Abstract
Purpose/Objectives:
In patients with gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue lymphoma (MALT lymphoma), the standard radiation therapy (RT) dose is ≥ 30 Gy. We report the outcome of patients treated with reduced 24 Gy compared with ≥ 30 Gy.
Materials/Methods:
We reviewed 32 patients diagnosed between 2007 and 2017 with gastric MALT lymphoma and treated with involved site radiation therapy (ISRT) using intensity modulated radiation therapy (IMRT). Response to therapy was based on post-RT endoscopic biopsy. Freedom from local treatment failure (FFTLF), freedom from treatment failure (FFTF), and overall survival (OS) outcomes were determined.
Results:
The median age of patients at diagnosis was 58 years. Antimicrobial therapy was administered to 11 patients pre-RT, with residual disease was documented in all 11patients. Rituximab (n=2) or Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP, n=1) was administered in 3 patients pre-RT; with biopsy proven persistent disease after therapy in all 3 cases. One patient received RT (36 Gy) and concurrent rituximab. Median RT dose was 30 Gy; 30–36 Gy in 66% (n=21) and 24 Gy in 34% (n=11). Post-RT biopsy documented CR in all patients. Failures occurred in the stomach, and duodenum at 3.6 and 4.5 years, respectively, after 30 Gy. At a median follow up of 55.2 months (73.8 for ≥ 30 Gy compared to 28.7 for 24 Gy; p<0.001), the 2-year FFLTF, FFTF and OS were 100%, 100% and 97%, respectively. There was no association between the lower 24 Gy dose with FFLTF (p=0.819), FFTF (p=0.819) or OS (p=0.469).
Conclusions:
Contemporary RT with ISRT using IMRT is associated with high CR rates for patients with gastric MALT lymphoma, even using reduced doses of 24 Gy. Additional follow-up and increased patient numbers are required to confirm equivalent disease control.
Keywords: Gastric MALT lymphoma, reduced dose, IMRT
Summary
We evaluated the outcome of patients with gastric mucosa-associated lymphoid tissue (gMALT) lymphoma treated with intensity modulated radiation therapy and involved site targeting. The 2-year freedom from local treatment failure rate was 100% and 2-year overall survival was 97%. There was no difference in outcome among patients treated with standard RT doses of 30–36 Gy compared to those who received reduced RT doses of 24 Gy.
Introduction
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is an indolent B-cell neoplasm that can occur in a variety of extranodal sites, with the stomach representing a common location (1),(2). Helicobacter pylori (H. pylori) infection is implicated in the pathogenesis of gastric MALT lymphoma (gMALT); resolution of disease can often be achieved with H. pylori eradication (2,3). In cases where gMALT persists or relapses after antibiotics or for disease not caused by infection, local therapy with radiation therapy (RT) is often considered.
Overall, the outcomes among patients with extranodal MALT lymphoma are excellent with deaths due to disease occurring only rarely (4). While relapses after initial therapy for limited stage MALT lymphoma can occur in roughly 35–60% of patients, for those with gMALT the relapse-free survival rates are superior(5–8).
Long-term series have reported local control rates in excess of 90% after RT for gMALT with standard RT doses of 30–36 Gy (6,8–11). Given the highly favorable outcomes of patients with gMALT coupled with excellent results after 30 Gy, dose de-escalation may be considered. A randomized trial compared 40–45 Gy to 24 Gy among indolent B-cell lymphoma patients, however, of 248 patients that received frontline RT only 17% had marginal zone lymphoma and it is unclear how many had gMALT(12). Furthermore, many series have reported outcomes after RT, however in those studies larger field sizes (including whole abdomen and extended-field RT) as well as traditional three-dimensional (3D) conformal RT delivery techniques were utilized(10,11). Therefore, we sought to evaluate the effect of dose on the outcome of patients treated with RT for gMALT lymphoma, as evaluated by 2- year disease control endpoints. Furthermore we explored outcomes using modern techniques including involved site radiation therapy (ISRT) and intensity modulated radiation therapy (IMRT) (13).
Methods
After obtaining Institutional Review Board approval we retrospectively reviewed the records of patients treated with RT for biopsy proven gMALT between October 2007 and October 2017. Patients were considered to have low grade gMALT lymphoma or gMALT lymphoma with a large cell component (defined as the presence of large cells within the neoplasm but of insufficient number to support the diagnosis of diffuse large B cell lymphoma [DLBCL]). Patients with a concurrent DLBCL diagnosis or without endoscopic assessment post-RT were excluded. Information regarding baseline patient, disease and treatment characteristics were recorded.
