Abstract
PURPOSE
Population-based studies of children and adolescents with Hodgkin lymphoma (HL) report a survival disadvantage in nonwhite—non-Hispanic black (NHB) and Hispanic—patients. Whether disparities persist after adjustment for clinical and treatment-related variables is unknown. We examined survival by race/ethnicity in children receiving risk-based, response-adapted, combined-modality therapy for HL in contemporary Children’s Oncology Group trials.
PATIENTS AND METHODS
This pooled analysis used individual-level data from 1,605 patients (younger than age 1 to 21 years) enrolled in phase III trials for low-risk (AHOD0431), intermediate-risk (AHOD0031), and high-risk (AHOD0831) HL from 2002 to 2012. Event-free survival (EFS) and overall survival (OS) were compared between non-Hispanic white (NHW) and nonwhite patients. Cox proportional hazards for survival were estimated for both de novo and relapsed HL, adjusting for demographics, disease characteristics, and therapy.
RESULTS
At median follow up of 6.9 years, cumulative incidence of relapse was 17%. Unadjusted 5-year EFS and OS were 83% (SE, 1.2%) and 97% (SE, < 1%), respectively. Neither differed by race/ethnicity. In multivariable analyses for OS, nonwhite patients had a 1.88× higher hazard of death (95% CI, 1.1 to 3.3). Five-year postrelapse survival probabilities by race were as follows: NHW, 90%; NHB, 66%; and Hispanic, 80% (P < .01). Compared with NHW, Hispanic and NHB children had 2.7-fold (95% CI, 1.2 to 6.2) and 3.5-fold (95% CI, 1.5 to 8.2) higher hazard of postrelapse mortality, respectively.
CONCLUSION
In patients who were treated for de novo HL in contemporary Children’s Oncology Group trials, EFS did not differ by race/ethnicity; however, adjusted OS was significantly worse in nonwhite patients, a finding driven by increased postrelapse mortality in this population. Additional studies examining treatment and survival disparities after relapse are warranted.
INTRODUCTION
Approximately 1,200 children and adolescents are diagnosed with Hodgkin lymphoma (HL) annually in the United States. Today, 5-year survival exceeds 95% with contemporary combined-modality therapy (CMT).1 For the 15% to 20% of patients with relapsed disease, more than 70% achieve remission with current salvage therapies, including hematopoietic cell transplantation (HCT).2-4 Despite continued advances, population-based5,6 and single-center studies7 suggest that non-Hispanic black (NHB) and Hispanic—versus non-Hispanic white (NHW) —race/ethnicity are associated with higher relapse rates and worse overall survival (OS). In 2008, Metzger et al7 reported higher incidence of late relapse and worse event-free survival (EFS) in black—versus white—children receiving risk-directed CMT at St Jude Children’s Research Hospital between 1990 and 2005. In a recent report from the SEER program, Grubb et al6 reported that black—versus white—patients with HL had inferior OS at 5 and 25 years. In multivariable analyses, Hispanic ethnicity independently predicted poor disease-specific survival (DSS).6
Parsing out reasons for observed disparities in HL has been challenging. Proposed hypotheses for cancer health disparities often relate to racial/ethnic differences in disease and host biology, or to systems-level factors that influence access to high-quality cancer care.8-10 These include differences in treatment location,11 health insurance, clinical trial enrollment,10,12 receipt of guideline-recommended therapy, and post-treatment surveillance.13,14
Existing studies that are suggestive of racial/ethnic disparities in childhood HL are limited by a lack of detailed clinical characteristics and therapeutic exposures in the current treatment era. Over the last two decades, the Children’s Oncology Group (COG) has conducted a series of phase III clinical trials evaluating dose-dense chemotherapy with response adaptation. To determine the effect of race/ethnicity on survival in the current treatment era, we examined EFS and OS in NHW, NHB, and Hispanic children receiving risk-stratified, response-adapted therapy for de novo HL in contemporary COG trials.
PATIENTS AND METHODS
Data Source and Cohort Selection
The analytic cohort included patients age 1 to 21 years with newly diagnosed classic HL (cHL) enrolled in one of three phase III COG trials in the United States between 2002 and 2012. Trials were defined by risk groups and included AHOD003115 (intermediate risk), AHOD043116 (low risk), and AHOD083117 (high risk). Primary objectives, treatment details, and results of these studies have been published. Each trial enrolled patients with newly diagnosed HL and evaluated dose-dense chemotherapy with augmentation of chemotherapy or radiation therapy (RT) on the basis of initial treatment response. Response was determined by central review, and RT administration guidelines were standardized across all patients. For patients enrolled in AHOD0431, a salvage therapy arm for recurrent disease was prespecified in the protocol and required reconsent for participation.16 The Central Institutional Review Board for the National Cancer Institute approved the original treatment protocols, as did the institutional review boards of participating COG institutions. Informed consent was obtained from participants or guardians at the time of enrollment.
