Abstract
The prevalence of refractive error and ocular disorders among infants and young children with severe behavioral problems and developmental disorders is not well defined, particularly in developing countries. We performed a retrospective review of ophthalmic examinations performed during a National Institutes of Health-funded cohort study of very young children in Peru with behavioral problems and at risk for developmental disorders. 222 children between the ages of 0 and 4 years (mean 2.2 ± 0.9 years) were examined and 100 (45.0%) had an abnormal ocular exam. Overall, the prevalence of refractive error was 33.3%, nystagmus was 12.2%, and strabismus was 10.9%. Among children with Down syndrome, refractive error ranged from 46.2% at age 2 to 85.7% at age 4. Refractive error and ocular disorders are highly prevalent even at a young age in children with behavioral problems and developmental disorders. Much of the visual impairment in this population is treatable; early identification and intervention can have a lifelong positive impact on neurodevelopment.
Keywords: vision impairment, behavioral disorder, refractive error, Down syndrome, autism spectrum disorder
INTRODUCTION
Intellectual and developmental disorders (IDD) affect an estimated 3-6% of the population worldwide and are twice as prevalent in low-income countries as compared to high-income countries (Lai, Tseng, Hou, & Guo, 2012; Maulik, Mascarenhas, Mathers, Dua, & Saxena, 2011). Adults with IDD have a significantly higher rate of refractive error and ocular disorders compared to the general population (Mackie et al., 1998; van Splunder, Stilma, Bernsen, & Evenhuis, 2004). Similarly, ocular disorders occur at twice the rate in children with IDD than as children without ocular disorders (Akinci et al., 2008). Children with Down syndrome (DS), for instance, have particularly elevated rates of ocular pathology: refractive error 30-62%, strabismus 3-57%, astigmatism 6-60%, and nystagmus 3-33% (Creavin & Brown, 2009).
Most visual impairment (VI) studies on individuals with IDD have been performed in adults and for specific developmental disorders only, such as Down syndrome. Studies on children with visual impairment are often limited to schools for the blind (C. E. Gilbert, Canovas, Hagan, Rao, & Foster, 1993; C. E. Gilbert, Canovas, Kocksch de Canovas, & Foster, 1994; C. Gilbert, Rahi, Eckstein, & Foster, 2009; Gogate et al., 2011; Limburg, Gilbert, Hon, Dung, & Hoang, 2012). Hence, we know the association of IDD with etiologies of severe pediatric vision impairment, but little about the prevalence in young children, particularly children with behavioral or developmental disorders. Furthermore, there are few studies on children before they reach school-age, particularly in developing countries.
Early detection of VI in children is critical for effective intervention and the prevention of amblyopia. Visual pathway formation is most active in the first years of life and it is well known that early treatment of ocular disorders and the correction of refractive error helps children reach their developmental goals (Reynell, 1978; Sonksen & Dale, 2002). Unfortunately, many children do not receive proper vision screening until they are school-aged. Children with ocular disorders and IDD benefit from timely referral to early interventionists and education specialists (Hatton, Ivy, & Boyer, 2013). The United Kingdom Down Syndrome Medical Interest Group (DSMIG) recommends a comprehensive eye exam by age 3 and found that earlier screening led to a statistically significant increase in earlier treatment (Stephen, Dickson, Kindley, Scott, & Charleton, 2007). In the United States, experts recommend that children with Down syndrome have an ophthalmologic exam by age 6 months because of a high rate of undetected refractive and accommodative abnormalities (Roizen, Mets, & Blondis, 1994).
As compared to developed countries, where surveillance and screening measures are in place, epidemiological studies of visual impairment in children with IDD in Latin America are limited (Furtado et al., 2012; Mercadante, Evans-Lacko, & Paula, 2009). Peru is representative of many middle-income countries. There are limited resources for children with IDD, unknown prevalence of disorders, and the concentration of medical and educational resources are in the capital city. Understanding the prevalence of ocular disorders in children with IDD enables the appropriation of public health resources and instruction of care providers on the importance of early ocular examination and interventions when necessary.
We sought to utilize a large sample of young children with IDD and aberrant behavior in order to identify the prevalence of visual impairment and ocular disorders in this population. The study sample consisted of the National Institutes of Health (NIH)-funded cohort “Early Prevention of Aberrant Behavior in Neurodevelopmental Disorders in Peru” at the Centro del Ann Sullivan del Peru (CASP), an internationally recognized educational facility for children with neurodevelopmental disabilities in Lima, Peru.