Staging and Workup
All patients underwent diagnostic endoscopy confirming disease. Initial imaging assessment was performed with computed tomography (CT) or positron emission tomography- computed tomography (PET-CT) imaging. The presence of a gastric fluorodeoxyglucose (FDG) avid abnormality and the maximum standardized uptake value (SUVmax) was recorded if PET-CT imaging was performed. Ann Arbor staging was used (14). H. pylori status was determined based on biopsy histology. The MALT International Prognostic Index (IPI) (based on age ≥ 70, stage III/IV and elevated serum lactate dehydrogenase) was recorded (15).
Treatment
In general, RT was administered as frontline therapy in cases of H. pylori negative gMALT, or after unsuccessful antibiotics or systemic therapy (ST). When ST was administered, patients were treated with single-agent rituximab or rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). All patients underwent CT simulation with four-dimensional (4D) CT or deep-inspiration breath hold (DIBH) after a 4-hour minimum fasting period. DIBH was conducted using a respiratory monitoring device and patient feedback system (Varian Real-time Position Management System, Palo Alto, CA). ISRT planning was utilized with the clinical target volume (CTV) including the stomach alone for stage I disease or stomach and involved lymph nodes (for stage II) according to the International Radiation Oncology Lymphoma Group (ILROG) guidelines (13). A planning target volume (PTV) margin of 0.5–1.5 cm was used with IMRT for radiation delivery. Daily low-dose CT-on-rails was utilized for image guidance (GE Medical Systems, Milwaukee, WI) (16). For patients treated in free breathing larger margins of 1.5 cm were generally used. Prescription doses of ≥30 Gy were considered standard-dose and 24 Gy was considered reduced-dose. In general, patients that were treated in late 2014 and beyond received doses of 24 Gy.
Disease assessment/Follow up
Endoscopy with multiple biopsies was performed 3–6 months after RT. Complete response (CR) was defined according to the European Gastro-Intestinal Lymphoma Study (EGILS) group, with complete histological response (ChR) as well as probable minimal residual disease (pMRD) representing CR(17). When residual gMALT was detected, repeat biopsies were performed on 3–6 month intervals until disease resolution.
Statistical methods and study endpoints
The median follow up was calculated from the start of RT based on the reverse Kaplan-Meier (KM) method(18). Freedom from treatment failure (FFTF) and freedom from local treatment failure (FFLTF) were defined from the RT start date to any disease relapse/progression or local relapse/progression in the stomach or surrounding lymph nodes, respectively. Censoring was performed at last follow-up or death. Overall survival (OS) was defined from the date of initiation of RT to the date of death from any cause. FFTF, FFLTF and OS were estimated by the KM method(19). Differences in survival outcomes between standard and reduced-dose cohorts were compared using the Log-rank (Mantel-Cox) test. Binary and categorical variables were presented as percentages. Pearson’s chi square and Fisher’s exact tests were used to assess for differences between the standard and reduced-dose RT cohorts as appropriate. For comparison of continuous variables, the student t-test was used. The mean of each dosimetric variable was determined for all patients and for the standard and reduced-dose groups. The means were compared using independent sample two-sided t-tests, with homogeneity of variance confirmed prior to this analysis (Levene’s test statistic was calculated for each comparison with p >0.05 for all tests of homogeneity unless otherwise specified). For instances where p ≤ 0.05 using the Levene’s statistic, Welch’s t-test for unequal variances was performed to assess for equality of means. Statistical analysis was performed with SPSS version 24 (SPSS Inc, Chicago, Il).
Results:
Forty-three consecutive patients with gMALT received RT. Seven patients were excluded because of a history of DLBCL (n=1), RT outside of our institution (n=3) or lack of EGD follow-up post-RT (n=3). An additional four patients were excluded due to systemic therapy administration prior to RT without confirmation of persistent disease at the time of RT initiation. The final cohort included 32 patients. Baseline patient and treatment characteristics are in Table 1. Most patients (n= 30, 94%) had stage I/II disease. Two patients had stage IV disease based on low burden bone marrow involvement (n=1) or multiple sites of extranodal disease (lung and stomach, n=1). H.pylori infection was identified in 7 (22%) patients. PET-CT imaging was conducted in 25 patients (78%) with abnormal gastric avidity noted in 10 (40%) and a median gastric SUVmax of 4.9 (range 3.3 – 27.4). The patient with SUVmax of 27.4 had a focus of large cells within the biopsy that was not adequate to support a DLBCL diagnosis.
Table 1.
