Abstract
PURPOSE
Fundamental gaps in knowledge regarding the risk of subsequent neoplasms (SNs) in children with pathogenic neurofibromatosis type 1 (NF1) variants exposed to radiation and/or alkylator chemotherapy have limited the use of these agents.
METHODS
We addressed these gaps by determining the SN risk in 167 NF1-affected versus 1,541 non–NF1-affected 5-year childhood cancer survivors from the Childhood Cancer Survivor Study and 176 nonoverlapping NF1-affected individuals with primary tumors from University of Alabama at Birmingham and Children’s Hospital of Philadelphia exposed to radiation and/or chemotherapy. Proportional subdistribution hazards multivariable regression analysis was used to examine risk factors, adjusting for type and age at primary tumor diagnosis and therapeutic exposures.
RESULTS
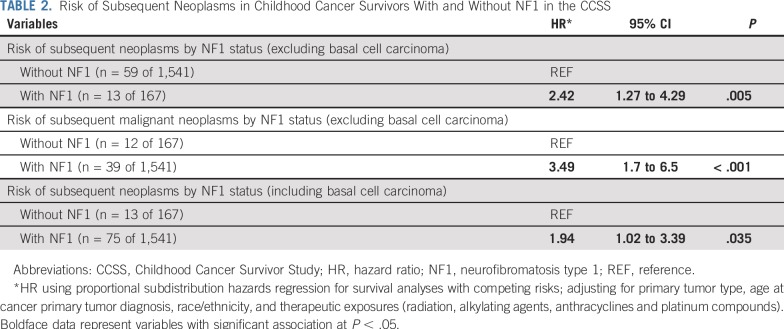
In the Childhood Cancer Survivor Study cohort, the 20-year cumulative incidence of SNs in NF1 childhood cancer survivors was 7.3%, compared with 2.9% in the non-NF1 childhood cancer survivors (P = .003), yielding a 2.4-fold higher risk of SN (95% CI, 1.3 to 4.3; P = .005) in the NF1-affected individuals. In the University of Alabama at Birmingham and Children’s Hospital of Philadelphia cohort, among NF1-affected individuals with a primary tumor, the risk of SNs was 2.8-fold higher in patients with irradiated NF1 (95% CI, 1.3 to 6.0; P = .009). In contrast, the risk of SNs was not significantly elevated after exposure to alkylating agents (hazard ratio, 1.27; 95% CI, 0.3 to 3.0; P = .9).
CONCLUSION
Children with NF1 who develop a primary tumor are at increased risk of SN when compared with non-NF1 childhood cancer survivors. Among NF1-affected children with a primary tumor, therapeutic radiation, but not alkylating agents, confer an increased risk of SNs. These findings can inform evidence-based clinical management of primary tumors in NF1-affected children.
INTRODUCTION
Individuals with neurofibromatosis type 1 (NF1) are at a four-fold to six-fold higher risk for developing primary tumors when compared with the general population.1-5 Previous small case series suggest that NF1-affected individuals with a primary tumor may be at increased risk of subsequent neoplasms (SNs).6,7 In one study, the prevalence of SNs was 11% among 64 NF1-affected children with primary tumors.6 With the exception of one patient with an SN, all others had received prior chemotherapy and/or radiation. In another case series of 58 NF1-affected patients with optic pathway glioma (OPG), the risk of SNs after exposure to radiation was three-fold higher when compared with the risk among those not exposed to radiation.7
Several questions remain unanswered. It is not known whether the risk of SNs is elevated in childhood cancer survivors with NF1 in comparison with those without NF1, after taking into account therapeutic exposures. In addition, among NF1-affected children with a primary tumor, it is not known whether the risk of SNs is higher among those who receive chemotherapy and/or radiation (ie, treated) when compared with those observed without any treatment or managed with surgery alone (ie, untreated). Finally, among the treated NF1 cohort, the risk of SN associated with radiation and/or alkylating agents is not known. Yet, despite the lack of conclusive evidence, radiation and alkylating agents are generally avoided or are used sparingly in patients with NF1. We address these gaps in knowledge by systematically analyzing large cohorts of NF1-affected and non–NF1-affected children with primary tumors.
