Abstract
Information about the tissue characteristics of abdominal aortic aneurysms (AAAs), some of which may be reflected in the serum, can help to elucidate AAA pathogenesis and identify new AAA biomarkers. This information would be beneficial not only for diagnostics and follow-up but also for potential therapeutic intervention. Therefore, the aim of our study was to compare the expression of structural proteins, immune factors (T and B lymphocytes, macrophages, neutrophils and pentraxin 3 (PTX3)), osteoprotegerin (OPG), microvessels and hypoxic cells in AAA and nonaneurysmal aortic walls. We examined specimens collected during surgery for AAA repair (n = 39) and from the abdominal aortas of kidney donors without AAA (n = 8). Using histochemical and immunohistochemical methods, we quantified the areas positive for smooth muscle actin, desmin, elastin, collagen, OPG, CD3, CD20, MAC387, myeloperoxidase, PTX3, and hypoxia-inducible factor 1-alpha and the density of CD31-positive microvessels. AAA samples contained significantly less actin, desmin, elastin and OPG, more collagen, macrophages, neutrophils, T lymphocytes, B lymphocytes, hypoxic cells and PTX3, and a greater density of vasa vasorum (VV) than those in non-AAA samples. Hypoxia positively correlated with actin and negatively correlated with collagen. Microvascular density was related to inflammatory cell infiltrates, hypoxia, PTX3 expression and AAA diameter. The lower OPG expression in AAAs supports the notion of its protective role in AAA remodeling. AAA contained altered amounts of structural proteins, implying reduced vascular elasticity. PTX3 was upregulated in AAA and colocalized with inflammatory infiltrates. This evidence supports further evaluation of PTX3 as a candidate marker of AAA. The presence of aortic hypoxia, despite hypervascularization, suggests that hypoxia-induced neoangiogenesis may play a role in AAA pathogenesis. VV angiogenesis of the AAA wall increases its vulnerability.
Introduction
Abdominal aortic aneurysms (AAAs) occur in 1–7% of the population over 50 years of age [1]. The pathomechanisms underlying the development of AAAs and AAA instability, which may induce AAA disruption, are still unclear. Therefore, the prevention and treatment of AAAs are insufficient. Furthermore, tools for monitoring AAAs and predicting their complications are limited.
Thus, it is important to identify the crucial structural changes and processes that lead to the development of AAAs and AAA instability. Some of these may be reflected in the serum, serve as biomarkers for diagnosing and monitoring AAAs, and predict their complications. Moreover, improved insights into the pathophysiological processes may help to identify novel therapeutic targets.
AAAs are characterized by decreased vascular elasticity. There are theories that inflammation and changes in microcirculation can contribute to the vascular remodeling of aneurysms [2–5]. Aortic inflammatory cells (T and B lymphocytes) and endothelial cells from invading neovessels express matrix metalloproteinases (MMP) and may substantially contribute to aneurysm instability [6]. Nonetheless, there have been inconsistent results regarding the vascularization of AAAs; while a study by Eberlová revealed lower microvascular density in AAAs, Rodella found a higher density of microvessels in the AAA aorta compared to the non-AAA aorta [2,4].
Among the factors that may be involved in the pathogenesis of AAAs are osteoprotegerin (OPG) and pentraxin 3 (PTX3). PTX3 is a molecule of the innate immune system that protects against infections, participates in the clearance of apoptotic cells, modulates inflammation and angiogenesis, and participates in extracellular matrix formation. PTX3 belongs to the same protein family as C-reactive protein (CRP). However, in contrast to CRP, it is produced locally in the inflamed tissue and in neutrophils [7]. There are indications that PTX3 may be superior to CRP as a biomarker of atherosclerotic cardiovascular diseases (CVD) (including acute coronary syndromes), possibly due to its ability to reflect vascular inflammation and due to the speed of its response [8–10]. Interestingly, the role of PTX3 in CVD may be protective, and PTX3 may represent a relevant therapeutic target [11,12]. Nevertheless, there is currently minimal knowledge about the role of PTX3 in AAAs.
OPG, a key regulator of bone remodeling, has also been implicated in the immune response and vascular diseases. OPG is secreted by osteoblasts, endothelial cells, human aortic vascular smooth muscle cells (VSMCs), dendritic cells, lymphocytes and plasma cells [13]. OPG inhibits vascular calcification by regulating the procalcific effects of receptor activator of nuclear factor kappa-B ligand in VSMCs [14,15]. The role of OPG in CVD has not yet been fully clarified. Clinical studies have shown that high OPG levels are related to the presence and progression of CVD, including AAAs [13,16,17]. However, animal models point to a protective role of OPG in CVD [18,19].
In order to improve insights into vessel wall alterations in AAAs, we compared the expression of structural proteins, osteoprotegerin, and pentraxin 3 and the presence of immune factors (T and B lymphocytes, neutrophils and macrophages), microvessels and hypoxic cells in AAA and non-aneurysmal aortic walls and to explore their relationships.
Materials and methods
Patients
In this study, we examined aortic tissue removed during open surgical repair of AAA from 39 patients, and corresponding aortic specimens from 8 individuals—cadaveric organ donors without aortic aneurysms. In the AAA group, the inclusion criteria were a diagnosis of AAA and open surgery at University Hospital in Pilsen. The exclusion criteria were malignancy in the anamnesis, infection or autoimmune disease, and renal and hepatic dysfunction. There were no inflammatory AAAs in our group of patients as well as no familial AAAs. Also all AAAs were non-ruptured. Control samples were from heart-beating donors after brain death diagnosed. The samples were taken together with other organs for transplantation according to the Transplantation Act valid in the Czech Republic. Thus, the samples did not suffer any warm ischemia. The aortas were flushed with a perfusion solution together with the organs, and immediately after explantation they were fixed in formalin solution. This study conforms to the principles outlined in the Declaration of Helsinki. The study was approved by the Ethical Committee of University Hospital and the Faculty of Medicine of Charles University in Pilsen on 12th August 2014, and all the AAA patients gave written informed consent.
