Abstract
PURPOSE
Enzalutamide, a potent androgen-receptor inhibitor, has demonstrated significant benefits in metastatic and nonmetastatic castration-resistant prostate cancer. We evaluated the efficacy and safety of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC).
METHODS
ARCHES (ClinicalTrials.gov identifier: NCT02677896) is a multinational, double-blind, phase III trial, wherein 1,150 men with mHSPC were randomly assigned 1:1 to enzalutamide (160 mg/day) or placebo, plus androgen deprivation therapy (ADT), stratified by disease volume and prior docetaxel chemotherapy. The primary end point was radiographic progression-free survival.
RESULTS
As of October 14, 2018, the risk of radiographic progression or death was significantly reduced with enzalutamide plus ADT versus placebo plus ADT (hazard ratio, 0.39; 95% CI, 0.30 to 0.50; P < .001; median not reached v 19.0 months). Similar significant improvements in radiographic progression-free survival were reported in prespecified subgroups on the basis of disease volume and prior docetaxel therapy. Enzalutamide plus ADT significantly reduced the risk of prostate-specific antigen progression, initiation of new antineoplastic therapy, first symptomatic skeletal event, castration resistance, and reduced risk of pain progression. More men achieved an undetectable prostate-specific antigen level and/or an objective response with enzalutamide plus ADT (P < .001). Patients in both treatment groups reported a high baseline level of quality of life, which was maintained over time. Grade 3 or greater adverse events were reported in 24.3% of patients who received enzalutamide plus ADT versus 25.6% of patients who received placebo plus ADT, with no unexpected adverse events.
CONCLUSION
Enzalutamide with ADT significantly reduced the risk of metastatic progression or death over time versus placebo plus ADT in men with mHSPC, including those with low-volume disease and/or prior docetaxel, with a safety analysis that seems consistent with the safety profile of enzalutamide in previous clinical trials in castration-resistant prostate cancer.
INTRODUCTION
Globally, prostate cancer was the most common cancer for men, with 1.4 million patients in 2016; mortality was 381,000.1 In the United States, 174,650 new cases of prostate cancer are expected in 2019, with 31,620 anticipated deaths.2 The majority of deaths from prostate cancer are due to metastatic disease, identified either at diagnosis or after relapse following local therapies.3
Metastatic hormone-sensitive prostate cancer (mHSPC), defined as patients with metastatic disease who have not yet received, or are continuing to respond to, hormone therapy, accounts for up to 5% of annual prostate cancer incidence in the United States.4 Androgen deprivation therapy (ADT) with a luteinizing hormone-releasing hormone agonist/receptor antagonist or bilateral orchiectomy has been the standard of care (SOC) for men with mHSPC.5 However, the majority of men with mHSPC who receive ADT alone progress to castration-resistant disease within 1 to 3 years, despite experiencing an initial response.5-7
Previous trials in men with mHSPC combining ADT with other treatments such as docetaxel chemotherapy6,8 or the selective androgen biosynthesis inhibitor abiraterone acetate,9-11 hereafter referred to as abiraterone, have demonstrated significant clinical benefits, including significantly improved overall survival (OS), and these combinations are now included in treatment guidelines as part of the SOC.12,13 Abiraterone plus ADT is approved in combination with prednisone for men with metastatic high-risk castration-sensitive prostate cancer,14,15 on the basis of the LATITUDE trial (ClinicalTrials.gov identifier: NCT01715285),10 which exclusively enrolled men with high-risk mHSPC and excluded previous chemotherapy.
The efficacy and safety of enzalutamide, a potent androgen-receptor (AR) inhibitor,16 has been demonstrated across the spectrum of castration-resistant prostate cancer (CRPC) by numerous, large-scale, randomized, controlled clinical trials.17-21 In addition, a phase II, open-label, single-arm study investigating enzalutamide monotherapy in patients with hormone-naïve prostate cancer demonstrated long-term reductions in prostate-specific antigen (PSA) levels, with minimal changes in overall bone mineral density and global health status.22-24
Two recent studies that investigated abiraterone in addition to ADT excluded men with prior docetaxel chemotherapy and did not include prospective evaluation of results by disease volume (high v low).10,11 ARCHES (ClinicalTrials.gov identifier: NCT02677896) aimed to assess efficacy and safety of enzalutamide plus ADT in men with mHSPC, regardless of prior docetaxel or disease volume. We hypothesized that enzalutamide, in combination with ADT, would prolong radiographic progression-free survival (rPFS) in men with mHSPC, compared with ADT alone.
METHODS
Study Design and Conduct
ARCHES is a multinational, double-blind, randomized, placebo-controlled, phase III trial. The study protocol was approved by local independent review boards and conducted according to provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation. All patients provided written informed consent. An independent Data Safety Monitoring Board (DSMB) evaluated unblinded safety data on an ongoing basis. Please refer to the Disclosures for full information on data sharing.
Patients and Treatments
Eligible patients were adult (defined according to local regulation) males with pathologically confirmed prostate adenocarcinoma, without neuroendocrine differentiation, signet-cell, or small-cell features, and an Eastern Cooperative Oncology Group performance status score of 0 or 1. Eligible patients had hormone-sensitive metastatic disease, either de novo or after recurrence after prior local therapy, documented by a positive bone scan, or metastatic lesions on computed tomography or magnetic resonance imaging. Enrollment was based on investigator-assessed metastases; after study entry, metastasis was evaluated by independent central review. Prior ADT and up to six cycles of prior docetaxel chemotherapy were permitted. Patients who experienced disease progression prior to randomization while receiving ADT and/or docetaxel were excluded. Additional details regarding inclusion/exclusion criteria are provided in the Data Supplement.
Patients were centrally randomized 1:1 to enzalutamide (160 mg/day) plus ADT or placebo plus ADT, stratified by disease volume (low v high) and prior docetaxel chemotherapy for prostate cancer (no cycles, one to five cycles, or six cycles). High-volume disease was defined as presence of metastases involving the viscera, or in the absence of visceral lesions, four or more bone lesions, one or more of which must have been in a bony structure beyond the vertebral column and pelvic bone, per CHAARTED (ClinicalTrials.gov identifier: NCT00309985) criteria.6 Treatment continued until occurrence of unacceptable toxicity, radiographic progression (confirmed by independent central review), or initiation of an investigational agent or new prostate cancer therapy. Subsequent therapy after treatment discontinuation was permitted per local practice. On the basis of the primary analysis results and DSMB recommendation of study continuation, eligible patients were offered the opportunity to transition to an open-label extension.