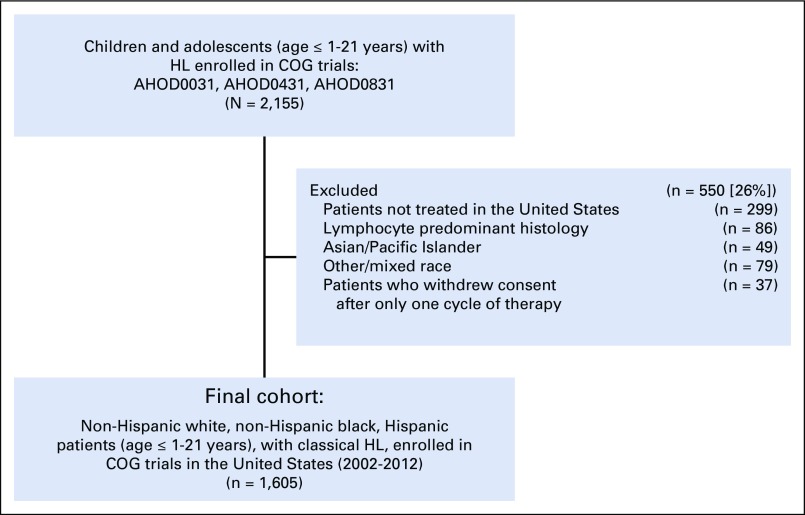
Between 2002 and 2012, 2,155 patients enrolled in COG trials for the treatment of de novo HL and 1,605 patients (76%) were included (Fig 1). Patients who were treated outside of the United States (n = 299), with lymphocyte predominant histology (n = 86), and who withdrew consent after one cycle (n = 37) were excluded. Race and ethnicity were documented by treating institutions at the time of study enrollment. Ethnicity was categorized as Hispanic or non-Hispanic. Race was categorized as black or African American (hereafter referred to as black), white or Caucasian (hereafter referred to as white), Asian/Pacific Islander, and other/mixed race. For the purpose of this study, analyses were restricted to patients who were NHW, NHB, and Hispanic. In total, 49 patients who were of Asian/Pacific Islander ethnicity and 79 patients who were of other/mixed race were excluded (Fig 1). For primary survival analyses, comparisons were made between NHW and nonwhite (NHB and Hispanic) patients.
FIG 1.
Cohort selection. COG, Children’s Oncology Group; HL, Hodgkin lymphoma.
Estimates of neighborhood socioeconomic status (SES) were assigned to patients using a linkage between individual patient zip code tabulation area and the US Census in the method of Krieger et al.18 Patients were categorized as either low or nonlow SES. Those residing in neighborhoods with more than 20% of households below the federal poverty level were defined as low SES.
Statistical Analysis
This was a pooled, secondary analysis of prospectively collected patient-level data. Baseline disease and demographic characteristics were compared across racial/ethnic groups using χ2 test for categorical variables and analysis of variance for continuous variables. EFS was measured from the date of study enrollment to the date of first relapse, subsequent malignant neoplasm (SMN), or death from any cause. Cumulative incidence of relapse was estimated with SMN and death as competing events. OS was measured from the date of study enrollment to the date of death. Patients with no events were censored at the time of last contact. Kaplan-Meier estimates of 5-year EFS and OS were compared across race/ethnicity.
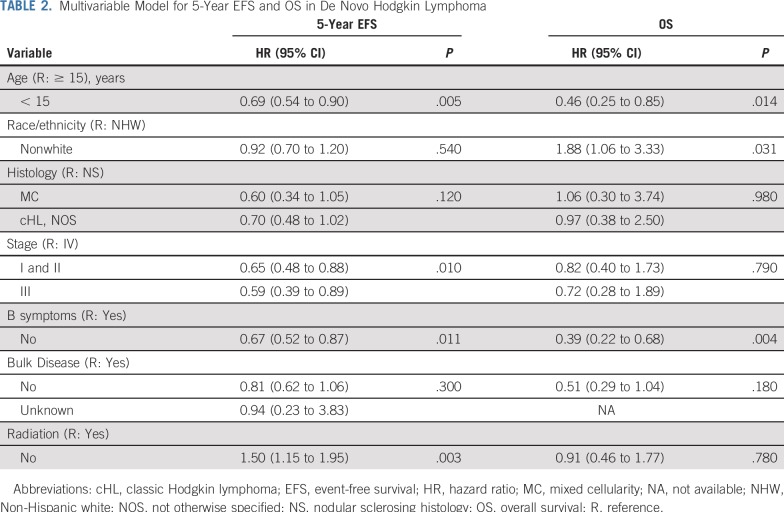
Multivariable cox proportional hazards regression analyses were used to identify risk factors for EFS and OS and are presented as hazard ratios (HRs) with 95% CIs. Preselected disease and clinical characteristics known to be associated with HL survival were included in final multivariable models, as were variables with P values less than .15 in multivariable regression models for EFS and OS. Backward selection was performed to determine the most robust model. Interactions between race/ethnicity and the following baseline characteristics were not statistically significant: age, stage, histology, bulk disease, and neighborhood SES. Final regression models were adjusted for age, HL histology, Ann Arbor stage, B symptoms, bulk disease, and RT. A P value of less than .05 was considered statistically significant.
Additional analyses were performed on NHW, NHB, and Hispanic patients with relapsed disease. Survival was measured from the date of first relapse, marking the end of upfront therapy, to the date of death. Multivariable models were adjusted for baseline characteristics at diagnosis, including age, histology, stage, B symptoms, bulk disease, RT during upfront therapy, and initial COG protocol. Multivariable models in the relapsed cohort were run both with and without the neighborhood SES variable to examine its impact on the overall hazard of death by race/ethnicity. For descriptive purposes, causes of death after relapse were categorized as HL related or tumor progression, toxicity or treatment related, and other/unknown. Analyses were conducted using SAS software (SAS/STAT User’s Guide, Version 9.3; SAS Institute, Cary, NC).
