 Ligand H4BATA forms highly stable complex with bismuth(iii) in 1–2 min at room temperature.
Ligand H4BATA forms highly stable complex with bismuth(iii) in 1–2 min at room temperature.
Abstract
A new benzoazacrown ligand H4BATA was synthesized and its complexation ability towards bismuth cations was evaluated. Binding of cation occurs at room temperature in a few minutes and formed complex exhibits the same level of inertness as highly stable complex with the well-known H4DOTA in biologically relevant and challenging media under in vivo conditions.
Introduction
At the present time, targeted alpha therapy with 225Ac and 213Bi has already shown promising results in the treatment of cancerous diseases such as bladder cancer, glioma, neuroendocrine tumors, etc.1,2 Chelating agents, such as H4DOTA and H5DTPA, are used to label target molecules.3,4 Complexes with azacrown ether H4DOTA are highly stable under challenging conditions in vitro and in vivo but the kinetics of radionuclide binding is quite slow and only elevated temperatures up to 100 °C facilitate complexation. In contrast, H5DTPA chelates cations instantaneously at room temperature but the formed complexes release radiometals in vitro and in vivo. In our work, we attempted to combine the macrocyclic effect of azacrown ethers with the mobility of acyclic ligands, from which the ligand H4BATA with a large macrocyclic cavity and 4 carboxylic pendant arms was synthesized (Scheme 1). In this ligand, the benzene ring plays a dual role: it gives greater rigidity to the macrocycle and can be used to add a functional group for conjugation with a biomolecule. Moreover, it can be expected that conjugation with a biomolecule through a substituent in the benzene ring will not alter the ability of the macrocycle to coordinate cations.
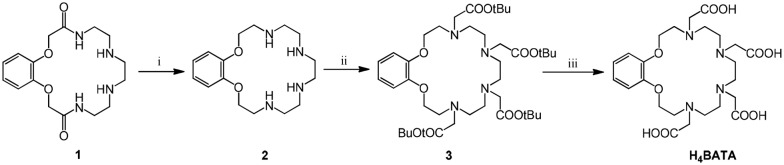
Scheme 1. Synthesis of the ligand H4BATA. Conditions: (i) BH3·THF; (ii) tert-butyl bromoacetate, K2CO3, MeCN; (iii) H2O.
Results and discussion
Synthesis of the ligand H4BATA was carried out according to Scheme 1. Macrocyclic bisamide 1, synthesized in accordance with a previous work,5 was reduced by BH3 in THF to give benzoazacrown 2 in high yield. Carboxylic pendant arms were introduced into the macrocycle in two steps. Compound 2 was N-alkylated with tert-butyl bromoacetate in MeCN under reflux in the presence of K2CO3 as the base to obtain 3, which was readily deprotected by hydrolysis in boiling water without any catalyst, in quantitative yield.
The protonation constants of H4BATA and its stability constants with Bi3+ were determined at 25 °C in a 0.1 M KNO3 aqueous solution using potentiometric titration.6,7 The calculated protonation constants and stability constants of Bi3+ complexes are presented in Table 1, which also lists the protonation and stability constants reported for H4DOTA.8 It was shown9 that the protonation of linear polyaminopolyacetic ligands proceeds by the successive addition of protons on all the nitrogen atoms after which the carboxylate groups can be protonated. On the other hand, the H4DOTA protonation scheme is different from the protonation scheme of its non-cyclic analogue, such as H4TTHA. This difference consists in the fact that two nitrogen atoms of DOTA turn out to be less basic than carboxylate groups.8 A comparison of the protonation constants determined for H4BATA and those reported for H4DOTA shows that all the four amino groups of H4BATA are more basic than the amino groups of H4DOTA. Thus, sequential protonation of H4BATA is closer to the protonation scheme of the non-cyclic analogue H4TTHA than to that of H4DOTA, more likely due to the large enough macrocyclic cavity of H4BATA. The latter does not provide rigidity making protonation of the neighboring amines easier than in the case of H4DOTA. Apart from H4TTHA,9 the obtained stepwise protonation constant values of H4BATA are close to the values of other polyaminopolyacetic ligands such as H4TETA, H5PEPA and H6HEHA.10 The value of log KH5L = 2.6 corresponds to one of the four carboxylic arms and is in line with protonation of the COOH group of EDTA, DTPA and other amines.9 Under the considered conditions, it was hard to reliably evaluate the formation of further protonated forms.
