Abstract
BACKGROUND
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial demonstrated that for patients with diabetes mellitus (DM) and multivessel coronary disease (MVD), coronary artery bypass grafting (CABG) is superior to percutaneous coronary intervention with drug-eluting stents (PCI-DES) in reducing the rate of major adverse cardiovascular and cerebrovascular events after a median follow-up of 3.8 years. It is not known, however, whether CABG confers a survival benefit after an extended follow-up period.
OBJECTIVES
The purpose of this study was to evaluate the long-term survival of DM patients with MVD undergoing coronary revascularization in the FREEDOM trial.
METHODS
The FREEDOM trial randomized 1,900 patients with DM and MVD to undergo either PCI with sirolimus-eluting or paclitaxel-eluting stents or CABG on a background of optimal medical therapy. After completion of the trial, enrolling centers and patients were invited to participate in the FREEDOM Follow-On study. Survival was evaluated using Kaplan-Meier analysis, and Cox proportional hazards models were used for subgroup and multivariate analyses.
RESULTS
A total of 25 centers (of 140 original centers) agreed to participate in the FREEDOM Follow-On study and contributed a total of 943 patients (49.6% of the original cohort) with a median follow-up of 7.5 years (range 0 to 13.2 years). Of the 1,900 patients, there were 314 deaths during the entire follow-up period (204 deaths in the original trial and 110 deaths in the FREEDOM Follow-On). The all-cause mortality rate was significantly higher in the PCI-DES group than in the CABG group (24.3% [159 deaths] vs. 18.3% [112 deaths]; hazard ratio: 1.36; 95% confidence interval: 1.07 to 1.74; p = 0.01). Of the 943 patients with extended follow-up, the all-cause mortality rate was 23.7% (99 deaths) in the PCI-DES group and 18.7% (72 deaths) in the CABG group (hazard ratio: 1.32; 95% confidence interval: 0.97 to 1.78; p = 0.076).
CONCLUSIONS
In patients with DM and MVD, coronary revascularization with CABG leads to lower all-cause mortality than with PCI-DES in long-term follow-up.
Keywords: coronary artery disease, coronary revascularization, diabetes
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial (1) demonstrated that, in patients with diabetes mellitus (DM) and multivessel coronary disease (MVD), coronary artery bypass graft (CABG) surgery is associated with a reduction in major adverse cardiovascular and cerebrovascular events at a median follow-up of 3.8 years when compared with percutaneous coronary intervention (PCI) using drug-eluting stents (DES). Earlier evidence from the BARI (Bypass Angioplasty Revascularization Investigation) and BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trials (2,3) first highlighted the benefits of CABG in this population. In BARI, CABG was related to lower mortality in diabetic patients when compared with PCI without stent implantation, and in BARI 2D, the rates of major cardiovascular events were lower in the CABG group when compared with medical therapy.
Follow-up beyond 5 years after coronary revascularization trials is unusual due to a lack of funding and logistical obstacles. Although the BARI (4) and COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial (5) investigators did report long-term results, the BARI trials preceded the use of DES in clinical practice, and COURAGE included patients without DM and did not compare outcomes of PCI with those of CABG. It is not known whether CABG results in improved long-term survival compared with PCI-DES in the diabetic population. The objective of the FREEDOM Follow-On study was to examine long-term all-cause mortality in patients with DM and MVD enrolled in the FREEDOM trial.
METHODS
PATIENTS.
The design and primary results of the FREEDOM trial have been reported previously (1,6). Between April 2005 and April 2010, FREEDOM enrolled 1,900 patients with DM and angiographically confirmed MVD, defined as a diameter stenosis of more than 70% in 2 or more major epicardial vessels involving at least 2 separate coronary artery territories and without left main coronary artery disease. Patients were deemed suitable for both PCI-DES and CABG based on the judgment of the local heart team.
In FREEDOM, the majority of patients (83%) had 3-vessel disease and most included involvement of the left anterior descending coronary artery (99%), with a mean SYNTAX score of 26.2 ± 8.6. In total, 140 centers globally participated in the FREEDOM trial (7).
TREATMENT AND THE ORIGINAL FREEDOM TRIAL FOLLOW-UP.