METHODS
For a neoplasm to be classified as an SN, one or more of the following criteria had to be satisfied: the SN had to be histologically distinct from the primary tumor, and the SN had to be diagnosed at least 6 months after the diagnosis of a primary tumor. This definition allowed us to differentiate patients with concurrent tumors and to address the following hypotheses.
Hypothesis 1: Risk of SNs Will Be Higher in Childhood Cancer Survivors With NF1 Compared With Those Without NF1, Independent of Therapeutic Exposure
We leveraged the resources offered by the large and well-annotated Childhood Cancer Survivor Study (CCSS) to identify 5-year survivors of childhood cancer with and without NF1. CCSS is a retrospective cohort with longitudinal follow-up that evaluates long-term health outcomes of this unique population (www.stjude.org/ccss). Eligibility included diagnosis and treatment at one of 27 collaborating institutions between 1970 and 1999 for select primary tumors at age younger than 21 years and survival of 5 years or more after diagnosis of a primary tumor.8 NF1 status of study participants was ascertained through self or proxy report (Data Supplement). Demographic and clinical data (primary tumor diagnosis, age at primary tumor diagnosis, chemotherapeutic agents, and site-specific radiation9) for the study participants were obtained from medical records. SNs were ascertained through self- or proxy-report questionnaires and/or death certificates. All SNs were validated with pathology reports. Participants, or parents of children younger than 18 years, provided informed consent.
Statistical analyses.
Given significant differences in the demographic and clinical characteristics of the CCSS patients with and without NF1 (younger age at diagnosis of primary tumor, an over-representation of primary CNS tumors, and shorter follow-up for the NF1 cohort), and the much larger number of patients in the non-NF1 childhood cancer survivor cohort, we conducted propensity score matching (1:10) on primary tumor diagnosis, age at primary tumor diagnosis, length of follow-up, as well as exposure to radiation and chemotherapeutic agents between childhood cancer survivors with and without NF1. After propensity score matching, the demographic and clinical variables and SNs were characterized by NF1 status. Each individual was assigned an indicator (yes/no) variable depending on exposure to radiation and chemotherapeutic agents. The effect of NF1 status on the development of SN was examined by fitting multivariable Fine and Gray proportional subdistribution hazards models, treating death as competing risk and adjusting for primary tumor type, age at diagnosis of primary tumor, race/ethnicity, and chemotherapy and radiation exposure, using time since cohort entry to development of SNs, death, or date of last contact as the period at risk. The association between SNs and NF1 status was determined by estimating the hazard ratio (HR) and its 95% CI; significance of HR was assessed by the Wald test. The proportional hazards assumption was formally tested by examining the interaction between NF1 status and time.
Hypothesis 2: Among NF1-Affected Children With a Primary Tumor, the Risk of SNs in the Treated Cohort Will Be Higher Compared With the Untreated Cohort
To test this hypothesis, we constructed an independent, nonoverlapping retrospective cohort of NF1-affected children with primary tumors from two centers with dedicated NF clinics (University of Alabama at Birmingham [UAB] and Children’s Hospital of Philadelphia [CHOP]). This large cohort of NF1-affected children with a primary tumor allowed us to capture events from primary tumor diagnosis to sentinel event and overcame the limitations of potential survival bias when using 5-year survivors (CCSS cohort). The UAB and CHOP NF1 and cancer registries were used to identify NF1-affected children with a primary tumor. Those under observation or managed with surgery alone were categorized as untreated. Information in the registry database included demographics and primary tumor type. Details of therapeutic exposures (specific chemotherapeutic agents and site-specific radiation), as well as details regarding SNs (type of SN and date of diagnosis), were abstracted from medical records. Pathology reports were reviewed to confirm the SNs. The UAB and CHOP Institutional Review Boards approved abstraction of medical records for the information pertaining to this study.
Statistical analyses.
The effect of treatment status (yes/no) on the development of SN was examined by fitting multivariable Fine and Gray proportional subdistribution hazards models, treating death as competing risk and adjusting for demographics.
