Abstract
Objective
We investigated the effects of sleeve gastrectomy (SG) on functional connectivity and associations with weight loss and eating-related cognitive control.
Methods
In a longitudinal study, 14 SG patients (13 F; 42.1 BMI pre-surgery) completed study visits 1-month pre- and 12-months post-surgery. Patients completed the Dutch Eating Behavior Questionnaire and resting state fMRI scanning to measure FC. Data were analyzed using a seed-to-voxel approach in the CONN Toolbox to investigate pre-/post-surgery changes (n=12) and to conduct predictive analysis (n=14).
Results
Seed-to-voxel analysis revealed changes in magnitude (decreases) and directionality (positively correlated to anticorrelated) of FC pre- to post-surgery within and between default mode network (DMN), salience network (SN), and frontoparietal network (FPN) nodes (FEW-p<0.05). Baseline FC of the nucleus accumbens (with insula) and hypothalamus (with precentral gyrus) predicted 12-month post-SG % total weight loss (FWE-p<0.05). Baseline FC of the hippocampus, FPN, and DMN nodes predicted improvement in cognitive control of eating behavior 12-months post-SG (FWE-p<0.05).
Conclusions
Our findings demonstrate changes in FC magnitude and directionality post- vs. pre-surgery within and between resting state networks and frontal, paralimbic, and visual areas in SG patients. Baseline FC predicted weight loss and changes in cognitive control of food intake behavior at 12 months. These could serve as predictive biomarkers for bariatric surgery.
Keywords: sleeve gastrectomy, functional connectivity, eating behavior, cognitive control
Introduction
While bariatric surgery is effective in achieving durable weight loss (1), there is considerable variability in weight outcomes post-surgery (2), warranting a more precise understanding of mechanisms underlying this phenomenon and identification of biomarkers which could improve long-term outcome prediction. Longitudinal studies using task-based fMRI have demonstrated changes in activity following bariatric surgery, predominantly reductions in response to palatable food images in food motivation and reward circuitry suggesting normalization in brain activity (3). Baseline food-cue reactivity in the hypothalamus, reward circuitry [including nucleus accumbens (NAcc)] and frontal regions, have been shown to be negatively correlated with food reward sensitivity and weight loss at 1- to 12-months following bariatric surgery procedures, including sleeve gastrectomy (SG) (3, 4, 5), suggesting task-based fMRI metrics could provide value in predicting outcomes, although other studies have not found relationships between baseline neuroimaging metrics and post-surgical weight loss (6). While task-based fMRI is helpful in investigating how brain regions respond to certain stimuli, resting-state fMRI allows insight into intrinsic brain network connectivity during rest and has been shown to confer unique predictive capacity of disease course (7) and treatment outcomes (8) in psychiatric conditions.
Studies using resting state fMRI (rsfMRI) to measure functional connectivity (FC) have demonstrated normalization of FC within established neural networks following bariatric surgery (9). However, it remains to be investigated whether changes in FC between key neural networks is altered by bariatric surgery and whether these data improve outcome prediction. Previous rsfMRI studies have reported aberrant FC in individuals with obesity (OB) compared to individuals with lean (LN) body mass index (BMI) (10, 11). Using independent component analysis (ICA), it was found that within the default mode network (DMN), the precuneus/posterior cingulate (PCC) showed increased FC strength, whereas anterior cingulate cortex (ACC) demonstrated reduced FC strength (10). The DMN network is linked to self-referential processing (12), with evidence of abnormal FC in this region in individuals with major depressive disorder (13). A similar study examined a range of RS networks (14) in a larger sample of individuals with OB and LN, reporting increased FC strength in the salience network (SN) in the OB group in the putamen (11). The SN is linked to integration of high-level sensory information with visceral, autonomic, and hedonic signals (15). Recent studies have investigated the effects of bariatric surgery on FC, with assessments of patients at 1- (16, 17), 3–4- (18, 19) and 12- (9, 20) months post-surgery. In a cross-sectional study, individuals with severe obesity who did not undergo surgery demonstrated increased FC strength within the DMN compared to Roux-en-Y gastric bypass (RYGB) patients scanned at least one year after surgery (20). The post-RYGB group showed similar FC strength within the DMN compared to a LN group, suggesting weight loss following RYGB and/or the surgery itself normalized aberrant functional connectivity associated with obesity (20). In a recent longitudinal study, connectivity between precuneus and anterior superior temporal gyrus decreased from pre- to 1-year post-RYGB, in comparison to no changes in individuals with lean BMIs scanned at a parallel interval (9). Within the RYGB group, there was a reduction in FC strength between the posterior cingulate cortex (PCC, a node in the DMN) with the anterior superior temporal gyrus and anterior temporal fusiform gyrus (9). FC pre-surgery between paracingulate gyrus and amygdala predicted 1-year BMI loss, such that greater baseline FC was associated with greater BMI loss (9). In sum, evidence suggests aberrant FC in individuals with obesity in the DMN and SN (10, 11), with normalization of FC in the DMN and SN following bariatric surgery (9, 20).
Data from behavioral studies indicate bariatric surgery leads to increased control of food intake (21), perhaps through networks supporting cognitive control, including the frontoparietal network (FPN), responsible for cognitive function and reward regulation (22), and the hippocampus, involved in memory and linked to cognitive functioning deficits in obesity (17, 23). While one study reported no FC differences between OB and LN groups in FPN (11), a recent study revealed higher degree centrality (indicating elevated connectedness between nodes in a local network) in the FPN in individuals with obesity, with FPN degree centrality positively correlated with BMI and disinhibited eating (23). These findings are corroborated by a clinical study demonstrating stronger FC between hippocampus and insula post- compared to pre-bariatric surgery, though changes in cognitive functioning were not examined (17).