After providing written informed consent, FREEDOM trial subjects were randomized to undergo either PCI-DES or CABG. Randomization was conducted in a 1:1 ratio with the use of permuted blocks with dynamic balancing within each study center. In the PCI-DES arm, sirolimus-eluting and paclitaxel-eluting stents were used in 51% and 43%, respectively, of the patients who underwent PCI, with only a minority of patients receiving second-generation DES. Dual antiplatelet therapy was recommended for at least 1 year after PCI-DES, which was accomplished in 78.1% of the patients. In the CABG group, 94.4% of the patients had a left internal thoracic-artery graft placed.
Patients from both intervention groups were mandated to receive guideline-driven optimal medical therapy, with a target low-density lipoprotein cholesterol lower than 70 mg/dl, blood pressure lower than 130/80 mm Hg, and glycated hemoglobin lower than 7% (8).
LONG-TERM FOLLOW-UP.
After the completion of the original FREEDOM trial in March 2012, patients and centers were invited to participate in the FREEDOM Follow-On study. A total of 25 centers agreed to participate in the Follow-On study (Online Table 1), and patients from these centers were consented to be contacted annually by phone or mail and/or to ascertain their vital status by reviewing the medical record or national death registries, based on their full name, date of birth, medical record number, and social insurance number. When a patient’s date of death was unavailable, patients were censored at the date of last contact. Clinical endpoints were adjudicated until the end of the original follow-up by an independent and blinded events committee. No adjudication was performed for death in the FREEDOM Follow-On study. The initial invitation to participate in the FREEDOM Follow-On Study was extended to all centers of the original FREEDOM trial. The highest-volume centers agreed to participate and contract with the coordinator center.
STUDY OVERSIGHT.
The FREEDOM Follow-On study was designed by the members of the FREEDOM trial Executive Committee, who were also responsible for the conduct, analysis and the decision to submit the paper for publication. The data collection for this extended follow-up study was performed by the investigators and study coordinators at each participating site, and the analysis of the data was performed by the study statistician (T.H.). The original FREEDOM protocol and the FREEDOM Follow-On protocol were approved by the local institutional ethics review board at each participating center.
STATISTICAL ANALYSES.
The primary outcome of the FREEDOM Follow-On Study was initially planned to be the same as that of the FREEDOM trial, i.e., a composite of all-cause mortality, nonfatal myocardial infarction and nonfatal stroke. Many centers were only able to collect data concerning mortality (vital status), which led to a change in the primary outcome of the present report to all-cause mortality alone. This primary analysis of all-cause mortality was performed according to the intention-to-treat (ITT) principle including all subjects enrolled in the FREEDOM trial (whole cohort). The analysis was repeated in the subset of FREEDOM subjects who were enrolled in the follow-on participant centers (extended follow-up cohort). A secondary non-ITT analysis was also performed in which patients were classified according to the initial revascularization procedure actually received. This was used as a sensitivity analysis for our results, allowing for assessment of the robustness of the follow-on study findings.
Baseline characteristics are summarized as mean ± SD for continuous variables and proportions for categorical variables by treatment group within extended and nonextended follow-up group. Continuous variables were compared by means of Student’s t-test or the Wilcoxon rank sum test for continuous variables and chi-square or Fisher exact test for categorical variables. All-cause mortality was described using Kaplan-Meier estimates and compared using the log-rank test. Multivariate time to all-cause mortality adjusted for covariates, subgroup, and interaction analyses were performed using Cox proportional-hazards regression. Nonproportionality was tested using interaction of treatment and survival time, and explored using log(−log[survival]) curves (9). A nonproportional hazard model allowing for a different treatment effect before and after the first 2 years of follow-up was also fitted and compared with the model with a constant treatment effect. Significant baseline variables between extended and without extended follow-up cohort comparison, or in the interaction test, were included in the multivariate analysis. A 2-sided alpha level of <0.05 was considered to indicate significance for the time to all-cause mortality analyses and subgroup interaction tests, and a p value <0.01 was considered statistically significant for the extended versus without extended follow-up cohort comparisons. SAS version 9.4 (SAS Institute, Cary, North Carolina) and R version 3.5.1 (R Foundation, Vienna, Austria) software were used to conduct the statistical analyses.
RESULTS
BASELINE CHARACTERISTICS.