End Points
The primary end point was rPFS, defined as the time from randomization to the first objective evidence of radiographic disease progression, as assessed by independent central review or death (defined as death from any cause within 24 weeks from study drug discontinuation), whichever occurred first. The cutoff of 24 weeks from study drug discontinuation (ie, the second long-term follow-up visit) for deaths (in the absence of disease progression) ensured a similar follow-up period as for monitoring of radiographic progression (ie, two 12-week radiologic assessment cycles post-treatment discontinuation). In addition, sensitivity analyses for rPFS were performed, including all deaths (in the absence of evidence of radiographic progression) regardless of timing, and radiographic progression documented by central review according to Prostate Cancer Working Group 2 criteria,25 to assess the robustness of the primary analysis; additional details are provided in Data Supplement Table A1. Key secondary end points were time to PSA progression, time to initiation of new antineoplastic therapy (including cytotoxic and hormone therapies), PSA undetectable rate, objective response rate, time to deterioration in urinary symptoms, and OS. Other secondary end points included time to first symptomatic skeletal event, time to castration resistance, patient-reported outcomes (PROs), time to deterioration of quality of life (QoL), and time to pain progression. Additional prespecified analyses, per a separate PRO statistical analysis plan (SAP), included QoL over time and sensitivity analyses of time to pain progression (using other clinically meaningful threshold criteria). Safety was also assessed. End point definitions are provided in Data Supplement Table A1.
Assessments
Efficacy assessments included sequential radiographic imaging performed at screening, at week 13, and every subsequent 12 weeks. Radiographic progression events were confirmed by independent central review; details regarding the definition of radiographic progression, including confirmatory scans required for new bone lesions observed over time, are provided in Data Supplement Table A2. PSA levels were measured at screening, at weeks 1, 5, and 13, every subsequent 12 weeks, and 30 days after the last dose or prior to initiation of new antineoplastic therapy for prostate cancer, whichever occurred first. PRO assessments, such as Functional Assessment of Cancer Therapy–Prostate,26 Quality of Life Prostate-Specific questionnaire 25,27 and Brief Pain Inventory–Short Form (BPI-SF), were completed at baseline, week 13, and every 12 weeks thereafter. Adverse events (AEs) were graded by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Statistical Analysis
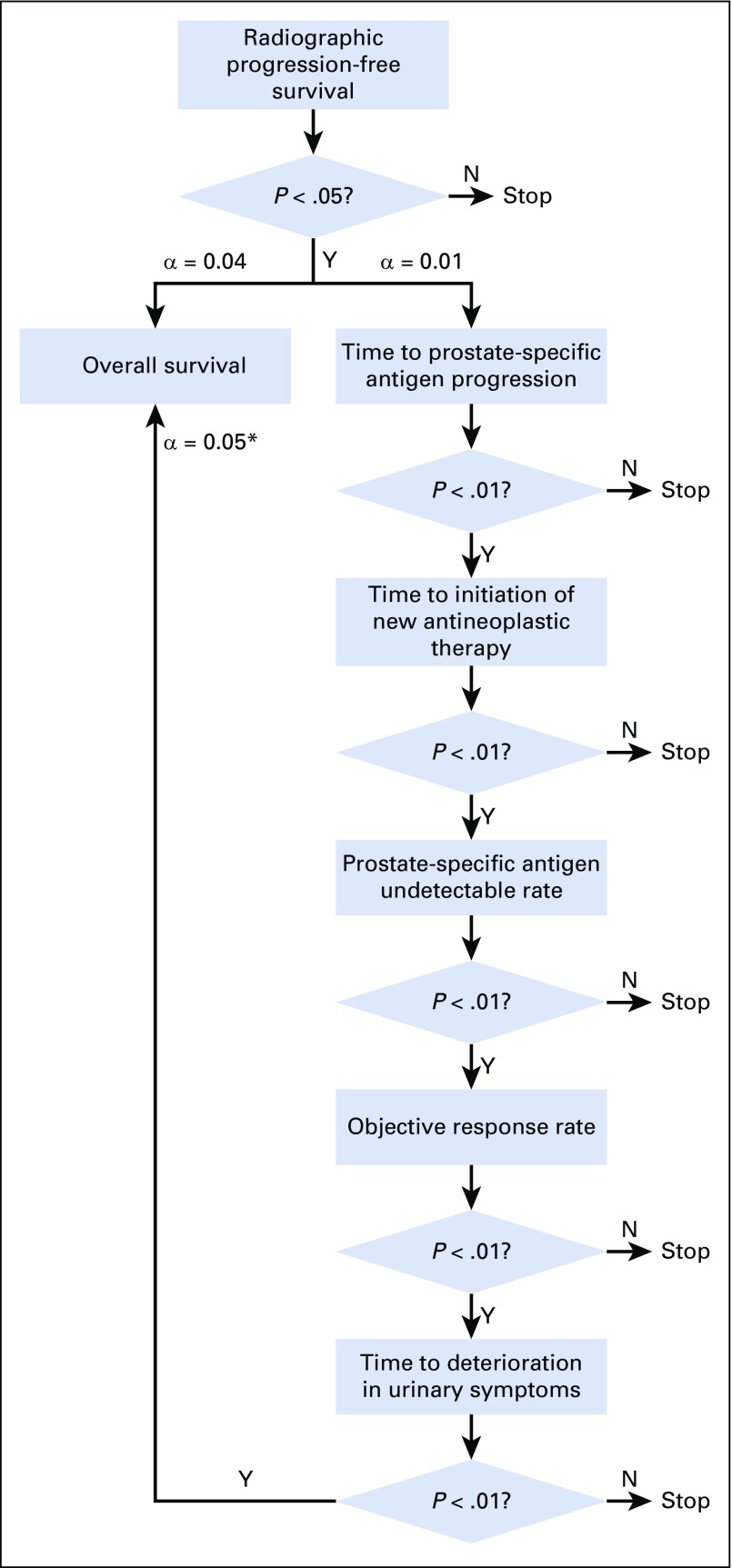
The final rPFS analysis was planned to occur after 262 events, to detect a hazard ratio (HR) of 0.67 with 90% power, on the basis of a two-sided log-rank test and 5% significance level. To adjust for multiplicity, a parallel testing strategy was applied to the key secondary end points (Appendix Fig A1, online only). Key secondary end points, other than OS, were sequentially tested at a 1% significance level. A final OS analysis will be performed when 342 deaths have occurred to provide 80% power to detect an HR of 0.73 at a 4% significance level. An interim OS analysis was performed at the time of the final rPFS analysis at a significance level calculated using the O’Brien-Fleming function to control the overall alpha. Additional details regarding the statistical analyses (original and final) are provided in the Data Supplement (Fig A1 and Table A1) and Protocol (including SAP).