In the current study, we investigated the effects of SG on FC strength using rsfMRI from pre- to 12-months post-SG, and relationships with percent total weight loss (%TWL) and cognitive control of eating. Specifically, we examined changes from pre- to 12-months post-SG in functional connectivity strength within the nodes of key neural networks (i.e., DMN, SN and FPN) and between nodes of these networks and areas outside these networks. We hypothesized decreased FC strength (magnitude and directionality) in the DMN, SN, and FPN post-surgery compared to pre-surgery. Further, based on our task-based fMRI findings in patients undergoing SG(3), we hypothesized that pre-surgery FC strength of (1) the NAcc, and (2) hypothalamus with other regions in the brain would be correlated with % TWL at 12-month post-surgery. Finally, in a set of exploratory analyses based on evidence of the role of the FPN and hippocampus in cognitive control and obesity (23), we examined whether baseline FC in these regions predicted 12-month change in self-reported cognitive control of eating behavior.
Methods
Participants
SG candidates were recruited from the Brigham and Women’s Hospital Center for Metabolic and Bariatric Surgery and the Massachusetts General Hospital Weight Center. Eligibility criteria: 21–55 years of age, BMI 35–60, and ability communicate in English. All procedures were approved by the Partners Healthcare Human Research Committee. Participants completed study visits within 1 month prior to surgery (baseline) and 12-months post-surgery. See Supporting Information for details on exclusion criteria and sample sizes for each set of analyses.
Experimental procedure
After a phone screening, participants were invited to complete assessments within one month prior to bariatric surgery and 12-months following bariatric surgery. Prior to each visit, participants were instructed to fast overnight (12 hours). After giving informed consent, participants were escorted to the MRI suite for the MRI session. Post-scan, participants completed behavioral questionnaires and anthropometric measurements.
Cognitive control of eating
Participants completed the Dutch Eating Behavior Questionnaire (DEBQ) (24) at both visits. The DEBQ is a validated and reliable instrument used to measure eating behavior in patients who have undergone bariatric surgery (25). The DEBQ consists of three subscales: emotional eating, restrained eating and external eating and has high internal consistency (25).
MRI acquisition
Structural and functional brain images were acquired on a Siemens 3T Skyra (Siemens Healthineers, Erlangen, Germany) using a 20-channel head coil (see Supporting Information for details).
Data analysis
Data preprocessing
Structural and functional images were preprocessed using standard spatial preprocessing steps in CONN Toolbox v17.b (26) (https://www.nitrc.org/projects/conn/), running in MATLAB R2017a environment (The MathWorks, Inc., Natick, Massachusetts, United States). Functional images were slice-time and motion corrected, realigned, co-registered to structural scans, normalized to MNI-space and spatially smoothed with an 8-mm FWHM Gaussian kernel.
Motion artifact detection and first-level FC analysis
ART (https://www.nitrc.org/projects/artifact_detect/) was used to identify and exclude outlier data points. Functional connectivity analysis was performed using the CONN Toolbox v17b, including denoising using aCompCor (27). See Supporting Information for additional details.
Second-level FC analysis
Second-level one-sample GLM analyses were performed to test primary hypotheses. Positive and negative correlations with seeds were investigated. A whole-brain height threshold of p<0.001 (uncorrected) was used to identify areas of significant functional connectivity. A false discovery rate (FDR) corrected threshold of p<0.05 at this height threshold was applied for all reported clusters.
Changes in functional connectivity from pre-surgery to 12-months post-surgery
Analyses comparing rsfMRI data at baseline (pre-surgery) and 12-month follow-up (post-surgery) were conducted on data from n=12 (of 15) participants with adequate rsfMRI data at both visits. Seed-to-voxel analysis was conducted to examine changes in FC strength. Seed-based correlations between network-based nodes in DMN, SN, and FPN and voxels across the rest of the brain were computed. All nodes (seeds) were defined by CONN (i.e., maps with fixed size and location; Table S1). Beta values were extracted from individual subjects at each visit for clusters meeting statistical thresholds as defined above and plotted to determine the nature of change (magnitude, directionality).
Correlation between pre-surgery FC and 12-month %TWL
Seed-to-voxel analysis was conducted with NAcc and hypothalamus as a priori seeds (pre-surgery) and 12-month %TWL as second covariate in n=14 participants with adequate rsfMRI data at baseline and weight loss data at 12-month follow-up. Left and right NAcc and hypothalamus were defined as spheres with 6-mm radius centered on previously published foci (3) and generated using MarsBar (28).
Correlation between pre-surgery FC and 12-month cognitive control of eating
Analyses focused on associations between baseline rsfMRI and self-report data (change in cognitive control of eating behavior and %TWL) were conducted on data from n=14 (of 19) participants with adequate rsfMRI data at baseline and with both weight loss and cognitive control of eating data at both visits. Seed-to-voxel analyses were conducted with a priori seeds including: DMN nodes, FPN nodes, and hippocampus with pre- to post-change in each DEBQ subscale as second covariate.
Results
Demographics
The study sample consisted of primarily non-Hispanic, Caucasian females. Demographic variables at baseline are presented in Table 1. The change in eating behavior from pre- to post-surgery assessed with the DEBQ and %TWL at 12-month follow-up are shown in Table 1. Data on DEBQ, BMI, and 12-month %TWL are reported in (3) on an overlapping sample and are presented for informational purposes. Two out of thirteen female participants were in perimenopause and one female participant was currently using hormonal contraception (Mirena IUD). The remaining women reported having regular menstrual cycles.
Table 1.
Demographic and Clinical Characteristics
| Baseline and 12-month follow-up characteristics | |
|---|---|
| Age (M±SD) | 37.6 ± 10.5 |
| Gender (F/M) | 13/1 |
| Race (%) | |
| Caucasian | 78.6 |
| African American | 7.1 |
| Other | 14.3 |
| Ethnicity (%) | |
| Hispanic | 21.4 |
| Non-Hispanic | 78.6 |
| Education (%) | |
| High school/GED | 14.3 |
| Some college | 21.4 |
| Bachelor’s degree | 42.9 |
| Master’s degree | 21.4 |
| BMI pre-surgery (M±SD)1 | 42.1 ± 4.7 |
| BMI 12-months post-surgery (M±SD)1 | 29.9 ± 3.9 |
| % TWL 12-months post-surgery (M±SD)1 | 29.0 ± 8.4 |
| Change in DEBQ subscale scores, pre- to post-surgery (M±SD)1 | |
| External eating | 1.1 ± 0.9 |
| Restrained eating | 0.3 ± 1.1 |
| Emotional eating | 1.4 ± 0.8 |
| Psychotropic medication2 | 4 (29%)2 |
Data on DEBQ, BMI, and 12-month %TWL are reported in Holsen et al. (2018) on an overlapping sample and are presented for informational purposes.