Hypothesis 3: Among Treated NF1-Affected Children With Primary Tumor, the Risk of SNs Will Be Higher for Those Exposed to Radiation and/or Alkylating Agents Compared With Those Treated With Other Therapies
To test this hypothesis, we restricted the analysis to only the NF1-affected children from UAB/CHOP who had been exposed to genotoxic agents.
Statistical analyses.
Among the treated NF1-affected children, the relation between radiation and/or alkylating agents and the risk of SNs was examined using multivariable Fine and Gray proportional subdistribution hazards models, treating death as competing risk and adjusting for demographics. Furthermore, given the fact that children with NF1 are at risk for developing multiple tumors (eg, plexiform neurofibromas) irrespective of therapeutic exposures, we conducted a subanalysis excluding plexiform neurofibromas as SNs.
All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC). Two-sided tests with P < .05 were considered statistically significant for Hypotheses 1 and 2. For Hypothesis 3, we were interested in the association between two exposures (alkylating agents and radiation) and SN risk; we considered two-sided tests with P < .025 (0.05 of 2) as statistically significant.
RESULTS
Risk of SNs in Childhood Cancer Survivors With and Without NF1 (CCSS)
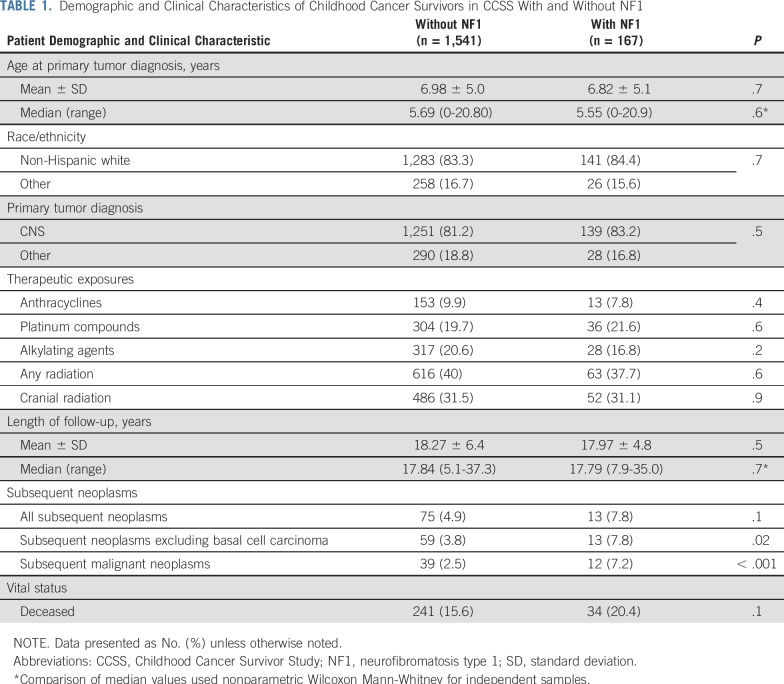
The demographic and clinical characteristics of the CCSS cohort of childhood cancer survivors identified with NF1 (n = 167) and without NF1 (n = 1,541) after propensity score matching are listed in Table 1. The two cohorts did not show significant differences with respect to demographics, primary tumor diagnoses, treatment exposures, and length of follow-up.
TABLE 1.