MATERIALS AND METHODS
Design
A retrospective review of ophthalmic data collected on children enrolled in the National Institutes of Health (NIH) - Fogarty International Center study “Early Prevention of Aberrant Behavior in Neurodevelopmental Disorders in Peru” (Grant No. HD 060500) was conducted. Institutional Review Board (IRB) approval was granted by the respective Peruvian and American institutions associated with the project (Centro Ann Sullivan del Peru, University of Kansas, and Duke University).
Population
The NIH “Early Prevention of Aberrant Behavior in Neurodevelopmental Disorders in Peru” study was conducted at the Centro Ann Sullivan del Peru (CASP) in Lima, Peru between September and December, 2010. A full description of the enrollment protocol, study population, and non-ophthalmic study findings have previously been reported (Mayo-Ortega et al., 2012; Schroeder et al., 2013, 2014). In summary, patients were recruited through a nationwide mass media campaign involving TV, radio, and newspaper advertisements. Parents who were concerned their child had a severe behavioral problem, such as stereotyped behavior, self-injury, or aggression, or a developmental disorder, such as Down syndrome, autism, or intellectual disability that might put that child at risk for behavioral problems, were invited to call the study coordinators at CASP.
Approximately 1,000 concerned parents called and an initial 341 were given an appointment to be screened in person with the Parental Concerns Questionnaire, which was designed specifically for the study using risk factors for severe behavioral problems identified in previous studies (Mayo-Ortega et al., 2012). This questionnaire was later validated via comparison with formal testing using the Repetitive Behavior Scale-Revised (RBS-R), Aberrant Behavior Checklist (ABC), and Behavior Problem Inventory(BPI-01) (Schroeder et al., 2013). Of the 341 parents interviewed, 262 had responses indicating concern for behavioral or developmental disorders and were invited to participate in the study.
There were 262 children enrolled in the cohort study, “Early Prevention of Aberrant Behavior in Neurodevelopmental Disorders in Peru” and 222 children received a comprehensive eye examination. As the eye exam was an optional component of the screening day, there are no data on why some parents did not elect to have their children examined. Anecdotal reports include parents not enrolling their children for belief their child was normally sighted and for already being under treatment of a known ocular disorder. The mean age at examination was 2.2 ± 0.9 years (range 0-4 years) of whom 64.9% were male and 35.1% were female. A family history of a neurodevelopmental disorder was reported in 61.1% of children and a family history of eye disease in 60.9%.
Cognitive delay, as measured by the Bayley Scales of Infant Development III, was present in 90% of children. The most common developmental disorder was DS (26.9%), followed by autism spectrum disorder (ASD) (21.2%), global developmental delay (17.3%), and language delay (16.3%). Children with DS were on average younger and smaller than the rest of the group, were born of older mothers, and had lower Bayley scores (Table 1). They were also commonly the second or later child, while most children with ASD or other disorders were first born. Autism spectrum disorder was a clinical diagnosis as previously described (Mayo-Ortega et al., 2012), and 90.9% of children suspected of having an ASD scored moderate or severe (>30) on the Childhood Autism Rating Scale (CARS). Overall, half the children were not enrolled in any school program and 6.5% attended a school for the blind.
Table 1:
Patient Demographics by Developmental Disorder
| Mean (SD) | |||||||
|---|---|---|---|---|---|---|---|
| Down Syndrome (n = 56) | Autism Spectrum Disorder (n = 44) | Global Developmental Delay (n = 36) | Language Delay (n = 34) | Other (n = 52) | Total (n = 222) | P-Value | |
| Age (years) | 1.86 (1.02) | 2.72 (0.71) | 2.37(0.84) | 2.60(0.70) | 1.95(0.82) | 2.19(0.93) | ANOVA 0.000 |
| Female | 51.8% | 11.4% | 44.4% | 32.4% | 34.6% | 26.9% | Fischer’s Exact 0.000 |
| Height (cm) | 78.13(9.9) | 93.56(9.89) | 87.53(8.24) | 93.09(8.35) | 83.93(9.58) | 87.50(11.25) | ANOVA 0.000 |
| Weight (kg) | 10.7(2.94) | 16.68(2.96) | 15.78(17.23) | 20.73(32.68) | 12.31(2.68) | 15.40(14.57) | ANOVA 0.018 |
| Head Circumference (cm) | 44.8(4.53) | 50.6(2.32) | 47.81(3.33) | 49.22(5.72) | 46.81(4.85) | 47.58(6.96) | ANOVA 0.000 |
| Gestational Age (weeks) | 37.69(2.57) | 38.18(2.68) | 36.97(4.56) | 37.68(3.69) | 38.00(2.85) | 37.04(4.69) | ANOVA 0.556 |
| Birth Weight (kg) | 3.07(0.58) | 3.33(0.53) | 2.89(0.67) | 3.15(0.68) | 3.15(0.69) | 3.15(0.73) | ANOVA 0.039 |