RESULTS
Patients and Treatment
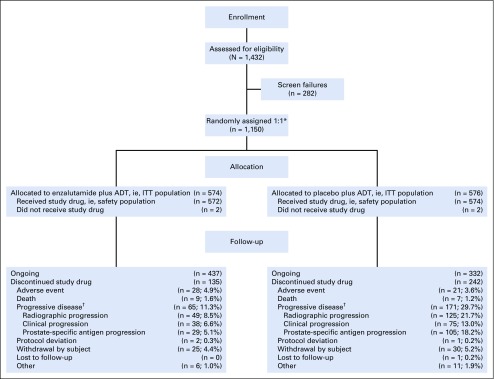
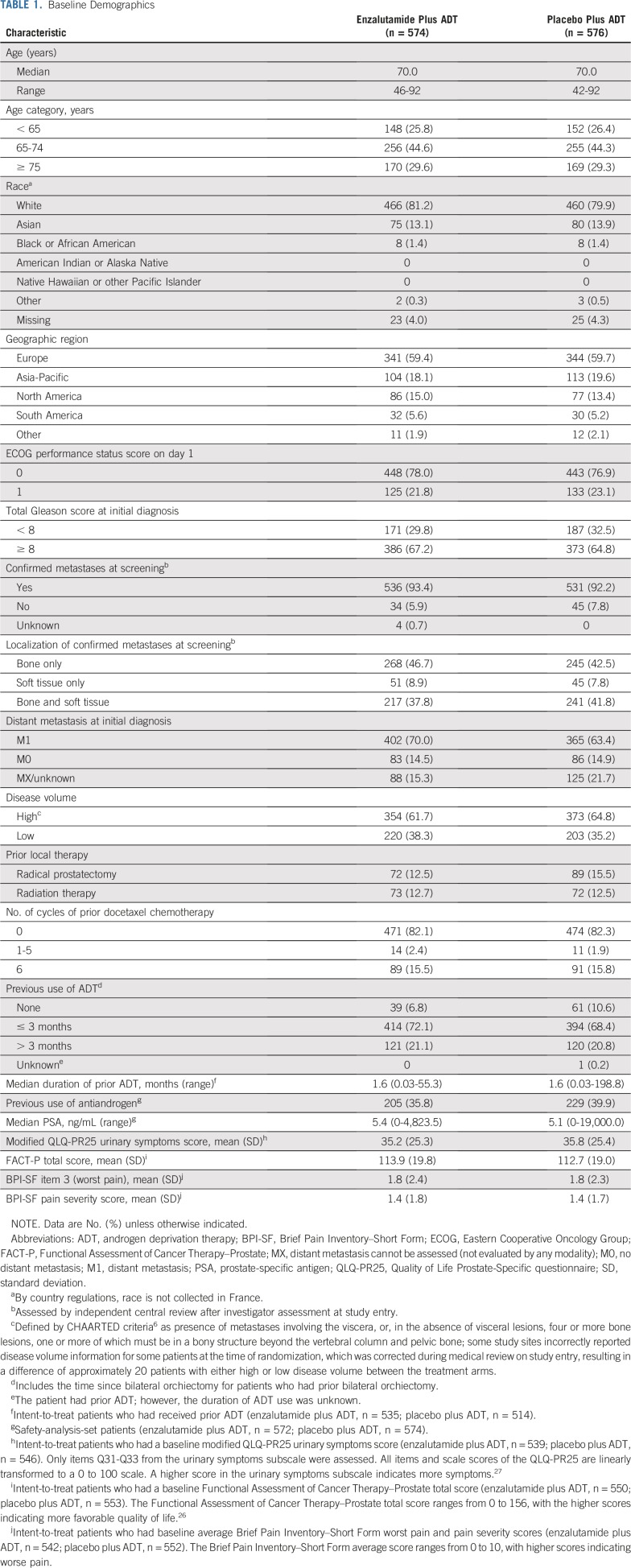
From March 21, 2016, to January 12, 2018, a total of 1,150 patients were randomly assigned 1:1 from 202 centers in North and Latin America, Europe, and Asia; 1,146 patients received at least one dose of the study drug (Fig 1). Baseline demographics were well balanced between treatment groups (Table 1); 727 patients (63.2%) had high-volume disease, and 205 (17.9%) received prior docetaxel chemotherapy. Use of concomitant antiandrogens as prostate cancer therapy during the study was reported by 34 patients (5.9%) in the enzalutamide plus ADT group and 43 patients (7.5%) in the placebo plus ADT group.
FIG 1.
CONSORT diagram. (*) Randomization 1:1 was stratified by volume of disease (low v high) and prior docetaxel therapy for prostate cancer (no cycles, one to five cycles, or six cycles); high volume of disease was defined as presence of metastases involving the viscera, or in the absence of visceral lesions, four or more bone lesions, one or more of which must have been in a bony structure beyond the vertebral column and pelvic bone, per CHAARTED (ClinicalTrials.gov identifier: NCT00309985) criteria.6 (†) Progressive disease types are not mutually exclusive; the same patient may be reported in multiple categories. ADT, androgen deprivation therapy; ITT, intent-to-treat.
TABLE 1.
Baseline Demographics
As of the data cutoff on October 14, 2018, median follow-up time was 14.4 months. Overall, 377 patients (32.8%) discontinued study treatment (enzalutamide plus ADT, n = 135 [23.5%]; placebo plus ADT, n = 242 [42.0%]). The primary reason for treatment discontinuation was progressive disease (enzalutamide plus ADT, n = 65 [11.3%] v placebo plus ADT, n = 171 [29.7%]), followed by patient withdrawal (n = 25 [4.4%] v n = 30 [5.2%], respectively; Fig 1).