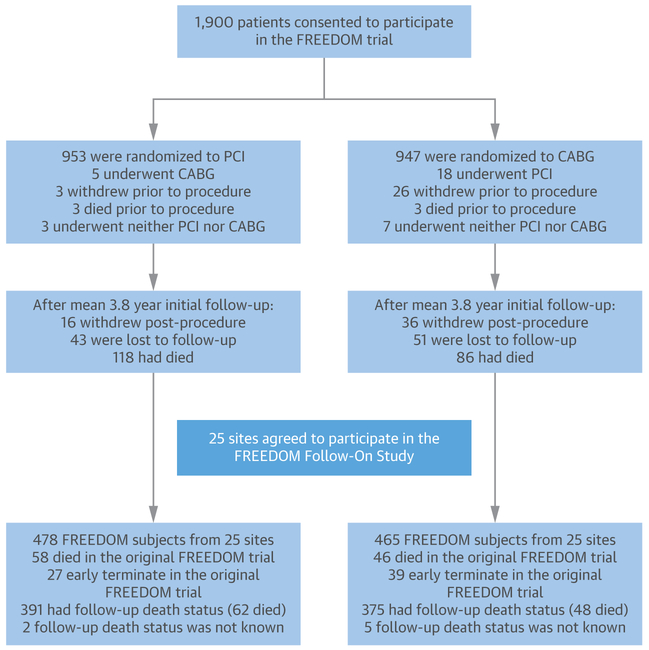
A total of 25 centers agreed to participate in the FREEDOM Follow-On study, resulting in the long-term follow-up of 943 patients (49.6% of the original whole cohort of the 1,900 patients in the FREEDOM trial). The detailed patient flow of the original trial and Follow-On study is shown in Figure 1.
FIGURE 1. Patient Flow in the Original FREEDOM Trial and the FREEDOM Follow-On Study.
After the completion of the initial FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, centers and patients were invited to participate in the FREEDOM Follow-On study. A total of 25 centers agreed to participate, resulting in a population of 943 patients. Excluding patients who died (n = 104), withdrew consent, or were lost to follow-up (n = 66) during the original FREEDOM follow-up, 773 patients were available to be followed. In the end, follow-up information was obtained for 766 patients (99% of the patients in the FREEDOM Follow-On). The expression “early terminate” in the bottom boxes refers to subjects who were lost to follow-up or withdrew consent before the beginning of the FREEDOM Follow-On study. As observed in the figure, there were numerical differences in the numbers of early termination and missing death status between the 2 treatment groups. CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention.
The baseline characteristics are summarized in Table 1, according to their randomized treatment group within extended and without extended follow-up cohorts. When compared with patients without extended follow-up, those with extended follow-up were less likely to have a history of stroke (p < 0.001) and to receive a radial graft (p = 0.003) and were more likely to have had a prior myocardial infarction (p < 0.001) and to be receiving oral glucose-lowering drugs at the time of the randomization (p < 0.001). However, none of the baseline characteristics was statistically significantly different between PCI-DES and CABG within the extended or without extended follow-up cohorts.
TABLE 1.
Baseline Characteristics of Patients According to Their Randomized Treatment and Follow-Up Cohort
| Extended Follow-Up |
No Extended Follow-Up |
p Value | |||
|---|---|---|---|---|---|
| PCI (n = 478) | CABG (n = 465) | PCI (n = 475) | CABG (n = 482) | ||
| Age at randomization, yrs | 63.4 ± 8.4 | 63.0 ± 9.1 | 62.9 ± 9.3 | 63.1 ± 9.4 | 0.52 |
| Male | 337 (70.5) | 314 (67.5) | 361 (76.0) | 344 (71.4) | 0.03 |
| Glycated hemoglobin, % | 7.9 ± 1.9 | 7.8 ± 1.7 | 7.6 ± 1.6 | 7.6 ± 1.6 | 0.03 |
| Current smoker | 61 (12.8) | 75 (16.1) | 80 (16.8) | 82 (17.0) | 0.13 |
| Hypertension | 395 (82.6) | 399 (85.8) | 411 (86.5) | 407 (84.4) | 0.44 |
| Previous stroke | 10 (2.1) | 7 (1.5) | 27 (5.7) | 21 (4.4) | <0.001 |
| Previous myocardial infarction | 141 (29.5) | 141 (30.3) | 109 (22.9) | 96 (19.9) | <0.001 |
| Recent acute coronary syndrome | 143 (29.9) | 127 (27.3) | 161 (33.9) | 152 (31.5) | 0.05 |