N=1 participant was taking clonazepam at the time of the baseline visit and did not take any psychotropic medications at the time of the 12-months follow-up visit; n=1 participant was taking fluoxetine at time of the baseline visit and lorazepam at 12-months follow-up; n=1 participant was taking sertraline at the time of baseline and at the 12-months follow-up visit; n=1 participant was taking escitalopram at time of the baseline visit and did not complete a 12-month follow-up visit.
Changes in FC strength from pre-surgery to 12-months post-surgery
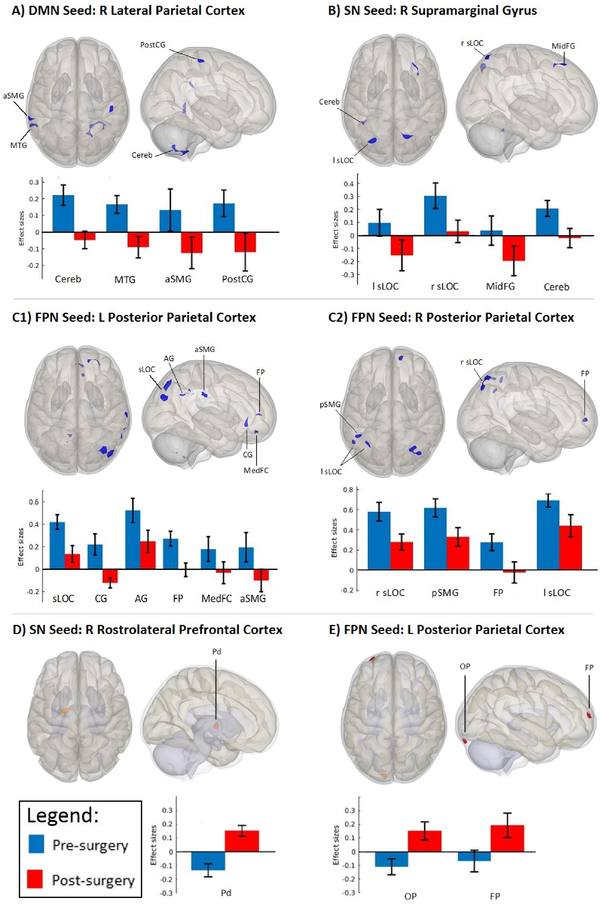
FC maps derived from the pre- vs. post-surgery contrast revealed two primary changes, revealed through extraction of beta weights individually from pre- and post-surgery timepoints for each subject and plotted as group means to discern the nature of the change. The first was magnitude of absolute change, in which the direction of correlation (positive or negative) remained consistent at both timepoints, but the magnitude of that correlation (i.e., increase/decrease) changed significantly. The second type involved change in directionality, in which correlations which were positive pre-surgery were altered post-surgery such that they became anticorrelations (negative correlations) post-surgery (or vice-versa: anticorrelations pre-surgery were revealed to be positive post-surgery). In detailing the effects of SG on FC below, results are organized according to these two types of changes in FC within each network.
Nodes showing decreases in magnitude and changes in directionality of FC from pre- to post-surgery
FC maps derived from DMN nodes showed changes in directionality between the right lateral parietal cortex (DMN node) with clusters in the cerebellum, middle temporal gyrus, supramarginal gyrus, and postcentral gyrus (Table 2, Figure 1A). Specifically, the right lateral parietal cortex showed positive correlations with the aforementioned clusters at pre-surgery and anticorrelations at post-surgery.
Table 2.
Changes in Functional Connectivity Strength from Pre-surgery to 12-months Post-surgery (Whole-Brain Seed Voxel Resting-State Functional Connectivity Analysis)
| Nodes showing decreased magnitude and changes in directionality (positively correlated to anticorrelated) of FC from pre- to post-surgery | ||||||
| Network/Seed 2,3 | Cluster | L/R | MNI (x, y, z) | Cluster size | ß | T (p-FDR)1 |
| Default Mode Network Seeds | ||||||
| R Lateral Parietal Cortex | Cerebellum | R | +12, −58, −56 | 197 | −0.27 | −11.20 (<0.001) |
| Middle Temporal Gyrus, temporoocippital part | L | −64, −48, +4 | 107 | −0.26 | −7.41 (0.005) | |
| Supramarginal Gyrus, anterior division | L | −68, −36, +28 | 76 | −0.26 | −6.03 (0.022) | |
| Postcentral Gyrus | R | +48, −26, +64 | 67 | −0.29 | −6.33 (0.029) | |
| Posterior Cingulate Cortex | Lateral Occipital Cortex, superior division | L | −32, −72, +36 | 100 | −0.26 | −6.69 (0.011) |
| Salience Network Seeds | ||||||
| R Supramarginal Gyrus | Lateral Occipital Cortex, superior division | L | −24, −70, +52 | 119 | −0.25 | −7.15 (0.002) |
| Lateral Occipital Cortex, superior division | R | +14, −66, +66 | 90 | −0.27 | −6.81 (0.006) | |
| Middle Frontal Gyrus | R | +26, +34, +54 | 68 | −0.23 | −6.44 (0.017) | |
| Cerebellum | L | −40, −50, −30 | 50 | −0.23 | −9.40 (0.043) | |
| Anterior Cingulate Cortex | Superior Parietal Lobule | L | −44, −52, +58 | 73 | −0.27 | −6.55 (0.021) |
| R Rostrolateral Prefrontal Cortex | Paracingulate Gyrus | R | +12, +44, −4 | 91 | −0.28 | −7.15 (0.010) |
| Frontoparietal Network Seeds | ||||||
| L Posterior Parietal Cortex | Lateral Occipital Cortex, superior division | R | +36, −72, +48 | 274 | −0.29 | −7.63 (<0.001) |
| Cingulate Gyrus, anterior division | +10, +30, −10 | 78 | −0.34 | −6.74 (0.042) | ||
| Angular Gyrus | R | +58, −54, +34 | 72 | −0.28 | −6.07 (0.042) | |
| Cerebellum | L | −8, −54, −52 | 66 | −0.28 | −6.03 (0.042) | |
| Frontal Pole | R | +30, +48, +6 | 63 | −0.28 | −7.42 (0.042) | |
| Frontal Medial Cortex | −2, +48, −20 | 62 | −0.21 | −8.40 (0.042) | ||
| Supramarginal Gyrus, anterior division | R | +68, −22, +30 | 57 | −0.30 | −5.68 (0.050) | |
| R Posterior Parietal Cortex | Lateral Occipital Cortex, superior division | R | +32, −64, +62 | 158 | −.030 | −9.00 (<0.001) |