Table 1. Overall (log βHhL, log βMHhL, and log βML) and stepwise (log KHhL) protonation constants and stability constants of H4BATA and its complexes with Bi3+ in aqueous solution at 25.0 °C and 0.1 M KNO3.
| Species |
H
4
BATA
|
H
4
DOTA
a
|
|||
| log βHhL | log βHhLM | log KHhL | log KHhL8 | log βLM11 | |
| HL | 11.9(1) | 11.9 | 11.08 | ||
| H2L | 21.9(1) | 10.0 | 9.23 | ||
| H3L | 30.3(1) | 8.4 | 4.24 | ||
| H4L | 35.5(1) | 5.2 | 4.18 | ||
| H5L | 38.1(1) | 2.6 | 1.88 | ||
| H2LBi | 35.9(4) | — | |||
| HLBi | 34.5(1) | — | |||
| LBi | 30.3(1) | 30.3 | |||
| LBiH–1 | 20.9(1) | — | |||
aLiterature data for H4DOTA8 are provided for comparison.
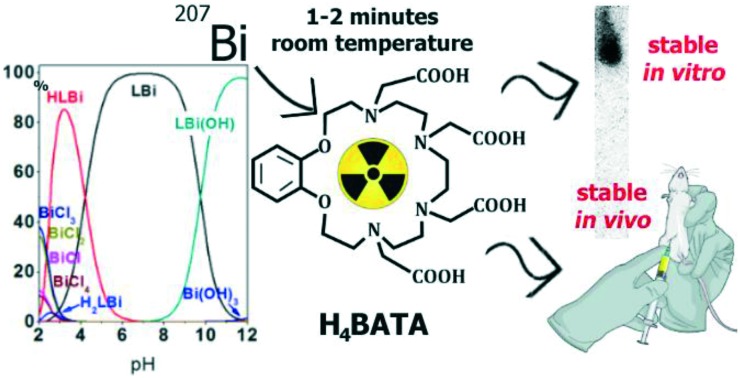
Potentiometric titration of H4BATA has been carried out in the presence of equimolar Bi3+ ions to determine the stability constants of the corresponding metal complexes. Protonated forms of the complexes H2BATABi+ and HBATABi and complexes BATABi– and BATABi(OH)2– have been detected over the studied pH range. To calculate the stability constants, the possibility of the formation of hydroxy and chloride complexes of bismuth(iii) was taken into account. The following values of the formation constants were used: log βBi(OH)2– = 12.4, log βBi(OH)2– = 23.48, log βBi(OH)3 = 31.9, log βBi(OH)4+ = 33.5 (ref. 12–15), log βBiCl2+ = 3.6, log βBiCl2+ = 5.6, log βBiCl3 = 7.0, and log βBiCl4– = 8.0.13
The species distribution diagram calculated for the complexes of H4BATA with Bi3+ shows the presence of BATABi– as the main component in the range of pH 5–9. HBATABi is mainly formed at lower pH while BATABi(OH)2– prevails in an alkaline medium (Fig. S2†).
It should be noted that the stability constant obtained for the BATABi– complex in a 0.1 M KNO3 aqueous solution (log KBATABi = 30.3) is equal to that reported for DOTABi– (log KDOTABi = 30.3).11 The pM values (pM = –log[Metal]free), calculated for a specific pH value taking into account the known values of the stability constants, are often considered as a better estimate of the complex stability. The pBi values obtained for the complexes of Bi3+ with H4BATA and H4DOTA in aqueous solution at pH = 7.4, [Bi3+] = 1 μM and [Ligand] = 9 μM are equal to 23.06 and 25.69 for H4BATA and H4DOTA respectively. This result shows that bismuth(iii) binds to H4BATA a little worse than to H4DOTA in aqueous solution at pH = 7.4 but still with high affinity.