Histological analysis
The specimens were fixed in formalin and embedded in paraffin, and each was cut into 28 serial 4-μm thick histological sections. The sections were stained using a battery of 14 methods to assess of overall morphology and markers of main tissue components, immune factors, hypoxia, osteoprotegerin and microvessels (Table 1). General protocol for immunohistochemical methods can be find here: dx.doi.org/10.17504/protocols.io.7uxhnxn. S1 Table provides details regarding the antibodies and pretreatment used in the immunohistochemical methods. The immunohistochemical reactions were visualized with diaminobenzidine (DAB+, Liquid; DakoCytomation, Glostrup, Denmark). The immunohistochemical sections were counterstained with Gill’s haematoxylin.
Table 1. Histological staining methods used in the study.
| Staining | Purpose and visualization of aortic wall components |
|---|---|
| Hematoxylin-eosin [20] | Overall morphology of the aortic wall |
| Verhoeff’s hematoxylin and green trichrome [21] | Overall morphology, differentiating connective tissue, smooth muscle |
| Picrosirius red (Direct Red 80, Sigma Aldrich, Munich, Germany) [22] | Type I and type III collagen when observed under circularly polarized light |
| Orcein (Tanzer’s orcein, Bowley Biochemical Inc., Danvers, MA, USA) | Elastic membranes, elastic fibres |
| Immunohistochemical detection of alpha-smooth muscle actin | Contractile phenotype of vascular smooth muscle cells |
| Immunohistochemical detection of desmin | Contractile phenotype of vascular smooth muscle cells |
| Immunohistochemical detection of MAC387 | Macrophages infiltrating the aortic wall |
| Immunohistochemical detection of myeloperoxidase | Neutrophilic granulocytes infiltrating the aortic wall |
| Immunohistochemical detection of CD3 | T-lymphocytes |
| Immunohistochemical detection of CD20 | B-lymphocytes |
| Immunohistochemical detection of CD31 | Endothelium of vasa vasorum |
| Immunohistochemical detection of HIF 1-alpha | Hypoxia-inducible factor 1-alpha, a marker of tissue hypoxia |
| Immunohistochemical detection of Pentraxin-3 | Pentraxin 3, a protein produced in response to inflammatory signals |
| Immunohistochemical detection of Osteoprotegerin | Osteoprotegerin, a calcium controlling protein |
For each staining method, 4 micrographs were randomly collected in a systematic and uniform manner; resulting in total 56 micrographs for each sample. We used 20× and 40× objectives mounted on an Olympus BX51 microscope to take the photomicrographs (see S2 Table). The unbiased sampling of the micrographs of the sections was performed as described in our previous studies on the aorta [23,24]. Briefly, starting in a randomly selected part of a histological section, the (x, y) distances between 4 micrographs uniformly covered the entire circumference of the tunica media, including the image fields bordering the adventitia or the lumen. This resulted in a fair sampling of the image fields, in which all of the components and structures were selected with a probability proportional to their areas on the histological slide. To quantify collagen by microscopy, we used a circular polarizing filter (Hama, Monheim, Germany) crossed with a quarter wave λ/4 filter below the analyser filter (U-GAN, Olympus, Tokyo, Japan) mounted on an Olympus CX41 microscope (Olympus, Tokyo, Japan). The advantages of this method are described elsewhere [22].
Histological quantification was performed as described previously [2,23–28]. Briefly, a stereological point grid (the PointGrid module of Ellipse software (ViDiTo, Košice, Slovakia), cf. [29]) was loaded and randomly superposed on the micrographs to quantify the area of actin, desmin, elastin, collagen staining and the immunopositivity of macrophages, neutrophils, T lymphocytes, B lymphocytes, hypoxic cells, PTX3, and OPG. The number of points that intersected the structure of interest was counted. The point grid method allowed for individual corrections of the reference space (i.e., tunica media or adventitia) for any possible artefacts, microcracks or the presence of the lumen vs. the vessel wall. Whenever possible, the entire wall was used as a meaningful reference space [30]. The area for each parameter was calculated for each protein by dividing the number of grid lines intersecting the structure of interest by the number of grid lines intersecting the reference space of the vascular wall, and the result was multiplied by 100. The vascularization of the wall was quantified using an unbiased counting frame positioned on the micrographs. The number of CD31-positive vasa vasorum (VV) profiles was divided by the sum of the areas of the counting frame and expressed as a two-dimensional density of microvessels (QA, quantity per area).
Statistics
In some cases, the surgeon took two samples from one patient. We processed both samples and used the average results for the statistical analysis. We used the chi-square test to assess differences in proportions among the groups. We compared continuous nonnormally distributed variables by the Mann-Whitney-Wilcoxon test. Correlations were assessed using the Spearman correlation coefficient and Kendall's tau coefficient. All tests were two-sided and performed with the Statistica Base 11 package (StatSoft, Inc., Tulsa, OK, USA). The level of statistical significance was set at 0.05. False discovery rate (FDR) was controlled using the Benjamini-Hochberg procedure carried out upon the results of all significance test performed within the study. At the baseline significance level of 0.05, the estimated FDR is 20%, indicating 80% of the presented significant results to be true positives. A conservative overall FDR of 5% would require the individual significance level of 0.005.
The complete primary morphometric data from our samples (see S3 Table) have been made publicly available for further analyses.
Results
Characteristics of the examined individuals are presented in Table 2. The AAA group showed a trend toward a higher proportion of males and a significantly higher mean age.