Demographic and Clinical Characteristics of Childhood Cancer Survivors in CCSS With and Without NF1
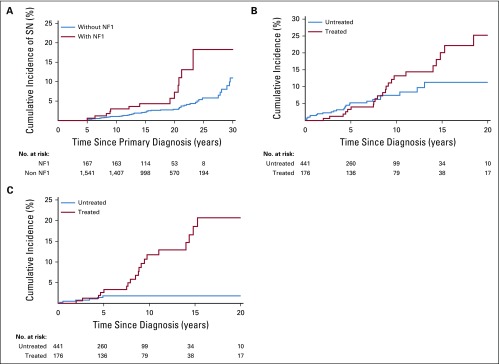
A total of 13 (7.8%) individuals in the CCSS NF1 cohort developed SNs; these included astrocytoma not otherwise specified (n = 3), acute lymphoblastic leukemia (n = 2), glioblastoma (n = 2), ependymoma (n = 1), malignant peripheral nerve sheath tumor (MPNST; n = 1), non-Hodgkin lymphoma (n = 1), malignant fibrous histiocytoma (n = 1), parotid gland mucoepidermoid carcinoma (n = 1), and thyroid carcinoma (n = 1; Data Supplement). The median latency from primary tumor diagnosis to SN was 17.2 years (range, 0.5 to 39.4 years) in the NF1-affected individuals versus 14.0 years (range, 0.8 to 23.2 years) in the non–NF1-affected individuals (P = .2). Although none of the NF1-affected individuals in the CCSS cohort developed a basal cell carcinoma (BCC), 16 (21.3%) of the 75 patients with SN in the non-NF1 cohort were diagnosed with BCC. Excluding BCC as an SN, we observed a significantly higher 20-year cumulative incidence of SNs in the NF1 cohort when compared with the non-NF1 cohort (7.3% v 2.9%; P = .003; Fig 1A). The proportional hazards assumption was satisfied (P = .9). Multivariable analysis (Table 2) found the NF1 cohort at a 2.4-fold higher risk of SN (P = .005) and a 3.5-fold higher risk of subsequent malignant neoplasms (P < .001) when compared with the non-NF1 cohort.
FIG 1.
(A) Cumulative incidence of subsequent neoplasms (SNs) in childhood cancer survivors with neurofibromatosis type 1 (NF1) versus survivors without NF1, excluding basal cell carcinoma as a SN. (B) Cumulative incidence of SNs in children with NF1 observed or treated with surgery alone (untreated) versus those treated with chemotherapy and/or radiation (treated), excluding basal cell carcinoma as a SN. (C) Cumulative incidence of SNs in children with NF1 observed or treated with surgery alone (untreated) versus those treated with chemotherapy and/or radiation (treated), excluding basal cell carcinoma and plexiform neurofibroma as a SN.
TABLE 2.
Risk of Subsequent Neoplasms in Childhood Cancer Survivors With and Without NF1 in the CCSS
SNs in the Treated Versus Untreated NF1-Affected Individuals With a Primary Tumor (UAB/CHOP)
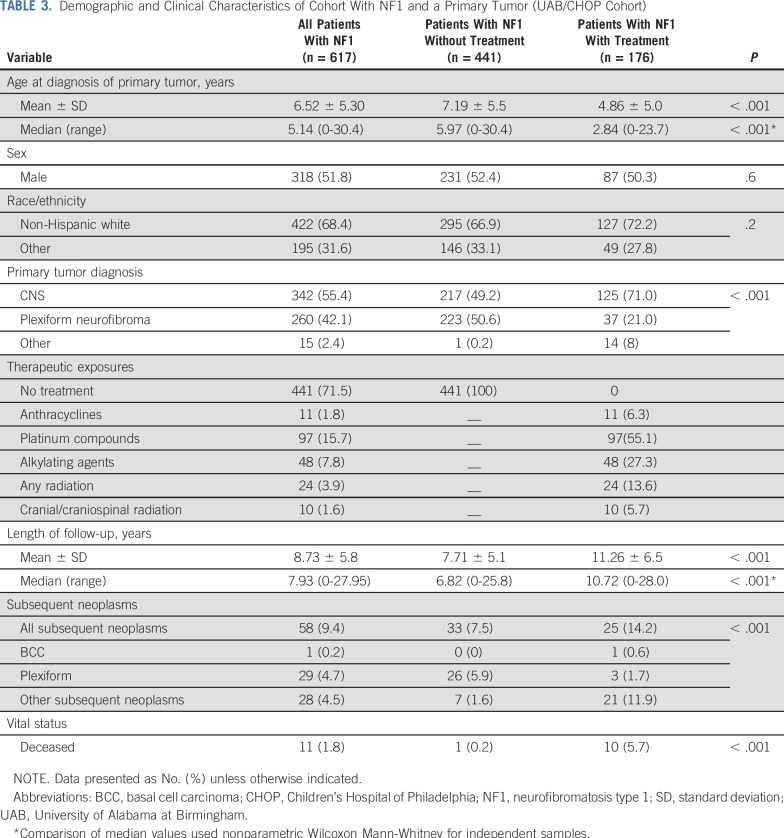
Of the 617 NF1-affected individuals in the UAB/CHOP cohort with a primary tumor, 71.5% did not receive radiation and/or chemotherapy. Table 3 lists the characteristics of treated versus untreated NF1-affected individuals with a primary tumor. In the treated group, there was an over-representation of primary CNS tumors (71% v 49.2%) and an under-representation of primary plexiform neurofibromas (21% v 50.6%; P < .001). The age at diagnosis of a primary tumor was younger for the treated group (median, 2.8 years v 6.0 years; P < .001). The median length of follow-up was longer for the treated group (10.7 years v 6.8 years).