RESULTS
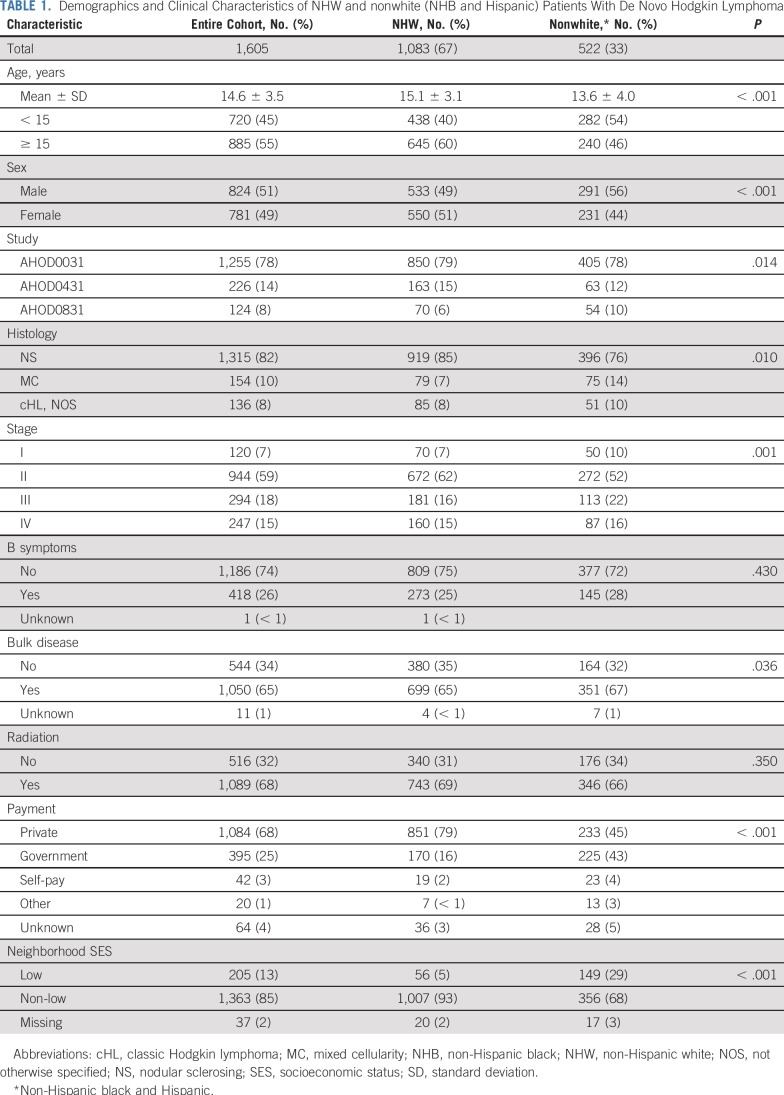
Baseline patient characteristics are listed in Table 1. Sixty-seven percent of the cohort (n = 1,083) was NHW. Among nonwhite patients (n = 522), 40% were NHB (n = 210) and 60% were Hispanic (n = 312). Median age at diagnosis was 14.6 years (± 3.5 years); a higher-proportion of nonwhite patients were younger than 15 years at diagnosis (P < .01). The proportion of patients with public/government versus private insurance differed significantly by race/ethnicity: 16% of NHW patients versus 41% and 44% of NHB and Hispanic patients, respectively, had public/government insurance (NHW v nonwhite, P < .01). Similarly, a higher proportion of nonwhite—versus NHW—patients resided in low-SES neighborhoods (P < .01). Nonwhite patients were more likely to present with stage III/IV disease—versus stage I/II disease (P < .01) —or bulky disease (P = .04). Sixty-eight percent of patients received RT, and this did not differ by race/ethnicity (Table 1).
TABLE 1.
Demographics and Clinical Characteristics of NHW and nonwhite (NHB and Hispanic) Patients With De Novo Hodgkin Lymphoma
Impact of Race/Ethnicity on Survival Outcomes
EFS and OS.
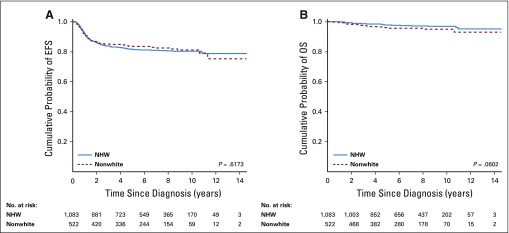
Median follow up was 6.9 years (range, 0.1 to 14.6 years). Pooled 5-year EFS was 82% (SE, 1.1%) and did not significantly differ between NHW (82%; SE, 1.5%) and nonwhite (84%; SE, 2.4%) patients in unadjusted (P = .62; Fig 2) or adjusted analyses (P = .60). Five-year cumulative incidence of relapse was 17% (SE, 1.0%). Cumulative SMN incidences at 10 and 12 years were 1.3% (SE, 0.4%) and 2.7% (SE, 1.0%), respectively. Pooled 5-year OS was 97% (SE, 0.5%) and did not differ between NHW (98%; SE, 0.5%) and nonwhite (96%; SE, 1.1%) patients in unadjusted analyses (P = .06; Fig 2). In multivariable analyses for OS, however, nonwhite race/ethnicity and age 15 years or older were significantly associated with a higher hazard of death. Compared with NHW patients, nonwhite children had a 1.88-fold increased risk of all-cause mortality (95% CI, 1.06 to 3.33; Table 2).
FIG 2.
Kaplan-Meier curves for (A) event-free survival (EFS) and (B) overall survival (OS) in non-Hispanic white (NHW) versus nonwhite (non-Hispanic black and Hispanic) patients with de novo Hodgkin lymphoma.
TABLE 2.
Multivariable Model for 5-Year EFS and OS in De Novo Hodgkin Lymphoma
Relapsed cohort.
