Abstract
Background
Even though the incidence of community-acquired Clostridium difficile infection (CDI) is reported to be increasing, few studies have reported on the healthcare costs of community-acquired CDI. We estimated cost of care for individuals with community-associated CDI and compared with that for matched controls without CDI in the time period of six months before to one year after CDI.
Methods
All individuals in the province of Manitoba, diagnosed with CDI between July 2005 and March 2015 were matched up to 4 individuals without CDI. Health care utilization and direct costs resulting from hospitalizations, physician reimbursement claims and prescriptions were determined from the population based provincial databases. Quantile regressions were performed to determine predictors of cost of individuals with community associated CDI.
Results
Of all CDIs, 30–40% in each period of the study had community-associated CDI; of which 12% were recurrent CDIs. The incremental median and 90th percentile cost of care for individuals with community-associated CDI was $800 and $16,000 respectively in the six months after CDI diagnosis. After adjustment for age, co-morbidities, sex, socioeconomic status and magnitude of health care utilization prior to CDI, the median incremental cost for recurrent CDI was $1,812 and that for a subsequent episode of CDI was $3,139 compared to those with a single community-associated CDI episode. The median cost for a prescription of Vancomycin was $316 (IQR 209–489).
Conclusions
Health care costs of an episode of community-associated CDI have been much more than the cost of antibiotic treatment. Our study provides population-based data for formal cost effectiveness analysis for use of newer treatments for community-associated CDI.
Introduction
Clostridium difficile (CD) is a Gram-positive anaerobic spore forming bacterium that produces toxins and can lead to clinically significant diarrhea and substantial morbidity and mortality[1]. Over the last decade several hospital based outbreaks with a newer more toxic strain have been reported in North America[2, 3] Moreover, an increasing occurrence of CD infections (CDI) has been reported over the last decade[4], including in ambulatory care settings[2]. The increasing incidence and associated morbidity and mortality have created an impetus to develop newer therapeutic approaches[1, 5]. While these newer agents are more expensive than traditional antibiotics, downstream health care savings could be substantive if the improved effectiveness of these regimens leads to rapid cure and/or a lower incidence of recurrent CDI and less associated morbidity. It is necessary to understand the traditional cost of initial and recurrent CDI before undertaking evaluations of the cost-effectiveness of novel, more expensive therapies.
Although up to 40% of CDI have been reported to be community-associated[6], few studies have evaluated health care costs associated specifically with community-associated CDI (ca-CDI)[7].
Recent guidelines, based on efficacy data, now recommend vancomycin or fidaxomicin rather than the traditionally used metronidazole, including for ca- CDI[8]. However, cost and utilization analyses were not addressed in these guidelines. Cost analyses from the perspective of payors of direct costs are essential to determine the cost-effectiveness of the recommendations.
Much of the information on the costs of CDI in North America has been reported by identifying CDI from International Classification of Diseases (ICD) codes for CDI in secondarily collected administrative health records or from single center studies[9–11]. However, the use of ICD codes to identify CDI has been reported to be inaccurate in several studies, overestimating the number of CDI cases relative to use of the toxin assay[12]; limited positive predictive value (71.6%; 95% Confidence Interval (CI): 62.1–86.6%)[13] and missing up to 30% of hospitalized CDI cases[14]. Reported per-patient costs of CDI vary by 2 orders of magnitude among different hospitals[15], which maybe at least partly due to the use of non-validated administrative data definitions of CDI to identify individuals with CDI. In addition, use of hospital discharge data does not allow assessment of CDI in the outpatient setting or determination of the precise date when a CDI infection was diagnosed.
In Manitoba, reporting of all positive CD assays was legally mandated since 2005[16] (https://www.gov.mb.ca/health/publichealth/act.html), which has allowed us to create a population-based comprehensive dataset of CDI cases and evaluate CDI epidemiology and costs of care on a population basis. In this manuscript, we report the estimated cost of care of individuals with ca-CDI compared with matched controls without CDI.
Methods
Data sources
Manitoba is a central Canadian province with a relatively stable population (population in 2014: 1.3 million)[17]. Manitoba Health is the provincial agency which oversees the delivery of universal health care in the province. Only members of the Canadian armed forces, national police and inmates of federal penitentiaries are excluded and covered by the federal government. Manitoba Health maintains several electronic administrative healthcare databases to monitor the services delivered and for re-imbursement to health care providers for the services rendered[18–21]. Up to 16 ICD-9 (prior to 2004) or 25 ICD-10 (since 2004) codes are recorded for each inpatient hospital stay, whereas each outpatient physician visit is coded with a single ICD-9 code. Records of all outpatient prescriptions dispensed since 1995 are recorded in the Drug Programs Information Network (DPIN) Database.
The Manitoba Health Public Health Branch Epidemiology and Surveillance Unit maintains a population-based CDI dataset since 2005, developed from the legally mandated universal reporting of documented CDI cases in the province to the unit. Diagnostic testing for CDI is performed in six public laboratories, of which 3 perform approximately 84% of the testing. Only loose stool, which takes shape of its container has been tested by the laboratories, thereby minimizing the detection of asymptomatic carriers (estimated to be 2–7% in the population[22, 23]).
Between 2005 and May 2013, the laboratories in Manitoba performed immunoassays for the glutamate dehydrogenase (GD) antigen and C. difficile toxins A and B, followed by the cytopathic effect (CPE) assay (using viable human fibroblasts) and/or culture for discordant results (i.e., GD antigen positive but C. difficile toxin A & B immunoassay negative)[24, 25]. The predominant assays used during the study years were the C. Diff Quik Chek test (Techlab Inc., Blacksburg, VA) for the GD antigen, and the Tox A/B Quik Chek test (Techlab Inc., Blacksburg, VA) for C. difficile toxins A and B. Since May 2013, the Nucleic Acid Amplification Test (NAAT, using a Health Canada cleared nucleic acid amplification assay for the detection of a segment of Clostridium difficile DNA known to be present in all known toxigenic strains of C. difficile, including A-B+ toxin types) is used for confirmation of CDI[25].