Table 2. Data of patients.
| AAA patient (n = 39) | Control group (n = 8) | p value | |
|---|---|---|---|
| Male n (%) | 32 (82%) | 4 (50%) | 0.057 |
| Age (years) | 70 (6.7) | 51 (10.4) | 0.00024 |
| Average size of aneurysm (mm) | 55 (11.8) | Not applicable | |
| Diabetes mellitus | 8 (18%) | 1 (13%) | NS |
| Hypertension | 27 (61%) | 3 (38%) | NS |
| Ischemic heart disease | 18 (41%) | 2 (25%) | NS |
| Peripheral artery disease | 12 (27%) | 4 (50%) | NS |
| Smoker—current | 22 (56%) | 3 (38%) | NS |
| Smoker—former | 9 (23%) | 2 (25%) | NS |
| Non-smoker | 8 (21%) | 3 (38%) | NS |
Values are the median (SD) or frequency (relative frequency). NS–not significant.
Comparisons of AAA and non-AAA samples are summarized in Table 3. AAA samples contained significantly less actin, desmin, elastin (Fig 1) and osteoprotegerin, more collagen, macrophages, neutrophils, T lymphocytes, B lymphocytes, hypoxic cells and PTX3, and a greater density of VV (Fig 2) than did non-AAA samples. Microvascular density was related to inflammatory cell infiltrates, PTX3 expression and hypoxia.
Table 3. Testing the differences between the AAA vs. the non-AAA samples of aortic wall.
| AAA | Non AAA | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Median | Lower Quartile | Upper Quartile | Median | Lower Quartile | Upper Quartile | p-value |
| AA(actin,int+media) | 2.1 | 1.8 | 3.1 | 15.3 | 11.1 | 17.0 | 0.000013 |
| AA(desmin,int+media) | 0.3 | 0.1 | 0.4 | 0.6 | 0.4 | 0.7 | 0.025 |
| AA(elastin,int+media) | 0.8 | 0.2 | 1.4 | 3.9 | 3.1 | 5.2 | 0.00015 |
| AA(collagen,int+media) | 9.0 | 3.2 | 13.4 | 1.2 | 0.7 | 5.1 | 0.0034 |
| AA(MAC387, wall) | 1.2 | 0.9 | 1.8 | 0.7 | 0.5 | 1.0 | 0.039 |
| AA(myeloperoxidase, wall) | 2.5 | 2.0 | 3.0 | 1.2 | 1.0 | 2.6 | 0.046 |
| AA(CD3, wall) | 0.4 | 0.3 | 0.5 | 0.2 | 0.1 | 0.3 | 0.009 |
| AA(CD20, wall) | 0.7 | 0.4 | 1.0 | 0 | 0 | 0.2 | 0.0001 |
| AA(HIF 1-alpha, wall) | 0.9 | 0.6 | 1.0 | 0.3 | 0.2 | 0.4 | 0.001 |
| AA(osteoprotegerin, wall) | 0.6 | 0.5 | 0.9 | 1.4 | 1.3 | 1.8 | 0.001 |
| AA(pentraxin-3, wall) | 0.7 | 0.5 | 0.9 | 0.4 | 0.3 | 0.5 | 0.007 |
| QA(CD31-positive microvessels,wall) (mm-2) | 74.4 | 52.4 | 98.1 | 37.2 | 24.5 | 38.9 | 0.0006 |
AA(component, space): Area fraction of the respective components within their reference spaces (%); QA: number of microvessel profiles per section area; int+media: data pooled from the intima and media; wall: data pooled the wall (i.e., from intima, media and adventitia). The abbreviations of all the parameters are explained in S2 Table.
Fig 1. Wall composition in AAA (left and middle columns) and non-AAA samples (right column).

In the AAA samples, the collagen was more abundant (A), the elastin was partially or mostly destroyed (B), the wall contained more vasa vasorum (D), expressed more hypoxia markers (E) and less contractile phenotype of vascular smooth muscle (C, F). Stained with picrosirius red (A), orcein (B), and immunohistochemistry with anti-smooth muscle actin antibody (C) for visualization of the contractile phenotype of vascular smooth muscle cells, anti-CD31 (D) for visualization of endothelium, anti-HIF 1-alpha (E) for visualization of tissue hypoxia and anti-desmin (F) for visualization of the contractile phenotype of vascular smooth muscle cells; nuclei were stained with Gill’s hematoxylin; scale bar 50 μm.
Fig 2. Wall composition in AAA (left and middle column) and non-AAA samples (right column).