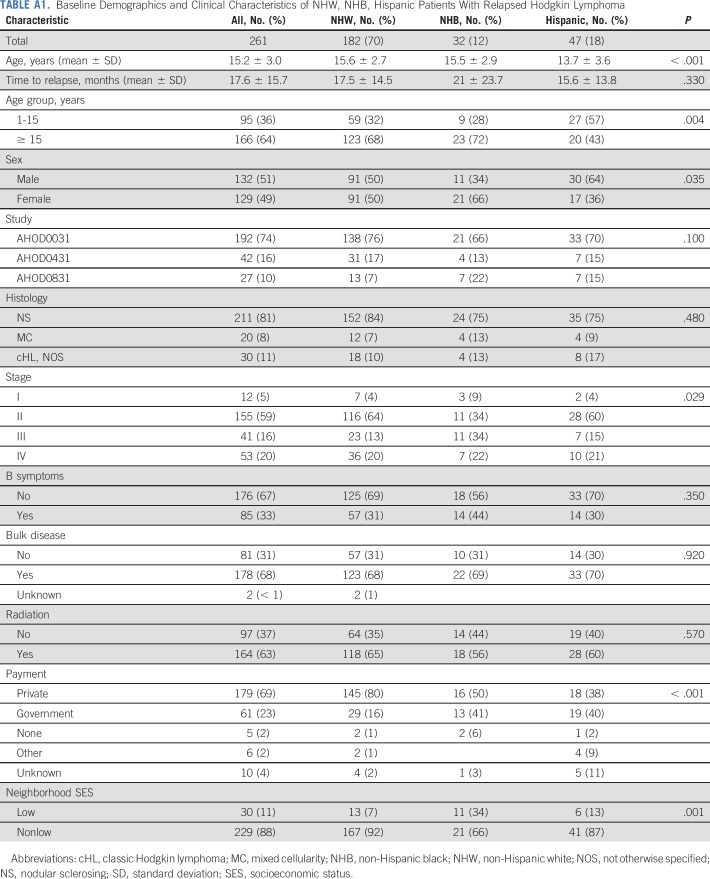
Median time to relapse was 1.0 year (range, 0.1 to 8.8 years) and did not differ by race/ethnicity. Among 261 participants with relapse events, 70% were NHW (n = 182) and 30% were nonwhite (NHB, n = 32; Hispanic, n = 47). Fifteen percent of patients with relapse events enrolled in subsequent COG trials for salvage therapy (NHW, n = 36; nonwhite, n = 4), including 15 patients who reconsented to the salvage treatment arm of AHOD0431. Baseline disease characteristics of the relapsed cohort are listed in Appendix Table A1 (online only).
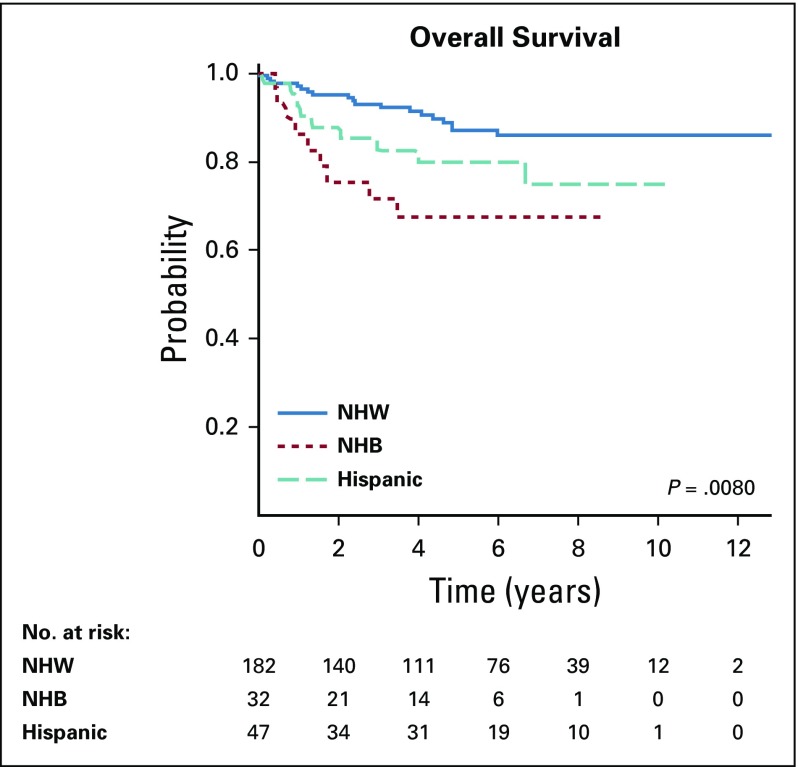
Median duration of follow up postrelapse was 4.8 years (range, 0 to 12.8 years) and did not significantly differ by race/ethnicity (P = .07). A total of 37 patients (14%) who experienced a first relapse died; pooled 5-year postrelapse survival was 83% (SE, 3.0%). Unadjusted survival estimates postrelapse differed significantly by race/ethnicity. Five-year postrelapse survival in NHW children was 87% (SE, 3.2%) versus 67% (SE, 12.2%) in NHB, and 80% (SE, 7.5%) in Hispanic patients (P = .008; Fig 3)
FIG 3.
Kaplan-Meier curves for postrelapse survival in non-Hispanic white (NHW), non-Hispanic black (NHB), and Hispanic patients. Time zero is the date of relapse.