Since 1984, all individuals in the province have been assigned a unique personal health identification number (PHIN). Scrambled anonymized PHINs were used to link the data in above databases for the current study.
CDI cases and controls
All individuals in the province, diagnosed with one or more episodes of CDI between July 2005 and March 2015 were matched with up to 4 individuals without CDI based on sex, age (± 5 years), area of residence (first 3 digits of the postal code) and duration of coverage with Manitoba Health prior to the CDI onset (approximated by the date of stool specimen collection; defined as the index date in the study) of each episode. Individuals with ca-CDI and their controls were included in the analysis for this report. Those younger than 18 years of age or residents of long-stay care facilities on the index date were excluded as we did not have access to person-level costing data from long-stay care facilities. All individuals were followed from one year prior to the index date to death, out-migration from the province, admission to a long-stay care facility or end of the study time period (March 2015).
Previously recommended and used definitions (S1 Table) for CDI[16, 26] were used in the study to determine the site of acquiring CDI and whether it was incident or recurrent, if it was severe and whether it was the first or a subsequent infection. Second/later episodes were defined as subsequent infections not meeting the definition of recurrent infections.
Costs
Direct costs resulting from hospitalizations, physician reimbursement claims and prescriptions were determined from the Manitoba Health databases. Inpatient hospitalization costs were calculated using data in each record from the Canadian Institute for Health Information (CIHI). Specifically, using a case mix methodology, CIHI groups together patients with similar clinical and resource-utilization characteristics, and uses this information to calculate a resource intensity weight (RIW) for each episode of care. This RIW reflects in hospital resource utilization, and is higher for more complex patients. An “average” resource-use patient would have a RIW = 1. Additionally, CIHI uses annual hospital budgets to calculate province-specific costs associated with an “average” hospital stay. This Cost per Standard Hospital Stay (CSHS) reflects the cost of one inpatient hospital stay with a RIW = 1 in a particular year. Thus, to derive inpatient hospitalization costs for each episode, RIW is multiplied by CSHS for each record in the hospital data. Case Mix Groups (CMGs), modeled after the American Diagnosis Related Groups (DRG's), represent a Canadian patient classification system used to group and describe types of inpatients with similar clinical characteristics and resource utilization discharged from acute-care hospitals.
RIW’s and financial data may change from one year to another due to changes in methodology or adjustments. However, CIHI provides grouper data for inpatient hospitalizations to make costing data over time periods comparable. The costs of outpatient (day) procedures were included, calculated using annual RIW and CSHS values. Physician visits costs were reimbursements for fee-for-service claims as listed in the MH provider re-imbursement claims dataset, and were applied for both inpatient and outpatient physician services. Prescription costs reflect charges paid by patients, the province and/or the insurance companies and include dispensing fee and the cost of the drugs. Although, inpatient dispensations from hospital pharmacies are not captured in DPIN, inpatient pharmacy costs are included in CSHS. All costs were adjusted to 2016 Canadian dollars using the Consumer Price Index (CPI).
Statistical analysis
Costs were calculated for 6-month time periods, starting from one year preceding each ca-CDI episode. We included cost estimates prior to the ca-CDI episode as many individuals with CDI have predisposing comorbid conditions, with associated higher costs of healthcare, irrespective of occurrence of CDI. Since migration and death reduce the time period of observation and therefore potentially reduce costs, all individuals had to be registered during the entire six month time periods that they contributed the data. Quantile regression models were selected because the study data included some very high cost patients leading to skewed distributions. The percentiles selected for modeling were the 50th (median), 75th and 90th. Covariates in the models included, in addition to group membership (i.e., case, control) age, sex, diabetes, dialysis, Charlson co-morbidity index (CCI) score, the socio-economic factor index (SEFI), and number of physician visits (categorised into quartiles) in the year preceding the time period evaluated. The CCI score (categorised as 0,1, 2, 3 or more) was calculated from diagnoses listed in hospitalizations records and physician claims in the year preceding the evaluated time period; diabetes and dialysis were not included in calculation of CCI score; rather, they were entered as separate covariates because they are relatively common diseases associated with CDI[27–29]. The SEFI is a validated measure of socio-economic status and is based on several neighborhood level measures of wealth[30]. SEFI scores can range from minus 3 to plus 7, with higher scores indicating greater deprivation. SEFI was included in the models as a continuous variable. Individuals were censored at the beginning of the time period in which they entered long-stay care, died or moved out of province.
Data management and analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). This study was approved by the University of Manitoba’s Health Research Ethics Board and the Health Information and Privacy Committee of Manitoba Health.
Results
There were 6,234 individuals aged 18 or older, who experienced 8,471 episodes of CDI between July 1, 2005 and March 31, 2015 in the province. 372 cases with CDI and 1188 controls without CDI were excluded because they were residents of long-stay care facilities prior to CDI diagnosis. After excluding CDI cases with no remaining matching controls, there were 5,852 cases experiencing their first recorded CDI and their 22,041 matching controls.
30–40% of individuals with CDI in each of the follow-up time periods had ca-CDI and were included in the analysis for this report. Their characteristics are provided in Table 1. Approximately 12% of ca-CDI episodes were recurrent CDIs. Individuals with ca-CDI were more likely to have diabetes, receive dialysis or have higher CCI score (i.e. co-morbidity burden) than their controls (Table 1).