TABLE 3.
Demographic and Clinical Characteristics of Cohort With NF1 and a Primary Tumor (UAB/CHOP Cohort)
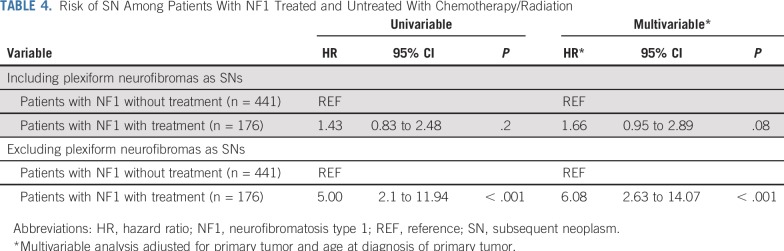
As shown in the Data Supplement, 24 of the 176 NF1-affected individuals in the treated group developed 24 SNs (glioma, n = 12; MPNST, n = 8; plexiform neurofibroma, n = 3; and malignant neuroblastic tumor, n = 1). In the untreated NF1 group, 33 of 441 patients developed 46 SNs (plexiform neurofibroma, n = 38; granular cell tumor, n = 3; glioma, n = 1; ganglioneuroblastoma, n = 1; MPNST, n = 1; malignant spindle cell sarcoma, n = 1; and Sertoli-Leydig cell tumor, n = 1). The median latency from primary tumor diagnosis to SN was 8.6 years (range, 2.0 to 18.4 years) in the treated NF1-affected individuals versus 4.2 years (range, 0.2 to 13.1 years) in the untreated group (P < .001). As shown in Figure 1B, a higher 10-year cumulative incidence of SNs was observed in the treated NF1 group when compared with the untreated NF1 group (13.2% v 7.4%). The assumption of proportional hazards was satisfied (P = .3). Multivariable analysis found the treated NF1 cohort to be at a 1.7-fold higher risk of SN (P = .08) than the untreated NF1 cohort (Table 4). After excluding plexiform neurofibroma as SNs, the 10-year cumulative incidence of SNs in the treated versus untreated group was 11.7% versus 1.8% (P < .001), respectively (Fig 1C), yielding a 6.1-fold higher risk of SN (P < .001) in the treated group (Table 4).
TABLE 4.