Patient and Disease Characteristics
| All Patients | Value or No. (%) | |||
|---|---|---|---|---|
| Characteristic | All Patients (n=32) |
Standard RT Dose (≥ 30Gy; n=21) | Lower RT Dose (≤ 24 Gy; n=11) |
P Value |
| Age, years, median (range) | 58 (32–88) | 58 (32–88) | 61 (35–72) | |
| Female | 13 (41%) | 9 (43%) | 4 (36%) | 1.00 |
| Disease stage | ||||
| I | 25 (78%) | 16 (76%) | 9 (82%) | .039 |
| II | 5 (16%) | 5 (24%) | 0 | |
| III | 0 | 0 | 0 | |
| IV | 2 (6%) | 0 | 2 (18%) | |
| B symptoms | ||||
| Yes | 2 (6%) | 1 (5%) | 1 (9%) | 0.577 |
| No | 30 (94%) | 20 (95%) | 10 (91%) | |
| MALT-IPI | ||||
| 0 | 28 (88%) | 19 (90%) | 9 (82%) | 0.281 |
| 1 | 3 (9%) | 1 (5%) | 2 (18%) | |
| Unknown | 1 (3%) | 1 (5%) | 0 | |
| Autoimmune disease | ||||
| Yes | 3 (9%) | 3 (14%) | 0 | 0.268 |
| No | 32 (89%) | 18 (86%) | 11 (100%) | |
| H. pylori infection | ||||
| Yes | 7 (22%) | 7 (33%) | 0 | 0.035 |
| No | 25 (78%) | 14 (67%) | 11 (100%) | |
| Large cell component | ||||
| Yes | 4 (13%) | 3 (14%) | 1 (9%) | 0.573 |
| No | 28 (88%) | 18 (86%) | 10 (91%) | |
| Location of dominant lesion | ||||
| Proximal 1/3 | 7 (22%) | 5 (24%) | 2 (18%) | 0.930 |
| Middle 1/3 | 17 (53%) | 11 (52%) | 6 (54%) | |
| Distal 1/3 | 8 (25%) | 5 (24%) | 3 (27%) | |
| Multifocal | ||||
| Yes | 11 (31%) | 8 (38%) | 1 (9%) | 0.090 |
| No | 25 (69%) | 13 (62%) | 10 (91%) | |
| Stomach thickening on CT | ||||
| Yes | 8 (75%) | 7 (33%) | 1 (9%) | 0.141 |
| No | 24 (75%) | 14 (67%) | 10 (91%) | |
| PET-CT performed | ||||
| Yes | 25 (78%) | 16 (76%) | 9 (82%) | 0.544 |
| No | 7 (22%) | 5 (24%) | 2 (18%) | |
| Stomach activity on PET? * | ||||
| Yes | 10 (40%) | 9 (56%) | 1 (11%) | .034 |
| No | 15 (60%) | 7 (44%) | 8 (91%) | |
Abbreviations: RT, Radiation therapy; MALT-IPI, mucosa associated lymphoid tissue international prognostic index; CT, computed tomography; PET-CT, Positron emission tomography–computed tomography;
Percentages for stomach activity on pet calculated based on patients that had PET-CT imaging performed
Anti-microbial and systemic therapy administered prior to RT
Antibiotics were given to 11 patients. Three of 11 patients had a CR to antibiotic therapy, but had a biopsy confirming H. pylori negative gMALT lymphoma relapse at 6.3, 30 and 71.1 months. Eight of the 11 patients that received antimicrobial therapy had persistent disease documented on endoscopic biopsy despite H. pylori eradication. One of these patients went on to receive Rituxan for persistent gMALT prior to RT. Overall, ST was given prior to RT to 3 patients including with R-CHOP (n=1) and rituximab (n=2). Among these 3 patients persistent gMALT after ST was confirmed by biopsy. An additional patient was treated with concurrent weekly rituximab. Overall, all 32 patients included in the analysis had residual disease documented just prior to RT by endoscopy.
Radiation Therapy
At the discretion of the treating physician, 21 patients (66%) received standard-dose RT of 30–36 Gy and 11 (34%) received a reduced-dose of 24 Gy (Table 2). All patients were treated with 1.5 – 2 Gy fractions. In the reduced-dose group, most patients were treated to 24 Gy in 2 Gy fractions (91%), but one patient received 24 Gy in 1.5 Gy fractions (9%). In the standard-dose cohort most patients were treated to a dose of 30 Gy (90%), whereas 2 patients received 36 Gy (10%). Fraction size of 1.5 Gy was used for most patients in the standard-dose group (80%). DIBH was utilized in 72% of patients in the cohort (n=23); all patients that received 24 Gy were treated with DIBH.
Table 2.