rPFS
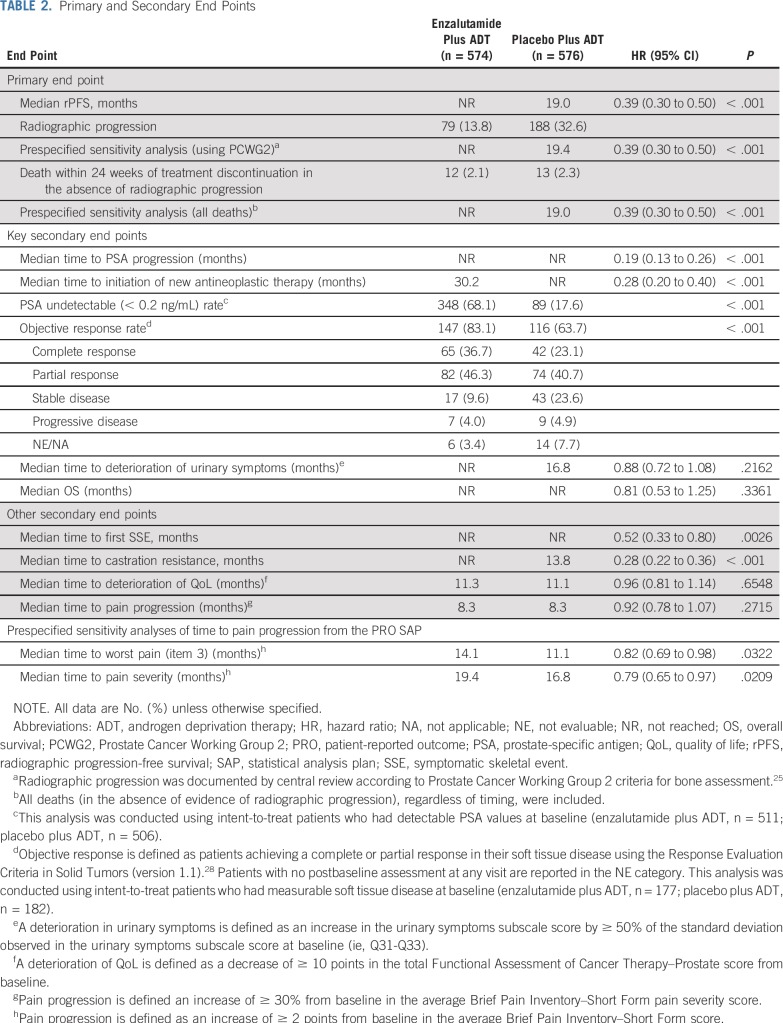
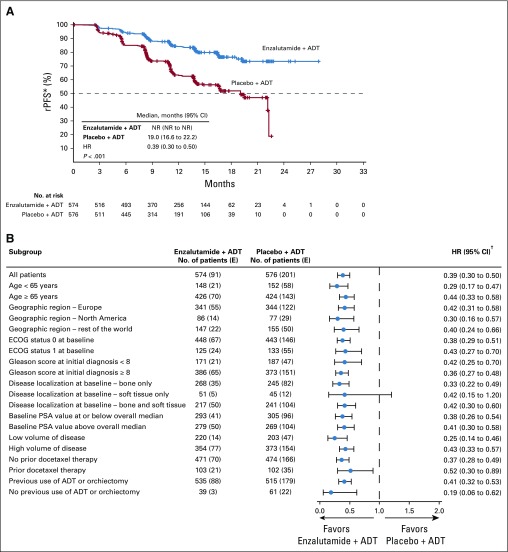
At data cutoff, 292 radiographic disease progression events or deaths without radiographic disease progression within 24 weeks of treatment discontinuation had occurred (enzalutamide plus ADT, n = 91 [15.9%]; placebo plus ADT, n = 201 [34.9%]; Table 2). Overall, enzalutamide plus ADT significantly reduced the risk of radiographic disease progression or death compared with placebo plus ADT by 61% (HR, 0.39; 95% CI, 0.30 to 0.50; P < .001; Fig 2A). Median rPFS was not reached (NR) with enzalutamide plus ADT (95% CI, NR to NR) versus 19.0 months (95% CI, 16.6 to 22.2 months) with placebo plus ADT. The treatment effect of enzalutamide plus ADT was consistent across all prespecified subgroups, including disease volume and prior docetaxel chemotherapy (Fig 2B). A sensitivity analysis of rPFS, including all deaths (in the absence of evidence of radiographic disease progression) regardless of timing, and a sensitivity analysis of radiographic progression documented by central review according to Prostate Cancer Working Group 2 criteria25 were both consistent with the primary analysis (Table 2).
TABLE 2.
Primary and Secondary End Points
FIG 2.
Kaplan-Meier estimate of (A) radiographic progression-free survival (rPFS) and (B) forest plot of rPFS for prespecified subgroups (intent-to-treat population). The dashed line at the 50th percentile indicates the median. Crosses indicate censored data. (*) For patients with no documented progression event, rPFS was censored on the date of the last radiologic assessment performed before the cutoff date. (†) 95% CIs provided are not adjusted for the number of subgroups summarized. ADT, androgen deprivation therapy; E, No. of events; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NR, not reached; PSA, prostate-specific antigen.
Secondary End Points
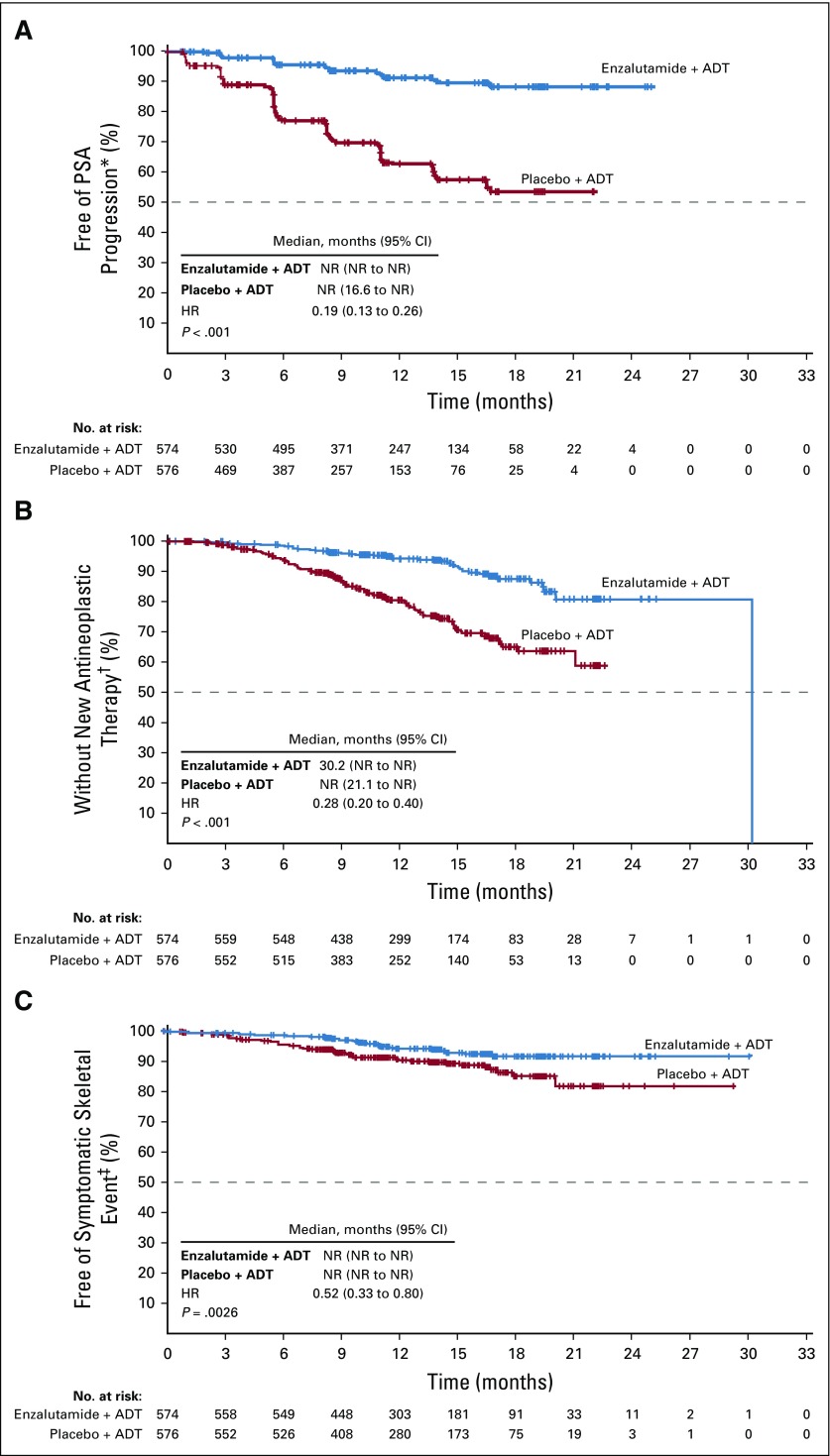
The superiority of enzalutamide plus ADT over placebo plus ADT was shown for the key secondary end points of time to PSA progression, time to initiation of new antineoplastic therapy, PSA undetectable rate, and objective response rate (Table 2; Fig 3). Although the median time to initiation of a new antineoplastic agent of 30.2 months in the enzalutamide arm is not a reliable estimate because it resulted from an event observed in the only remaining patient at risk, the treatment effect was robust, as evidenced by the HR of 0.28 (95% CI, 0.20 to 0.40; P < .001). Of the patients who initiated new antineoplastic therapy, the most common therapy was abiraterone (n = 13; 28.3%) followed by docetaxel (n = 11; 23.9%) in the enzalutamide plus ADT group and docetaxel (n = 52; 39.1%) followed by abiraterone and enzalutamide (n = 28 each; 21.1%) in the placebo plus