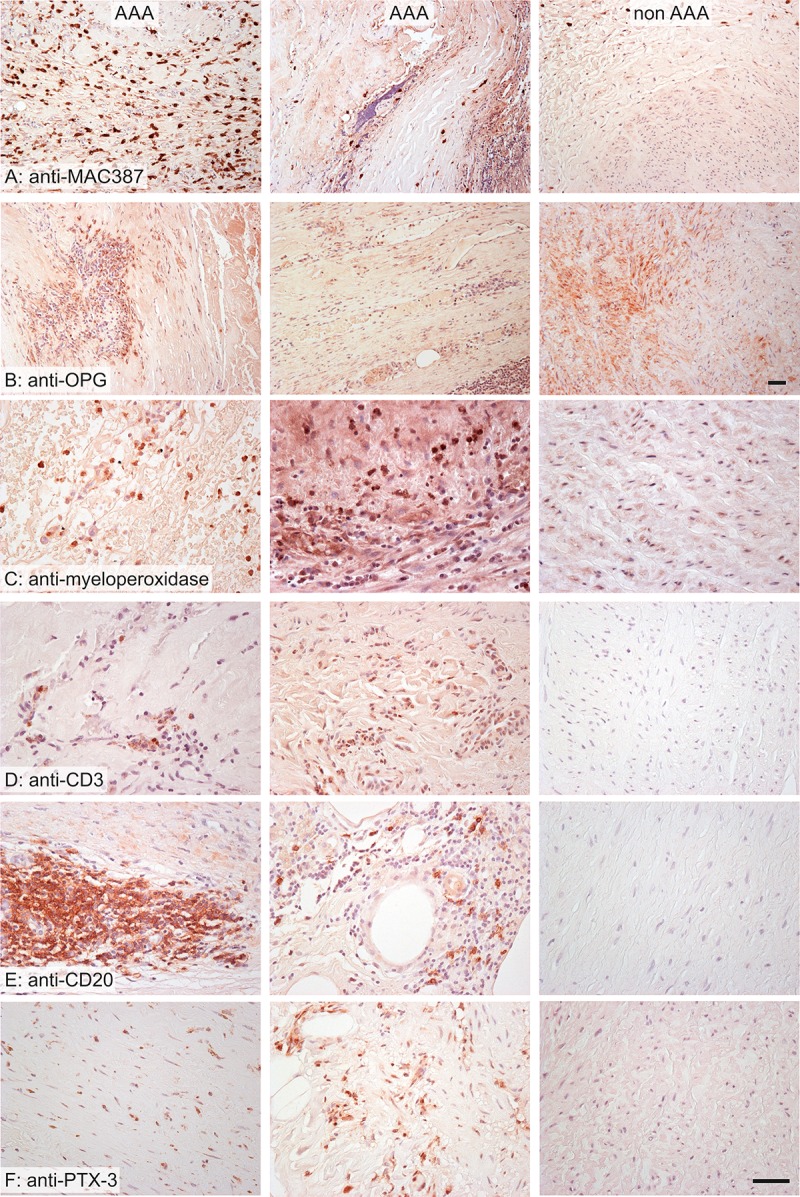
The AAA samples were more infiltrated by macrophages (A), neutrophils (B), T lymphocytes (D) and B lymphocytes (E). The distribution of osteoprotegerin (OPG) was more diffuse in non-AAA (C). The expression of pentraxin 3 was greater in AAA samples (F). Stained immunohistochemistry with anti-MAC387 antibody (A) for visualization of macrophages, anti-osteoprotegerin (B), anti-myeloperoxidase (C) for visualization of neutrophilic granulocytes, anti-CD3 (D) for visualization of T lymphocytes, anti-CD20 (E) for visualization of B lymphocytes, anti-pentraxin 3 (F); nuclei were stained with Gill’s hematoxylin; scale bar 50 μm.
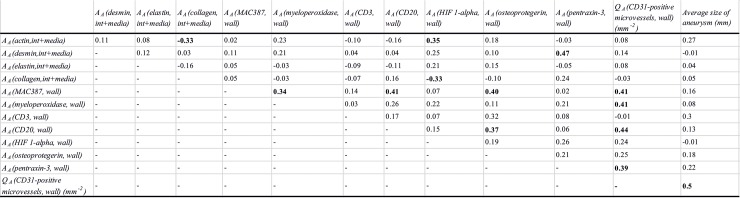
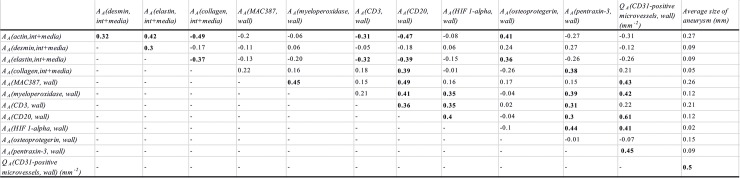
The correlations among the quantitative parameters among AAA patients are presented in Fig 3 and those among all examined individuals are presented in Fig 4.
Fig 3. Correlations of the examined histological parameters, in AAA samples.
All correlations significant at p<0.05 are highlighted in boldface. AA(component, space): Area fraction of the respective components within their reference spaces; QA: number of microvessel profiles per section area; int+media: data pooled from the intima and media; wall: data pooled from the wall (i.e., from intima, media and adventitia). Abbreviations of all the examined parameters are explained in S2 Table.
Fig 4. Correlations of the examined histological parameters, in all samples.
All correlations significant at p<0.05 are highlighted in boldface. AA(component, space): Area fraction of the respective components within their reference spaces; QA: number of microvessel profiles per section area; int+media: data pooled from the intima and media; wall: data pooled from the wall (i.e., from intima, media and adventitia). The abbreviations of all the parameters are explained in S2 Table.
Microvascular density was moderately positively correlated with hypoxia, neutrophils, macrophages, B lymphocytes and the clinically measured size of aneurysms in all samples and with neutrophils, macrophages, B lymphocytes and the aneurysm size in AAA samples.
OPG was positively moderately correlated with actin and elastin in all samples. OPG was positively moderately correlated with macrophages and B lymphocytes in the wall of AAA.
Markers of hypoxic cells were positively moderately correlated with neutrophils, T and B lymphocytes and patient age in all samples, while in AAA samples, the hypoxic cells were moderately positively correlated with actin and negatively correlated with collagen.
Men had fewer hypoxic cells than women (p = 0.001) in the AAA samples. Age had only a weak effect on the vascular density (r = 0.3; p = 0.032) in AAA samples. There was no influence of smoking on the evaluated histological parameters. The qualitative histological findings are shown as a composite in Fig 5. Our primary data from histological quantitative analysis are shown in S3 Table.
Fig 5. Qualitative findings in AAA samples.
A—The basic pattern of the aortic wall was destroyed by newly formed vessels surrounded by inflammatory infiltrates. The elastin fibers (black) were compressed and destroyed. B–The inflammatory cells penetrated among the smooth muscle cells (brown) into the tunica media. C–Lymphocytes (brown) occurred mostly in aggregates resembling lymphoid follicles. D—Rarely, the macrophages (brown) penetrated diffusely the whole wall of the AAA. E–The expression of osteoprotegerin (brown) occurred mostly in the areas infiltrated by leukocytes. F–Similarly, the positivity of pentraxin 3 (brown) also occurred in areas infiltrated by leukocytes. Stained with Verhoeff’s hematoxylin and green trichrome (A) and immunohistochemistry with an antibody against smooth muscle actin (B) for visualization of the contractile phenotype of vascular smooth muscle cells, anti-CD20 (C) for visualization of B lymphocytes, anti-MAC387 (D) for visualization of macrophages, anti-osteoprotegerin (anti-OPG) (E) and anti-pentraxin 3 (anti-PTX3) (F); nuclei were stained with Gill’s hematoxylin; scale bar 100 μm (A, B), 500 μm (C, D) and 50 μm (E, F).