Table 1. Characteristics of CDI cases and controls without community associated CDI by time period of follow-up before and after CDI diagnosis.
| Time | 1 year—6 months prior | 6 months—1 day prior | CDI—6 months post | 6 months—1 year post | ||||
|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | Case | Control | |
| N | 1,887 | 7,307 | 1,887 | 7,307 | 1,637 | 6,321 | 1,503 | 5,740 |
| Male (%) | 37.5 | 37.7 | 37.5 | 37.7 | 37.5 | 37.4 | 37.8 | 37.6 |
| Dialysis (%) | 1.8 | 0.1 | 2.0 | 0.1 | 1.4 | 0.1 | 1.3 | 0.1 |
| Diabetes diagnosis (%) | 15.0 | 12.4 | 15.6 | 13.0 | 14.4 | 12.7 | 14.5 | 13.0 |
| Charlson Co-morbidity Index score (without Diabetes or Renal failure) (%) | ||||||||
| 0 | 68.3 | 81.9 | 66.7 | 80.9 | 68.3 | 81.2 | 65.8 | 81.2 |
| 1 | 17.6 | 11.2 | 17.4 | 11.9 | 16.8 | 11.8 | 16.8 | 11.5 |
| 2 | 9.2 | 5.1 | 10.3 | 5.3 | 9.7 | 5.3 | 10.5 | 5.4 |
| 3+ | 4.9 | 1.8 | 5.6 | 2.0 | 5.2 | 1.7 | 6.9 | 1.9 |
| Age (years) | ||||||||
| Median | 56 | 55 | 56 | 55 | 55 | 54 | 56 | 55 |
| Lower Quartile | 41 | 40 | 41 | 41 | 40 | 40 | 41 | 41 |
| Upper Quartile | 70 | 69 | 71 | 69 | 68 | 67 | 69 | 67 |
| Ambulatory Care Contacts in the year prior to the evaluated time period (n) | ||||||||
| Median | 10 | 6 | 10 | 6 | 12 | 6 | 15 | 6 |
| Lower Quartile | 5 | 3 | 5 | 3 | 6 | 2 | 9 | 3 |
| Upper Quartile | 19 | 12 | 19 | 12 | 20 | 12 | 24 | 12 |
| SEFI Socio-economic Measure | ||||||||
| Median | -0.18 | -0.18 | -0.18 | -0.18 | -0.21 | -0.19 | -0.21 | -0.19 |
| Lower Quartile | -0.70 | -0.69 | -0.70 | -0.69 | -0.72 | -0.70 | -0.72 | -0.71 |
| Upper Quartile | 0.27 | 0.28 | 0.27 | 0.28 | 0.26 | 0.27 | 0.26 | 0.27 |
| Recurrence (% of all CDI cases) | ||||||||
| Yes | 12.7 | 12.7 | 12.2 | 11.9 | ||||
| No, but later infection (i.e. 2 or more episodes) | 5.9 | 5.9 | 5.8 | 5.7 | ||||
| No (incident only/ single episode) |
81.4 | 81.4 | 82.0 | 82.4 | ||||
The median cost of care for individuals with ca- CDI was higher than the median cost for their controls in all of the time periods, including even 6 months to a year before CDI diagnosis; the incremental cost for individuals with ca-CDI compared to those without CDI was highest in the six months after ca-CDI diagnosis—an increase of median cost of $800 and 90th percentile cost of approximately $16,000 among those with ca-CDI as compared to the six month cost before ca-CDI (Table 2). The costs remained stable among those without CDI.
Table 2. Cost of healthcare for CDI cases and controls without community-associated CDI by time period of follow-up before and after CDI diagnosis (2016 constant Canadian dollars).
| Number of Individuals | Total annual cost | Median | 75th percentile | 90th percentile | |
|---|---|---|---|---|---|
| 12–6 m prior to index date | |||||
| CDI cases | 1887 | 15,961,986 | 682 | 2088 | 8110 |
| Controls | 7307 | 19,503,288 | 317 | 863 | 2156 |
| Incremental adjusted CDI Cost (from multivariable model) | 137 | 312 | 1473 | ||
| 6 m– 1 day prior to index date | |||||
| CDI cases | 1887 | 13,596,512 | 933 | 2566 | 8554 |
| Controls | 7307 | 21,062,756 | 319 | 926 | 2339 |
| Incremental adjusted CDI Cost (from multivariable model) | 330 | 610 | 1895 | ||
| CDI– 6 m post CDI | |||||
| CDI cases | 1637 | 26,905,230 | 1561 | 6107 | 16358 |
| Controls | 6321 | 18,478,716 | 313 | 875 | 2211 |
| Incremental adjusted CDI Cost (from multivariable model) | 734 | 3916 | 8186 | ||
| 6–12 m post CDI | |||||
| CDI cases | 1503 | 11,645,036 | 722 | 2133 | 8009 |
| Controls | 5740 | 14,974,800 | 307 | 856 | 2180 |
| Incremental adjusted Cost (from multivariable model) | 68 | 192 | 1397 | ||
Incremental adjusted CDI Cost from multivariate model, adjusted for differences between those with and without CDI, such as presence of comorbidities, which influence costs.
In the multivariable quantile regression analysis, the incremental costs in the six months after ca-CDI diagnosis for the individuals with ca-CDI as compared to those without CDI had an estimated median of $734, 75th percentile of $3,916 and 90th percentile of $8,186, which were approximately $400, $3300 and $6000 more than in the six months before ca-CDI (Table 3).
Table 3. Estimates from multivariable quantile regression models for additional cost of healthcare (in 2016 Canadian dollars) for individuals with community-associated CDI by time period of follow-up before and after CDI diagnosis.
| Median (95% CI) costs | 75th percentile (95% CI) costs | 90th percentile (95% CI) costs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before CDI | After CDI | Before CDI | After CDI | Before CDI | After CDI | |||||||
| 1y – 6m | 6m – 1d | diagnosis– 6m | 6m – 1y | 1y – 6m | 6m – 1d | CDI– 6m | 6m – 1y | 1y – 6m | 6m – 1d | CDI– 6m | 6m – 1y | |
| CDI vs without CDI | 137 | 330 | 734 | 68 | 312 | 610 | 3916 | 192 | 1473 | 1895 | 8186 | 1397 |
| (124, 150) | (317, 344) | (717, 752) | (47, 88) | (276, 348) | (573, 648) | (3875, 3957) | (149, 236) | (1355, 1591) | (1761, 2029) | (8072, 8300) | (1260, 1535) | |
Multivariable regression analysis was performed for ca-CDI cases alone, with categorisation of ca-CDI as single episode, with recurrent episode and second/later episode and incremental costs were compared to those with a single episode alone. In this analysis, although the incremental median costs in the first six months after the ca-CDI diagnosis were higher for second/later episode than recurrent episode, ($3,139 and $1,812 respectively), the 75th and 90th percentile costs were higher for those with recurrent CDIs (75th percentile recurrent $7,086, later episode $5,113; 90th percentile recurrent $25, 256, later episode $20,013)(Table 4).