Risk of SN Among Patients With NF1 Treated and Untreated With Chemotherapy/Radiation
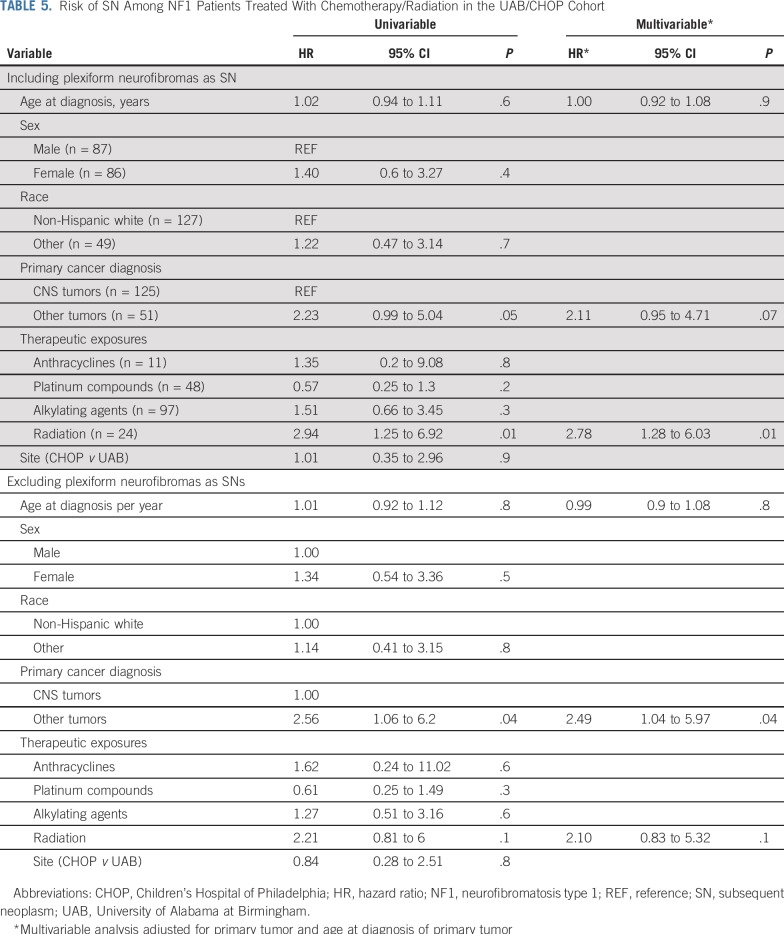
Risk of SNs Among NF1-Affected Patients With a Primary Tumor Treated With Chemotherapy/Radiation (UAB/CHOP)
The risk of SNs was 2.8-fold higher among those treated with radiation when compared with those not exposed to radiation (P = .01; Table 5). Excluding plexiform neurofibroma as an SN yielded similar associations, although the magnitude of association between radiation and SN (HR, 2.1; P = .1) was attenuated (Table 5). Of the 24 patients with SNs in the UAB/CHOP cohort, seven had received radiation therapy, and six of these seven SNs developed in the radiation field. We conducted a sensitivity analysis, where we excluded the patients who had developed the SN outside the radiation field and found that the risk of SNs was 2.27-fold higher among those exposed to radiation when compared with those not exposed to radiation (95% CI, 1.04 to 4.97; P = .04; Data Supplement).
TABLE 5.
Risk of SN Among NF1 Patients Treated With Chemotherapy/Radiation in the UAB/CHOP Cohort
Alkylating agents were not associated with an increased risk of SNs in the univariable analysis (HR, 1.27; P = .6; Table 5). Inclusion of alkylating agents into the multivariable model resulted in further mitigation of the association between alkylating agents and SNs (HR, 1.0; P = .9; Data Supplement).
DISCUSSION
This systematic analysis demonstrates that NF1-affected children with a primary tumor are at a significantly higher risk of developing SNs when compared with their non-NF1 counterpart. Furthermore, among NF1-affected children with primary tumors, those exposed to chemotherapy and/or radiation are at a higher risk of developing SNs when compared with those who were observed or managed with surgery alone. Finally, among the treated NF1-affected children with primary tumors, radiation is associated with an increased risk for SNs, but alkylator chemotherapy is not.
The relative role of radiation therapy in improving long-term control of NF1-associated tumors is unknown. There has been uneven use of this modality in circumstances that might prompt use in non–NF1-affected patients because of concerns of higher risk of long-term complications in NF1-affected patients, such as radiation-induced malignant transformation or vasculopathy.6,7,10,11 Furthermore, alkylators are used sparingly in NF1-affected individuals, because of concern for therapy-related leukemia, limiting treatment options for OPG. Yet these clinical management decisions have been based on case reports and small case series6,7; the magnitude of risk of SNs in NF1-affected children with a primary tumor compared with those without NF1, taking into account therapeutic exposures, was previously unknown. We harnessed a unique resource of childhood cancer survivors from the CCSS to address this knowledge gap.12-15 After adjusting for demographic, clinical, and therapeutic exposures, we were able to determine that NF1-affected childhood cancer survivors were at a 2.4-fold higher risk of developing SNs and 3.5-fold higher risk of developing subsequent malignant neoplasms when compared with non–NF1-affected childhood cancer survivors. These findings inform the need for health care providers taking care of childhood cancer survivors with NF1 to be sensitized to the higher risk of SNs in this population.