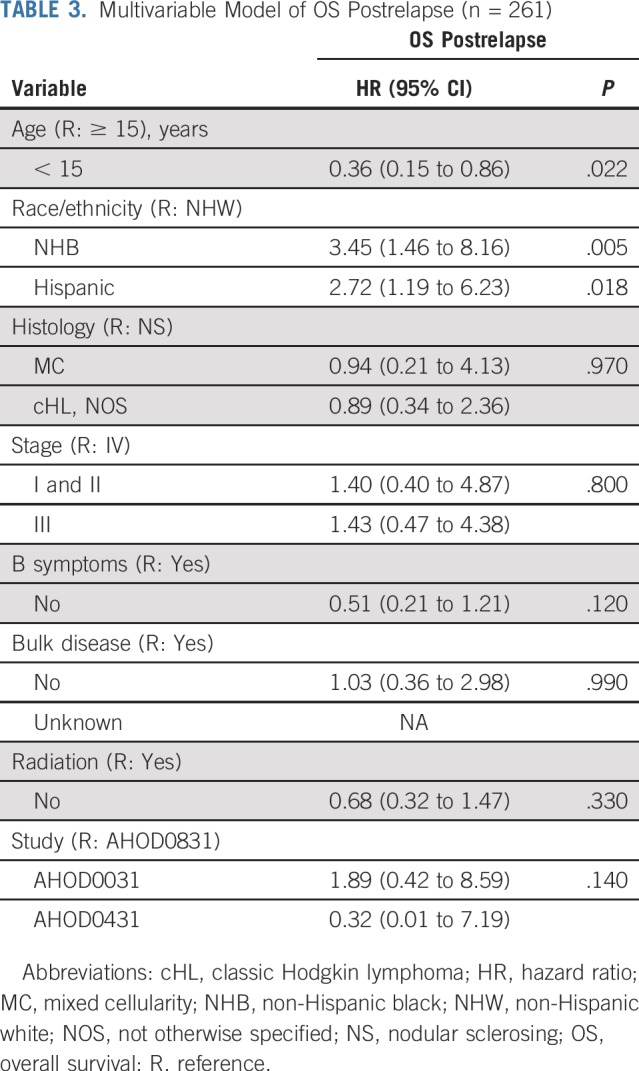
In multivariable analyses, differences in postrelapse survival between NHW, NHB, and Hispanic patients remained statistically significant (Table 3). Compared with NHW patients, NHB patients had nearly 3.5-fold higher hazards of postrelapse mortality (HR, 3.45; 95% CI, 1.46 to 8.16; Table 3). Hispanic children also had significantly higher hazards of death after relapse compared with NHW children (HR, 2.72; 95% CI, 1.19 to 6.23; Table 3). Including neighborhood SES in regression models did not mitigate observed differences in overall or postrelapse mortality risk by race/ethnicity.
TABLE 3.
Multivariable Model of OS Postrelapse (n = 261)
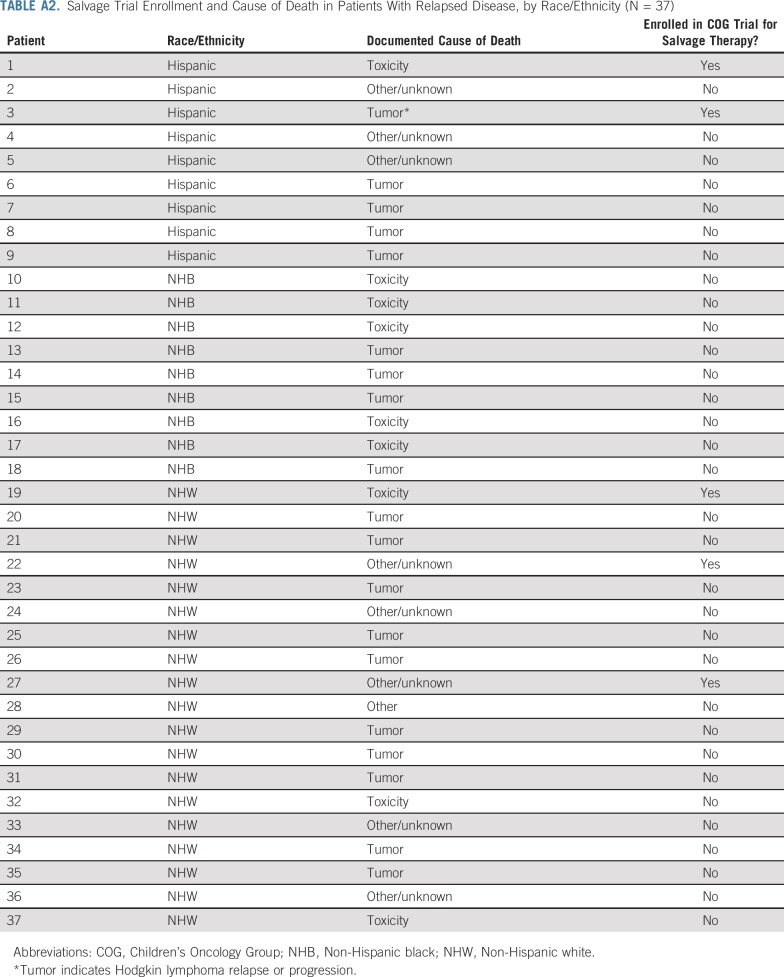
Among deaths in the relapse cohort, progression of HL was the documented cause in 51% (NHW: n = 10; nonwhite: n = 9). Treatment-related toxicity was the documented cause of death in 24% (NHW: n = 3; nonwhite: n = 6), and nine patients had unknown cause of attribution. Finally, among the 37 patients who died postrelapse, five received salvage therapy in the setting of a COG clinical trial (Appendix Table A2, online only).