Table 4. Multivariable quantile regression models for potential predictors of cost of care (in 2016 Canadian dollars) among individuals with community-associated CDI by time period of follow up (before and after initial CDI) *.
| Median (95% CI) costs | 75th percentile (95% CI) costs | 90th percentile (95% CI) costs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before CDI | After CDI | Before CDI | After CDI | Before CDI | After CDI | ||||||||
| 1y – 6m | 6m – 1d | CDI– 6m | 6m – 1y | 1y – 6m | 6m – 1d | CDI– 6m | 6m – 1y | 1y – 6m | 6m – 1d | CDI– 6m | 6m – 1y | ||
| Intercept | 153 | 296 | 491 | 274 | 344 | 699 | 1463 | 378 | 891 | 1137 | 5008 | 962 | |
| (60, 246) | (166, 425) | (153, 830) | (89, 459) | (104, 584) | (385, 1013) | (107, 2819) | (-100, 856) | (93, 1689) | (-158, 2431) | (994, 9022) | (-1423, 3347) | ||
| Type of CDI | Recurrent vs Single Episode |
28 | 157 | 1812 | 21 | 107 | 4 | 7086 | -43 | 1386 | 380 | 25256 | 628 |
| (-53, 109) | (47, 267) | (1559, 2064) | (-90, 132) | (-102, 316) | (-263, 270) | (6074, 8098) | (-330, 243) | (691, 2080) | (-718, 1478) | (22261, 28252) | (-800, 2057) | ||
| Second/later vs Single episode | 172 | 186 | 3139 | 329 | 508 | 1348 | 5113 | 1312 | 5436 | 3574 | 20013 | 12156 | |
| (59, 286) | (32, 339) | (2782, 3496) | (172, 486) | (215, 801) | (975, 1721) | (3683, 6542) | (908, 1717) | (4462, 6410) | (2037, 5110) | (15782, 24244) | (10139, 14173) | ||
| Male vs Female | -3 | 31 | 28 | -94 | 108 | 141 | 1061 | -56 | 810 | 1329 | 3975 | 604 | |
| (-58, 52) | (-44, 105) | (-142, 198) | (-167, -21) | (-33, 250) | (-40, 322) | (381, 1742) | (-244, 133) | (338, 1282) | (584, 2074) | (1961, 5989) | (-336, 1544) | ||
| Diabetes Diagnosis | 717 | 904 | 1245 | 634 | 1962 | 1669 | 1070 | 925 | 6467 | 3445 | -1317 | 3929 | |
| (638, 796) | (799, 1009) | (994, 1495) | (527, 740) | (1759, 2165) | (1414, 1925) | (66, 2074) | (651, 1198) | (5792, 7143) | (2392, 4498) | (-4289, 1655) | (2563, 5295) | ||
| Dialysis | 15044 | 5752 | 21650 | 5566 | 48162 | 14550 | 60410 | 38966 | 108427 | 29536 | 197689 | 63974 | |
| (14840, 15247) | (5488, 6017) | (20935, 22365) | (5244, 5889) | (47638, 48685) | (13908, 15193) | (57545, 63275) | (38135, 39798) | (106684, 110169) | (26889, 32183) | (189209, 206170) | (59826, 68123) | ||
| SEFI | -7 | -15 | 59 | 0 | 21 | 71 | 227 | 26 | 153 | 34 | 818 | 114 | |
| (-38, 24) | (-56, 27) | (-35, 153) | (-40, 40) | (-57, 100) | (-30, 171) | (-149, 602) | (-79, 130) | (-109, 415) | (-381, 449) | (-295, 1930) | (-405, 634) | ||
| Age (yrs) (reference category 60–69) |
18–39 | -110 | -121 | -318 | -180 | -161 | -260 | -679 | -127 | -528 | -311 | -1119 | -457 |
| (-197, -23) | (-239, -3) | (-582, -55) | (-292, -67) | (-385, 63) | (-546, 26) | (-1735, 378) | (-417, 163) | (-1273, 217) | (-1488, 866) | (-4246, 2008) | (-1904, 989) | ||
| 40–49 | -83 | -108 | -262 | -136 | -195 | -255 | -781 | -62 | -355 | -139 | -1510 | -492 | |
| (-178, 13) | (-238, 21) | (-553, 29) | (-259, -13) | (-440, 49) | (-570, 59) | (-1949, 386) | (-379, 255) | (-1170, 460) | (-1436, 1159) | (-4965, 1945) | (-2074, 1090) | ||
| 50–59 | 21 | -32 | -243 | 41 | -8 | -17 | -665 | 189 | -339 | 159 | 2326 | 655 | |
| (-68, 110) | (-152, 87) | (-508, 22) | (-73, 155) | (-238, 221) | (-307, 273) | (-1726, 397) | (-105, 482) | (-1102, 424) | (-1037, 1355) | (-816, 5468) | (-810, 2120) | ||
| 70–79 | 231 | 332 | 979 | 33 | 183 | 189 | 2794 | 444 | 985 | 127 | 6712 | 429 | |
| (135, 326) | (203, 462) | (684, 1274) | (-91, 158) | (-62, 429) | (-125, 504) | (1613, 3975) | (122, 765) | (168, 1802) | (-1170, 1424) | (3216, 10207) | (-1174, 2032) | ||