| Supramarginal Gyrus, posterior division | L | −48, −50, +56 | 65 | −0.29 | −7.59 (0.041) | |
| Frontal Pole | R | +12, +56, +6 | 64 | −0.30 | −7.18 (0.041) | |
| Lateral Occipital Cortex, superior division | L | −34, −58, +58 | 58 | −0.27 | −7.80 (0.041) | |
| Lateral Occipital Cortex, superior division | L | −52, −58, +44 | 58 | −0.25 | −7.49 (0.041) | |
| L Lateral Prefrontal Cortex | Occipital Pole | R | +14, −94, −18 | 96 | −0.24 | −7.15 (0.009) |
| Nodes showing changes in directionality (anticorrelated to positively correlated) of FC from pre- to post-surgery | ||||||
| Network/Seed | Cluster | L/R | MNI (x, y, z) | Cluster size | ß | T (p-FDR)1 |
| Salience Network Seeds | ||||||
| R Rostrolateral Prefrontal Cortex | Pallidum | L | −16, −10, +2 | 117 | 0.29 | 9.24 (0.007) |
| Frontoparietal Network Seeds | ||||||
| L Posterior Parietal Cortex | Occipital Pole | L | −10, −94, −22 | 91 | 0.26 | 7.80 (0.010) |
| Frontal Pole | L | −32, +62, +16 | 62 | 0.26 | 6.93 (0.031) | |
Height threshold p<0.001, p-uncorrected and cluster threshold p<0.05, cluster-size p-FDR corrected.
These set of nodes were tested bilaterally and significant results are presented in the current table.
Complete list with tested nodes is presented in Table S1, Supporting Information.
Figure 1.
Quantitative connectivity of DMN, SN and FPN with significant clusters (derived from seed-to-voxel whole-brain correlation analyses; whole brain height threshold of p<0.001, cluster-size p-FDR-corrected threshold of p<0.05). Bar graphs indicate average connectivity values (i.e., mean connectivity z-scores) extracted from largest clusters for each condition (i.e., pre- and post-surgery) to assess the directionality and magnitude of the observed changes. (A) Change in directionality pre- (positively correlated) to post-surgery (anticorrelated) between Default Mode Network (Seed: Right Lateral Parietal Cortex) and Right Cerebellum (Cereb), Left Middle Temporal Gyrus (MTG), Left Supramarginal Gyrus, anterior division (aSMG), and Right Postcentral Gyrus (PostCG). (B) Change in directionality pre- (positively correlated) to post-surgery (anticorrelated) between Salience Network (Seed: Right Supramarginal Gyrus) and Left Lateral Occipital Cortex, superior division (l sLOC), Right Middle Frontal Gyrus (MidFG) and Left Cerebellum (Cereb). This same SN seed demonstrated reduced magnitude of positive correlation post-surgery (vs. pre-surgery) with the Right Lateral Occipital Cortex, superior division (r sLOC). (C1) Reduced magnitude of positive correlations post-surgery (vs. pre-surgery) between Frontoparietal Network (Seed: Left Posterior Parietal Cortex) and Right Lateral Occipital Cortex, superior division (sLOC) and Right Angular Gyrus (AG). This same FPN seed showed a change in directionality pre- (positively correlated) to post-surgery (anticorrelated) with the Cingulate Gyrus, anterior division (CG), Right Frontal Pole (FP), Frontal Medial Cortex (MedFC), and Right Supramarginal Gyrus, anterior division (aSMG). (C2) Reduced magnitude of positive correlations post-surgery (vs. pre-surgery) between Frontoparietal Network (Seed: Right Posterior Parietal Cortex) and Lateral Occipital Cortex, superior division (sLOC; Left and Right), Left Supramarginal Gyrus, posterior division (pSMG). This same FPN seed demonstrated anticorrelations post-surgery (vs. positive correlations pre-surgery) with the Right Frontal Pole (FP). (D) Change in directionality pre- (anticorrelated) to post-surgery (positively correlated) between Salience Network (Seed: Right Rostrolateral Prefrontal Cortex) and Pallidum (Pd) (E) Change in directionality pre- (anticorrelated) to post-surgery (positively correlated) between Frontoparietal Network (Seed: Left Posterior Parietal Cortex) and Left Occipital pole (OP), Left Frontal Pole (FP).
Within the SN, FC maps derived from SN nodes demonstrated changes in directionality and magnitude. Changes in directionality were observed between right supramarginal gyrus (SMG, an SN node) and a cluster encompassing the left lateral occipital cortex and cerebellum (Table 2, Figure 1B), and with a cluster in the FPN (middle frontal gyrus). Specifically, the SMG showed positive correlations with these clusters pre-surgery and anticorrelations post-surgery. Reduced magnitude of a positive correlation was found between right SMG and right lateral occipital cortex.