Discussion
The aim of this study was to identify markers to recognize the presence and vulnerability of AAA because screening is not performed using Ultrasonography or Computer Tomography in most countries. Our AAA samples contained a higher microvessel density than non-AAA samples, which is in accordance with the findings of Rodella et al. [4]. As in the study by Eberlová et al., the microvessel density was correlated with the AAA diameter and the inflammatory infiltrates [2]. The number of microvessel profiles correlated positively with hypoxia and immune factors (B lymphocytes, macrophages, neutrophils and PTX3). The number of microvessel profiles correlated positively with hypoxia and immune factors (B lymphocytes, macrophages, neutrophils and PTX3). This correlation might be due to the stimulating effects of inflammatory and hypoxic states on angiogenesis [31]. In human AAA, the adventitial VV has been observed to be stenotic in both small and large AAA, with the sac tissue in these AAA being ischemic and hypoxic. Hypoperfusion of the vascular wall vessel could have critical effects on the development of infrarenal AAA [5]. This hypothesis was verified in a rodent model of AAA when VV blood flow was blocked through the tight ligation of the aorta over the catheter [32]. However, the reason for ischemia formation is still unclear. There were indications that the reduced oxygenation of the aortic wall might be due to reduced oxygen diffusion from the lumen due to intraluminal thrombus (ILT) [33]. Notably, more recent analyses indicate that changes in adventitial VV occurred, irrespective of the presence of ILT. Adventitial VV may play an independent role in the perfusion and oxygenation of the aortic wall, with VV stenosis contributing to the ischemia of the aortic wall itself [34]. Furthermore, increased aortic stiffness and hypertension may narrow the lumen of VV. Greater vascular density in AAA was found in older AAA patients. This result is in accordance with the findings [35] that showed an increased VV diameter and area within thoracic aorta in aged subjects. The AAA samples contained a greater number of hypoxic cells than did the non-AAA samples. The number of hypoxic cells correlated positively with immune factors (T and B lymphocytes and neutrophils). There are indications from other research that HIF 1-alpha is pivotal for AAA progression toward rupture [36]. Several factors related to aneurysm susceptibility (including angiotensin II and nicotine) cause the upregulation of MMP-2 and MMP-9 through aberrantly induced HIF 1-alpha and promote aneurysmal progression [37]. We demonstrated that men had fewer hypoxic cells than women in AAA patients. In a study of samples from patients with pulmonary arterial hypertension, the expression of HIF 1-alpha was higher in female than in male pulmonary artery smooth muscle cells [38]. There are differences in HIF 1-alpha signaling between female and male vascular smooth muscle cells [38]. This difference should be analyzed futher, since these findings could be useful for understanding the pathomechanisms of AAA in different genders.
We demonstrated a statistically significantly higher expression of PTX3 in AAA than in non-AAA patients. The PTX3 correlated positively with collagen, hypoxia and immune cells (T and B lymphocytes and neutrophils). These results corresponded with the findings from studies in which the parameters from AAA were compared to samples of ascending aorta without AAA [39,40]. The findings from these studies were not compared with other structural components of AAA tissue. These authors also analyzed PTX3 serum levels. There was no difference between the serum levels of AAA and non-AAA patients. PTX3 expression negatively correlated with the maximum diameter of the aneurysm [39]. In a recent study, PTX3 serum levels in AAA patients were higher than those in non-AAA patients. Serum levels were AAA diameter-independent [41]. The PTX3 expression in our tissue samples also did not correlate with aneurysm diameter. Peak levels of PTX3 in patients with acute aortic dissection were associated with the amount of transient pleural fluid accumulation, which may be associated with inflammatory vascular permeability [42]. Based on our results, we hypothesized that PTX3 might play a role in the pathogenesis of AAA. However, the histological analysis reflected the outcome of the AAA remodeling. Therefore, more evidence is necessary.
The structure of PTX3 is similar to that of CRP, which is from the same pentraxin superfamily. CRP was also suggested as a biomarker of AAA [43–46]. In contrast to PTX3, which is expressed in circulating neutrophils as well as a variety of cells in inflamed tissue (including endothelial cells, fibroblasts, VSMCs and inflammatory cells) [40,47,48]. CRP is synthesized by hepatocytes upon stimulation by systemic proinflammatory cytokines. Therefore, PTX3 is a more accurate marker of the actual inflammatory state and mirrors local inflammation that does not necessarily lead to an increase in systemic cytokines (e.g., in vascular inflammation, even without systemic inflammation) [49]. PTX3 is also less influenced by total cholesterol, high-density lipoprotein, hemoglobin, smoking, obesity or gender [49,50]. Our study provides evidence of the distribution of PTX3, OPG and hypoxic cells within AAAs, but further analyses relating histological data to biochemical markers are needed.
The AAA samples contained less elastin and more macrophages, neutrophils, T and B lymphocytes and a greater density of VV. These data support the finding of the increasing gene expression of MMP-9, which degrades elastin and plays a role in the proliferation and migration of VSMCs, and intercellular adhesion molecule-1, which promotes leukocyte adhesion to and migration through endothelial cells [6,51].
The AAA samples contained a smaller amount of OPG. These findings from our quantitative analyses support a potential preventive effect of OPG [19].