The fast formation of the complex is essential for radiopharmaceutical and, in particular, for therapeutic radioisotopes of 212,213Bi which have fairly short half-lives (61 and 46 minutes respectively). Therefore, the chelator must be able to rapidly coordinate with Bi3+ under mild conditions, must be suitable for use in combination with biological vectors, and must provide a high yield of labeling. Thus, the labeling efficacy was determined at room temperature and at 80 °C after 1 hour of incubation. The H4BATA labeling by 207Bi was quantified using thin layer chromatography (TLC) with further measurements of radioactivity on each half of a plate using a γ-scintillation counter. For this purpose, into a solution of H4BATA (1.0 μM to 770 μM) buffered at pH 6.1 or 8.0, a solution of [207Bi]BiCl3 was added. The radiolabeled ligand has an Rf = 0.9 as determined by TLC under the experimental conditions and the free Bi3+ stays at the start line with an Rf = 0.
The radiolabeling yield immediately after preparation (that is, after 2–3 minutes) and during 1 hour of equilibration did not change regardless of temperature: 44% at pH 6.1 and 1 μM H4BATA concentration at 80 °C versus 49% at room temperature (Table 2). According to the literature,16 the slow kinetics of complexation by H4DOTA is due to the slow introduction of a cation into the cage formed by the macrocycle and carboxylic arms, with simultaneous deprotonation of the macrocyclic N-atoms. In the case of H4BATA, fast complexation can be provided by a much less rigid azacrown-ether cavity due to the larger sized 18-crown-6 of H4BATA compared to the 12-crown-4 of H4DOTA. This is the reason why all the labeling experiments were performed at room temperature without additional equilibration time.
Table 2. Labeling yields of H4BATA with 207Bi.
| c(H4BATA), μM | 1 | 5 | 10 | 77 | 100 | 300 | 500 | 770 |
| pH 6 | 49 ± 5% | 64 ± 6% | 88 ± 5% | 96 ± 4% | 95 ± 5% | 95 ± 5% | 97 ± 3% | 95 ± 5% |
| pH 8 | 61 ± 6% | 63 ± 5% | 70 ± 5% | 76 ± 4% | 78 ± 6% | — | — | 95 ± 5% |
Since the pH value could influence complexation, we evaluated the labeling yields for several concentrations, and no significant differences were observed except for a slight decrease in yield at higher pH values. The lower labeling yield at higher pH is due to the extremely hydrolysable character of Bi3+. The labeling yield reached >96% at pH 6 and 77–100 μM of H4BATA (Table 2). This large value has not changed in other buffer systems, such as PBS, bicarbonate and acetate buffers, which means that none of these buffer systems have an effect on complexation. This allows the use of any of these buffer systems, if necessary.
To further assess the in vitro stability of Bi3+ complexes, the following conditions were chosen: 500 μM of H4BATA and 50 Bq of 207Bi in 0.01 M MES buffer (pH 6.1) at room temperature. No dissociation of the complexes in saline (0.9% NaCl) was detected after at least 2 days (Fig. S3†). In addition, the complexes were stable in the presence of a large excess of biologically significant cations: Ca2+ (5 mM), Mg2+ (5 mM), Zn2+ (0.1 mM) and Cu2+ (0.1 mM). It has been shown (Fig. S3†) that the complexes are stable in the presence of these cations for at least 1 day.
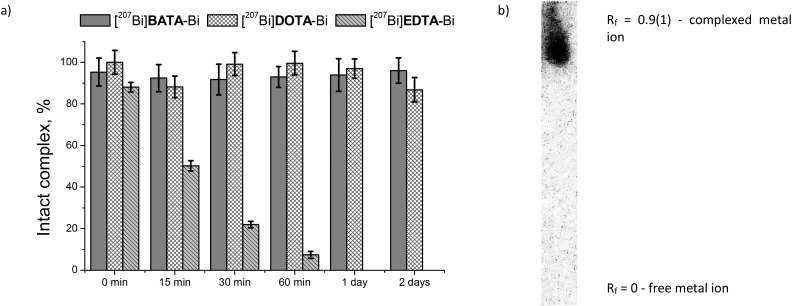
The stability of the Bi3+ complexes in serum was studied in a 100-fold excess (by volume) of fetal bovine serum by precipitation of the protein-bound fraction, as described elsewhere.17,18 We did not observe the release of radionuclides from the complex within the course of the experiment, which is in line with our pBi calculation (Fig. 1). In addition, for an adequate comparison with the reference complexes, we conducted experiments on serum stability under the same conditions with [207Bi]DOTA-Bi, which is known to be highly stable (Fig. 1a), and added similar data from the literature concerning the easily dissociated complex with the acyclic chelator [207Bi]EDTA-Bi.17 The supernatant after protein precipitation was analyzed by TLC, and it was proven that the radioactive species in this fraction are the same as those immediately after the radiolabeling (Rf = 0.9) (Fig. 1b). The results show the absence of cation rechelation by serum proteins from the complex with H4BATA, and that with H4DOTA, in contrast to the easily dissociating chelate with H4EDTA (Fig. 1a).