Within the FPN, FC maps derived from FPN nodes demonstrated changes in directionality and magnitude. Changes in directionality were observed between left posterior parietal cortex (PPC, an FPN node) with clusters in the SN (cingulate gyrus), DMN (anterior SMG), frontal pole, and frontal medial cortex (Table 2, Figure 1C1). Specifically, the left PCC showed positive correlations with these clusters pre-surgery and anticorrelations post-surgery. Similarly, the right PCC showed positive correlations with the frontal pole pre-surgery and anticorrelations post-surgery (Figure 1C2). Reduced magnitude of correlation was observed between FPN nodes with clusters in visual cortex (lateral occipital cortex) and DMN (angular gyrus and posterior SMG; Figure 1C), with PPC positively correlated with these clusters pre-surgery, and a reduction in the positive correlation post-surgery.
Nodes showing changes in directionality of FC from pre- to post-surgery
Significant shifts in FC directionality from anticorrelation to positive correlation were noted for clusters in SN and FPN networks. The right rostrolateral prefrontal cortex (SN node) was anticorrelated with the pallidum at pre-surgery and positively correlated at post-surgery (Table 2, Figure 1D). A similar change in directionality was observed between the left PPC and frontal/occipital poles (Figure 1E).
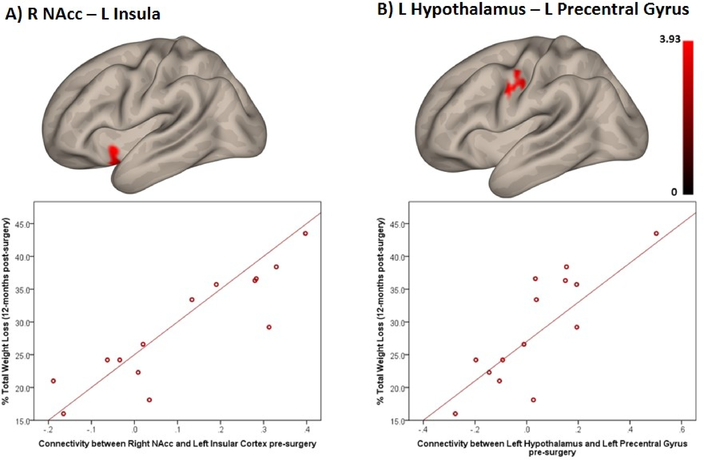
Correlation between pre-surgery FC strength and 12-month %TWL
Pre-surgery FC strength between striatal and homeostatic regions and other regions in the brain was positively associated with weight loss 12-months after surgery. Specifically, pre-surgery FC strength between right NAcc and left insular cortex was positively associated with 12-month %TWL (Table 3; Figure 2A). Additionally, pre-surgery FC strength between left hypothalamus and left precentral gyrus was positively associated with 12-month %TWL (Figure 2B).
Table 3.
Positive Correlations Between Pre-Surgery Functional Connectivity and 12-month %TWL
| Seed | MNI (x, y, z) | Cluster | MNI (x, y, z) | Cluster size | ß | T (p-FDR)1 |
|---|---|---|---|---|---|---|
| R Nucleus Accumbens2 | 15, 20, −8 | L Insular Cortex | −42, +12, −10 | 119 | 0.02 | 6.75 (<0.001) |
| L Temporal Pole | 46 | |||||
| L Frontal Orbital Cortex | 32 | |||||
| L Hypothalamus2 | −6, −10, −5 | L Precentral Gyrus | −42, −10, +34 | 116 | 0.02 | 5.16 (0.024) |
Height threshold p<0.001, p-uncorrected and cluster threshold p<0.05, cluster-size p-FDR corrected.
There were no significant correlations found for the Left Nucleus Accumbens (−12, 14, −11) or for the Right Hypothalamus (9, −10, −5).
Figure 2.
Positive association between pre-surgery functional connectivity and % Total Weight Loss 12-months post-surgery (note: color scale indicates T-values; the X-axis indicate average connectivity values). (A) Right Nucleus Accumbens and Left Insular Cortex (p-FDR-corrected<0.001). (B) Left Hypothalamus and Left Precentral Gyrus (p-FDR-corrected=0.024).
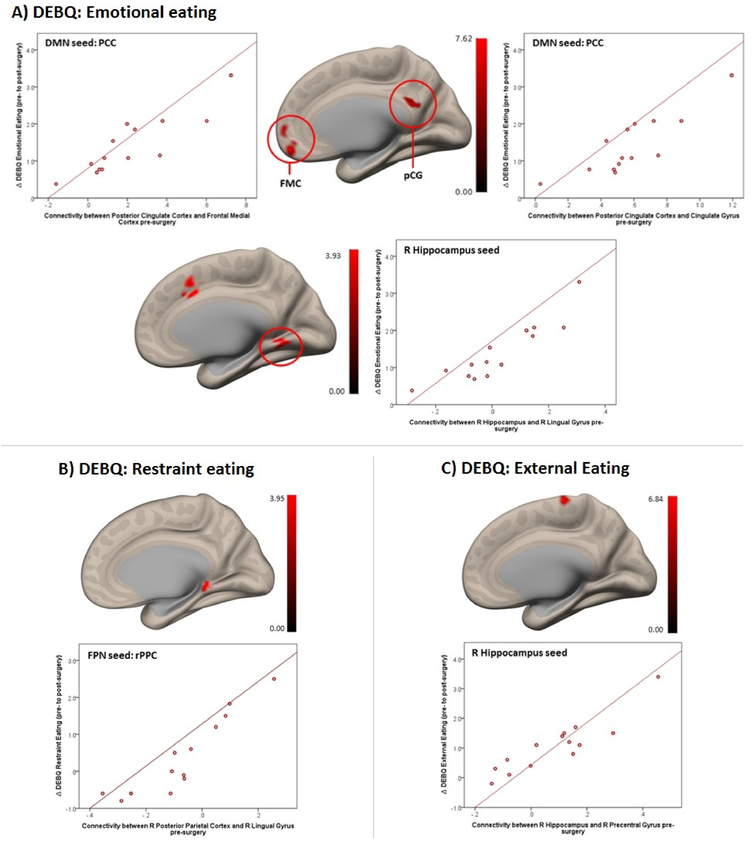
Correlation between pre-surgery FC and change in cognitive control of eating
At the network level, pre-surgery FC between DMN and FPN nodes with clusters in frontal cortex, cingulate, and lingual gyri, was positively associated with improvement in cognitive control of eating behavior 12-months post-surgery (Table S2; Figure 3). Positive correlations were found between pre-surgery FC within the DMN (medial frontal cortex with precuneus cortex and SMG) and change in emotional eating. In addition, pre-surgery FC of the PCC with bilateral frontal medial cortex and with posterior cingulate gyrus were positively associated with change in emotional eating (Figure 3A). For restraint eating, pre-surgery FC strength between right posterior parietal cortex (FPN node) with left cerebellum and right lingual gyrus (Figure 3B) were associated with change in self-report of this eating domain. Finally, baseline FC between right hippocampus and right precentral gyrus (Figure 3C) was positively correlated with changes in eating in response to external cues. FC between right hippocampus with right lingual gyrus, right paracingulate gyrus, and frontal pole (Figure 3C) positively predicted changes in emotional eating.