A limitation of the present study is that the results were based on AAA samples harvested during an open surgical repair of AAA sac. However, many AAA patients undergo endovascular aortic repair [52], where no morphological tissue samples can be removed for research purposes. Moreover, also harvesting of control samples of healthy aorta from organ donors has limitations as the interest of the waiting organ recipients is prioritized over harvesting of samples for research purposes. In addition, bias may have been introduced since the morphometry of our samples was based on 1–2 histological sections per each quantitative parameter. Also, our quantification used two-dimensional routine sections only, whereas some structures (e.g. the vasa vasorum) are three-dimensional and would require more sophisticated quantification techniques, e.g. confocal microscopy or X-ray microtomography. Moreover, also the present study relies on histological analysis, but its conclusions should be further verified using established quantitative techniques (Enzyme-Linked Immunosorbent Assay or western blots) to demonstrate the differences in protein expression more convincingly.
Conclusion
AAA tissue samples contained significantly less actin, desmin, elastin and osteoprotegerin, more collagen, macrophages, neutrophils, T lymphocytes, B lymphocytes, hypoxic cells and PTX3, and a greater density of VV than did non-AAA samples. PTX3 and hypoxia correlated with each other and with T and B lymphocytes and neutrophils. Microvascular density was related to inflammatory cell infiltrates, PTX3 expression, hypoxia and average size of aneurysms. AAA contained altered amounts of structural proteins, implying reduced vascular elasticity. This remodeling of the AAA wall occurred under significant tissue hypoxia, despite a greater density of microvessels than of non-aneurysmal aorta. PTX3 was upregulated in AAA, and its colocalization with the inflammatory infiltrates supports the theory of a potential role for PTX3 as a marker of vascular inflammation. The presence of aortic hypoxia, despite hypervascularization, suggests hypoxia-induced neoangiogenesis that may play a role in AAA pathogenesis. VV angiogenesis of the AAA wall increases its vulnerability.
Supporting information
(DOC)
(DOC)
(XLS)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was mainly supported by the Ministry of Health of the Czech Republic, Project No. AZV 15-32727A. The study received additional support from the National Sustainability Program I No. LO1503, from the Charles University Research Fund – Progres Q39, by project No. CZ.02.1.01/0.0/0.0/16_019/0000787 “Fighting INfectious Diseases” and No. CZ.02.1.01/0.0/0.017_048/0007280 “Application of Modern Technologies in Medicine and Industry”, awarded by the Ministry of Education, Youth and Sport of the Czech Republic, financed by European Regional Development Fund.
References
- 1.Kühnl A, Erk A, Trenner M, Salvermoser M, Schmid V, Eckstein H-H. Incidence, Treatment and Mortality in Patients with Abdominal Aortic Aneurysms: An Analysis of Hospital Discharge Data from 2005–2014. Dtsch Aerzteblatt Online. 2017; 10.3238/arztebl.2017.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eberlová L, Tonar Z, Witter K, Křížková V, Nedorost L, Korabečná M, et al. Asymptomatic Abdominal Aortic Aneurysms Show Histological Signs of Progression: a Quantitative Histochemical Analysis. Pathobiol J Immunopathol Mol Cell Biol. 2013;80: 11–23. 10.1159/000339304 [DOI] [PubMed] [Google Scholar]
- 3.Billaud M, Hill JC, Richards TD, Gleason TG, Phillippi JA. Medial Hypoxia and Adventitial Vasa Vasorum Remodeling in Human Ascending Aortic Aneurysm. Front Cardiovasc Med. 2018;5 10.3389/fcvm.2018.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodella LF, Rezzani R, Bonomini F, Peroni M, Cocchi MA, Hirtler L, et al. Abdominal Aortic Aneurysm and Histological, Clinical, Radiological Correlation. Acta Histochem. 2016;118: 256–262. 10.1016/j.acthis.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Zaima N, Sasaki T, Sano M, Yamamoto N, Saito T, et al. Hypoperfusion of the Adventitial Vasa Vasorum Develops an Abdominal Aortic Aneurysm. PloS One. 2015;10: e0134386 10.1371/journal.pone.0134386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeps C, Pelisek J, Seidl S, Schuster T, Zimmermann A, Kuehnl A, et al. Inflammatory Infiltrates and Neovessels Are Relevant Sources of MMPs in Abdominal Aortic Aneurysm Wall. Pathobiol J Immunopathol Mol Cell Biol. 2009;76: 243–252. 10.1159/000228900 [DOI] [PubMed] [Google Scholar]
- 7.Cieślik P, Hrycek A. Long Pentraxin 3 (PTX3) in the Light of Its Structure, Mechanism of Action and Clinical Implications. Autoimmunity. 2012;45: 119–128. 10.3109/08916934.2011.611549 [DOI] [PubMed] [Google Scholar]
- 8.Bonacina F, Baragetti A, Catapano AL, Norata GD. Long Pentraxin 3: Experimental and Clinical Relevance in Cardiovascular Diseases. Mediators Inflamm. 2013;2013: 1–10. 10.1155/2013/725102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yigit S, Sari S, Canbolat IP, Arat Ozkan A, Ersanli MK, Gurmen T. Pentraxin-3 a Novel Biomarker for Predicting Coronary Artery Disease. Eur Heart J. 2013;34: P3108–P3108. 10.1093/eurheartj/eht309.P3108 [DOI] [Google Scholar]