ADT group (Data Supplement Table A3). At this interim OS analysis, data were immature, with 84 deaths (enzalutamide plus ADT, n = 39; placebo plus ADT, n = 45); median duration of OS was NR in either treatment group (HR, 0.81; 95% CI, 0.53 to 1.25; P = .3361; Table 2; Data Supplement Fig A2). Enzalutamide plus ADT also significantly reduced the risk of a first symptomatic skeletal event (Table 2; Fig 3) and castration resistance (Table 2; Data Supplement Fig A3).
FIG 3.
Kaplan-Meier estimates of time to (A) prostate-specific antigen (PSA) progression, (B) initiation of new antineoplastic therapy, and (C) first symptomatic skeletal event (intent-to-treat population). The dashed line at the 50th percentile indicates the median. Crosses indicate censored data. (*) In patients with no PSA progression, time to PSA progression was censored on the date of the last PSA sample taken. Patients without PSA progression before two or more consecutive missed PSA assessments were censored on the date of last PSA assessment before the assessments missed. (†) In patients with no new antineoplastic therapy initiated for prostate cancer after randomization, time to start of new antineoplastic therapy was censored on the last visit date or the date of randomization, whichever occurred last. The median for the enzalutamide plus androgen deprivation therapy (ADT) group was not a reliable estimate because it resulted from an event observed in the only remaining patient at risk at approximately 30 months, leading to the vertical drop at the end of the Kaplan-Meier curve. The hazard ratio (HR; 95% CI) is a more accurate depiction of the differences between treatment arms. (‡) In patients with no symptomatic skeletal event by the time of the data cutoff point, time to symptomatic skeletal event was censored on the last visit date or the date of randomization, whichever occurred last. HR, hazard ratio; NR, not reached.
Mean Functional Assessment of Cancer Therapy–Prostate total score, as a global indicator of QoL, was high at baseline for both treatment groups (Table 1) and remained high over time (Data Supplement Fig A4). Enzalutamide plus ADT did not significantly affect time to deterioration in urinary symptoms or QoL compared with placebo plus ADT (Table 2). Although the analysis of time to pain progression, with progression defined as a 30% or greater increase from baseline in average BPI-SF pain severity score, was not delayed (Table 2), prespecified sensitivity analyses from the PRO SAP, using a clinically significant 2-point or greater increase from baseline in average BPI-SF score as the progression threshold, demonstrated that enzalutamide plus ADT delayed time to pain progression for worst pain and pain severity versus placebo plus ADT (Table 2; Data Supplement Fig A5).