| 80+ | 487 | 269 | 2483 | 174 | 813 | 456 | 6454 | 222 | 985 | 2785 | 13278 | 1665 | |
| (386, 588) | (136, 401) | (2155, 2811) | (36, 313) | (553, 1073) | (133, 778) | (5140, 7768) | (-135, 579) | (121, 1850) | (1455, 4114) | (9389, 17168) | (-117, 3446) | ||
| CCI score (reference: 0) |
3+ | 1592 | 2193 | 1817 | 1591 | 4852 | 5449 | 5590 | 5205 | 25994 | 6111 | 3139 | 5182 |
| (1460, 1723) | (2024, 2361) | (1424, 2209) | (1441, 1740) | (4514, 5191) | (5040, 5858) | (4017, 7163) | (4820, 5590) | (24867, 27121) | (4427, 7795) | (-1517, 7795) | (3261, 7103) | ||
| 2 | 835 | 805 | 1191 | 782 | 3189 | 3369 | 6223 | 1829 | 12390 | 8685 | 11948 | 5482 | |
| (736, 934) | (675, 935) | (894, 1488) | (659, 905) | (2934, 3445) | (3054, 3685) | (5033, 7413) | (1512, 2147) | (11540, 13240) | (7384, 9985) | (8425, 15470) | (3897, 7068) | ||
| 1 | 342 | 142 | 321 | 176 | 805 | 236 | 788 | 169 | 4116 | 1743 | 3598 | 195 | |
| (268, 417) | (40, 244) | (91, 552) | (78, 275) | (613, 997) | (-10, 483) | (-135, 1712) | (-85, 422) | (3477, 4755) | (726, 2759) | (863, 6332) | (-1071, 1461) | ||
| Physician Usage (by quartile of use) (reference: Lowest Quartile) |
Highest | 1081 | 1349 | 1529 | 736 | 2351 | 2759 | 4062 | 2455 | 5704 | 8496 | 6515 | 6330 |
| (993, 1168) | (1229, 1469) | (1218, 1840) | (561, 911) | (2126, 2575) | (2468, 3050) | (2817, 5307) | (2004, 2906) | (4956, 6452) | (7297, 9695) | (2830, 10201) | (4078, 8581) | ||
| Third | 349 | 332 | 391 | 191 | 635 | 621 | 851 | 391 | 1318 | 1655 | -38 | 638 | |
| (264, 433) | (215, 449) | (87, 695) | (16, 366) | (418, 851) | (337, 905) | (-367, 2068) | (-60, 842) | (597, 2039) | (486, 2825) | (-3642, 3566) | (-1610, 2887) | ||
| Second | 117 | 148 | 118 | 64 | 253 | 176 | 256 | 121 | 393 | 198 | -1368 | 270 | |
| (33, 200) | (31, 264) | (-197, 433) | (-121, 249) | (37, 468) | (-107, 459) | (-1006, 1518) | (-357, 598) | (-325, 1111) | (-969, 1365) | (-5103, 2368) | (-2113, 2654) | ||
* Each time period analysis was limited to those surviving to the end of the period.
Intercepts represent the cost when all variables are set to reference values for example, the intercept provides the median cost for a 60–69 year old female subject without, diabetes or dialysis, who had median SEFI score and no comorbidities contributing to Charlson comorbidity score and low previous ambulatory care use.
The 75th and 90th percentile regression analyses suggest the incremental costs for the most expensive 25 percent of individuals with recurrent ca-CDI are $7,086 per person and that for the most expensive 10 percent of individuals with recurrent ca-CDI are $ 20,013 per person in the first six months after the initial ca-CDI.
Increasing age, male sex, higher CCI score (co-morbidities), diabetes, dialysis and prior ambulatory care use were associated with increased cost of care among those with ca-CDI, both before and after the ca-CDI index date; the incremental costs with presence of each these factors were much higher in the six months after ca-CDI diagnosis.
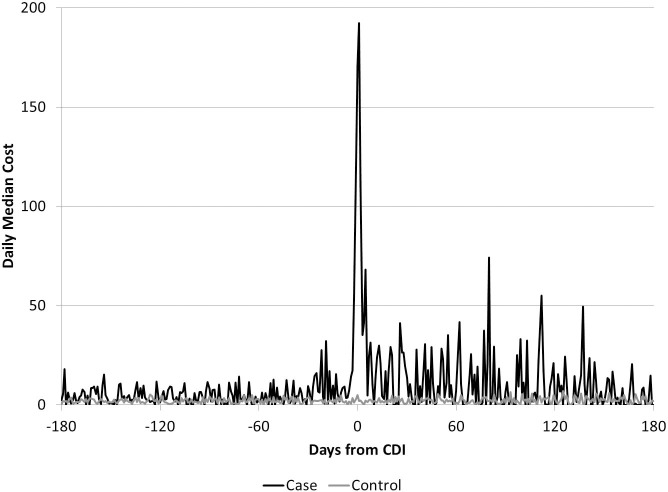
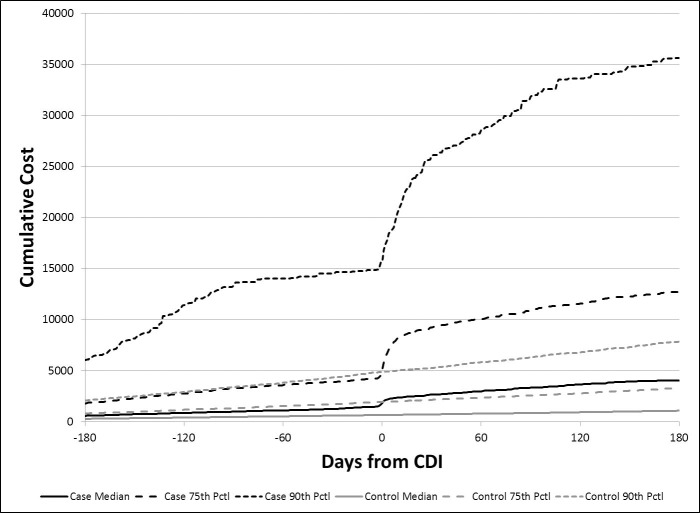
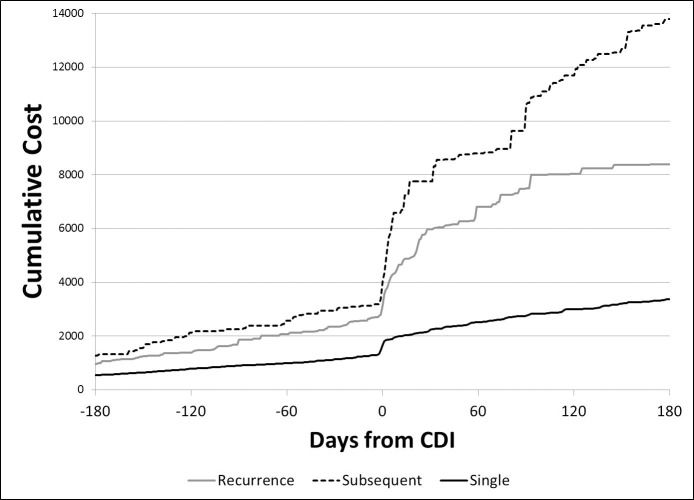
The plot of daily median costs among those with and without ca-CDI (Fig 1) suggests that although the cost differential was highest soon after diagnosis, it continued for several months. The cumulative costs are displayed in Figs 2 and 3 and suggest cumulative median costs among those with subsequent CDI episodes were higher than among those with recurrent episodes (Fig 3)
Fig 1. Daily median costs for CDI cases and controls without community-associated CDI, over six months before and after index date.