DISCUSSION
In this analysis of 1,605 children and adolescents receiving upfront therapy for HL in contemporary COG trials in the United States, we observed no difference in relapse rate, SMN, or EFS between NHW and nonwhite patients. Results of our analyses suggest that, in patients with access to cooperative group trials, uniform risk-directed therapy with a response-adapted, combined-modality approach eliminates the EFS gap by race/ethnicity in children with HL. This is notable, as nonwhite children were more likely to present with advanced stage disease, have public insurance, and live in high-poverty areas.
Nationally representative studies of children and adolescent/young adults (age 15 to 39 years) with cancer report that nonwhite race/ethnicity, public insurance, and low SES are associated with advanced stage at presentation and worse DSS.19,20 In an analysis of the Florida Cancer Registry, Grubb et al6 reported that black children with HL were more likely than white children to present with advanced disease (41% v 29%; P = .02), but were less likely to receive RT (34% v 45%; P < .01). The difference in therapy was hypothesized to influence the significantly worse DSS in black patients. In another population-based analysis of 58,000 adolescent/young adults from the California Cancer Registry, Keegan et al13 reported that nonwhite patients were more likely to have public or no insurance and advanced-stage HL at diagnosis. Despite being more likely to present with advanced-stage HL, black and Hispanic patients were less likely than white patients to receive CMT.13 In this population-based cohort, not receiving RT was an independent predictor of higher mortality.
Racial differences in presenting characteristics of patients in the COG cohort mirrored those reported in population-based studies. Specifically, more nonwhite patients in COG were from high-poverty neighborhoods and presented with advanced-stage disease. Unlike prior studies, however, NHW and nonwhite patients in our cohort were equally likely to receive CMT, possibly narrowing the gap in EFS observed in prior reports. In addition to receiving risk-directed CMT, patients enrolled in COG studies received dose-dense chemotherapy with a response-adapted approach. This is in contrast to the St Jude cohort in which patients received risk-based CMT without response adaptation. Similar to our cohort, a higher proportion of black—versus white—patients from the St Jude cohort were from low-income households and presented with advanced-stage HL (P < .05). In contrast to our findings, however, black—versus white—patients at St Jude had inferior 5-year EFS (black v white: 71% v 84%; P = .01). In the COG cohort, EFS did not significantly differ by race/ethnicity in unadjusted or adjusted analyses of pooled data across risk groups. In addition to differences in treatment intensity between the historic St Jude approach and the contemporary COG approach, use of response-adapted therapy and higher rate of RT in COG trials may have reduced the risk of later relapse in our patients with advanced-stage disease.
We observed a significant difference in OS by race/ethnicity in the COG cohort, with nonwhite patients having an 88% higher hazard of death compared with NHW patients after adjustment for clinical and treatment factors in multivariable analyses. This survival difference was largely driven by differential hazards of postrelapse mortality between racial/ethnic groups. Compared with NHW patients, NHB and Hispanic patients in our cohort had between 2.7-fold and 3.5-fold higher hazards of postrelapse mortality, regardless of adjustment for neighborhood SES.
In recent decades, numerous novel agents and advances in the field of HCT have led to improved outcomes for patients with relapsed HL.21-24 The success of salvage regimens, however, depends not only on disease biology and treatment tolerability, but also on patients having access to these interventions. NHB patients in the United States are consistently underrepresented in early-phase cooperative group studies where many novel agents are trialed.25 In the experience from St Jude, although black—versus white—children had higher relapse rates, similar access to salvage therapy within the single institution may have resulted in comparable OS between groups. Baseline differences in neighborhood SES or insurance status between NHW and nonwhite patients in COG may have affected salvage treatment options and the location of care postrelapse. However, differences in postrelapse survival across NHW and nonwhite patients in our cohort cannot be entirely explained by SES, as adjustment for neighborhood SES did not reduce the hazard of mortality in nonwhite children.
In addition to being less likely to enroll in early-phase trials, NHB and Hispanic patients are historically less likely than NHW patients to undergo HCT. Studies of patients with HL from the Center for International Blood and Marrow Transplant Research database report that nonwhite patients comprise a significantly smaller proportion of transplantation populations than white patients.26 Thus, it is also possible that NHB and Hispanic patients in our cohort were less likely to undergo HCT after relapse. Some patients in the COG cohort also died of treatment-related mortality (TRM) and toxicities after relapse. Although our study did not examine differences in TRM by race/ethnicity, other studies in pediatric oncology have suggested that TRM rates differ by race and treatment location.7,27
This analysis is subject to some limitations. Although we have data on front-line therapy, cumulative chemotherapy doses, and RT adherence, response-adaptation rates were not analyzed given similar EFS across racial/ethnic groups. Details about salvage treatment beyond upfront therapy, including the receipt of HCT, were not uniformly available, which limited our ability to control for these factors in analyses of postrelapse outcomes. We also did not have information about whether patients participated in clinical trials through other cooperative groups postrelapse. Future studies to address these limitations may include analyses of salvage trials, registries linked to insurance claims, or data from other national and international consortia, including the Center for International Blood and Marrow Transplant Research. We did not have individual-level SES data, which could have provided more granular detail about the impact of poverty on outcomes. For this cohort, information about genetic ancestry28 was not available, and race/ethnicity were not exclusively documented by self-report. Thus, it is possible that this study may have been subject to potential misclassification of race/ethnicity. Whereas sociodemographic characteristics of NHB and Hispanic patients in our cohort trended in the same direction, we were unable to adjust for sociocultural factors, which also can affect health outcomes. In future trials, prospective collection of data on health beliefs and acculturation, in addition to self-reported race/ethnicity, will be imperative. Given the absence of validated biomarkers of outcome in HL,29 racial/ethnic differences in tumor biology beyond histology were not addressed.30 We did not examine host pharmacogenomics or polymorphisms that affect drug metabolism, which may contribute to differences in tumor response or toxicities.31 Despite these limitations, the strength of this study is the large, well-characterized cohort of children and adolescents with HL for whom we had complete clinical and initial treatment data from contemporary phase III clinical trials.