Figure 3.
Positive association between pre-surgery functional connectivity (in the DMN, FPN and hippocampus) and increase in cognitive control of eating from pre- to post surgery (note: color scale indicates T-values; X-axis on scatterplot indicates average connectivity values). (A) DEBQ Emotional Eating subscale associated with FC between the Posterior Cingulate Cortex (DMN) with Frontal Medial Cortex (FMC; L/R) (p-FDR corrected<0.001) and PCC (DMN) with Cingulate Gyrus, posterior division (pCG) (p-FDR-corrected<0.001) (upper panel) and with FC between the right Hippocampus with R Lingual Gyrus/Paracingulate Gyrus (p-FDR-corrected=0.019) (lower panel). (B) DEBQ Restraint Eating subscale associated with FC between the Right Posterior Parietal Cortex (FPN) with Right Lingual Gyrus (p-FDR-corrected=0.037). (C) DEBQ External Eating subscale associated with FC between the Right Hippocampus with Right Precentral Gyrus (p-FDR-corrected=0.018).
Discussion
Results of this longitudinal study on SG-induced modifications of functional brain connectivity reveal four main findings: 1) FC within nodes of the DMN changed from positively correlated at baseline to anticorrelated 12-months post-surgery; 2) alterations in FC within nodes of SN and FPN networks, and between SN and FPN nodes and clusters in SN, DMN, FPN and visual areas, included pre- to post-surgery effects on magnitude (reduced FC post-surgery) and direction (positively correlated pre-surgery to anticorrelated post-surgery, and vice-versa); 3) pre-surgery NAcc-insula and hypothalamus-precentral gyrus connectivity predicted 12-month post-surgery %TWL; and 4) pre-surgery FC of DMN nodes, FPN nodes and hippocampus with frontal, lingual, precentral, and cingulate regions predicted improvement in cognitive control of eating behavior at 12-months post-surgery. Consistent with prior task-based fMRI studies and emerging rsfMRI reports, these data support the substantial impact of bariatric surgery on brain networks involved in evaluation of salient stimuli and internal mental processing and offer novel evidence of changes in cognitive control networks, suggesting baseline FC of these networks might predict clinically-relevant outcomes following SG, and bariatric surgery more broadly.
We found that FC between the DMN nodes with clusters in the DMN and paralimbic, somatosensory regions were altered to such a degree that positive correlations pre-surgery “switched” to become anticorrelated post-surgery. Although reduced FC between DMN nodes and anterior middle temporal gyrus has been reported in RYGB patients scanned at similar timepoints (9), to our knowledge we are the first to identify anticorrelations in the DMN in SG patients 1-year post-surgery. DMN connectivity has been associated with self-awareness and introspection (12), and both DMN intra-network correlations and DMN inter-network anticorrelations have been proposed to be integral to consciousness (29). Our findings could indicate reorganization of DMN connectivity following bariatric surgery to restore these inter-network anticorrelations in support of more efficient processing during self-reflective thought and heightened attention to internal states (12).
Salience network hyper-connectivity in individuals with obesity may underlie the over-integration of multisensory information and appetite in individuals with obesity (11), with previous studies reporting attenuation and normalization of FC in SN 1- to 12-months following bariatric surgery (9, 16). Our results are consistent with these findings and with task-based functional connectivity studies demonstrating altered FC in the salience network and visual areas in obese vs. lean individuals during a food processing task (30). More specifically, augmented FC in the salience network was found in individuals with obesity in response to low-caloric food, and while we did not utilize a food processing task, our findings demonstrate reduced connectivity post-surgery in the same network/areas (30). In combination with novel evidence of shifts from positive to anticorrelated activity between SMG and FPN, these adaptations may indicate better coordinated functioning between motivational salience and cognitive control networks, even at rest, which could extend to normalized activity during processing of food-related stimuli after SG. Overall, our findings of robust changes in DMN-SN FC with regions involved in internal monitoring and integration of multisensory data with visceral, autonomic, and hedonic signals (15) 12-months post-surgery are consistent with behavioral data suggesting reduced craving and attention to internal appetitive state (31) post-bariatric surgery.
Of note, positive shifts in FC were also observed in our sample, with directionality changes favoring positive correlations between the rostrolateral prefrontal cortex (implicated in reward prediction) and pallidum (involved in hedonic food processing) post-surgery, compared to anticorrelations pre-surgery. These findings may reflect resolution of disruptions in coordinated activity underlying inaccurate inferences about rewarding properties of food, perhaps facilitating behavioral adaptations which support healthier choices around food intake.
