary of Defense (Personnel and Readiness) for Gulf War Illnesses Medical Readiness and Military Deployments. [Google Scholar]
- Worek F, Thiermann H, Wille T. (2016) Oximes in organophosphate poisoning: 60 years of hope and despair. Chem Biol Interact. November 25;259(Pt B):93–98. doi: 10.1016/j.cbi.2016.04.032. [DOI] [PubMed] [Google Scholar]
- World malaria report 2017. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
- Yan D, Zhang Y, Liu L, Yan H. (2016) Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep. September 1;6:32222. doi: 10.1038/srep32222. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. (2008) Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008. April 1;228(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL, Lein PJ. (2011) Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci. May;121(1):146–59. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Naughton SX, Han Z, He M, Zheng YG, Terry AV Jr., Bartlett MG. (2018) Mass Spectrometric Quantitation of Tubulin Acetylation from Pepsin-Digested Rat Brain Tissue Using a Novel Stable-Isotope Standard and Capture by Anti-Peptide Antibody (SISCAPA) Method. Anal Chem February 6;90(3):2155–2163. doi: 10.1021/acs.analchem.7b04484. [DOI] [PubMed] [Google Scholar]
- Zaganas I, Kapetanaki S, Mastorodemos V, Kanavouras K, Colosio C, Wilks MF, Tsatsakis AM. (2013) Linking pesticide exposure and dementia: what is the evidence? Toxicology. May 10;307:3–11. doi: 10.1016/j.tox.2013.02.002. Review. [DOI] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB 3rd, Lee VM, Brunden KR. (2012) The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. March 14;32(11):3601–11. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Jain P, Tsao C, Sinclair A, Sun F, Hung HC, Bai T, Wu K, Jiang S. (2016) Butyrylcholinesterase nanocapsule as a long circulating bioscavenger with reduced immune response. J Control Release. May 28;230:73–8. doi: 10.1016/j.jconrel.2016.04.008. [DOI] [PubMed] [Google Scholar]