Treatment Characteristics
| All Patients | Value or No. (%) | |||
|---|---|---|---|---|
| Characteristic | All Patients (n=32) |
Standard RT Dose (≥ 30 Gy; n=21) | Lower RT Dose (≤ 24 Gy; n=11) |
P Value |
| Antibiotics Given Prior to RT | ||||
| Yes | 11 (34%) | 11 (52%) | 0 (8%) | .003 |
| No | 21 (66%) | 10 (48%) | 11 (100%) | |
| Systemic Therapy Prior to RT | ||||
| R-CHOP | 1 (3%) | 1 (5%) | 0 | 0.734 |
| Rituxan | 2 (6%) | 1 (5%) | 1 (9%) | |
| None | 29 (91%) | 19 (90%) | 10 (91%) | |
| Concurrent rituximab | ||||
| Yes | 1 (3%) | 1 (5%) | 0 | 1.00 |
| No | 31 (97%) | 20 (95%) | 12 (100%) | |
| Radiation Dose | ||||
| 24 Gy | 11 (34%) | 0 | 12 (100) | <0.001 |
| 30 Gy | 18 (56%) | 19 (90%) | 0 | |
| 36 Gy | 2 (6%) | 2 (10%) | 0 | |
| Fraction Size | ||||
| 1.5 Gy | 18 (56%) | 17 (80%) | 1 (9%) | <0.001 |
| 1.8 Gy | 2 (6%) | 2 (10%) | 0 | |
| 2.0 Gy | 12 (38%) | 2 (10%) | 10 (91%) | |
| Deep Inspiration Breathhold | ||||
| Yes | 23 (72%) | 12 (57%) | 11 (100%) | 0.013 |
| No | 9 (28%) | 9 (43%) | 0 | |
| Median follow-up time*, months (95% Cl) | 55.2 (32.4–78.1) | 73.8 (71.7–75.8) | 28.7 (23.0–34.3) | <0.001 |
Abbreviations: RT, Radiation therapy; R-CHOP, rituxan, cyclophosphamide, doxorubicin, vincristine, prednisone.
Follow up time calculated from the start of radiation therapy
Outcomes
The median follow-up for all patients was 55.2 months (95% Confidence Interval [CI], 32.4–78.1 months) and was shorter for patients treated with reduced-dose RT (28.7 months) versus standard-dose RT (73.8 months, p<0.001, Table 2). All patients had a CR following RT, including 31 patients who experienced a ChR and one patient that was treated with 24 Gy that had pMRD. CR (n=29) or pMRD (n=1) was achieved at the time of first post-RT endoscopy for 30 of 32 patients (94%) at a median time of 3.5 months (range 0.8–17.2 months). Two patients had a partial response at the time of first post-RT endoscopy performed at 3.5 and 4.7 months, but in subsequent endoscopies had a documented CR at 9.1 and 25.5 months without additional intervening therapy.
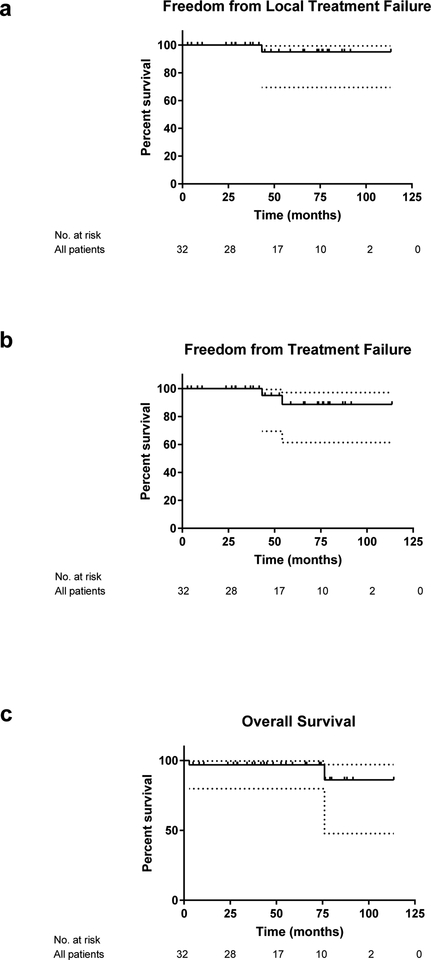
The 2 year FFLTF, FFTF and OS for the entire cohort was 100%, 100%, and 97% respectively (Figure 1a–c). There were two deaths. One patient died of unknown causes 6.3 years after RT to 36 Gy. This patient was in CR at the follow-up visit preceding death. A second patient from a brain infection 3 months after completion of 30 Gy with remission of gMALT confirmed by endoscopy one month prior to death. A total of 2 relapses occurred, 1 distant and 1 local. One patient who initially presented with gMALT with regional lymph node involvement relapsed in the duodenum 4.5 years after 30 Gy to the stomach and involved lymph nodes. This patient had a negative biopsy from the duodenum at diagnosis. The patient was observed and remained on surveillance at the time of last follow-up. One local failure occurred in the gastric body 3.6 years after completion of 30 Gy to the stomach for stage I disease. The patient has been observed since that time.