| Maternal Age (years) | 35.56(5.98) | 30.07(6.31) | 29.22(6.78) | 29.64(5.98) | 28.73(6.99) | 28.69(7.04) | ANOVA 0.000 |
| Birth Order | 2.38(1.42) | 1.53(0.83) | 1.94(1.08) | 1.97(0.97) | 1.53(0.78) | 1.89(1.30) | ANOVA 0.000 |
| Bayley Score | 39.24(12.6) | 58.09(10.5) | 49.33(16) | 60.67(9.58) | 49.44(14.41) | 53.18(14.16) | ANOVA 0.000 |
Procedures
All children underwent a battery of pediatric, neurologic, psychosocial, and behavior exams performed by trained professionals on mass-screening days as previously described (Mayo-Ortega et al., 2012). Each child had a full medical history taken, including gestational health, maternal, family, and educational histories, measurements including height, weight, and head circumference, and testing with the Bayley Scales of Infant Development (BSID-III) and, if ASD was suspected, the Childhood Autism Rating Scale (CARS) was included. A comprehensive ophthalmic examination was offered to all children on the same day, but was not mandatory and the data collected were not part of the original NIH-Fogarty study. CASP has a long history of collaborating with international ophthalmologists and providing eye exams for its students. Knowing that many children in the study would likely have never had an eye exam, two Peruvian and one American ophthalmologist experienced in the examination of children with IDD volunteered to exam them.
The ophthalmic examination consisted of visual acuity measured by preferential looking (LEA GRATING acuity), a comprehensive dilated eye exam, portable slit lamp examination, oculomotor evaluation, retinal and optic nerve examination by indirect ophthalmoscopy, and cycloplegic refraction. Binocular acuity measurements were performed, as a measure of overall visual function, by two trained technicians at a distance of 60 cm with LEA GRATING acuity (Good-Lite, US), a preferential looking test. All children were examined in the presence of their parents or guardians and without the use of anesthesia. The examination findings were recorded on paper ophthalmic forms.
A follow-up ophthalmic examination was performed at one year on selected children, who were then classified into one of four visual function categories:
Normal visual function. The child has refraction within normal range, normal function, does not need any interventions directly for use of vision or has very mild refractive errors and the child could function without glasses.
Visual impairment that can be improved. The child has significant abnormalities on eye exam either refractive, oculomotor, or ocular pathology. Can be improved with glasses, surgery, or medication. If proper interventions not instituted, will continue with visual impairment. More than likely vision will be child’s main learning media.
Severe visual impairment that more than likely cannot be improved with glasses, surgery, or other means. Vision may not be available as main learning media, but useful for mobility and some functioning with assistance in the environment.
Blind. No functional vision. Vision not available as a learning media or useful for mobility. Child will need to learn skills associated with positive outcomes in persons who are blind.
Statistical Analysis and Definitions
The chart review and data entry were performed by a medical professional with a reliability observer and 10% resample rate. All statistical analysis was performed using STATA 12 (StataCorp, College Station, Texas, USA). Data analysis included Pearson’s chi-square comparative analysis of proportions, t-test for difference in means, analysis of variance (ANOVA), and single and multiple logistic regression.
Refractive error as measured by cycloplegic examination was defined for myopia as less than or equal to −0.50 diopters (D), hyperopia as greater than or equal to +2.0D, astigmatism as greater than or equal to 1.0D, and anisometropia as greater than a 1.5D difference between eyes in hyperopes and 3.0D difference in myopes. The thresholds for refractive error were based on previous studies (Akinci et al., 2008; Nepal, Koirala, Adhikary, & Sharma, 2003; Ying et al., 2014).
Age-appropriate acuity levels for LEA GRATINGs in children with developmental disorders have not been published. Cutoffs for pre-verbal children can be used as an estimate (Martini, Netto, Morcillo, Gagliardo, & de Oliveira, 2014; Mody et al., 2012). We used the lower confidence interval, two standard deviations below the mean, in these studies to provide a minimal framework for statistical analysis of a “passing” level of cycles per centimeter (CPCM): < 2 months - 0.5CPCM, < 4 months - 1.0CPCM, < 6 months - 2CPCM, and > 6 months - 4CPCM.