| Total cholesterol, mg/dl | 166.4 ± 72.1 | 166.6 ± 45.8 | 171.7 ± 109.6 | 166.8 ± 44.7 | 0.75 |
| LDL cholesterol, mg/dl | 91.4 ± 32.7 | 93.4 ± 37.6 | 93.4 ± 37.9 | 92.9 ± 36.8 | 0.98 |
| HDL cholesterol, mg/dl | 38.5 ± 10.9 | 38.9 ± 10.8 | 39.5 ± 10.9 | 40.0 ± 12.0 | 0.03 |
| Triglycerides median, mg/dl | 184.4 ± 307.8 | 177.2 ± 136.7 | 203.3 ± 496.6 | 178.5 ± 127.4 | 0.95 |
| BMI, kg/m2 | 29.5 ± 5.5 | 29.7 ± 5.2 | 29.7 ± 5.2 | 29.9 ± 5.4 | 0.31 |
| Creatinine clearance, ml/min | 89.0 ± 50.0 | 86.5 ± 34.1 | 90.9 ± 37.8 | 90.2 ± 45.4 | 0.10 |
| Microalbuminuria, mg/dl | 94.6 ± 227.4 | 117.0 ± 295.7 | 89.1 ± 364.6 | 76.3 ± 188.1 | 0.27 |
| Left ventricular ejection fraction | |||||
| Percent | 65.8 ± 12.2 | 66.2 ± 10.4 | 65.6 ± 12.0 | 67.1 ± 10.6 | 0.52 |
| <40% | 11/335 (3.3) | 4/322 (1.2) | 10/306 (3.3) | 7/328 (2.1) | 0.65 |
| EuroSCORE | 0.17 | ||||
| Mean | 2.5 ± 2.0 | 2.7 ± 2.2 | 2.8 ± 2.7 | 2.8 ± 2.8 | |
| Median (interquartile range) | 1.8 (1.2–3.0) | 1.9 (1.2–3.2) | 2.0 (1.3–3.2) | 2.1 (1.3–3.3) | |
| SYNTAX score | 0.04 | ||||
| Mean | 25.5 ± 8.5 | 26.2 ± 9.4 | 26.9 ± 8.2 | 26.1 ± 8.1 | |
| Median (interquartile range) | 25.0 (20.0–31.0) | 25.5 (19.0–32.0) | 27.0 (21.0–31.5) | 26.0 (20.0–31.0) | |
| Category | 0.08 | ||||
| Low | 180/474 (38.0) | 173/459 (37.7) | 149/475 (31.4) | 167/479 (34.9) | |
| Intermediate | 210/474 (44.3) | 186/459 (40.5) | 228/475 (48.0) | 220/479 (45.9) | |
| High | 84/474 (17.7) | 100/459 (21.8) | 98/475 (20.6) | 92/479 (19.2) | |
| 3-vessel disease | 386/474 (81.4) | 379/459 (82.6) | 394/474 (83.1) | 414/480 (86.3) | 0.12 |
| Chronic total occlusion | 147/2,729 (5.4) | 162/2,759 (5.9) | 176/2,835 (6.2) | 167/2,903 (5.8) | 0.35 |
| Use of insulin | 150/477 (31.4) | 141/465 (30.3) | 172/475 (36.2) | 152/482 (31.5) | 0.17 |
| Use of oral glucose-lowering drugs | 386/477 (80.9) | 382/465 (82.2) | 350/475 (73.7) | 347/482 (72.0) | <0.001 |
| Total no. of lesions stented across all stages | 3.4 ± 1.3 | — | 3.7 ± 1.5 | — | 0.01 |
| Surgery off-pump | — | 82/440 (18.6) | — | 83/453 (18.3) | 0.87 |
| No. of graft vessels | — | 2.9 ± 0.8 | — | 2.9 ± 0.8 | 0.74 |
| Left internal thoracic artery graft | — | 415/440 (94.3) | — | 428/453 (94.5) | >0.99 |
| Bilateral-internal thoracic artery graft | — | 54/440 (12.3) | — | 56/453 (12.4) | >0.99 |
| Left internal thoracic artery and radial graft | — | 28/440 (6.4) | — | 56/453 (12.4) | 0.003 |
Values are mean ± SD, n (%), or n/N (%), unless otherwise indicated. p values for comparisons between patients with and without extended follow-up. There were no significant differences between the treatment arms (CABG vs. PCI) within each follow-up cohort.
BMI = body mass index; CABG = coronary artery bypass grafting; HDL = high-density lipoprotein; LDL = low-density lipoprotein; PCI = percutaneous coronary intervention.
FOLLOW-UP OF THE EXTENDED COHORT.