To understand the excess risk of SNs among children with NF1 and a primary tumor exposed to any treatment when compared with their untreated counterparts, we used the UAB/CHOP NF registries. The modest excess risk of SNs observed in the treated patients with NF1 as compared with the untreated NF1 patients increased to six-fold on excluding plexiform neurofibroma as an SN, emphasizing the fact that treatment of patients with NF1 with a primary tumor increases the risk of SNs that are distinct from the types of multiple tumors typically observed in patients with NF1 (ie, plexiform neurofibromas). The excess risk observed in our cohort is consistent with the findings from a previous study, where the risk of SNs after exposure to radiation for OPG was reported to be three-fold higher when compared with the risk among the unexposed.7 These findings allow us to quantify the magnitude of excess risk of SNs after exposure to genotoxic agents in NF1-affected children with a primary tumor.
A previous study of NF1-affected individuals with a primary tumor6 exposed to chemotherapy and/or radiation reported an 11% prevalence of SN. In our UAB/CHOP cohort, 14% of the NF1-affected children treated with radiation and/or chemotherapy for a primary tumor had developed an SN, yielding a higher risk of SNs among those exposed to radiation when compared with those not exposed; this excess risk persisted on exclusion of plexiform neurofibroma as an SN. Although 67% of the clinically overt subsequent plexiform neurofibromas developed within the radiation field, given the retrospective nature of the study, we did not have the ability to perform serial prospective whole-body magnetic resonance imaging scans to enumerate and follow all potential tumors, which would be required to definitively determine the incidence of new tumors and/or growing tumors both within and outside the radiation field. On the other hand, alkylators were not associated with a significant increase in SN risk. Of note, among the SNs, meningioma (radiation related) and therapy-related leukemia (alkylator related) were notably absent.
Findings from this study should be interpreted in the context of certain limitations. First, the NF1 status for the CCSS study was self-reported. We attempted to overcome this limitation by applying stringent criteria to identify NF1-affected individuals and had two independent reviewers assign the NF1 status. Second, medical record abstraction carries the risk of missing certain sentinel events if they are not recorded, as could have happened in the NF1 cohort from UAB and CHOP. Third, when comparing treated patients with NF1 with untreated patients with NF1, there could have been unmeasured confounding factors that were not accounted for. Thus, it is possible that patients who developed an SN had a more severe phenotype of NF1, and this may have contributed to the results; however, when we restricted the analysis to NF1-affected individuals who had received treatment, there was a higher risk of SNs associated with radiation. In addition, given the retrospective nature of the NF1 cohort, the decision to treat with surgery alone or radiation or chemotherapy was provider dependent or as a result of inherent differences in the primary tumor type; we were unable to capture these details in this analysis. Furthermore, it is not possible to account for the number of concurrent tumors at the time of diagnosis of the primary tumor or the grade of the primary tumor, and this variable could not be adjusted for in the comparison of untreated and treated NF1 cohorts. Fourth, because of the small number and heterogeneity within the SNs, it was difficult to examine a dose-response relationship between radiation exposure and SN risk.
Despite these limitations, we provide a comprehensive report showing that NF1-affected children with a primary tumor carry an excess risk for developing an SN when compared with non-NF1 childhood cancer survivors. This excess risk is likely in part due to the natural propensity of NF1-affected individuals to develop multiple tumors across the life span, in part due to the therapeutic exposures and in part due to the interaction between the therapeutic exposures and the NF1 status. We were unable to determine precisely the underlying cause of the excess risk in this study. Among NF1-affected children, exposure to radiation therapy is associated with an increased risk of SNs, whereas exposure to alkylating agents is not. These findings may potentially affect treatment selection for certain tumors, especially as newer drug choices become available. In addition, the findings provide evidence for close monitoring of the tissue/organs within the radiation field for patients with NF1 treated with radiation therapy for a primary tumor. The duration of the at-risk period is not yet determined, because it would be important to follow these patients for several decades. However, findings from childhood cancer survivors without NF1 suggest that childhood cancer survivors with a history of radiation therapy need to be under active surveillance for SNs well into their sixth decades.13 Finally, these findings present an opportunity to explore the underlying pathogenesis of the higher risk of SNs in NF1-affected individuals, especially after exposure to radiation, and to identify those at highest (and lowest) risk, such that treatment and long-term follow-up of NF1-affected children with a primary tumor could be personalized.