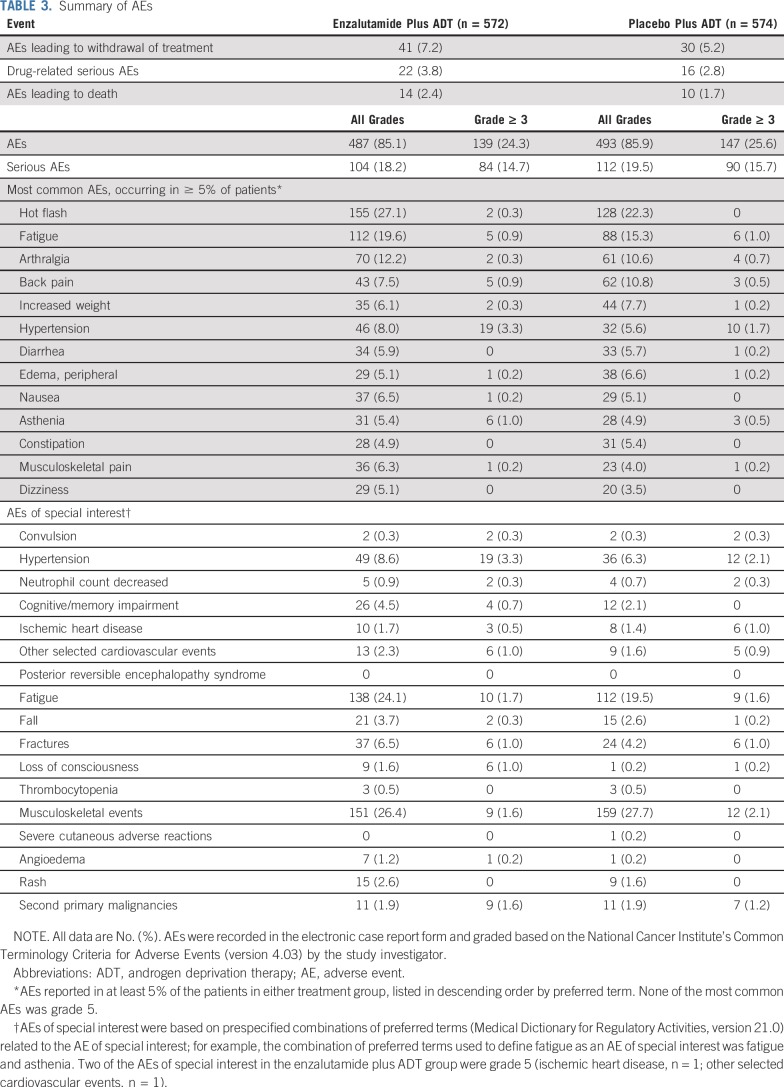
Safety
Median treatment duration was 12.8 months (range, 0.2 to 26.6 months) in the enzalutamide plus ADT group and 11.6 months (range, 0.2 to 24.6 months) in the placebo plus ADT group. Grade 3 or greater AEs, serious AEs, and AEs leading to treatment discontinuation were reported in similar proportions of patients in both treatment groups (Table 3). There were no unexpected AEs; of the 14 AEs (2.4%) leading to death in the enzalutamide plus ADT group and 10 (1.7%) in the placebo plus ADT group, none were assessed by the investigator to be related to treatment in the enzalutamide plus ADT group, whereas one event (general physical health deterioration) was assessed by the investigator to be related in the placebo plus ADT group.
TABLE 3.
Summary of AEs
DISCUSSION
In this phase III trial involving men with mHSPC, adding enzalutamide to ADT significantly reduced the risk of radiographic disease progression or death by 61% compared with placebo plus ADT (HR, 0.39; P < .001). Significant improvements with enzalutamide plus ADT were also observed in secondary efficacy end points. OS data are immature and will be analyzed when 342 deaths have occurred. Preliminary safety analysis showed an acceptable safety profile that seems consistent with that in previously reported clinical trials involving patients with CRPC,17,18 with maintenance of QoL at the high level reported at baseline. These efficacy and safety results prompted the DSMB to recommend crossing patients treated with placebo plus ADT over to enzalutamide plus ADT.
Importantly, the significant reduction in the risk of radiographic disease progression or death with enzalutamide plus ADT in this study (P < .001) was observed in all prespecified subgroups, including men with or without prior docetaxel chemotherapy and those with a low or high volume of metastatic disease. These data support the consideration of enzalutamide in addition to ADT for men with mHSPC, including patients with prior docetaxel treatment and regardless of disease volume. Although OS data remain immature, these findings have clear clinical implications for the current management of these patients.
PROs from assessments of daily living have also been shown to predict survival in prostate cancer.29 In this population of men with mHSPC, we observed maintenance of high QoL over time, similar to that observed in the population with nonmetastatic castration-resistant disease.30 Baseline average BPI-SF scores were low overall, with almost half of patients reporting scores of zero. Consequently, no significant difference between treatment groups in risk of pain progression, defined as a 30% or greater increase in average BPI-SF pain severity score, was observed. However, when using a more clinically meaningful definition of pain progression (≥ 2-point threshold)31 during the prespecified sensitivity analyses from the PRO SAP, enzalutamide plus ADT showed a delay in pain progression versus placebo plus ADT. Ultimately, no significant difference between treatment groups in risk of deterioration of urinary symptoms or QoL was observed, suggesting there was no negative impact on PROs due to the addition of enzalutamide to ADT. Additional analyses of the PROs are ongoing and are also planned as part of the long-term follow-up.
Currently, ARCHES is the first trial to demonstrate clinically meaningful benefits of potent AR inhibition with a second-generation nonsteroidal antiandrogen (enzalutamide) in combination with ADT, including a subgroup of men with mHSPC after docetaxel chemotherapy. Whereas some previous studies focused on patients with high risk and entirely excluded patients with previous chemotherapy,6-8,10,11 the specific inclusion of patients with prior docetaxel chemotherapy in ARCHES provides unique insight into this important patient subgroup with unmet clinical needs.
Both rPFS and metastasis-free survival are accepted by the US Food and Drug Administration as primary efficacy end points in metastatic CRPC and nonmetastatic CRPC, respectively.32,33 However, although rPFS has not yet been established as a surrogate for OS in mHSPC, it is an acceptable regulatory end point, and reducing the risk of radiographic progression or death is of clinical importance, given the strong positive correlation reported for rPFS and OS in patients with metastatic CRPC34,35 and the direct impact of additional metastatic progression in this setting on patient management. Furthermore, rPFS requires shorter follow-up periods and fewer patients compared with OS as a result of the higher event rate, accelerating trial completion.36 It is also in the interest of patients to unblind trials earlier, on the basis of robust rPFS evidence, especially when supported by strong secondary end points, to allow crossover to active treatment. Therefore, ARCHES was accelerated, with rPFS analysis conducted after only 262 events, despite an immature OS analysis. At the time of manuscript submission, a phase III study investigating the addition of enzalutamide versus a first-generation nonsteroidal antiandrogen, such as bicalutamide, to ADT, with or without docetaxel chemotherapy, in men with mHSPC37 is currently ongoing and will provide additional data on the clinical benefits of enzalutamide plus ADT, including the impact on OS.
Several therapies have recently been shown to be effective in men with mHSPC; therefore, ADT alone may no longer be an appropriate control arm in this patient population. However, docetaxel plus ADT only became part of the global SOC for mHSPC in 2016, after patients were already enrolling in ARCHES,12 and thus, docetaxel could not have been considered as part of the comparator arm in the current study. Furthermore, patients with high-volume disease who had completed prior docetaxel were eligible for trial entry by study design, and for those with low-volume disease, the benefit of early treatment with docetaxel combined with ADT has not been established.13,38,39
In conclusion, in comparison with placebo, the addition of enzalutamide to ADT for men with mHSPC provided clinically meaningful improvements across key efficacy end points while maintaining the high level of QoL reported at baseline. Enzalutamide was generally well tolerated, with a preliminary safety analysis seeming to be consistent with the safety profile of enzalutamide in previous clinical trials in CRPC. Enzalutamide plus ADT should therefore be considered as a treatment option for men with mHSPC, including those with low-volume disease or who had received prior docetaxel. Additional studies are necessary to clarify whether combination or sequential approaches with AR-targeted therapies or chemotherapy are favored for initial management.