The median duration of follow-up for the whole cohort of all patients during the original FREEDOM trial was 3.8 years (range 0.0 to 6.9 years; interquartile range: 2.5 to 4.9 years; mean 3.6 ± 1.6 years). The median duration of follow-up in the original FREEDOM trial for subjects that were later included in the extended follow-up cohort was 3.6 years (range 0.0 to 6.9 years; interquartile range: 2.5 to 5.0 years; mean 3.6 ± 1.6 years). Summing up the follow-up period during the original FREEDOM trial and the follow-up period during the FREEDOM Follow-On Study, patients from the extended follow-up cohort were followed by 7.5 years (range 0.0 to 13.2 years; interquartile range: 5.0 to 9.0 years; mean 6.7 ± 3.1 years). This is an increase of 3.9 years in the median follow-up or an increase of 3.1 ± 2.3 years on the average follow-up.
SURVIVAL ANALYSIS.
Whole cohort.
A total of 314 patients died during the entire follow-up period: 204 deaths occurred during the original FREEDOM trial and 110 in the FREEDOM Follow-On study. Figure 2A shows survival curves for up to 8 years of follow-up for the whole cohort of all patients enrolled in the FREEDOM trial. The follow-up event rate at 8 years was 24.3% (159 deaths) in the PCI-DES group, as opposed to 18.3% (112 deaths) in the CABG group (unadjusted hazard ratio [HR]: 1.36; 95% confidence interval [CI]: 1.07 to 1.74; p = 0.01). The treatment-time interaction was not statistically significant (p = 0.27). The log(−log[survival function]) curve shows the curves overlap in the first 2 years and then they become parallel (Online Figures 1A to 1C). Allowing that a nonconstant treatment HR on all-cause death suggested a lag in the treatment effect, a treatment difference was absent during the first 2 years (HR: 1.04; 95% CI: 0.73 to 1.50; p = 0.82), but PCI had a higher risk of death after the second year (HR: 1.69; 95% CI: 1.22 to 2.36; p = 0.002). The comparison of the model fit with and without a constant HR resulted in a p value of 0.051. The results from the non-ITT analyses were similar.
FIGURE 2. Kaplan-Meier Estimates of Survival in the 2 Treatment Groups.
(A) Survival curves for the whole cohort with all patients enrolled in the FREEDOM trial, according to treatment group (n = 1,900). (B) Survival curves only for patients from the extended follow-up cohort, according to treatment group (n = 943). CI = confidence interval; HR = hazard ratio; other abbreviations as in Figure 1.
Extended follow-up cohort.
With consideration of only patients from centers that participated in FREEDOM Follow-On study (943 patients), there were 99 deaths (event rate 23.7%) in the PCI-DES group and 72 deaths (event rate 18.7%) in the CABG group (unadjusted HR: 1.32; 95% CI: 0.97 to 1.78; p = 0.076)over 8 years of follow-up (Figure 2B). The results from the non-ITT analyses were similar. Of note, 17 centers (representing 415 patients) also recorded data regarding myocardial infarction (proportion of events: 4.7% in the PCI group [10 events] vs. 4.0% [8 events] in the CABG group) and stroke (proportion of events: 2.3% in the PCI group [5 events] vs. 1.5% [3 events] in the CABG group) during the extended follow-up period.
EXTENDED VERSUS WITHOUT EXTENDED FOLLOW-UP COHORT IN THE ORIGINAL TRIAL.
To explore whether the 5-year follow-up during the original FREEDOM trial was different between patients that were later included in the extended follow-up cohort versus patients without extended follow-up, survival curves for these 2 cohorts are shown in Online Figures 2A and 2B. The treatment effects comparing PCI-DES versus CABG were comparable between the 2 cohorts.
MULTIVARIABLE AND SUBGROUP ANALYSES.
Whole cohort.
After controlling for key baseline characteristics, the Cox regression analysis of the whole cohort of all FREEDOM trial patients demonstrated that the HR for death from any cause during the entire post-revascularization follow-up period in the PCI-DES group versus the CABG group was 1.38 (95% CI: 1.08 to 1.76; p = 0.01). Figure 3 shows the treatment effect in the various subgroups of interest in the whole cohort of all FREEDOM trial patients. The survival benefit of CABG versus PCI-DES was consistent across most subgroups. Patients younger than the median age at study entry (63.3 years) tended to derive preferential benefit from CABG, as well as those with a history of smoking and patients from centers in North America (p values for interaction, respectively: 0.001, 0.01, and 0.02).