Fig. 1. a) Rechelation of 207Bi3+ by serum proteins from the complexes with H4EDTA,17H4DOTA and H4BATA; b) radiographic image of the TLC plate of the supernatant fraction after 2 days of incubation (95% of the complex).
Being part of the targeted radiopharmaceutical radioactive chelate itself is expected not to display any affinity to tissues. In order to estimate the biodistribution of the ligand itself at the very first level, we developed a simple deep learning model. The model was based on the Anatomical Therapeutic Chemical classification system,19 where the top-level (one-letter) groups were taken as labels for classification (details in the ESI†). The accuracy of the model predictions on the external experimental test set of 312 molecules is about 80% which can be considered as a good prediction quality (and outperforms the existing models like described in ref. 20) and allows us to use the model as a kind of fingerprint describing drug-like molecular behavior.
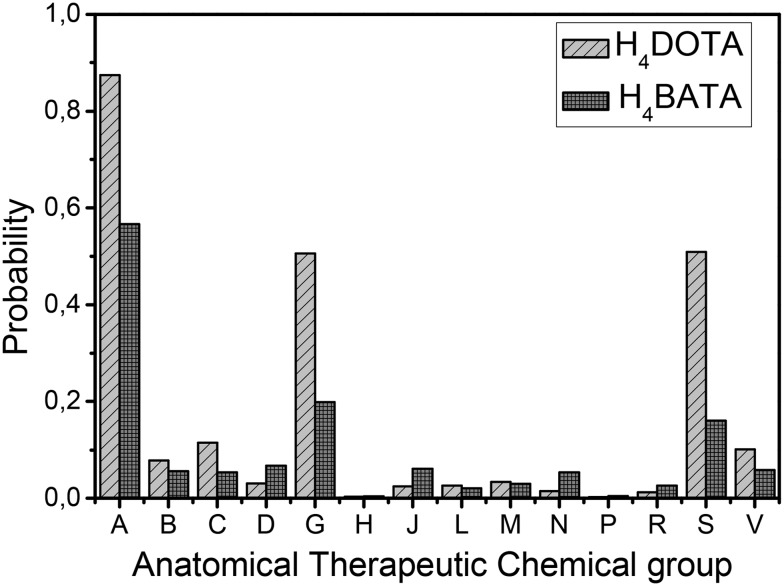
We applied the model to H4BATA and to H4DOTA as a reference point (Fig. 2). The presented distribution can be interpreted as the Anatomical Therapeutic Chemical profile of drug-like molecules (ATC-profile). According to our model, H4BATA should behave very similarly to H4DOTA, indicating qualitatively the same affinity to A (alimentary tract and metabolism), G (genitourinary system) and S (sensory organs) groups. Moreover H4BATA provides even less activity concerning the above mentioned groups. Thus, we assumed a similar biodistribution for both ligands with a similar predicted ATC profile. Additionally, we could expect even less H4BATA accumulation compared to that of H4DOTA.
Fig. 2. ATC profiles of H4DOTA and H4BATA: predicted probabilities of ATC group attributions.
The fast complex formation at room temperature, high values of stability constants, stability of the complexes in vitro and theoretically estimated behavior of H4BATAin vivo prompted us to conduct a biodistribution study on normal mice to evaluate the stability of [207Bi]BATA-Bi in vivo.