RESULTS
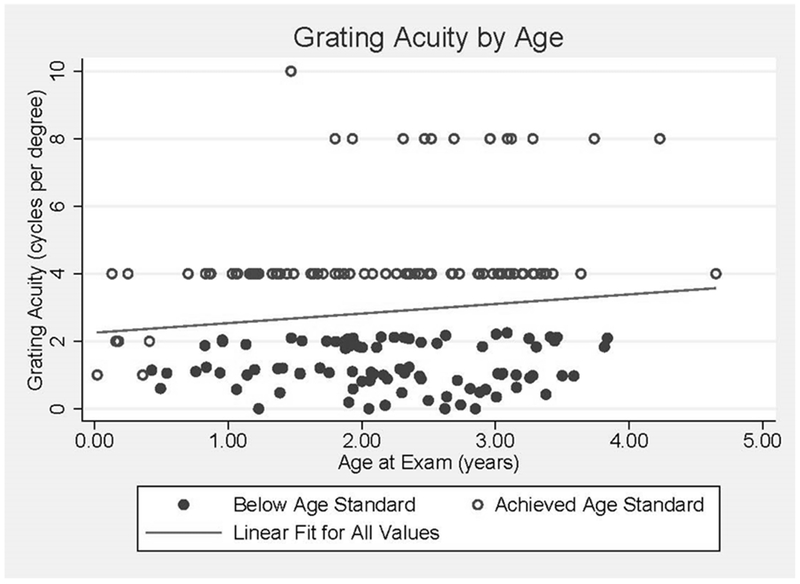
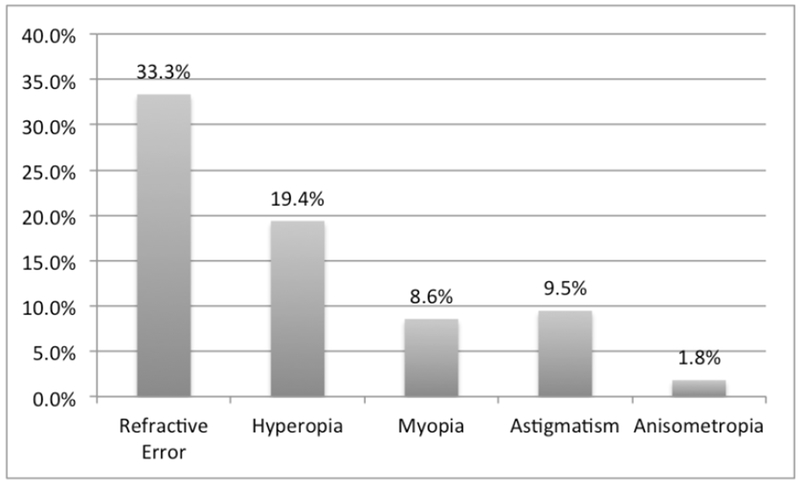
Grating acuity was able to be measured in 172 (80.6%) children. The mean overall LEA GRATING acuity was 2.9 ± 1.7 CPCM and varied by age (Table 2). Overall, 90 (50.6%) children passed a minimal age-standard (Figure 1). A complete dilated eye exam was performed on 203 of 222 (91.4%) children. The remaining 19 children had incomplete examinations due to limitation in cooperation with the exam, administration of eye drops, or time constraints. Refractive error was found in 74 children (33.3%), of whom hyperopia was the most common diagnosis: 58.1% of cases of refractive error and 19.4% of all children (Figure 2). Anisometropia, a significant refractive difference between the eyes and a risk factor for amblyopia, was found in 1.8% of children. Glasses were prescribed to 36 children (16.2%). Patching to treat amblyopia was recommended for 4 children (1.8%).
Table 2:
Grating Acuity by Age
| Age (Years) | CPCM (Mean ± Std Dev) |
|---|---|
| <1 | 2.3 ± 1.2 |
| 1-1.99 | 2.9 ± 1.6 |
| 2-2.99 | 2.7 ± 1.8 |
| 3-3.99 | 3.2 ± 1.7 |
| >=4 | 6.0 ± 2.0 |
| Overall | 2.9 ± 1.7 |
Abbreviation: CPCM (cycles per centimeter)
Figure 1:
Visual Acuity (LEA GRATING Paddles) by Age, All Children
Figure 2:
Prevalence and Form of Refractive Error Among All Children
Strabismus was found in 10.9% of children. Esotropia was the more common diagnosis (21 children, 9.5%) compared to exotropia (3 children, 1.4%). The type of esotropia (e.g. accommodative estotropia) was not recorded. Nystagmus was present in 27 children (12.2%). There were three cases of retinopathy of prematurity (ROP). Optic nerve abnormalities, including atrophy (ONA) and optic nerve hypoplasia (ONH), were present in 10 children (4.5%). Cerebral vision impairment (CVI) was present in 10 (4.5%) children (Figure 3). A clinical diagnosis of CVI was recorded for children whose visual impairments could not be explained by their ocular examination alone, and had a history compatible with CVI (Dutton, 2013). Overall, 100 (45.0%) children had a refractive error or an ocular disorder. Follow-up recommendations were recorded for 79 children of whom 50.6% of children were recommended to follow-up with an ophthalmologist within 6 months.