CONCLUSION
In the controlled setting of cooperative group clinical trials, treatment with dose-dense, response-adapted therapy for patients with newly diagnosed HL eliminates racial/ethnic differences in EFS. This observation suggests that current approaches to risk stratification with response-adapted regimens are highly effective for both NHW and nonwhite children with HL. Despite comparable EFS across NHW and nonwhite patients in our cohort, OS differed significantly after adjusting for baseline clinical, disease, and treatment variables. This striking difference in the risk of all-cause mortality between NHW and nonwhite patients raises the possibility that differences in salvage therapy or supportive care outside of the cooperative group clinical trials may be driving the disparities observed at the population level.12
Critical areas of future study and policy initiatives to reduce racial disparities in HL should focus on facilitating clinical trial enrollment in NHB and Hispanic populations, both in the upfront and relapsed settings. Additional analyses examining racial/ethnic differences in salvage therapy, including clinical trial participation rates, are needed. Concerns about radiation-associated late effects, over the next decade, will lead to less RT use. Thus, access to clinical trials will be especially important as more novel agents are introduced. Finally, studies should also examine why nonwhite patients are more likely to present with advanced-stage disease, a finding consistently reported in both registry- and consortium-based analyses.
In the coming years, the field of pediatric and adolescent HL will focus on investigating novel agents to both improve survival outcomes and reduce the risk of long-term treatment-related morbidities.32 Given the findings of this work, it is imperative that simultaneous efforts are focused on improving health equity, expanding clinical trial participation, and identifying drivers of racial/ethnic disparities in children with relapsed disease.
Appendix
TABLE A1.
Baseline Demographics and Clinical Characteristics of NHW, NHB, Hispanic Patients With Relapsed Hodgkin Lymphoma
TABLE A2.
Salvage Trial Enrollment and Cause of Death in Patients With Relapsed Disease, by Race/Ethnicity (N = 37)
Footnotes
Presented at the International Symposium on Hodgkin Lymphoma, Cologne, Germany, October 27-29, 2018; and at the 59th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 9-12, 2017.
Supported by the Children’s Oncology Group Chair Grant No. U10-CA98543, the Children’s Oncology Group Statistics and Data Center Grant No. U10-CA098413, the National Clinical Trials Network Operations Center Grant No. U10-CA180886, and the National Clinical Trials Network Statistics and Data Center Grant No. U10-CA180899. Also supported by the St Baldrick’s Foundation, the Lymphoma Research Foundation, and by a KL2 Mentored Career Development Award Grant No. KL2-TR001874 from the Irving Institute for Clinical and Translational Research at Columbia University Irving Medical Center.
AUTHOR CONTRIBUTIONS
Conception and design: Justine M. Kahn, Kara M. Kelly, Smita Bhatia, Tara O. Henderson, Cindy L. Schwartz, Sharon M. Castellino
Provision of study materials or patients: Debra L. Friedman, Cindy L. Schwartz, Frank G. Keller, Kara M. Kelly
Collection and assembly of data: Justine M. Kahn, Kara M. Kelly, Rizvan Bush, Qinglin Pei, Debra L. Friedman, Frank G. Keller, Sharon M. Castellino
Data analysis and interpretation: Justine M. Kahn, Kara M. Kelly, Qinglin Pei, Rizvan Bush, Debra L. Friedman, Frank G. Keller, Tara O.Henderson, Cindy L. Schwartz, Smita Bhatia, Sharon M. Castellino
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survival by Race and Ethnicity in Pediatric and Adolescent Patients With Hodgkin Lymphoma: A Children’s Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kara M. Kelly
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Tara O. Henderson
Research Funding: Seattle Genetics
Other Relationship: Seattle Genetics
Sharon M. Castellino
Research Funding: Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kelly KM, Hodgson D, Appel B, et al. Children’s Oncology Group’s 2013 blueprint for research: Hodgkin lymphoma. Pediatr Blood Cancer. 2013;60:972–978. doi: 10.1002/pbc.24423. [DOI] [PubMed] [Google Scholar]
- 2.LaCasce AS, Bociek RG, Sawas A, et al. Brentuximab vedotin plus bendamustine: A highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132:40–48. doi: 10.1182/blood-2017-11-815183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole PD, McCarten K, Metzger M, et al. Phase 2 trial of brentuximab vedotin and gemcitabine for pediatric and young adult patients with relapsed or refractory Hodgkin lymphoma (HL): A Children’s Oncology Group (COG) report. J Clin Oncol. 2017;35(suppl; abstr 7527) doi: 10.1016/S1470-2045(18)30426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar A, Hayward J, Kirkwood A, et al. Treatment outcome in children and adolescents with relapsed Hodgkin lymphoma: Results of the UK HD3 relapse treatment strategy. Br J Haematol. 2014;165:534–544. doi: 10.1111/bjh.12768. [DOI] [PubMed] [Google Scholar]
- 5.Evens AM, Antillón M, Aschebrook-Kilfoy B, et al. Racial disparities in Hodgkin’s lymphoma: A comprehensive population-based analysis. Ann Oncol. 2012;23:2128–2137. doi: 10.1093/annonc/mdr578. [DOI] [PubMed] [Google Scholar]
- 6.Grubb WR, Neboori HJ, Diaz AD, et al. Racial and ethnic disparities in the pediatric Hodgkin lymphoma population. Pediatr Blood Cancer. 2016;63:428–435. doi: 10.1002/pbc.25802. [DOI] [PubMed] [Google Scholar]