Fig 2. Cumulative median, 75th percentile, and 90th percentile costs for community-associated CDI cases and controls without CDI.
Fig 3. Cumulative median costs for those with single episode of community-associated CDI, recurrent and one or more subsequent CDI episodes.
The median cost of all antibiotic prescriptions for those with first episode was $8 (mean cost $89) and for that with recurrent/second or later episode of CDI was $85 (mean cost $474). The median cost for a prescription of vancomycin was $316 (Interquartile range (IQR) $209-$489) and for metronidazole it was $4 (IQR $3-$5).
Discussion
In this province wide population-based study, we are reporting markedly incremental costs of those with recurrent and repeated CDIs after an initial ca-CDI as compared to those with single ca-CDI episode. We report incremental costs of care of individuals with ca-CDI even before ca-CDI diagnosis, predictors of increased cost among those with ca-CDI and that ca-CDI increases cost of care much more in older individuals. These data would be valuable for cost-effectiveness studies evaluating benefits of newer therapies to treat ca-CDI and prevent recurrent and repeat CDIs after the initial ca-CDI. These data also provide economic rationale for prevention of ca-CDI from the perspective of payors of health care services.
Nana et al. estimated median attributable six months costs of ca-CDI of $7,394 (2014 Canadian dollars), using administrative health care data in Ontario for CDIs between 2003 and 2010[31]. However, they were not able to include individuals who did not visit Emergency departments and/or were not admitted (i.e. who only had physician office visits for CDI) and hence likely reported costs of the more severe ca-CDI cases i.e. costs of ca-CDI leading to hospitalization. They were also limited by the limitations of using ICD codes to identify CDI. We are not aware of other prior studies evaluating incremental cost of care of individuals with ca-CDI. A systematic review published in 2014, estimated CDI attributable costs expressed in US dollars, as $6,774-$10,212 for CDI requiring admission and $2,992-$29,000 for hospital-acquired CDI[32]. Other estimates of the per-patient cost of CDI in the US have varied markedly between individual hospitals[15]. Levy et al. (2015) estimated the burden of CDI in Canada, including direct and indirect (productivity loss) costs using projections of CDI and costs from the literature. At the episode level, they estimated that the incremental hospitalization cost, excluding pharmacotherapy, was $11,928 for each initial infection, and $15,330 for each recurrent infection (in 2012 Canadian dollars)[33]. Our estimates are lower, likely as we included milder cases and individuals who never presented to the EDs or hospitals. Even then, as noted in Table 2, there are substantial cumulative total costs associated with ca- CDI.
Our study suggests that even when the analysis is adjusted for several baseline characteristic differences among those predisposed to develop ca-CDI and those who do not develop CDI, the cost of care is higher even before ca-CDI episode. We believe, this differential cost at baseline should be considered in any cost effectiveness study evaluating the effect of different treatments for CDI and not merely limit to matching those with and without CDI as that may not adjust for underlying cost differential among people predisposed to CDI vs not.
Our study found pharmaceutical costs were a small component of increased costs among those with recurrent/subsequent episode of CDI. Therefore, the increased costs are more likely due to increase in other health care use. The additional mean pharmaceutical costs were similar to that of cost of a prescription for vancomycin, which was approximately $400. This alone would support the use of vancomycin as the preferred agent of recurrent/subsequent episode of ca-CDI, with no net increase in pharmaceutical costs.
There are no data on cost-effectiveness of different strategies for ca-CDI; our study provides cost data using traditional approach for such analyses. While similar recurrence rates have been reported with use of vancomycin and metronidazole[34], because of its lower efficacy for clinical response, metronidazole is no longer recommended and instead vancomycin or fidaxomicin are recommended as first line treatments for all cases of CDI[8]. Fidaxomicin has been reported to be noninferior to vancomycin for clinical cure (fidaxomicin, 88% vs vancomycin, 86%) and associated with much lower recurrence rate(15.4% vs. 25.3%, P = 0.005)[35]. Even though fidaxomicin is much more expensive than vancomcycin (current (April 2019) mean cost in Manitoba for the recommended 10 day prescription for fidaxomicin is $2016.16, including dispensing fee), fidaxomicin was a more cost-effective therapy than metronidazole or vancomycin for initial CDI, in 2 of 3 studies identified in a recent systematic review[36]. However, there are no cost-effectiveness data for ca-CDI.
We are reporting increasing age, male sex, higher CCI score (co-morbidities), diabetes, dialysis and higher prior ambulatory care use are associated with increased incremental cost of care (median as well as higher percentiles) among those with ca-CDI. Purely on economic basis, therefore, individuals with these factors should particularly be considered for use of therapies with higher efficacy for clinical cure and/or lower recurrent episodes.
Our results should be viewed in the context of strengths and limitations of our study. We are reporting direct costs of care in a population-based setting of ca-CDI. There are many studies on CDI costing, but health care costs of this sub-group of individuals with CDI has not been previously extensively investigated, likely because of limited truly population-based datasets of ca-CDI We were able to identify CDI, using a laboratory confirmed cases dataset. We are providing costs by initial and subsequent CDI and predictors of cost in the population setting. However, in the time period of this study, metronidazole was the standard of care for first episode of mild CDI, which is no longer recommended. Our study does suggest that health care costs of ca-CDI were much more than the cost of an average prescription of vancomycin and therefore higher efficacy of vancomycin could potentially lower overall costs of ca-CDI by reducing the subsequent health care utilization—this will need to be analyzed in cost effectiveness studies. We included dialysis patients in the ca-CDI category, although some dialysis units are located in the hospitals; however, most are in ambulatory care centers and the recommended definition in the time period of the study included them in the community associated category.