ACKNOWLEDGMENT
The senior academic authors and employees of the study sponsors were responsible for the study design. Data analyses were performed by the study sponsors and provided to all authors. A professional medical writer, funded by the sponsors, assisted in the preparation of the manuscript. All authors had full access to the data, were involved in drafting the manuscript, and made the decision to submit it for publication. The authors assume responsibility for the accuracy and completeness of the data and for the adherence of the trial to the protocol. All of the authors and participating institutions have agreements with the sponsors regarding data confidentiality. We thank the patients who volunteered to participate in this trial and the investigators and trial staff who cared for them; and Stephanie Rippon, MBio, and Lauren Smith from Complete HealthVizion, for medical writing and editing assistance, funded by the sponsor companies.
DATA SHARING
Access to anonymized, individual, participant-level data collected during the trial, in addition to supporting clinical documentation, is planned for trials conducted with approved product indications and formulations, as well as compounds terminated during development. Conditions and exceptions are described under the Sponsor Specific Details for Astellas on www.clinicalstudydatarequest.com. Study-related supporting documentation is redacted and provided if available, such as the protocol and amendments, statistical analysis plan, and clinical study report. Access to participant-level data is offered to researchers after publication of the primary manuscript (if applicable) and is available as long as Astellas has legal authority to provide the data. Researchers must submit a proposal to conduct a scientifically relevant analysis of the study data. The research proposal is reviewed by an Independent Research Panel. If the proposal is approved, access to the study data is provided in a secure data-sharing environment after receipt of a signed Data-Sharing Agreement.
Appendix
FIG A1.
Multiplicity adjustment strategy. (*) Overall survival will be tested at 0.05 only if all the other five secondary end points analyses are statistically significant at 0.01, otherwise it will be tested at 0.04. N, no; Y, yes.
Footnotes
Presented in part at the ASCO 2019 Genitourinary Cancers Symposium, San Francisco, CA, February 14 to 16, 2019; and European Association of Urology 2019 Annual Congress, Barcelona, Spain, March 15 to 19, 2019.
Supported by Astellas Pharma and Pfizer.
See accompanying Editorial on page 2957
See accompanying article on page 2961
Clinical trial information: NCT02677896.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew J. Armstrong, Benoit Baron
Provision of study materials or patients: Andrew J. Armstrong, Russell Z. Szmulewitz, Daniel P. Petrylak, Jeffrey Holzbeierlein, Arnauld Villers, Arun Azad, Antonio Alcaraz, Boris Alekseev, Taro Iguchi, Neal D. Shore, Lucy Chen, Arnulf Stenzl
Collection and assembly of data: Andrew J. Armstrong, Russell Z. Szmulewitz, Arnauld Villers, Arun Azad, Antonio Alcaraz, Boris Alekseev, Lucy Chen, Arnulf Stenzl
Data analysis and interpretation: Andrew J. Armstrong, Russell Z. Szmulewitz, Daniel P. Petrylak, Jeffrey Holzbeierlein, Arnauld Villers, Arun Azad, Antonio Alcaraz, Boris Alekseev, Taro Iguchi, Neal D. Shore, Brad Rosbrook, Jennifer Sugg, Benoit Baron, Lucy Chen, Arnulf Stenzl
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Andrew J. Armstrong
Honoraria: Sanofi, Dendreon, Janssen Oncology
Consulting or Advisory Role: Bayer, Sanofi, Dendreon, Medivation, Janssen Biotech, Pfizer, Astellas Scientific and Medical Affairs, Clovis Oncology, AstraZeneca
Speakers' Bureau: Dendreon, Sanofi, Bayer
Research Funding: Dendreon (Inst), Sanofi (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Medivation (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Genentech (Inst), Active Biotech (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst)
Travel, Accommodations, Expenses: Dendreon, Janssen Biotech, Bayer, Astellas Scientific and Medical Affairs
Russell Z. Szmulewitz
Honoraria: Astellas Pharma
Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
Research Funding: AbbVie, Astellas Pharma, Incyte, Macrogenics, Janssen Oncology
Patents, Royalties, Other Intellectual Property: Patent licensed by University of Chicago of which I am co-inventor to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Travel, Accommodations, Expenses: Corcept Therapeutics
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, Tyme
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Ada Cap, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, Urogen Pharma
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Ada Cap (Inst), Bristol-Myers Squibb (Inst)
Expert Testimony: Celgene, Sanofi
Jeffrey Holzbeierlein
Research Funding: Astellas Pharma (Inst), MDxHealth (Inst)
Arnauld Villers
Consulting or Advisory Role: Astellas Pharma
Research Funding: Astellas Pharma (Inst), Ipsen (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag
Arun Azad
Honoraria: Janssen, Astellas Pharma, Novartis, Tolmar, Amgen, Pfizer, Bayer, Telix Pharmaceuticals
Consulting or Advisory Role: Astellas Pharma, Novartis, Janssen, Sanofi, AstraZeneca, Pfizer, Bristol-Myers Squibb, Tolmar, Telix Pharmaceuticals
Speakers' Bureau: Astellas Pharma, Novartis, Amgen, Bayer
Research Funding: Astellas Pharma, Merck Serono (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi, Merck Serono
Antonio Alcaraz
Travel, Accommodations, Expenses: Astellas, Olympus, Ipsen, Janssen, Bayer
Boris Alexeev
Consulting or Advisory Role: AstraZeneca, Astellas Pharma, Bayer, Bristol-Myers Squibb, Ferring, Janssen, Merck, Sanofi, Pfizer
Speakers' Bureau: Janssen, Sanofi, Ferring, Astellas Pharma, Pfizer, AstraZeneca, Bayer, Merck, Bristol-Myers Squibb
Research Funding: AstraZeneca, Merck, Sanofi, Bayer, Astellas Pharma, Janssen, Bristol-Myers Squibb, Bavarian Nordic, Pfizer, Icon Clinical Research
Travel, Accommodations, Expenses: AstraZeneca, Astellas, Bayer, BMS, Janssen, MSD, Pfizer, Sanofi
Taro Iguchi
Consulting or Advisory Role: Astellas Pharma, Bayer Yakuhin, Janssen Pharmaceutical
Speakers' Bureau: Astellas Pharma, Bayer Yakuhin, Janssen Pharmaceutical, Sanofi
Research Funding: Astellas Pharma, Bayer Yakuhin
Neal D. Shore
Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring, Medivation/Astellas, Amgen, Pfizer, AstraZeneca, Genentech, Myovant Sciences
Speakers' Bureau: Janssen, Bayer, Dendreon
Brad Rosbrook
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Jennifer Sugg
Employment: Astellas Pharma
Stock and Other Ownership Interests: AstraZeneca
Benoit Baron
Employment: Astellas Pharma (during conduct of the study)
Consulting or Advisory Role: Astellas Pharma
Travel, Accommodations, Expenses: Astellas Pharma
Lucy Chen
Employment: Astellas Pharma
Honoraria: Abbvie (I), Reflexion Medical (I)
Consulting or Advisory Role: AbbVie (I), Reflexion Medical (I)
Travel, Accommodations, Expenses: AbbVie (I), Reflexion Medical (I)
Arnulf Stenzl
Consulting or Advisory Role: Ipsen Pharma, Roche, Janssen, Alere, Bristol-Myers-Squibb, Stebabiotech
Research Funding: Karl Storz AG, Astellas Pharma, AstraZeneca, Medivation, Janssen
Patents, Royalties, Other Intellectual Property: Patent A290/99: Implantable incontinence device; AT00/0001: C-Trap, implantable device to treat urinary incontinence; 2018/6579: Gene-expression signature for subtype and prognostic prediction of renal cell carcinoma
Expert Testimony: GBA photodynamic therapy of prostate cancer
Travel, Accommodations, Expenses: Janssen, Ipsen Pharma, Sanofi Aventis, CureVac, Ferring
No other potential conflicts of interest were reported.