- 10.Buda V, Andor M, Tomescu MC, Cristescu C, Voicu M, Citu I, et al. P2637ACE Inhibitors and ARBs Decrease More Powerful the PTX-3 Plasma Levels of Hypertensive Patients with Endothelial Dysfunction Compared with Other Anti-Hypertensive Drugs, in a Chronic Treatment. Eur Heart J. 2017;38 10.1093/eurheartj/ehx502.P2637 [DOI] [Google Scholar]
- 11.Nakamura A, Miura S, Shiga Y, Norimatsu K, Miyase Y, Suematsu Y, et al. Is Pentraxin 3 a Biomarker, a Player, or Both in the Context of Coronary Atherosclerosis and Metabolic Factors? Heart Vessels. 2015;30: 752–761. 10.1007/s00380-014-0553-0 [DOI] [PubMed] [Google Scholar]
- 12.Norata GD, Garlanda C, Catapano AL. The Long Pentraxin PTX3: A Modulator of the Immunoinflammatory Response in Atherosclerosis and Cardiovascular Diseases. Trends Cardiovasc Med. 2010;20: 35–40. 10.1016/j.tcm.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 13.Koole D, Hurks R, Schoneveld A, Vink A, Golledge J, Moran CS, et al. Osteoprotegerin Is Associated with Aneurysm Diameter and Proteolysis in Abdominal Aortic Aneurysm Disease. Arterioscler Thromb Vasc Biol. 2012;32: 1497–1504. 10.1161/ATVBAHA.111.243592 [DOI] [PubMed] [Google Scholar]
- 14.Callegari A, Coons ML, Ricks JL, Rosenfeld ME, Scatena M. Increased Calcification in Osteoprotegerin-Deficient Smooth Muscle Cells: Dependence on Receptor Activator of NF-κB Ligand and Interleukin 6. J Vasc Res. 2014;51: 118–131. 10.1159/000358920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, et al. Osteoprotegerin Inhibits Vascular Calcification without Affecting Atherosclerosis in Ldlr(-/-) Mice. Circulation. 2008;117: 411–420. 10.1161/CIRCULATIONAHA.107.707380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, et al. Serum Osteoprotegerin Levels Are Associated with the Presence and Severity of Coronary Artery Disease. Circulation. 2002;106: 1192–1194. 10.1161/01.cir.0000031524.49139.29 [DOI] [PubMed] [Google Scholar]
- 17.Moran CS, McCann M, Karan M, Norman P, Ketheesan N, Golledge J. Association of Osteoprotegerin With Human Abdominal Aortic Aneurysm Progression. Circulation. 2005;111: 3119–3125. 10.1161/CIRCULATIONAHA.104.464727 [DOI] [PubMed] [Google Scholar]
- 18.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, et al. Osteoprotegerin Inactivation Accelerates Advanced Atherosclerotic Lesion Progression and Calcification in Older ApoE-/- Mice. Arterioscler Thromb Vasc Biol. 2006;26: 2117–2124. 10.1161/01.ATV.0000236428.91125.e6 [DOI] [PubMed] [Google Scholar]
- 19.Bumdelger B, Kokubo H, Kamata R, Fujii M, Yoshimura K, Aoki H, et al. Osteoprotegerin Prevents Development of Abdominal Aortic Aneurysms. PloS One. 2016;11: e0147088 10.1371/journal.pone.0147088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. [Edinburgh]: Churchill Livingstone; 2008. [Google Scholar]
- 21.Kocová J. Overall Staining of Connective Tissue and the Muscular Layer of Vessels. Folia Morphol. 1970;18: 293–295. [PubMed] [Google Scholar]
- 22.Rich L, Whittaker P. Collagen and Picrosirius Red Staining: a Polarized Light Assessment of Fibrillar Hue and Spatial Distribution. Braz J Morphol Sci. 2005;2005: 97–104. [Google Scholar]
- 23.Kubíková T, Kochová P, Brázdil J, Špatenka J, Burkert J, Králíčková M, et al. The Composition and Biomechanical Properties of Human Cryopreserved Aortas, Pulmonary Trunks, and Aortic and Pulmonary Cusps. Ann Anat—Anat Anz. 2017;212: 17–26. 10.1016/j.aanat.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 24.Tonar Z, Tomášek P, Loskot P, Janáček J, Králíčková M, Witter K. Vasa Vasorum in the Tunica Media and Tunica Adventitia of the Porcine Aorta. Ann Anat—Anat Anz. 2016;205: 22–36. 10.1016/j.aanat.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 25.Houdek K, Moláček J, Třeška V, Křížková V, Eberlová L, Boudová L, et al. Focal Histopathological Progression of Porcine Experimental Abdominal Aortic Aneurysm is Mitigated by Atorvastatin. Int Angiol J Int Union Angiol. 2013;32: 291–306. [PubMed] [Google Scholar]
- 26.Mouton PR. Principles and Practices of Unbiased Stereology: an Introduction for Bioscientists. Baltimore: Johns Hopkins University Press; 2002. [Google Scholar]
- 27.Tonar Z, Kubíková T, Prior C, Demjén E, Liška V, Králíčková M, et al. Segmental and Age Differences in the Elastin Network, Collagen, and Smooth Muscle Phenotype in the Tunica Media of the Porcine Aorta. Ann Anat Anat Anz Off Organ Anat Ges. 2015;201: 79–90. 10.1016/j.aanat.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 28.Tonar Z, Egger GF, Witter K, Wolfesberger B. Quantification of Microvessels in Canine Lymph Nodes. Microsc Res Tech. 2008;71: 760–772. 10.1002/jemt.20619 [DOI] [PubMed] [Google Scholar]
- 29.Howard V, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Liverpool: QTP; 2010. [Google Scholar]
- 30.Witter K, Tonar Z, Schöpper H. How many Layers has the Adventitia?—Structure of the Arterial Tunica Externa Revisited. Anat Histol Embryol. 2017;46: 110–120. 10.1111/ahe.12239 [DOI] [PubMed] [Google Scholar]