In conclusion, we report costs for care of individuals with ca- CDI in a province wide setting that highlight the need of prevention of ca-CDI as well as therapies which can lead to rapid cure and prevent recurrence in community setting also of CDI. Health care costs of an episode of ca- CDI are much more than the cost of antibiotic treatment. Our study provides population-based data for use in future studies that evaluate the cost-effectiveness of various CDI therapies for ca-CDI.
Supporting information
(DOCX)
Data Availability
The data used in this analysis are owned by the Government of Manitoba. We received special access privileges to the data that others would not have, without permission. We were given permission to use the data to conduct the analysis. However we do not have permission to share the data. Researchers interested in replicating our results, can apply to the ministry of health to access the data: Health Information Privacy Committee, Manitoba Health, Seniors and Active Living, 4043 - 300 Carlton Street, Winnipeg MB R3B 3M9 (email: hipc@gov.mb.ca). Instructions can be found at: http://www.gov.mb.ca/health/hipc/submission.html. We believe that interested researchers will be able to replicate the results of our study by following protocol outlined in the Methods section of the manuscript.
Funding Statement
Supported by a research grant from the Merck Investigator Studies Program to HS. The opinions expressed in this paper are those of the authors. The study sponsor had no role in study design, the collection, analysis, and interpretation of data, the writing of the report; and the decision to submit the manuscript for publication.
References
- 1.To KB, Napolitano LM. Clostridium difficile Infection: Update on Diagnosis, Epidemiology, and Treatment Strategies. Surgical infections. 2014;15(5):490–502. Epub 2014/10/15. 10.1089/sur.2013.186 . [DOI] [PubMed] [Google Scholar]
- 2.Le Monnier A, Zahar JR, Barbut F. Update on Clostridium difficile infections. Medecine et maladies infectieuses. 2014;44(8):354–65. Epub 2014/08/27. 10.1016/j.medmal.2014.04.002 . [DOI] [PubMed] [Google Scholar]
- 3.Lessa FC, Gould CV, McDonald LC. Current Status of Clostridium difficile Infection Epidemiology. Clinical Infectious Diseases. 2012;55(suppl 2):S65–S70. 10.1093/cid/cis319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. American journal of infection control. 2014;42(10):1028–32. Epub 2014/10/04. 10.1016/j.ajic.2014.06.011 . [DOI] [PubMed] [Google Scholar]
- 5.Dupont HL. Diagnosis and management of Clostridium difficile infection. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(10):1216–23; quiz e73. Epub 2013/04/02. 10.1016/j.cgh.2013.03.016 . [DOI] [PubMed] [Google Scholar]
- 6.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, et al. The Epidemiology of Community-Acquired Clostridium difficile Infection: A Population-Based Study. The American journal of gastroenterology. 2011;107:89 10.1038/ajg.2011.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanwa N, Kendzerska T, Krahn M, Kwong JC, Daneman N, Witteman W, et al. The economic impact of Clostridium difficile infection: a systematic review. The American journal of gastroenterology. 2015;110(4):511–9. Epub 2015/04/08. 10.1038/ajg.2015.48 . [DOI] [PubMed] [Google Scholar]
- 8.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2018;66(7):987–94. Epub 2018/03/22. 10.1093/cid/ciy149 . [DOI] [PubMed] [Google Scholar]
- 9.Choi KB, Suh KN, Muldoon KA, Roth VR, Forster A. Hospital Acquired Clostridium difficile Infection (HA-CDI): An institutional costing analysis. The Journal of hospital infection. 2019. Epub 2019/01/29. 10.1016/j.jhin.2019.01.019 . [DOI] [PubMed] [Google Scholar]
- 10.Mollard S, Lurienne L, Heimann SM, Bandinelli PA. The Burden of Clostridium difficile Infection During Inpatient Stays in the United States between 2012 and 2016. The Journal of hospital infection. 2019. Epub 2019/01/29. 10.1016/j.jhin.2019.01.020 . [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues R, Barber GE, Ananthakrishnan AN. A Comprehensive Study of Costs Associated With Recurrent Clostridium difficile Infection. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2017;38(2):196–202. Epub 2016/11/08. 10.1017/ice.2016.246 . [DOI] [PubMed] [Google Scholar]
- 12.Dubberke ER, Butler AM, Nyazee HA, Reske KA, Yokoe DS, Mayer J, et al. The impact of ICD-9-CM code rank order on the estimated prevalence of Clostridium difficile infections. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(1):20–5. Epub 2011/06/10. 10.1093/cid/cir246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto M, Ohl ME, Schweizer ML, Perencevich EN. Accuracy of administrative code data for the surveillance of healthcare-associated infections: a systematic review and meta-analysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(5):688–96. Epub 2013/11/13. 10.1093/cid/cit737 . [DOI] [PubMed] [Google Scholar]
- 14.Singh H, Nugent Z, Yu BN, Lix LM, Targownik L, Bernstein C. Hospital discharge abstracts have limited accuracy in identifying occurrence of Clostridium difficile infections among hospitalized individuals with inflammatory bowel disease: A population-based study. PloS one. 2017;12(2):e0171266 Epub 2017/02/16. 10.1371/journal.pone.0171266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pak TR, Chacko KI, O'Donnell T, Huprikar SS, van Bakel H, Kasarskis A, et al. Estimating Local Costs Associated With Clostridium difficile Infection Using Machine Learning and Electronic Medical Records. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2017;38(12):1478–86. Epub 2017/11/07. 10.1017/ice.2017.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert PJ, Dyck M, Thompson LH, Hammond GW. Population-based surveillance of Clostridium difficile infection in Manitoba, Canada, by using interim surveillance definitions. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2009;30(10):945–51. Epub 2009/09/08. 10.1086/605719 . [DOI] [PubMed] [Google Scholar]