All in vivo experiments were carried out in accordance with the ARRIVE guideline and in accordance with the EU Directive 2010/63/EU for animal experiments. The experimental protocol was approved by the Institute Commission for Animals. A total of 12 normal male BALB/c mice were used. A 100 μL solution of the radiolabeled ligand (pH 6.1, 0.01 M MES) was injected intraperitoneally. The mice were euthanized after 1 and 6 h. The blood and major organs were harvested and wet weighed and radioactivity was measured by gamma-spectrometry (with an HPGe-detector GR3818, Canberra Ind.). The percent injected dose per gram (% ID per g) was determined for each tissue. The mean value and standard deviation for each tissue are presented in Table 3. It can be seen that a biodistribution profile and rapid elimination from the body demonstrated by complex with H4BATA are similar to complex with H4DOTA. Detected radioactivity in all tissues including the whole body reached a maximum of 3% ID per g (in the kidney) and this value decreased to 2% ID per g after 6 h post-injection, identifying renal clearance of the complex [207Bi]BATA-Bi. After 6 h, no sufficient accumulation in other tissues was observed: only 0.1% ID per g was detected in the liver, and lower doses were found in the rest of the organs. Moreover, in some cases, the radioactivity of both [207Bi]DOTA-Bi and [207Bi]BATA-Bi in organs did not exceed the minimum detectable level, therefore it was impossible to quantify accumulation. Compared to the results obtained with the [207Bi]EDTA-Bi complex,17 we can conclude that the radionuclide injected as the [207Bi]BATA-Bi complex demonstrates high inertness in vivo as a chelate compared with the “gold standard” in radiopharmacy [207Bi]DOTA-Bi.
Table 3. Biodistribution (% ID per g) of the Bi3+ complexes with H4DOTA and H4BATA in normal mice a .
| Tissue | [207Bi]DOTA-Bi |
[207Bi]BATA-Bi |
||
| Time points (h) | ||||
| 1 | 6 | 1 | 6 | |
| Kidneys | 1.83 ± 0.42 | 1.10 ± 0.23 | 3.03 ± 0.52 | 1.97 ± 0.14 |
| Blood | 1.22 ± 0.29 | b | 1.14 ± 0.63 | b |
| Femur | 0.97 ± 0.60 | 0.14 ± 0.07 | 0.58 ± 0.23 | 0.07 ± 0.01 |
| Liver | 0.18 ± 0.02 | 0.07 ± 0.01 | 0.40 ± 0.29 | 0.11 ± 0.02 |
| Heart | 0.07 ± 0.01 | b | 0.10 ± 0.08 | 0.02 ± 0.01 |
| Lungs | 0.16 ± 0.03 | 0.03 ± 0.01 | 0.22 ± 0.13 | 0.06 ± 0.01 |
| Spleen | 0.12 ± 0.01 | 0.04 ± 0.02 | 0.19 ± 0.13 | 0.08 ± 0.02 |
| Brain | 0.05 ± 0.01 | b | 0.02 ± 0.01 | b |
| The rest of body | 0.47 ± 0.09 | 0.15 ± 0.01 | 1.1 ± 0.5 | 0.25 ± 0.06 |
a n = 3 for each point.
bRadioactivity is lower than the minimum detectable level.
Comparison of the experimental data with the predicted ATC-profile confirmed the initial assumption of a similar biodistribution of ligands. According to this, we can suggest that the biodistribution of both radioactive complexes reflects a biodistribution of ligands with low radioactivity release in vivo.
Unlike qualitative results, the quantitative prediction appeared to be much worse (H4DOTA complex's accumulation was consistently lower than that of the complex with H4BATA), and the model at the present stage can be used only for comparative evaluation in this way. Although the ATC classification was not originally designed to demonstrate therapeutic equivalence, the results showed that in some cases the ATC-profile was useful for estimating the biodistribution and the very first evaluation of drug behavior in vivo.
Conclusions
Our studies have shown that the ligand H4BATA, having a large macrocyclic cavity and bearing 4 carboxylic pendant arms, is characterized by the fast formation of a very stable complex with bismuth(iii) already at room temperature in a wide pH range. This complex exhibits high stability in vitro in the experiments in biologically significant media comparable to the stability of complex with H4DOTA. Furthermore this complex formed in 1–2 minutes at room temperature possesses the same inertness in vivo and high clearance rate as [207Bi]DOTA-Bi.