Figure 1. Treatment outcomes after radiation therapy among all patients.
Freedom from local treatment failure (FFLTF, a), freedom from treatment failure (FFTF, b) and overall survival (OS, c) among 32 patients treated with definitive RT for gastric MALT lymphoma.
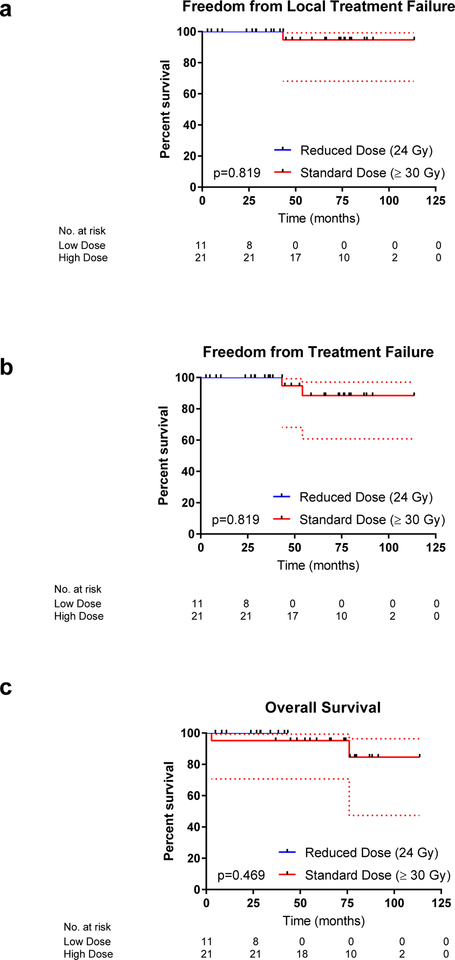
The small number of events in this cohort precluded a formal univariate analysis. When FFLTF, FFTF and OS were assessed by the KM method and compared by the Log-rank test according to RT dose (standard versus reduced) there was no significant difference in outcome (p=0.819, p=0.819 and p=0.469respectively; Figure 2a–c). When the analysis was limited to patients with stage I/II gMALT lymphoma (n=30), there was no difference in FFLTF (p=0.819), FFTF (p=0.819) or OS (p=0.513) between the standard (n=21) and reduced dose cohorts (n=9). These comparisons however are likely underpowered due to limited cohort size and small number of events.
Figure 2. Treatment outcomes after radiation therapy among patients according to radiation dose.
Freedom from local treatment failure (FFLTF, a), freedom from treatment failure (FFTF, b) and overall survival (OS, c) among 21 patients treated with definitive RT for gastric MALT lymphoma to a standard dose of 30–36 Gy compared to 11 patients that were treated with reduced-dose RT (24 Gy).
One second malignancy occurred in a patient with stage I gMALT who developed small lymphocytic lymphoma in a pelvic lymph node outside of the radiation field 6.1 years after completion of 30 Gy. No instances of late cardiovascular or renal dysfunction were observed in the follow up period.
Dosimetric Analysis
Mean doses to the right and left kidneys, liver and heart are presented in Table 3. The mean doses to all organs at risk were low and within accepted organ tolerance. Patients treated with reduced-dose RT had significantly lower mean doses administered to the liver (p=0.008) and heart (p=0.028).
Table 3.
Dosimetric Analysis
| All Patients | Dose in Gy | |||
|---|---|---|---|---|
| Characteristic | All Patients (n=32) |
Standard Dose (≥ 30 Gy; n=21) | Reduced Dose (≤ 24 Gy; n=11) |
P Value |
| Mean Right Kidney Dose (SD) | 2.4 (1.7) | 2.5 (1.5) | 2.3 (2.1) | 0.713 |
| Mean Left Kidney Dose (SD) | 3.6 (2.3) | 3.6 (2.1) | 3.6 (2.7) | 0.995 |
| Mean Liver Dose (SD) | 9.6 (2.6) | 10.4 (2.1) | 7.9 (2.8) | 0.008 |
| Mean Heart Dose (SD) | 2.9 (2.2) | 3.4 (2.5) | 2.0 (0.8) | 0.028* |
Levene’s test for equality of variance was <0.05, therefore t test for equality of means was performed with equal variance not assumed (Welch’s T-test for unequal variances).