- 17.Manitoba Health HLaS. Manitoba Health, Healthy Living and Seniors Population Report June 1, 2015. Winnipeg, Manitoba: Government of Manitoba; 2015. [Google Scholar]
- 18.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. AnnPharmacother. 1998;32(11):1152–7. [DOI] [PubMed] [Google Scholar]
- 19.Roos LL, Mustard CA, Nicol JP, McLerran DF, Malenka DJ, Young TK, et al. Registries and administrative data: organization and accuracy. MedCare. 1993;31(3):201–12. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JR, Young TK, Roos LL, Gelskey DE. Estimating the burden of disease. Comparing administrative data and self-reports. MedCare. 1997;35(9):932–47. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. AmJEpidemiol. 1999;149(10):916–24. [DOI] [PubMed] [Google Scholar]
- 22.Rea MC, O'Sullivan O, Shanahan F, O'Toole PW, Stanton C, Ross RP, et al. Clostridium difficile Carriage in Elderly Subjects and Associated Changes in the Intestinal Microbiota. Journal of clinical microbiology. 2012;50(3):867–75. 10.1128/JCM.05176-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galdys AL, Nelson JS, Shutt KA, Schlackman JL, Pakstis DL, Pasculle AW, et al. Prevalence and duration of asymptomatic Clostridium difficile carriage among healthy subjects in Pittsburgh, Pennsylvania. Journal of clinical microbiology. 2014;52(7):2406–9. Epub 2014/04/25. 10.1128/JCM.00222-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfa MJ, Swan B, VanDekerkhove B, Pang P, Harding GK. The diagnosis of Clostridium difficile-associated diarrhea: comparison of Triage C. difficile panel, EIA for Tox A/B and cytotoxin assays. Diagnostic microbiology and infectious disease. 2002;43(4):257–63. Epub 2002/08/02. 10.1016/s0732-8893(02)00413-3 . [DOI] [PubMed] [Google Scholar]
- 25.Walkty A, Lagace-Wiens PR, Manickam K, Adam H, Pieroni P, Hoban D, et al. Evaluation of an algorithmic approach in comparison with the Illumigene assay for laboratory diagnosis of Clostridium difficile infection. Journal of clinical microbiology. 2013;51(4):1152–7. Epub 2013/02/01. 10.1128/JCM.03203-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2007;28(2):140–5. Epub 2007/02/01. 10.1086/511798 . [DOI] [PubMed] [Google Scholar]
- 27.Phatharacharukul P, Thongprayoon C, Cheungpasitporn W, Edmonds PJ, Mahaparn P, Bruminhent J. The Risks of Incident and Recurrent Clostridium difficile-Associated Diarrhea in Chronic Kidney Disease and End-Stage Kidney Disease Patients: A Systematic Review and Meta-Analysis. Digestive diseases and sciences. 2015;60(10):2913–22. Epub 2015/05/20. 10.1007/s10620-015-3714-9 . [DOI] [PubMed] [Google Scholar]
- 28.Qu HQ, Jiang ZD. Clostridium difficile infection in diabetes. Diabetes research and clinical practice. 2014;105(3):285–94. Epub 2014/07/13. 10.1016/j.diabres.2014.06.002 . [DOI] [PubMed] [Google Scholar]
- 29.Piper MS, Saad RJ. Diabetes Mellitus and the Colon. Current treatment options in gastroenterology. 2017;15(4):460–74. Epub 2017/10/25. 10.1007/s11938-017-0151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chateau D, Metge C, Prior H, Soodeen RA. Learning from the census: the Socio-economic Factor Index (SEFI) and health outcomes in Manitoba. Canadian journal of public health = Revue canadienne de sante publique. 2012;103(8 Suppl 2):S23–7. Epub 2012/01/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanwa N, Sander B, Krahn M, Daneman N, Lu H, Austin PC, et al. A population-based matched cohort study examining the mortality and costs of patients with community-onset Clostridium difficile infection identified using emergency department visits and hospital admissions. PloS one. 2017;12(3):e0172410 Epub 2017/03/04. 10.1371/journal.pone.0172410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. The Journal of hospital infection. 2014;88(1):12–21. Epub 2014/07/06. 10.1016/j.jhin.2014.04.011 . [DOI] [PubMed] [Google Scholar]
- 33.Levy AR, Szabo SM, Lozano-Ortega G, Lloyd-Smith E, Leung V, Lawrence R, et al. Incidence and Costs of Clostridium difficile Infections in Canada. Open forum infectious diseases. 2015;2(3):ofv076 Epub 2015/07/21. 10.1093/ofid/ofv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens VW, Nelson RE, Schwab-Daugherty EM, Khader K, Jones MM, Brown KA, et al. Comparative Effectiveness of Vancomycin and Metronidazole for the Prevention of Recurrence and Death in Patients With Clostridium difficile Infection. JAMA internal medicine. 2017;177(4):546–53. Epub 2017/02/07. 10.1001/jamainternmed.2016.9045 . [DOI] [PubMed] [Google Scholar]
- 35.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. The New England journal of medicine. 2011;364(5):422–31. Epub 2011/02/04. 10.1056/NEJMoa0910812 . [DOI] [PubMed] [Google Scholar]
- 36.Le P, Nghiem VT, Mullen PD, Deshpande A. Cost-Effectiveness of Competing Treatment Strategies for Clostridium difficile Infection: A Systematic Review. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2018;39(4):412–24. Epub 2018/02/22. 10.1017/ice.2017.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The data used in this analysis are owned by the Government of Manitoba. We received special access privileges to the data that others would not have, without permission. We were given permission to use the data to conduct the analysis. However we do not have permission to share the data. Researchers interested in replicating our results, can apply to the ministry of health to access the data: Health Information Privacy Committee, Manitoba Health, Seniors and Active Living, 4043 - 300 Carlton Street, Winnipeg MB R3B 3M9 (email: hipc@gov.mb.ca). Instructions can be found at: http://www.gov.mb.ca/health/hipc/submission.html. We believe that interested researchers will be able to replicate the results of our study by following protocol outlined in the Methods section of the manuscript.