Figure 3: Prevalence of Ocular Disorders Among All Children.
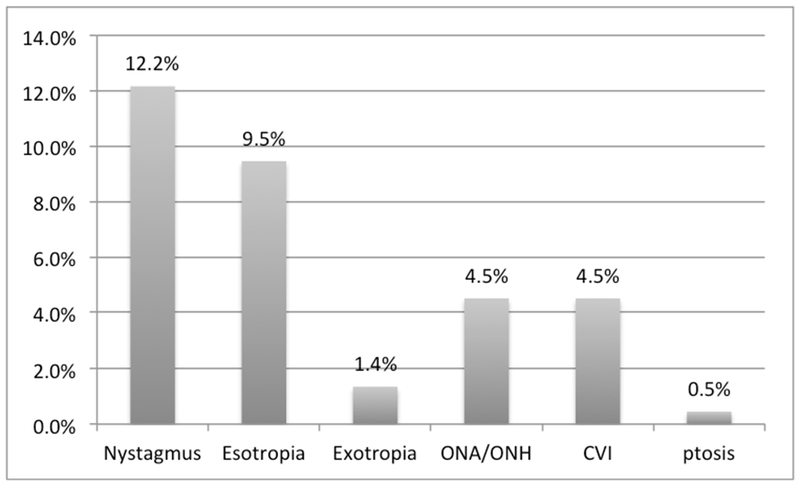
Abbreviations: ONA (optic nerve atrophy), ONH (optic nerve hypoplasia), CVI (cerebral vision impairment).
In comparing the two largest clinical diagnosis groups, DS and ASD, the prevalence of ocular conditions was markedly different (Figure 4). Over two-thirds of children with DS had an ocular condition (73.2%) compared to 22.7% in children with suspected ASD (p < .0001). Refractive error was reported in 31 (55.5%) children with DS and 7 (15.9%) children with suspected ASD (p < .0001). The most common diagnosis was hyperopia: 19 (33.9%) children with DS and 5 (11.4%) children with suspected ASD (p = .0087). Nystagmus was found in 17 (30.3%) children with DS and in no child suspected of ASD (p < .0001). The prevalence of refractive error in children with DS under 12 months was 46.2% and increased to 87.5% by age 3.
Figure 4: Refractive Error and Ocular Disorders by Diagnosis.
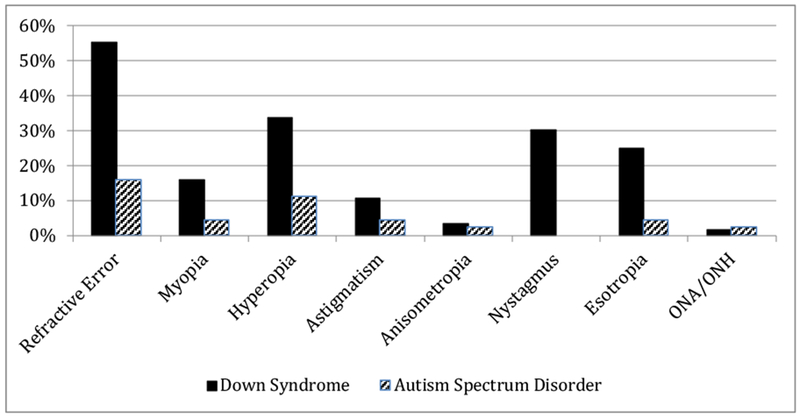
Abbreviation: ONA (optic nerve atrophy), ONH (optic nerve hypoplasia), CVI (cerebral vision impairment); There were no cases of exotropia, CVI, or ptosis.
In simple logistic regression of baseline demographics and clinical measurements on the outcome of refractive error, only the Bayley developmental score was found to be associated with refractive error. There was an inverse relationship of greater odds of refractive error with lower Bayley score (p = .018). However, after adjustment for clinical diagnosis, Bayley score was no longer associated with refractive error (p =.667).
A follow-up exam at one year was performed in 148 children. Classification of children into a visual impairment scale (1-4), demonstrated that 20.3% of children had mild VI, 5.4% moderate VI, and 7.4% severe VI. Children with Down syndrome had a high rate of mild VI (51.3%), children with suspected ASD were mostly normal (91.2%) and the remaining children had the highest rate of moderate (9.3%) and severe (13.3%) VI (Table 3).