Discussion:
In the current study we report the outcome of 32 gMALT patients treated with contemporary RT techniques, including ISRT and IMRT. We observed low rates of disease relapse and minimal toxicity after RT. There was no significant difference in the frequency of local or distant disease failure among the patients treated with reduced-dose RT compared with the standard-dose cohort, although the follow-up was shorter for patients treated with 24 Gy and the analysis was limited by small cohort size with few events.
Gastric MALT patients tend to have a particularly excellent outcome with fewer relapses after therapy compared to other MALT primary disease locations (6–8). In a series of 244 patients with early stage extranodal marginal zone lymphoma treated with RT at Memorial Sloan Kettering Cancer Center (MSKCC) between 1992 and 2012, the cumulative incidence of disease specific death was only 2% at 10 years(6). Relapses among these patients, however, were not uncommon. Relapse-free survival, while only 57% at 10 years for all patients, was found to be superior for patients with a gMALT. Similarly in a study reporting outcomes among 167 patients treated with RT for limited stage MALT lymphoma at Princess Margaret Hospital, the 10-year cause specific survival rate was 98%. While the 10-year recurrence-free rate was 76% for all patients, among the 25 patients with gMALT the 10 year recurrence-free rate was 95%. In both studies most patients were treated to 30 Gy. These reports suggest that gMALT may have a favorable biologic profile and illustrate the success of this treatment strategy with moderate RT doses.
The International Extranodal Lymphoma Study Group (IELSG) conducted a large multi-center study to evaluate the outcome of 102 patients with stage I/II gMALT treated with RT between 1981 and 2004(11). The 10-year freedom from treatment failure was 88%. RT was administered via 3D techniques to either the stomach with involved lymph nodes in 60% or the entire abdomen followed by a stomach boost in 40%. The median total RT dose was 40 Gy; no patient received a dose less than 26 Gy. There was no association between radiation field size or dose with treatment failure. In this study the actuarial incidence of a second malignancy was 14% at 10 years. It was reported that 3 pelvic malignancies in the whole abdomen cohort would likely have been avoided with a limited field. Late renal failure was not observed, but 8 patients died secondary to cardiovascular causes. The authors concluded that RT to the whole abdomen should be abandoned and treatment to the stomach and perigastric lymph nodes was associated with excellent treatment outcomes with minimal long term toxicity.
Doses of 30–45 Gy have been used in the past to treat patients with gMALT and small fraction sizes of 1.5 Gy have been employed in an effort to reduce long-term normal tissue toxicity(20). In our study, most patients who received a dose of 30–36 Gy had treatment administered in 1.5 Gy daily fractions over 4 weeks (20 out of 24 patients), a common regimen that was adopted after MSKCC reported successful outcomes with this dose and fractionation(20). The equivalent dose in 2 Gy fractions of this regimen is approximately 29 Gy. On the other hand, 24 Gy was delivered in 12 daily fractions of 2 Gy in most patients in the reduced-dose cohort just over 2 weeks (11 of 12 patients). As 2 Gy fractions are commonly used across tumor histologies and seem to be safe, increased late toxicity is not expected with this approach. Furthermore, this dose and fractionation scheme would be expected to be favored by many patients due to the reduced number of daily treatments.
Given that gMALT is rarely life threatening and that antibiotics can often provide cure, it is essential that additional treatment options provide disease control with minimal long-term effects. Limiting RT field size through the utilization of modern techniques, coupled with lower radiation doses, represent current approaches to eradicate disease while being mindful of the normal tissue radiation exposure. The results of a randomized trial conducted in the United Kingdom suggests that 24 Gy is effective for low-grade B-cell lymphoma (12). Retrospective studies among patients with low-grade orbital B-cell lymphoma, suggest that 24–25 Gy provides excellent disease control(21,22) and that even 4 Gy may have efficacy in many cases(23,24). These studies provide the basis for treatment with doses lower that 30 Gy in gMALT patients. In the current study among the patients treated with a reduced 24 Gy dose, CR was achieved in 100%; no local or distant relapses were observed.
A recently reported randomized trial (HELYX II) evaluated the efficacy of salvage RT for patients that harbored persistent lymphoma after H. pylori eradication or for those who presented with H. pylori negative disease (25). Twenty nine patients with low grade gMALT lymphoma stages IE and II1E were randomized to receive RT to the stomach to a dose of 25.2 or 36 Gy (both in 1.8 Gy fractions). Of the randomized 29 patients, 22 patients (12 in the 36 Gy arm and 10 in the 25.2 Gy arm) completed 1-year follow up and were included in the analysis. At a median follow up time of 79 months, there were no recurrences in either arm. Our data supports the conclusion of this randomized study, that reduced RT doses can be effective standard doses in limited stage gMALT lymphoma.