FIGURE 3. Subgroup Analysis of All-Cause Mortality for the Whole Cohort.
The treatment effect compared PCI with drug-eluting stents versus CABG, both on top of optimal medical therapy. HRs for LVEF <40%: 2.70 (95% CI: 0.58 to 12.50); absence of LAD involvement: 1.88 (95% CI: 0.78 to 4.49); and positive history of stroke: 1.83 (95% CI: 0.57 to 5.86). LAD = left anterior descending artery; LVEF = left ventricular ejection fraction; other abbreviations as in Figures 1 and 2.
EXTENDED FOLLOW-ON COHORT.
Similar trends were observed when performing multivariate analyses (HR: 1.32; 95% CI: 0.97 to 1.79; p = 0.076), and when performing subgroup analyses only with patients from centers with extended follow-up (Online Figure 3).
DISCUSSION
In this long-term follow-up study comparing coronary revascularization either with PCI-DES or CABG in patients with DM and MVD enrolled in the FREEDOM trial, CABG was associated with a significant reduction in all-cause mortality at 8 years (Central Illustration). These results are consistent with the 10-year outcomes of the DM subgroup of the BARI trial (2,4), even after considering the advances in the PCI technique (BARI patients underwent angioplasty alone without stent placement) and improvements in medical therapy. It is critical that guideline-driven optimization of medical therapy is adopted as the cornerstone in the management of DM patients with MVD (8).
CENTRAL ILLUSTRATION. Survival Curves According to the Revascularization Strategy in the FREEDOM Follow-On Study.
KapLan-Meier estimates and survival curves including all patients enrolled in the FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial (whole cohort of patients). Coronary artery bypass grafting results in a Long-term survival benefit in patients with diabetes and multivessel coronary disease when compared with revascularization with percutaneous coronary intervention with drug-eluting stents.
FREEDOM was the first adequately powered randomized trial to compare PCI-DES with CABG in patients with DM and MVD. Since the publication of the original FREEDOM trial in 2012, clinical practice guidelines from major international cardiovascular societies have recommended CABG over PCI as the revascularization method of choice in patients with DM and MVD (10-13). Other clinical trials of patients with DM were with shorter follow-up duration but also reported superior results with CABG when compared to PCI with bare-metal stents (14,15), first-generation DES (16-19), and second-generation DES (20). A patient-level pooled analysis (21) of the 5,034 patients with DM randomized in the FREEDOM, BARI-2D, and COURAGE trials also reported lower mortality rates associated with CABG in comparison with PCI (HR: 0.76; 95% CI: 0.60 to 0.96; p = 0.024) after a median 4.5 years of follow-up. In addition, Head et al. (22) recently published a patient-level analysis of 11 trials comparing CABG and PCI, in which 34.2% of the PCI procedures were performed with newer-generation DES. In the subgroup of patients with DM and MVD (n = 3,266), PCI was associated with a higher risk of 5-year all-cause mortality (HR: 1.48; 95% CI: 1.19 to 1.84; p = 0.0004).
Although further advances in PCI have been made since the FREEDOM trial, data over the past 5 years continue to support CABG over PCI in patients with stable CAD and DM. A registry-based analysis of all revascularization procedures performed in patients with DM and MVD in the province of British Columbia, Canada, reported that CABG was associated with a lower mortality rate in comparison with PCI (7.8% vs. 12.2%; p < 0.01) after a median follow-up of 3.3 years (23). Additionally, the BEST trial randomized 880 patients with MVD (approximately 40% with DM) and demonstrated a significantly higher rate of the primary composite outcome of death, myocardial infarction, or target vessel revascularization after PCI with everolimus-eluting stents when compared with CABG (20). Furthermore, the rates of spontaneous myocardial infarction and revascularization due to new lesions were significantly higher in the PCI versus CABG arms—protection from both of which have been suggested to be the mechanism of benefit with CABG in patients with diffuse atherosclerosis such as DM (24).