Footnotes
Presented at the Joint Global Neurofibromatosis Conference, Paris, France, November 5, 2018.
Supported by National Cancer Institute Grant No. P50 CA196519-01 (D.W.C., K.S.). The Childhood Cancer Survivor Study was supported by National Cancer Institute Grant No. CA55727 (G.T.A.). Support to St Jude Children’s Research Hospital was provided by the Cancer Center Support Grant No. CA21765 (G.T.A., L.L.R.) and the American Lebanese-Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Smita Bhatia, F. Lennie Wong, Michael J. Fisher
Financial support: Smita Bhatia, D. Wade Clapp, Kevin Shannon
Administrative support: Smita Bhatia, Lindsey Hageman, D. Wade Clapp, Kevin Shannon
Provision of study material or patients: Smita Bhatia, Gregory T. Armstrong, Michael J. Fisher
Collection and assembly of data: Smita Bhatia, Lindsey Hageman, Kandice Smith, Alejandro Paz, Joseph E. Andress, Joseph P. Neglia, Michael Arnold, Lucie M. Turcotte, Peter de Blank, Wendy Leisenring, Leslie L. Robison, Michael J. Fisher
Data analysis and interpretation: Smita Bhatia, Yanjun Chen, F. Lennie Wong, Michael J. Fisher
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Subsequent Neoplasms After a Primary Tumor in Individuals With Neurofibromatosis Type 1
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Bruce Korf
Consulting or Advisory Role: SpringWorks Therapeutics, AstraZeneca, Genome Medical, Envision Genomics, Accolade Pharmaceuticals
Research Funding: Novartis
Patents, Royalties, Other Intellectual Property: Patent application related to treatment of NF1
Travel, Accommodations, Expenses: SpringWorks Therapeutics, AstraZeneca
Kevin Shannon
Stock and Other Ownership Interests: AbbVie, Mirat
Patents, Royalties, Other Intellectual Property: Genentech has licensed mouse leukemias generated in my laboratory for preclinical testing through the University of California. They have paid a licensing fee for access to these materials.
Michael J. Fisher
Travel, Accommodations, Expenses: AstraZeneca, SpringWorks Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain. 1988;111:1355–1381. doi: 10.1093/brain/111.6.1355. [DOI] [PubMed] [Google Scholar]
- 2.Matsui I, Tanimura M, Kobayashi N, et al. Neurofibromatosis type 1 and childhood cancer. Cancer. 1993;72:2746–2754. doi: 10.1002/1097-0142(19931101)72:9<2746::aid-cncr2820720936>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Sørensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986;314:1010–1015. doi: 10.1056/NEJM198604173141603. [DOI] [PubMed] [Google Scholar]
- 4.Lin AL, Gutmann DH. Advances in the treatment of neurofibromatosis-associated tumours. Nat Rev Clin Oncol. 2013;10:616–624. doi: 10.1038/nrclinonc.2013.144. [DOI] [PubMed] [Google Scholar]
- 5.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 6.Maris JM, Wiersma SR, Mahgoub N, et al. Monosomy 7 myelodysplastic syndrome and other second malignant neoplasms in children with neurofibromatosis type 1. Cancer. 1997;79:1438–1446. [PubMed] [Google Scholar]
- 7.Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: Substantial risks after radiotherapy. J Clin Oncol. 2006;24:2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 8.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 10.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68:932–938. doi: 10.1212/01.wnl.0000257095.33125.48. [DOI] [PubMed] [Google Scholar]
- 12.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turcotte LM, Whitton JA, Friedman DL, et al. Risk of subsequent neoplasms during the fifth and sixth decades of life in the Childhood Cancer Survivor Study Cohort. J Clin Oncol. 2015;33:3568–3575. doi: 10.1200/JCO.2015.60.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2011;29:3056–3064. doi: 10.1200/JCO.2011.34.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]