The FPN is involved in cognitive control through regulation of visual, limbic, and motor systems (22), consisting of brain regions that, depending on task requirements, rapidly change their FC with task-specific neural networks (22). Recently, FC strength of the FPN was found to be correlated with eating disinhibition (23), while activity in response to food stimuli in the angular gyrus was reduced following behavioral weight loss (32). Our findings of reductions in magnitude and shifts from positive to anticorrelated activity both within the FPN and between FPN and SN/DMN clusters align with these previous data, suggesting attenuation of coordinated activity between regions involved in cognitive control and those critical to visual/attentional processing. Data demonstrating shifts in directionality of the FPN suggest dynamic attenuations [i.e. “toggling” (29)] between FPN inter-network correlations with networks engaged in intrinsic and externally directed attentional/visual processes. These findings imply that adaptive changes in the dynamic interactions between the FPN with the DMN and SN might be related to coordinated regulation of the FPN and visual/attentional processes that are associated with weight loss.
In an earlier a task-based fMRI study, we showed that pre-SG activity in NAcc and hypothalamus during desire for palatable food predicted %TWL at 12 months (3). In the current study, we found that pre-SG FC between (a) NAcc and insular cortex and (b) hypothalamus with precentral gyrus correlated positively with %TWL at 12-months post-surgery. As part of the brain’s hedonic system, the NAcc is involved in reward processing, while the insular cortex plays a role in viscerosensory and somatosensory signaling. Task-based fMRI studies have shown that individuals with obesity exhibit increased insular activity in response to food cues (33), but reduced insular FC (10). Our findings suggest a potential mechanism through which stronger baseline coordinated activity between these regions might allow for improved awareness of the internal state due to more efficient processing in the insular cortex (34), and improved long-term changes in the control of food reward. Previous work demonstrated increased FC of the precuneus with precentral gyrus and motor areas during an appetite control task in individuals with obesity, suggesting compensatory coordinated activity between inhibitory control and motor regions during voluntary appetite regulation (35). Our results of pre-surgery FC between hypothalamus and precentral gyrus predicting %TWL might indicate that baseline coordinated activity between homeostatic control and motor control regions provides more effective regulation of eating-related motor planning synced to homeostatic appetitive drive, and eventually weight loss.
Finally, our exploratory analyses revealed that stronger baseline FC in DMN and FPN was associated with enhanced cognitive control of eating behavior post-surgery. Pre-surgery FC of the hippocampus with lingual gyrus [involved in food cue processing (36)], and precentral gyrus was positively associated with reduced eating in response to emotions and external cues post-surgery. The PCC coordinates internally-directed attention (37), while the precuneus is involved in self-centered mental imagery and consciousness/awareness (38). Increased FC in this region has been reported in individuals with obesity (10). Our results suggest stronger baseline FC among these networks might provide neural infrastructure to support behavioral changes in food-related decision-making following surgery which promote weight loss.
Limitations of our study include a relatively small sample size and lack of a control group. Additionally, our sample consisted primarily of female participants, preventing investigation of sex differences. Future studies would benefit from a larger, gender-balanced sample with comparison to a BMI-matched control group. While our results convey clinical relevance, these results may not generalize to men, individuals with Type 2 diabetes, or RYGB patients. Prior weight loss treatments (including required minimal weight loss prior to surgery) and data related to possible genetic predispositions were not systematically recorded in this sample. Hence, we cannot determine whether previous or recent weight loss and genetic factors contributed to the effects we are reporting. Change in cognitive control of eating was measured via self-report, and future studies would benefit from objective measures of eating behavior. Although a minority of participants was taking psychotropic medication at the time of one of the study visits, which could have impacted our primary variables of interest, close inspection of each participant’s rsfMRI and %TWL data demonstrated absence of any systematic bias on the overall group results from these participants. Additionally, inclusion of women in perimenopause and taking hormonal contraception, as well as scheduling challenges, prevented controlling for variation in gonadal hormone changes across the menstrual cycle in female participants. However, the literature is mixed with respect to the potential effect of such variation on network connectivity (39, 40), and we maintain that any effects would be minimal in comparison to the overall impact of surgery on resting state networks. Furthermore, previous rsfMRI studies examining functional connectivity following bariatric surgery reported changes between/within networks, including positive correlation between paracingulate gyrus-amygdala connectivity and weight loss at one year after RYGB reported by Olivo and colleagues (9), which we did not observe. Lack of correspondence with these prior findings are likely attributable to differences in surgical procedure (RYGB or adjustable gastric banding vs. vertical SG), duration of follow-up (1–6 months vs. one year after surgery), experimental procedures (scanning participants at fasted vs. sated state), or data processing and analytical approaches. Finally, our study is largely descriptive and would benefit from replication.
In summary, our study identified key alterations in brain network connectivity 1 year following SG and is the first to specify the nature of these shifts, defining changes in magnitude and in directionality. These shifts could indicate increased efficiency in network coordination to support behavioral adaptations related to food intake decision-making to facilitate weight loss. Along with evidence of brain network FC predicting weight loss and cognitive control of eating outcomes, these findings support the significant effects of SG (and bariatric surgery more broadly) on the brain and development of multi-modal imaging biomarkers to predict bariatric surgery outcomes. Further, while the practical implications of the results of our study are still preliminary, these data provide mechanistic insight into the role of the brain in bariatric surgery efficacy, and as such, a foundation for interventional work in the future. For example, identification of baseline network connectivity related to post-surgical outcomes could inform clinical adjunctive treatments (i.e., neuromodulation techniques) designed to enhance weight loss outcomes.
Supplementary Material
Study Importance.
What is already known about this subject?
Bariatric surgery is a highly effective intervention to achieve weight loss, however, there remains significant variability in outcomes.
Resting state fMRI data have demonstrated normalization in functional connectivity after gastric bypass in networks associated with internal mental processing and integration of sensory with interoceptive/hedonic signals.
What does your study add?
We noted significant changes in magnitude and directionality of functional connectivity within and between nodes in several key networks from pre- to 12-months-post sleeve gastrectomy.
We identified connectivity between nodes in brain networks related to cognitive control and regulation of food-reward which predicted improvement in cognitive control of eating and weight loss 12-months post-surgery.
Acknowledgements
The authors are grateful to study participants for volunteering their time, Vanessa Calderon, Mark Gorman, and Noreen Harrington for coordinating subject recruitment, and Max Curran for programming the fMRI paradigm.