The functionalization of H4BATA on the phenyl core may allow the preparation of conjugates with biomolecules including heat-sensitive molecules and lead to the creation of therapeutic radiopharmaceuticals containing short-lived bismuth radioisotopes.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors thank the Russian Science Foundation for financial support (project No. 18-73-10035). The authors are grateful to Adelya Khayrullina for assistance in computational modeling. The research was carried out using the equipment of the shared research facilities of HPC computing resources at Lomonosov Moscow State University. This work was partly performed using the facilities provided by M. V. Lomonosov Moscow State University Program of Development.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00251k
References
- Autenrieth M. E., Seidl C., Bruchertseifer F., Horn T., Kurtz F., Feuerecker B., Alessandria C. D., Pfob C., Nekolla S., Apostolidis C., Mirzadeh S., Gschwend J. E., Schwaiger M., Scheidhauer K., Morgenstern A. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1364–1371. doi: 10.1007/s00259-018-4003-6. [DOI] [PubMed] [Google Scholar]
- Morgenstern A., Apostolidis C., Kratochwil C., Sathekge M., Krolicki L., Bruchertseifer F. Curr. Radiopharm. 2018;11:200–208. doi: 10.2174/1874471011666180502104524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarin A., Valverde I. E., Mindt T. L. Med. Chem. Commun. 2016;7:1640–1646. [Google Scholar]
- Price E. W., Cawthray J. F., Adam M. J., Orvig C. Dalton Trans. 2014;43:7176–7190. doi: 10.1039/c4dt00239c. [DOI] [PubMed] [Google Scholar]
- Zubenko A. D., Egorova B. V., Kalmykov S. N., Shepel N. E., Karnoukhova V. A., Fedyanin I. V., Fedorov Y. V., Fedorova O. A. Tetrahedron. 2019;75:2848–2859. [Google Scholar]
- Gans P., Sabatini A., Vacca A. Talanta. 1996;43:1739–1753. doi: 10.1016/0039-9140(96)01958-3. [DOI] [PubMed] [Google Scholar]
- Lima L. M. P., Beyler M., Oukhatar F., Le Saec P., Faivre-Chauvet A., Platas-Iglesias C., Delgado R., Tripier R. Chem. Commun. 2014;50:12371–12374. doi: 10.1039/c4cc05529b. [DOI] [PubMed] [Google Scholar]
- Desreux J. F., Merciny E., Loncin M. F. Inorg. Chem. 1981;20:987–991. [Google Scholar]
- Letkeman P., Martell A. E. Inorg. Chem. 1979;18:1284–1289. [Google Scholar]
- Kodama M., Koike T., Mahatma A. B., Kimura E. Inorg. Chem. 1991;30:1270–1273. [Google Scholar]
- Csajbok Ä., Baranyai Z., Brucher E., Kiraly R., Muller-Fahrnow A., Platzek J., Raduchel B., Schaefer M. Inorg. Chem. 2003;42:2342–2349. doi: 10.1021/ic0261272. [DOI] [PubMed] [Google Scholar]
- Hataye I., Suganuma H., Ikegami H., Kuchiki T. Bull. Chem. Soc. Jpn. 1982;55:1475–1479. [Google Scholar]
- Rai D., Yui M., Schaef H. T., Kitamura A. J. Solution Chem. 2010;39:999–1019. [Google Scholar]
- Cukrowski I., Hancock R. D., Luckay R. C. Anal. Chim. Acta. 1996;319:39–48. [Google Scholar]
- Baes C. F. and Mesmer R. E., The Hydrolysis of Cations, John Wiley & Sons, Inc, New York, 1976. [Google Scholar]
- Moreau J., Guillon E., Pierrard J. C., Rimbault J., Port M., Aplincourt M. Chem. – Eur. J. 2004;10:5218–5232. doi: 10.1002/chem.200400006. [DOI] [PubMed] [Google Scholar]
- Egorova B. V., Matazova E. V., Mitrofanov A. A., Aleshin G. Y., Trigub A. L., Zubenko A. D., Fedorova O. A., Fedorov Y. V., Kalmykov S. N. Nucl. Med. Biol. 2018;60:1–10. doi: 10.1016/j.nucmedbio.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Torres S., Martins J. A., André J. P., Neves M., Santos A. C., Prata M. I. M., Geraldes C. F. G. C. Radiochim. Acta. 2007;95:343–349. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology .
- Chen L., Zeng W.-M., Cai Y.-D., Feng K.-Y., Chou K.-C. PLoS One. 2012;7:1–7. doi: 10.1371/journal.pone.0035254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.