It is logical that patients treated with reduced prescription doses would also have lower RT doses administered to surrounding organs. Indeed, we did observe that the mean doses to the liver and heart were significantly lower among the reduced-dose cohort. However, it is important to note that there were no patients with stage II disease in the reduced dose 24 Gy cohort, which likely resulted in smaller treatment volumes compared to the standard dose group. This may have contributed to the lower OAR doses in the 24 Gy cohort. The mean doses to the kidneys were less than 5 Gy in all patients, illustrating the minimal risk for renal dysfunction as a complication of treatment with IMRT. Indeed, while late kidney failure has historically been considered as a potential late toxicity after treatment for gMALT, even in older series where whole abdominal RT was administered, renal failure was rare (11,26–28). It is our institutional practice to treat gMALT patients with IMRT, daily volumetric imaging and DIBH. When utilizing IMRT with limited PTV margins of 0.7–1.0 cm, considerable inter-fractional variation in stomach volume can occur, even with long periods of fasting, therefore daily CT imaging is helpful for maintaining target coverage (16). Given the proximity of the stomach to the diaphragm, gastric motion can be influenced by respiration (29). DIBH permits limitation of respiratory induced gastric motion which facilitates the utilization of smaller PTV margins(30). Furthermore, DIBH appears to provide physical distance between the base of the heart and the superior aspect of the stomach which results in reduced radiation cardiac exposure(31). Patients treated with 24 Gy were more likely to have received RT with DIBH (p=0.006) therefore the significantly lower OAR doses may have not been attributed solely to the lower prescription dose. A previous study that assessed the motion of whole stomach among patients with gastric lymphoma treated to the entire stomach in free breathing and aligned for treatment with bony anatomy, found that a uniform margin of 2.2 cm was required adequate gastric target coverage (≥95%)(32). Therefore, when DIBH and daily volumetric imaging are not readily available, clinicians must be mindful of the appropriate margins needed in order to ensure consistent target coverage.
While the data presented are promising, there are several limitations to our study in addition to its retrospective nature and limited patient number. The follow-up was significantly shorter for the patients treated with 24 Gy, therefore continued monitoring will be important to confirm disease control. In addition, one patient that was treated in the standard-dose cohort to 30 Gy received concurrent rituximab. As observation is being increasingly offered to patients with gMALT (3,4), minimally toxic therapies are of increased interest. To this end we are conducting a prospective trial to evaluate the efficacy of 4 Gy in 2 fractions in the definitive management of patients with gMALT (XXXXXXXXXXXXXXXXXXXXXXXXXX).
This study confirms previous reports that patients with gMALT have excellent outcomes after primary therapy with RT. Our data suggests that patients with gMALT may have successful treatment outcomes with IMRT and involved site targeting to reduced doses of 24 Gy. Longer follow up and additional studies with larger patient cohorts will be necessary to examine the efficacy of this reduced dose regimen as well as the efficacy of doses lower than 24 Gy.
Funding:
Supported in part by Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Conflicts of interest: Dr. Pinnix received research support from Merck & Co, is a consultant for Global Oncology One and receives a stipend from the International Journal of Radiation Oncology Biology and Physics for an editorial position.