Funding
This work was funded by the Harvard Nutrition Obesity Research Center (P30 DK040561), Global Foundation for Eating Disorders, and the Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR025758 and financial contributions from Harvard University and affiliated academic health care centers). Support for a portion of LMH’s time was provided by NIMH K01 HD019222 and BWH BRI Fund for Research Excellence.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health.
Supporting information is available at the Obesity website.
References
- 1.Chang S, Stoll C, Song J, Varela J, Eagon C, Colditz G. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg 2014;149: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310: 2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holsen L, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes 2018;42: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ness A, Bruce J, Bruce A, Aupperle R, Lepping R, Martin L, et al. Pre-surgical cortical activation to food pictures is associated with weight loss following bariatric surgery. Surg Obes Relat Dis 2014;10: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 5.Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J, et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 2012;209: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, et al. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis 2012;8: 602–608. [DOI] [PubMed] [Google Scholar]
- 7.Shapero BG, Chai XJ, Vangel M, Biederman J, Hoover CS, Whitfield-Gabrieli S, et al. Neural markers of depression risk predict the onset of depression. Psychiatry Res Neuroimaging 2019;285: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, et al. Brain connectomics predict response to treatment in social anxiety disorder. Mol Psychiatry 2016;21: 680–685. [DOI] [PubMed] [Google Scholar]
- 9.Olivo G, Zhou W, Sundbom M, Zhukovsky C, Hogenkamp P, Nikontovic L, et al. Resting-state brain connectivity changes in obese women after Roux-en-Y gastric bypass surgery: A longitudinal study. Sci Rep 2017;7: 6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33: 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-García I, Jurado MÁ, Garolera M, Segura B, Sala-Llonch R, Marqués-Iturria I, et al. Alterations of the salience network in obesity: A resting-state fMRI study. Hum Brain Mapp 2013;34: 2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008;1124: 1–38. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord 2012;139: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 2005;360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27: 2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiemerslage L, Zhou W, Olivo G, Stark J, Hogenkamp PS, Larsson EM, et al. A resting-state fMRI study of obese females between pre‐and postprandial states before and after bariatric surgery. Eur J Neurosci 2017;45: 333–341. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Ji G, Li G, Hu Y, Liu L, Jin Q, et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes 2018. [DOI] [PubMed] [Google Scholar]
- 18.Lepping RJ, Bruce AS, Francisco A, Yeh HW, Martin LE, Powell JN, et al. Resting‐state brain connectivity after surgical and behavioral weight loss. Obesity 2015;23: 1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Shan H, Liang S, Nie B, Liu H, Duan S, et al. Sleeve gastrectomy recovering disordered brain function in subjects with obesity: A longitudinal fMRI study. Obes Surg 2018;28: 2421–2428. [DOI] [PubMed] [Google Scholar]
- 20.Frank S, Wilms B, Veit R, Ernst B, Thurnheer M, Kullmann S, et al. Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. Int J Obes 2014;38: 341–348. [DOI] [PubMed] [Google Scholar]
- 21.Mathus-Vliegen EM. Long-term health and psychosocial outcomes from surgically induced weight loss: Results obtained in patients not attending protocolled follow-up visits. Int J Obes 2006;31: 299. [DOI] [PubMed] [Google Scholar]
- 22.Cole MW, Repovs G, Anticevic A. The frontoparietal control system: A central role in mental health. Neuroscientist 2014;20: 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park B-y, Seo J, Park H. Functional brain networks associated with eating behaviors in obesity. Sci Rep 2016;6: 23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eating Disord 1986;5: 295–315. [Google Scholar]
- 25.Brunault P, Rabemampianina I, Apfeldorfer G, Ballon N, Couet C, Réveillère C, et al. The Dutch Eating Behavior Questionnaire: Further psychometric validation and clinical implications of the French version in normal weight and obese persons. Presse Méd 2015;44: e363–e372. [DOI] [PubMed] [Google Scholar]
- 26.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2: 125–141. [DOI] [PubMed] [Google Scholar]
- 27.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 2002;16: S497. [Google Scholar]
- 29.Threlkeld ZD, Bodien YG, Rosenthal ES, Giacino JT, Nieto-Castanon A, Wu O, et al. Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex 2018;106: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullmann S, Pape A-A, Heni M, Ketterer C, Schick F, Häring H-U, et al. Functional network connectivity underlying food processing: Disturbed salience and visual processing in overweight and obese adults. Cereb Cortex 2013;23: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 31.Leahey TM, Bond DS, Raynor H, Roye D, Vithiananthan S, Ryder BA, et al. Effects of bariatric surgery on food cravings: Do food cravings and the consumption of craved foods “normalize” after surgery? Surg Obes Relat Dis 2012;8: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdaugh DL, Cox JE, Cook EW, Weller RE. fMRI reactivity to high-calorie food pictures short- and long-term outcome in a weight-loss program. Neuroimage 2012;59: 2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41: 636–647. [DOI] [PubMed] [Google Scholar]
- 34.Kringelbach ML, de Araujo IET, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage 2004;21: 781–788. [DOI] [PubMed] [Google Scholar]
- 35.Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L. Neural circuits for cognitive appetite control in healthy and obese individuals: An fMRI study. PLoS ONE 2015;10: e0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aviram-Friedman R, Astbury N, Ochner CN, Contento I, Geliebter A. Neurobiological evidence for attention bias to food, emotional dysregulation, disinhibition and deficient somatosensory awareness in obesity with binge eating disorder. Physiol Behav 2018;184: 122–128. [DOI] [PubMed] [Google Scholar]
- 37.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014;137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006;129: 564–583. [DOI] [PubMed] [Google Scholar]
- 39.De Bondt T, Smeets D, Pullens P, Van Hecke W, Jacquemyn Y, Parizel PM. Stability of resting state networks in the female brain during hormonal changes and their relation to premenstrual symptoms. Brain Res 2015;1624: 275–285. [DOI] [PubMed] [Google Scholar]
- 40.Weis S, Hodgetts S, Hausmann M. Sex differences and menstrual cycle effects in cognitive and sensory resting state networks. Brain Cogn 2019;131: 66–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.