In the original FREEDOM trial, the mortality curves for PCI-DES versus CABG began to separate during the second year of follow-up. With long-term follow-up, the curves continued to separate, making this difference more pronounced. Of note, the same mortality trend was observed in the survival curves comprised of only patients with extended follow-up and the lack of statistical significance in this cohort is likely to be due to lack of power. FREEDOM Follow-On demonstrated important trends for greater benefit of CABG in those patients who were younger, had a history of smoking, and who were enrolled in North American centers; all of which deserve further evaluation. In the meta-analysis by Head et al. (22), no interaction was found between age and revascularization method after 5 years of follow-up. For younger patients, the greater benefit of CABG in FREEDOM Follow-On supports the hypothesis that the number of life-years gained can only be evaluated by extending follow-up beyond 5 years and introduces age as a potential key determinant in the shared decision-making process around the optimal mode of revascularization in patients with DM and MVD. History of smoking was evaluated in the cohort with DM in the BARI trial, and, after 7 years of follow-up, unlike our findings, no differential survival benefit was present in smokers versus nonsmokers according to the mode of revascularization (25). Finally, the FREEDOM Follow-On study corroborates the trend observed in the original FREEDOM trial that regional differences may also be a determinant of clinical outcomes following coronary revascularization.
STUDY LIMITATIONS.
First, the cohort of patients with extended follow-up included only one-half (49.6%) of the population in the original FREEDOM trial. However, it is important to consider that the FREEDOM trial randomization was stratified by site, and a very high overall follow-up rate (99%) was attained in the participating centers of the FREEDOM Follow-On study. Moreover, there were only few baseline differences between the cohorts with and without extended follow-up, which do not seem to have a major influence in the long-term survival, although theoretically unmeasured confounders may also intervene in the results. Second, although efforts were made to contact all of the patients in participating centers by phone or by mail, there was a small proportion of patients that were lost to follow-up or withdrew consent before and during the FREEDOM Follow-On study, and this was numerically higher in the CABG group, as depicted in Figure 1. Also, for a minority of patients from both treatment groups, vital status was obtained through local administrative registries. However, this comprises only a small percentage of the entire population (<10%) and, even considering that these data sources may be less robust, it is unlikely to change the overall study results. Third, in the last decade, stent platforms have continued to evolve as newer-generation stents have been adopted into practice. These newer stents were largely unavailable during the enrollment period of the FREEDOM trial. The results of FREEDOM must be interpreted based on the totality of the evidence demonstrating that changes in PCI platforms have not significantly altered the effect size of the long-term survival advantage of CABG over PCI (22). Medical treatment options for DM have also evolved in the last decade as newer hypoglycemic agents are also associated with reductions in major adverse cardiovascular and cerebrovascular event rates (26,27). The combination of these newer glucose-lowering medications with a contemporary revascularization strategy has yet to be studied. Finally, data concerning concurrent medical therapy, additional revascularization procedures or other clinically relevant endpoints, such as myocardial infarction and stroke, were available only at selected centers during the extended follow-up period and, therefore, not included in the current analysis. We are unable to assess the contribution of incomplete revascularization to survival in FREEDOM since this was not assessed at the time of angiographic core laboratory evaluation. Nonetheless, this study provides the longest follow-up data on all-cause mortality after randomization of patients with DM and MVD, without left main disease, to PCI-DES or CABG.
CONCLUSIONS
In the present FREEDOM Follow-On study, we performed a long-term survival analysis of the patients enrolled in the FREEDOM trial. In patients with DM and MVD and without left main disease, CABG remains superior to PCI-DES in reducing all-cause mortality at a follow-up of 8 years. These data support current recommendations that CABG be considered the preferred revascularization strategy for such patients.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: SurgicaL revascularization of patients with diabetes and MVD is associated with Lower mortality compared to PCI.
TRANSLATIONAL OUTLOOK: Advances in medical therapy, PCI technology, and CABG techniques over the decade since the FREEDOM trial began should be taken into account when considering revascularization of patients with diabetes and coronary artery disease.
Acknowledgments
The original FREEDOM trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI), with grants from Cordis Corporation, a Johnson & Johnson Company at the time, Boston Scientific (providing the stents), Eli Lilly (providing abciximab and an unrestricted research grant), Sanofi, and Bristol-Myers Squibb (providing clopidogrel). The FREEDOM Follow-On study was sponsored by the Joseph and Vicky Safra Foundation. No company or agency provided additional funding for the extended follow-up, and none participated in the review, analysis, and decision to submit the manuscript for publication. Dr. Farkouh has received research grants from Amgen and Novo Nordisk. Dr. Dangas’s spouse has served on the advisory board for Boston Scientific and Abbott Vascular; and has served as a consultant to Abbott Vascular. Dr. Shah has served on the advisory board of Philips Volcano; and has served as a consultant to Terumo. Dr. Stefanini has received a research grant (to his institution) from Boston Scientific; and has received speaker/consultant fees from B.Braun, Biosensors, and Boston Scientific. Dr. Sharma has served on the Speakers Bureau for Abbott Vascular, Boston Scientific, Cardiovascular Systems, Inc., and TriReme. Dr. Cohen has received research grant support to his institution and consulting income (modest) from Medtronic; research grant support from Abbott Vascular; and research grant support to his institution from Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Deepak L. Bhatt, MD, MPH, served as Guest Editor for this paper.