Table 3:
Visual Impairment Scale by Developmental Disorder
| Visual Impairment Scale | Down Syndrome | Autism | Other | All |
|---|---|---|---|---|
| 1 | 18 | 31 | 50 | 99 |
| 2 | 20 | 2 | 8 | 30 |
| 3 | 1 | 0 | 7 | 8 |
| 4 | 0 | 1 | 10 | 11 |
| Total | 39 | 34 | 75 | 148 |
DISCUSSION
This is the first study, of which we are aware, to report the prevalence of refractive error and ocular disorders in infants and young children with severe behavioral disorders and developmental disabilities in Latin America. Examining this patient population is difficult and it is rare to be able to study so many children at one time. Previous studies have reported on the significant visual impairment found at older ages when neuro-ophthalmic development is complete. Early diagnosis is essential for early intervention. Behavioral research has shown the effect of vision impairment on early development is profound and by the first year of life visually handicapped children already begin to developmentally fall behind similar sighted children (Reynell, 1978; Sonksen & Dale, 2002).
The mean age of participants in our study was 2.2 years and few participants had ever before had an eye exam; however over 90% of children were able to complete a dilated eye exam. We found 45% of children under the age of 4 had refractive error or a diagnosable ocular disorder. Similarly, in the United Kingdom, among a cohort of 439 children with severe visual impairment, 65% were diagnosed at younger than 1 year of age, and in Turkey 77% of children with IDD had an ocular disorder (Rahi, Cumberland, Peckham, & Group, 2010; Akinci et al., 2008).
The only comparable studies in Latin America were among older children enrolled in schools for the blind, which intrinsically have an elevated prevalence of severe eye disease (C. E. Gilbert et al., 1993, 1994). Our study sampled a population from a nationwide media campaign and provides a more representative sample of eye disease in young children with behavioral problems and developmental disorders. However, it was not a cross-sectional study and we cannot generalize our results to estimate a country wide prevalence. Our study is most resembling of the population that may be cared for by a pediatrician, neurologist, family practitioner, or early interventionist and for whom it is important to be aware of the frequent comorbid visual impairment with systemic disorders.
In our study, 1in 3 children presented with refractive error. Our population of infants and children had predominately hyperopic refractive error. Hyperopia is more common in infants. For comparison, the population based “Refractive Error in Childhood” study in Chile found the prevalence of hyperopia (> 2.0D) in 5-year-olds to be 24.7% and myopia (< −0.5D) 3.4% (Maul, Barroso, Munoz, Sperduto, & Ellwein, 2000). In the Vision in Preschoolers Study (USA), hyperopia (>3.25D) ranged from 5.5% in Asians to 11.9% in non-Hispanic whites (Ying et al., 2014).
Children with intellectual disability also have higher rates of strabismus, nystagmus, and amblyopia (Akinci et al., 2008; Erkkilä, Lindberg, & Kallio, 1996; Kang & Kim, 2010). The most common ocular conditions in our study were strabismus (10.9%) and nystagmus (12.2%). CVI and optic nerve abnormalities were each present in 4.5% of children. Our results are in line with studies of older children from India, Nepal, and Korea (Ghising et al., 2007; Gogate et al., 2011; Kang & Kim, 2010), highlighting that these conditions are both present and diagnosable at a younger age.
Children with syndromic causes of IDD have even a higher prevalence of nystagmus, strabismus, and refractive error compared to children with non-syndromic IDD (Akinci et al., 2008). In particular, children with DS are known to have a high rate of refractive error and strabismus (Creavin & Brown, 2009; Stephen et al., 2007). In our study, children with DS had the highest rate of both refractive error and ocular disorders. The prevalence of refractive error in children with DS is reported to double from infancy to childhood (Mackie et al., 1998; Roizen et al., 1994). We also found over half of children with DS had refractive error and the prevalence increased to 87.5% by age 3.
Grating acuity or preferential looking tests are effective in screening nonverbal and preverbal children and are frequently used with children with IDD (Mayer, Fulton, & Hansen, 1982). Age appropriate estimates using LEA GRATING acuity are suggested in the literature for Brazilian infants up to 4 months (Martini et al., 2014) and Indian children up to 3 years (Mody et al., 2012). We used these guidelines to determine that only 50.6% reached the lower end of an age appropriate cutoff. Any implication on overall visual impairment should take into account the complete ophthalmic exam. Nevertheless, we found 80% can successfully complete this exam. Grating acuity, although not a stand-alone screening device, is an excellent tool for assessing visual function and monitoring response to therapy in young children with IDD.
This is one of the largest samples of very young children with IDD ever screened for ophthalmic disorders and the first, to our knowledge, to look specifically at children with behavioral problems, providing a rare estimate of the burden of disease in this population. Additionally, these results contribute to the relatively sparse data on children of any age with IDD in Latin America. Our results highlight the importance of screening. We found that even in a young age group, there was an extremely high prevalence of eye disease, including refractive error, nystagmus, and strabismus and the risk for amblyopia. It is well known that early identification and treatment can prevent permanent vision loss and disability. We recommend that any child newly diagnosed with a IDD, regardless of age, be screened for ocular disorders and VI. Middle and developing countries, like Peru, need additional trained screeners, ophthalmologists and early intervention specialists experienced with children with visual impairment and IDD. Training in this area is widely needed and could benefit from international collaboration.