- 31.Holmes DR, Liao S, Parks WC, Thompson RW. Medial Neovascularization in Abdominal aortic Aneurysms: a Histopathologic Marker of Aneurysmal Degeneration with Pathophysiologic Implications. J Vasc Surg. 1995;21: 761–771; discussion 771–772. 10.1016/s0741-5214(05)80007-2 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka H, Unno N, Yata T, Kugo H, Zaima N, Sasaki T, et al. Creation of a Rodent Model of Abdominal Aortic Aneurysm by Blocking Adventitial Vasa Vasorum Perfusion. J Vis Exp. 2017; 10.3791/55763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, et al. Association of Intraluminal Thrombus in Abdominal Aortic Aneurysm with Local Hypoxia and Wall Weakening. J Vasc Surg. 2001;34: 291–299. 10.1067/mva.2001.114813 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Zaima N, Sasaki T, Hayasaka T, Goto-Inoue N, Onoue K, et al. Adventitial Vasa Vasorum Arteriosclerosis in Abdominal Aortic Aneurysm. PloS One. 2013;8: e57398 10.1371/journal.pone.0057398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popescu MR, Butcovan D, Folescu R, Motoc A, Zamfir CL. Thoracic Aorta Dissection—Assessment of Aortic Adventitia Involvement. Rom J Leg Med. 2013;21: 207–2014. doi: 0.4323/rjlm.2013.207 [Google Scholar]
- 36.Gäbel G, Northoff BH, Weinzierl I, Ludwig S, Hinterseher I, Wilfert W, et al. Molecular Fingerprint for Terminal Abdominal Aortic Aneurysm Disease. J Am Heart Assoc. 2017;6 10.1161/JAHA.117.006798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai S-H, Huang P-H, Hsu Y-J, Peng Y-J, Lee C-H, Wang J-C, et al. Inhibition of Hypoxia Inducible Factor-1α Attenuates Abdominal Aortic Aneurysm Progression through the Down-Regulation of Matrix Metalloproteinases. Sci Rep. 2016;6: 28612 10.1038/srep28612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Docherty CK, Nilsen M, MacLean MR. Influence of 2-Methoxyestradiol and Sex on Hypoxia-Induced Pulmonary Hypertension and Hypoxia-Inducible Factor-1-α. J Am Heart Assoc. 2019;8: e011628 10.1161/JAHA.118.011628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada H, Naito Y, Oboshi M, Iwasaku T, Morisawa D, Okuhara Y, et al. Pentraxin 3 Expression in Human Abdominal Aortic Aneurysm. Circulation. 2014;2014: A15060. [Google Scholar]
- 40.Sawada H, Naito Y, Oboshi M, Soyama Y, Nishimura K, Eguchi A, et al. Increment of Pentraxin3 Expression in Abdominal Aortic Aneurysm. Int J Cardiol. 2015;195: 281–282. 10.1016/j.ijcard.2015.05.177 [DOI] [PubMed] [Google Scholar]
- 41.Molacek J, Treska V, Zeithaml J, Hollan I, Topolcan O, Pecen L, et al. Blood Biomarker Panel Recommended for Personalized Prediction, Prognosis, and Prevention of Complications Associated with Abdominal Aortic Aneurysm. EPMA J. 2019;10: 125–135. 10.1007/s13167-019-00173-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arao K, Fujiwara T, Taniguchi Y, Jinnouchi H, Sasai H, Matsumoto M, et al. Implications of Pentraxin 3 Levels in Patients with Acute Aortic Dissection. Heart Vessels. 2015;30: 211–217. 10.1007/s00380-014-0470-2 [DOI] [PubMed] [Google Scholar]
- 43.Folsom Aaron R., Yao Lu, Alonso Alvaro, Lutsey Pamela L., Missov Emil, Lederle Frank A., et al. Circulating Biomarkers and Abdominal Aortic Aneurysm Incidence. Circulation. 2015;132: 578–585. 10.1161/CIRCULATIONAHA.115.016537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriksson AE, Lindqvist M, Sihlbom C, Bergström J, Bylund D. Identification of Potential Plasma Biomarkers for Abdominal Aortic Aneurysm Using Tandem Mass Tag Quantitative Proteomics. Proteomes. 2018;6 10.3390/proteomes6040043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moris D, Mantonakis E, Avgerinos E, Makris M, Bakoyiannis C, Pikoulis E, et al. Novel Biomarkers of Abdominal Aortic Aneurysm Disease: Identifying Gaps and Dispelling Misperceptions. BioMed Res Int. 2014;2014: 1–13. 10.1155/2014/925840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urbonavicius S, Urbonaviciene G, Honoré B, Henneberg EW, Vorum H, Lindholt JS. Potential Circulating Biomarkers for Abdominal Aortic Aneurysm Expansion and Rupture—a Systematic Review. Eur J Vasc Endovasc Surg. 2008;36: 273–280. 10.1016/j.ejvs.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 47.Fornai F, Carrizzo A, Forte M, Ambrosio M, Damato A, Ferrucci M, et al. The Inflammatory Protein Pentraxin 3 in Cardiovascular Disease. Immun Ageing A. 2016;13: 25 10.1186/s12979-016-0080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabiati M, Svezia B, Verde A, Caselli C, Del Ry S. P3401Pentraxin 3, a Novel Inflammatory Marker in Heart Failure Patients: Its Expression in Circulating Leukocytes as a Function of Clinical Severity. Eur Heart J. 2017;38 10.1093/eurheartj/ehx504.P3401 [DOI] [Google Scholar]
- 49.Liu H, Guan S, Fang W, Yuan F, Zhang M, Qu X. Associations Between Pentraxin 3 and Severity of Coronary Artery Disease. BMJ Open. 2015;5: e007123 10.1136/bmjopen-2014-007123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb. 2018;25: 846–984. 10.5551/jat.GL2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong PJ, Johanning JM, Calton WC, Delatore JR, Franklin DP, Han DC, et al. Differential Gene Expression in Human Abdominal Aorta: Aneurysmal versus Occlusive Disease. J Vasc Surg. 2002;35: 346–14. 10.1067/mva.2002.121071 [DOI] [PubMed] [Google Scholar]
- 52.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular Versus Open Repair of Abdominal Aortic Aneurysm in 15-years’ Follow-up of the UK Endovascular Aneurysm Repair Trial 1 (EVAR Trial 1): a Randomised Controlled Trial. The Lancet. 2016;388: 2366–2374. 10.1016/S0140-6736(16)31135-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.