ABBREVIATIONS AND ACRONYMS
- CABG
coronary artery bypass graft
- DES
drug-eluting stents
- DM
diabetes mellitus
- ITT
intention to treat
- MVD
multivessel coronary disease
- PCI
percutaneous coronary intervention
Footnotes
APPENDIX For supplemental figures and a table, please see the online version of this paper.
REFERENCES
- 1.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–84. [DOI] [PubMed] [Google Scholar]
- 2.The BARI Investigators. Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease. N Engl J Med 1997;96:1761–9. [DOI] [PubMed] [Google Scholar]
- 3.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007;49:1600–6. [DOI] [PubMed] [Google Scholar]
- 5.Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 2015;373:1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farkouh ME, Dangas G, Leon MB, et al. Design of the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) Trial. Am Heart J 2008;155:215–23. [DOI] [PubMed] [Google Scholar]
- 7.Bansilal S, Farkouh ME, Hueb W, et al. The Future REvascularization Evaluation in patients with Diabetes mellitus: optimal management of Multivessel disease (FREEDOM) trial: clinical and angiographic profile at study entry. Am Heart J 2012;164:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkouh ME, Boden WE, Bittner V, et al. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol 2013;61:1607–15. [DOI] [PubMed] [Google Scholar]
- 9.Allison PD. Survival Analysis Using SAS. A Practical Guide. Cary, NC: SAS Institute Inc, 1995. [Google Scholar]
- 10.Patel MR, Calhoon JH, Dehmer GJ, et al. Appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease. J Am Coll Cardiol 2017;69:2212–41. [DOI] [PubMed] [Google Scholar]
- 11.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 12.Teo KK, Cohen E, Buller C, et al. Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology/Canadian Society of Cardiac Surgery position statement on revascularization—multivessel coronary artery disease. Can J Cardiol 2014;30:1482–91. [DOI] [PubMed] [Google Scholar]
- 13.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 focused update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. J Am Coll Cardiol 2014;64:1929–49. [DOI] [PubMed] [Google Scholar]
- 14.Abizaid A, Costa MA, Centemero M, et al. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation 2001;104:533–8. [DOI] [PubMed] [Google Scholar]
- 15.Lima EG, Hueb W, Garcia RM, et al. Impact of diabetes on 10-year outcomes of patients with multivessel coronary artery disease in the Medicine, Angioplasty, or Surgery Study II (MASS II) trial. Am Heart J 2013;166:250–7. [DOI] [PubMed] [Google Scholar]
- 16.Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–75. [DOI] [PubMed] [Google Scholar]
- 17.Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010;55:432–40. [DOI] [PubMed] [Google Scholar]
- 18.Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013;61:808–16. [DOI] [PubMed] [Google Scholar]
- 19.Kappetein AP, Head SJ, Morice MC, et al. , for the SYNTAX Investigators. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013;43:1006–13. [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Ahn JM, Kim YH, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–12. [DOI] [PubMed] [Google Scholar]
- 21.Mancini GB, Farkouh ME, Brooks MM, et al. Medical treatment and revascularization options in patients with type 2 diabetes and coronary disease. J Am Coll Cardiol 2016;68:985–95. [DOI] [PubMed] [Google Scholar]
- 22.Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939–48. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan K, Abel JG, Park JE, et al. Surgical versus percutaneous coronary revascularization in patients with diabetes and acute coronary syndromes. J Am Coll Cardiol 2017;70:2995–3006. [DOI] [PubMed] [Google Scholar]
- 24.Domanski MJ, Farkouh ME. Type 1 diabetes, coronary disease complexity, and optimal revascularization strategy. J Am Coll Cardiol 2017;70:1452–4. [DOI] [PubMed] [Google Scholar]
- 25.The BARI Investigators. Seven-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) by treatment and diabetic status. J Am Coll Cardiol 2000;35:1122–9. [DOI] [PubMed] [Google Scholar]
- 26.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.