Interventions in the study cohort were provided to maximize use of vision depending on the diagnosis. Local Peruvian pediatric ophthalmologists agreed to provide follow-up and children were given glasses prescriptions, amblyopia therapy instituted, and surgery scheduled if needed. Follow-up appointments to monitor response to amblyopia treatment are a critical component of visual rehabilitation and providing access to care and coordination with other care providers essential. Low vision rehabilitation specialists and early intervention specialists need to become an integral part of the care team and with emphasis on maximizing quality of vision and visual functioning to enhance developmental outcomes. Visual function and ocular health should be evaluated in any child with aberrant behaviors or neuro-developmental disabilities. This is often recommended, but rarely followed up in practice.
There are limitations to our study and possible sources of bias that could affect internal and external validity. Even in an optimal setting, examination of very young children with developmental disabilities is a challenging task and measurements can vary on the day and disposition of the child, particularly in a cohort with behavioral problems. As a retrospective review, our data are limited to what was recorded at the time of the initial cohort study. Forty children in the cohort study did not receive an eye exam for reasons unknown and 79 of the 222 children did not have a recorded follow-up examination. Our sample had a relatively high percentage of children with DS, a group known to frequently have ocular disorders, and this may have skewed the prevalence in the overall group. Also, the study population was screened for children with behavioral problems with developmental disorders, a group at risk of ocular self-injury (Collacott, Cooper, Branford, & McGrother, 1998). However, there was no evidence of ocular self-injury as the cause of ocular pathology in our study. Another concern was that children with severe vision impairment could be mistaken by their parents to have aberrant behavior when their behavior was actually due to visual impairment. Our specialist examination and battery of neurodevelopmental tests, however, found that nearly every child had some degree of IDD.
Further research and attention is needed on children with IDD and VI. In particular, studies of visual impairment and interventions in children with problem behavior are needed to help us understand how vision impairment is a factor in the display of those aberrant behaviors and how interventions can impact behavior. Outcome studies for interventions in children with visual impairment and IDD are sparse. Unfortunately, there are no published tools to measure the outcomes of ophthalmic interventions in this population. Such tools are needed so that we can study how early interventions affect the long-term visual outcomes, developmental outcomes, and quality of life measures for the child and family.
In summary, in this descriptive cohort of infants and young children with severe behavioral problems and developmental disorders, we report a high prevalence of refractive error and ocular disorders. Nearly, half of the children in the cohort are affected and one in eight have moderate to severe visual impairment. This population needs early intervention services as well as close ophthalmic follow-up. It is of the upmost importance that very young children with IDD be referred for a comprehensive eye exam. Awareness of the burden of ocular disorders in this population will hopefully bring attention to this underserved group and further research in the area.
Acknowledgments:
Joseph Zunt MD, MPH for mentorship and critical revision of manuscript
Supported by the Fogarty International Research Grant No. HD 060500
REFERENCES
- Akinci A, Oner O, Bozkurt OH, Guven A, Degerliyurt A, & Munir K (2008). Refractive errors and ocular findings in children with intellectual disability: A controlled study. Journal of American Association for Pediatric Ophthalmology and Strabismus, 12(5), 477–481. 10.1016/j.jaapos.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collacott RA, Cooper SA, Branford D, & McGrother C (1998). Epidemiology of self-injurious behaviour in adults with learning disabilities. The British Journal of Psychiatry, 173(5), 428–432. 10.1192/bjp.173.5.428 [DOI] [PubMed] [Google Scholar]
- Creavin AL, & Brown RD (2009). Ophthalmic abnormalities in children with Down syndrome. Journal of Pediatric Ophthalmology and Strabismus, 46(2), 76–82. [DOI] [PubMed] [Google Scholar]
- Dutton GN (2013). The spectrum of cerebral visual impairment as a sequel to premature birth: an overview. Documenta Ophthalmologica, 127(1), 69–78. 10.1007/s10633-013-9382-1 [DOI] [PubMed] [Google Scholar]