REFERENCES
- 1.Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Helgstrand JT, Røder MA, Klemann N, et al. Diagnostic characteristics of lethal prostate cancer. Eur J Cancer. 2017;84:18–26. doi: 10.1016/j.ejca.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Solo K, Valant J, et al. Prevalence of prostate cancer clinical states and mortality in the United States: Estimates using a dynamic progression model. PLoS One. 2015;10:e0139440. doi: 10.1371/journal.pone.0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Jenkins C, Tannock IF. Should docetaxel be standard of care for patients with metastatic hormone-sensitive prostate cancer? Pro and contra. Ann Oncol. 2015;26:1660–1667. doi: 10.1093/annonc/mdv245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 8.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 11.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mottet N, van den Bergh RCN, Briers E, et al: EAU Guidelines. Presented at the EAU Annual Congress Copenhagen, Denmark, March 16 to 20, 2018 (ed 2018). Arnhem, The Netherlands, EAU Guidelines Office, 2018. https://uroweb.org/wp-content/uploads/EAU-ESUR-ESTRO-SIOG-Guidelines-on-Prostate-Cancer-large-text-V2.pdf. [Google Scholar]
- 13. National Comprehensive Cancer Network: NCCN Clinical practice guidelines in oncology (NCCN Guidelines). Prostate cancer. Version 4. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 14. Zytiga (abiraterone acetate) highlights of prescribing information. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ZYTIGA-pi.pdf.
- 15. Zytiga (abiraterone acetate) summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/zytiga-epar-product-information_en.pdf.
- 16.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 19.Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): A randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17:153–163. doi: 10.1016/S1470-2045(15)00518-5. [DOI] [PubMed] [Google Scholar]
- 20.Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J Clin Oncol. 2016;34:2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tombal B, Borre M, Rathenborg P, et al. Long-term antitumor activity and safety of enzalutamide monotherapy in hormone naïve prostate cancer: 3-year open label followup results. J Urol. 2018;199:459–464. doi: 10.1016/j.juro.2017.08.103. [DOI] [PubMed] [Google Scholar]
- 23.Tombal B, Borre M, Rathenborg P, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: Primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014;15:592–600. doi: 10.1016/S1470-2045(14)70129-9. [DOI] [PubMed] [Google Scholar]
- 24.Tombal B, Borre M, Rathenborg P, et al. Long-term efficacy and safety of enzalutamide monotherapy in hormone-naïve prostate cancer: 1- and 2-year open-label follow-up results. Eur Urol. 2015;68:787–794. doi: 10.1016/j.eururo.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skaltsa K, Longworth L, Ivanescu C, et al. Mapping the FACT-P to the preference-based EQ-5D questionnaire in metastatic castration-resistant prostate cancer. Value Health. 2014;17:238–244. doi: 10.1016/j.jval.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.van Andel G, Bottomley A, Fosså SD, et al. An international field study of the EORTC QLQ-PR25: A questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44:2418–2424. doi: 10.1016/j.ejca.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Braun DP, Gupta D, Staren ED. Predicting survival in prostate cancer: The role of quality of life assessment. Support Care Cancer. 2012;20:1267–1274. doi: 10.1007/s00520-011-1213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. doi: 10.1016/S1470-2045(18)30898-2. Tombal B, Saad F, Penson D, et al: Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic castration-resistant prostate cancer (PROSPER): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 20:556-569, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Basch E. Patient-related outcomes for metastatic prostate cancer. Lancet Oncol. 2015;16:477–478. doi: 10.1016/S1470-2045(15)70171-3. [DOI] [PubMed] [Google Scholar]
- 32. Endocyte announces FDA acceptance of radiographic progression free survival (rPFS) as an alternative primary endpoint of the VISION trial in addition to overall survival (OS). https://investor.endocyte.com/news-releases/news-release-details/endocyte-announces-fda-acceptance-radiographic-progression-free.
- 33.Beaver JA, Kluetz PG, Pazdur R. Metastasis-free survival – A new end point in prostate cancer trials. N Engl J Med. 2018;378:2458–2460. doi: 10.1056/NEJMp1805966. [DOI] [PubMed] [Google Scholar]
- 34.Rathkopf DE, Beer TM, Loriot Y, et al. Radiographic progression-free survival as a clinically meaningful end point in metastatic castration-resistant prostate cancer: The PREVAIL randomized clinical trial. JAMA Oncol. 2018;4:694–701. doi: 10.1001/jamaoncol.2017.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol. 2015;33:1356–1363. doi: 10.1200/JCO.2014.55.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halabi S, Vogelzang NJ, Ou SS, et al. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol. 2009;27:2766–2771. doi: 10.1200/JCO.2008.18.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov: Enzalutamide in first line androgen deprivation therapy for metastatic prostate cancer (ENZAMET). https://clinicaltrials.gov/ct2/show/NCT02446405?term=NCT02446405&rank=1.
- 38.Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: Impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70:256–262. doi: 10.1016/j.eururo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]