- 7.Metzger ML, Castellino SM, Hudson MM, et al. Effect of race on the outcome of pediatric patients with Hodgkin’s lymphoma. J Clin Oncol. 2008;26:1282–1288. doi: 10.1200/JCO.2007.14.0699. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2011;29:76–82. doi: 10.1200/JCO.2010.29.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia S, Sather HN, Heerema NA, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 11.Wolfson J, Sun CL, Wyatt L, et al. Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: Impact of care at specialized cancer centers on survival outcome. Cancer Epidemiol Biomarkers Prev. 2017;26:312–320. doi: 10.1158/1055-9965.EPI-16-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatia S. Disparities in cancer outcomes: Lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keegan TH, DeRouen MC, Parsons HM, et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult Hodgkin lymphoma: A population-based study. Cancer Epidemiol Biomarkers Prev. 2016;25:264–273. doi: 10.1158/1055-9965.EPI-15-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedmann AM, Wolfson JA, Hudson MM, et al. Relapse after treatment of pediatric Hodgkin lymphoma: Outcome and role of surveillance after end of therapy. Pediatr Blood Cancer. 2013;60:1458–1463. doi: 10.1002/pbc.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: A report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32:3651–3658. doi: 10.1200/JCO.2013.52.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller FG, Castellino SM, Chen L, et al. Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: A report from the Children’s Oncology Group. Cancer. 2018;124:3210–3219. doi: 10.1002/cncr.31519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly KM, Cole PD, Chen L, et al. Phase III study of response adapted therapy for the treatment of children with newly diagnosed very high risk Hodgkin lymphoma (stages IIIB/IVB) (AHOD0831): A report from the Children's Oncology Group. Blood. 2015;126(abstr):3927. doi: 10.1111/bjh.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N, Chen JT, Waterman PD, et al. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeRouen MC, Parsons HM, Kent EE, et al. Sociodemographic disparities in survival for adolescents and young adults with cancer differ by health insurance status. Cancer Causes Control. 2017;28:841–851. doi: 10.1007/s10552-017-0914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keegan TH, Clarke CA, Chang ET, et al. Disparities in survival after Hodgkin lymphoma: A population-based study. Cancer Causes Control. 2009;20:1881–1892. doi: 10.1007/s10552-009-9382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole PD, Schwartz CL, Drachtman RA, et al. Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractory Hodgkin’s disease: A Children’s Oncology Group report. J Clin Oncol. 2009;27:1456–1461. doi: 10.1200/JCO.2008.20.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Palmer J, Martin P, et al. Post transplant outcome of a multicenter phase II study of brentuximab vedotin as first line salvage therapy in relapsed/refractory HL prior to AHCT. Blood. 2016;126(abstr):519. [PubMed] [Google Scholar]
- 24.Akhtar S, El Weshi A, Rahal M, et al. High-dose chemotherapy and autologous stem cell transplant in adolescent patients with relapsed or refractory Hodgkin’s lymphoma. Bone Marrow Transplant. 2010;45:476–482. doi: 10.1038/bmt.2009.197. [DOI] [PubMed] [Google Scholar]
- 25.Christian MC, Trimble EL. Increasing participation of physicians and patients from underrepresented racial and ethnic groups in National Cancer Institute-sponsored clinical trials. Cancer Epidemiol Biomarkers Prev. 2003;12:277s–283s. [PubMed] [Google Scholar]
- 26.Myers RM, Hill BT, Shaw BE, et al. Long-term outcomes among 2-year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large B-cell lymphoma. Cancer. 2018;124:816–825. doi: 10.1002/cncr.31114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn JM, Cole PD, Blonquist TM, et al. An investigation of toxicities and survival in Hispanic children and adolescents with ALL: Results from the Dana-Farber Cancer Institute ALL Consortium protocol 05-001. Pediatr Blood Cancer. 2018;65:e26871. doi: 10.1002/pbc.26871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh KM, de Smith AJ, Welch TC, et al. Genomic ancestry and somatic alterations correlate with age at diagnosis in Hispanic children with B-cell acute lymphoblastic leukemia. Am J Hematol. 2014;89:721–725. doi: 10.1002/ajh.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly KM. Hodgkin lymphoma in children and adolescents: Improving the therapeutic index. Hematology (Am Soc Hematol Educ Program) 2015;2015:514–521. doi: 10.1182/asheducation-2015.1.514. [DOI] [PubMed] [Google Scholar]
- 30.Glaser SL, Gulley ML, Clarke CA, et al. Racial/ethnic variation in EBV-positive classical Hodgkin lymphoma in California populations. Int J Cancer. 2008;123:1499–1507. doi: 10.1002/ijc.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y, Sun CL, Li L, et al. Genetic susceptibility to therapy-related leukemia after Hodgkin lymphoma or non-Hodgkin lymphoma: Role of drug metabolism, apoptosis and DNA repair. Blood Cancer J. 2012;2:e58. doi: 10.1038/bcj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansell SM. Hodgkin lymphoma: MOPP chemotherapy to PD-1 blockade and beyond. Am J Hematol. 2016;91:109–112. doi: 10.1002/ajh.24226. [DOI] [PubMed] [Google Scholar]