References
- 1.Ferreri AJ, Zucca E. Marginal-zone lymphoma. Crit Rev Oncol Hematol 2007;63:245–56. [DOI] [PubMed] [Google Scholar]
- 2.Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (malt lymphoma). CA Cancer J Clin 2016;66:153–71. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric malt lymphoma after eradication of helicobacter pylori: A multicentre cohort follow-up study of 420 patients in japan. Gut 2012;61:507–13. [DOI] [PubMed] [Google Scholar]
- 4.Teckie S, Qi S, Chelius M, et al. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol 2017;28:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe S, Oda I, Inaba K, et al. A retrospective study of 5-year outcomes of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to helicobacter pylori eradication therapy. Jpn J Clin Oncol 2013;43:917–22. [DOI] [PubMed] [Google Scholar]
- 6.Teckie S, Qi S, Lovie S, et al. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys 2015;92:130–7. [DOI] [PubMed] [Google Scholar]
- 7.Goda JS, Gospodarowicz M, Pintilie M, et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010;116:3815–24. [DOI] [PubMed] [Google Scholar]
- 8.Tsang RW, Gospodarowicz MK, Pintilie M, et al. Stage i and ii malt lymphoma: Results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys 2001;50:1258–64. [DOI] [PubMed] [Google Scholar]
- 9.Ohkubo Y, Saito Y, Ushijima H, et al. Radiotherapy for localized gastric mucosa-associated lymphoid tissue lymphoma: Long-term outcomes over 10 years. J Radiat Res 2017;58:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruskone-Fourmestraux A, Matysiak-Budnik T, Fabiani B, et al. Exclusive moderate-dose radiotherapy in gastric marginal zone b-cell malt lymphoma: Results of a prospective study with a long term follow-up. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2015;117:178–82. [DOI] [PubMed] [Google Scholar]
- 11.Wirth A, Gospodarowicz M, Aleman BM, et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: A retrospective, multi-centre, international extranodal lymphoma study group study. Ann Oncol 2013;24:1344–51. [DOI] [PubMed] [Google Scholar]
- 12.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-hodgkin lymphoma: A randomised phase iii trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- 13.Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-hodgkin lymphoma-target definition and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys 2014;89:49–58. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thieblemont C, Cascione L, Conconi A, et al. A malt lymphoma prognostic index. Blood 2017;130:1409–1417. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Milgrom SA, Dabaja BS, et al. Daily ct guidance improves target coverage during definitive radiation therapy for gastric malt lymphoma. Pract Radiat Oncol 2017;7:e471–e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruskone-Fourmestraux A, Fischbach W, Aleman BM, et al. Egils consensus report. Gastric extranodal marginal zone b-cell lymphoma of malt. Gut 2011;60:747–58. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, De Stavola BL, Love SB, et al. Review of survival analyses published in cancer journals. Br J Cancer 1995;72:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EMP. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 1958;53:471–481. [Google Scholar]
- 20.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998;16:1916–21. [DOI] [PubMed] [Google Scholar]
- 21.Goda JS, Le LW, Lapperriere NJ, et al. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: Efficacy and toxicity. Int J Radiat Oncol Biol Phys 2011;81:e659–66. [DOI] [PubMed] [Google Scholar]
- 22.Tran KH, Campbell BA, Fua T, et al. Efficacy of low dose radiotherapy for primary orbital marginal zone lymphoma. Leuk Lymphoma 2013;54:491–6. [DOI] [PubMed] [Google Scholar]
- 23.Fasola CE, Jones JC, Huang DD, et al. Low-dose radiation therapy (2 gy × 2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys 2013;86:930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinnix CC, Dabaja BS, Milgrom SA, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal b-cell lymphoma. Head Neck 2018;40:1335. [DOI] [PubMed] [Google Scholar]
- 25.Schmelz R, Miehlke S, Thiede C, et al. Sequential h. Pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric malt lymphoma stages ie & ii1e: A prospective randomized trial. J Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 26.Aviles A, Nambo MJ, Neri N, et al. Mucosa-associated lymphoid tissue (malt) lymphoma of the stomach: Results of a controlled clinical trial. Med Oncol 2005;22:57–62. [DOI] [PubMed] [Google Scholar]
- 27.Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: Data of patients registered within the german multicenter study (git nhl 02/96). J Clin Oncol 2005;23:7050–9. [DOI] [PubMed] [Google Scholar]
- 28.Maor MH, North LB, Cabanillas FF, et al. Outcomes of high-dose unilateral kidney irradiation in patients with gastric lymphoma. Int J Radiat Oncol Biol Phys 1998;41:647–50. [DOI] [PubMed] [Google Scholar]
- 29.Wysocka B, Kassam Z, Lockwood G, et al. Interfraction and respiratory organ motion during conformal radiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 2010;77:53–9. [DOI] [PubMed] [Google Scholar]
- 30.Hu W, Ye J, Wang J, et al. Incorporating breath holding and image guidance in the adjuvant gastric cancer radiotherapy: A dosimetric study. Radiat Oncol 2012;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christopherson KG J; Milgrom S; Wong P; Ning M; Nastoupil L; Neelapu S; Fowler N; Fanale M; Westin J; Khoury J; Dabaja B; Pinnix C. Primary gastric diffuse large b-cell lymphoma treated with abbreviated chemoimmunotherapy and contemporary radiation therapy has excellent outcomes with minimal toxicity. American Society for Radiation Oncology (ASTRO) annual Meeting; San Antonio, Texas: 2018. [Google Scholar]
- 32.Johnson ME, Pereira GC, El Naqa IM, et al. Determination of planning target volume for whole stomach irradiation using daily megavoltage computed tomographic images. Pract Radiat Oncol 2012;2:e85–e88. [DOI] [PubMed] [Google Scholar]