- Emerson E, & Robertson J (2011). The Estimated Prevalence of Visual Impairment among People with Learning Disabilities in the UK Improving Health and Lives: Learning Disabilities Observatory Report for RNIB and SeeAbility. [Google Scholar]
- Erkkilä H, Lindberg L, & Kallio AK (1996). Strabismus in children with cerebral palsy. Acta Ophthalmologica Scandinavica,74(6), 636–638. [DOI] [PubMed] [Google Scholar]
- Furtado JM, Lansingh VC, Carter MJ, Milanese MF, Peña BN, Ghersi HA, … Silva JC (2012). Causes of Blindness and Visual Impairment in Latin America. Survey of Ophthalmology, 57(2), 149–177. 10.1016/j.survophthal.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Ghising R, Shakya S, Rizyal A, Shrestha R, Shrestha S, & Wang-Harris S (2007). Prevalence of refractive error in mentally retarded students of Kathmandu Valley. Nepal Medical College Journal, 9(4), 262–265. [PubMed] [Google Scholar]
- Gilbert CE, Canovas R, Hagan M, Rao S, & Foster A (1993). Causes of childhood blindness: results from west Africa, south India and Chile. Eye, 7, 184–188. 10.1038/eye.1993.39 [DOI] [PubMed] [Google Scholar]
- Gilbert CE, Canovas R, Kocksch de Canovas R, & Foster A (1994). Causes of blindness and severe visual impairment in children in Chile. Developmental Medicine and Child Neurology, 36(4), 326–333. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Rahi J, Eckstein M,& Foster A (1995). Hereditary disease as a cause of childhood blindness: regional variation. Results of blind school studies undertaken in countries of Latin America, Asia and Africa. Ophthalmic Genetics, 16(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Gogate P, Soneji FR, Kharat J, Dulera H, Deshpande M, & Gilbert C (2011). Ocular disorders in children with learning disabilities in special education schools of Pune, India. Indian Journal of Ophthalmology, 59(3), 223–228. 10.4103/0301-4738.81036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Ivy SE, & Boyer C (2013). Severe visual impairments in infants and toddlers in the United States. Journal of Visual Impairment& Blindness (Online), 107(5), 325. [Google Scholar]
- Kang H, & Kim HY (2010). Ophthalmologic Evaluation in Pediatric Developmental Delay. Journal of the Korean Ophthalmological Society, 51(12), 1625 10.3341/jkos.2010.51.12.1625 [DOI] [Google Scholar]
- Lai D-C, Tseng Y-C, Hou Y-M,& Guo H-R (2012). Gender and geographic differences in the prevalence of intellectual disability in children: Analysis of data from the national disability registry of Taiwan. Researchin Developmental Disabilities, 33(6), 2301–2307. 10.1016/j.ridd.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Limburg H, Gilbert C, Hon DN, Dung NC, & Hoang TH (2012). Prevalence and Causes of Blindness in Children in Vietnam. Ophthalmology, 119(2), 355–361. 10.1016/j.ophtha.2011.07.037 [DOI] [PubMed] [Google Scholar]
- Mackie RT, McCulloch DL, Saunders KJ, Day RE, Phillips S, & Dutton GN (1998). Relation between neurological status, refractive error, and visual acuity in children: a clinical study. Developmental Medicine and Child Neurology, 40(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Martini G, Netto AA, Morcillo AM, Gagliardo HGRG, & de Oliveira DF (2014). The LEA Grating Test in assessing detection grating acuity in normal infants less than 4 months of age. Journal of American Associationfor Pediatric Ophthalmology and Strabismus, 18(6), 563–566. 10.1016/j.jaapos.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Maul E, Barroso S, Munoz SR, Sperduto RD, & Ellwein LB (2000). Refractive error study in children: results from La Florida, Chile. American Journal of Ophthalmology, 129(4), 445–454. 10.1016/S0002-9394(99)00454-7 [DOI] [PubMed] [Google Scholar]
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, & Saxena S (2011). Prevalence of intellectual disability: A meta-analysis of population-based studies. Research in Developmental Disabilities, 32(2), 419–436. 10.1016/j.ridd.2010.12.018 [DOI] [PubMed] [Google Scholar]
- Mayer DL, Fulton AB, & Hansen RM (1982). Preferential looking acuity obtained with a staircase procedure in pediatric patients. Investigative Ophthalmology & Visual Science, 23(4), 538–543. [PubMed] [Google Scholar]
- Mayo-Ortega L, Oyama-Ganiko R, Leblanc J, Schroeder SR, Brady N, Butler MG, … Marquis J (2012). Mass Screening for Severe Problem Behavior Among Infants and Toddlers in Peru. Journal of Mental Health Research in Intellectual Disabilities, 5(3-4), 246–259. 10.1080/19315864.2011.590626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante MT, Evans-Lacko S,& Paula CS (2009). Perspectives of intellectual disability in Latin American countries: epidemiology, policy, and services for children and adults. Current Opinion in Psychiatry, 22(5), 469–474. 10.1097/YCO.0b013e32832eb8c6 [DOI] [PubMed] [Google Scholar]


