Abstract
Mucin-secreting goblet cell metaplasia and hyperplasia (GCMH) is a common pathological phenotype in many human respiratory diseases, including asthma, chronic obstructive pulmonary disease, cystic fibrosis, primary ciliary dyskinesia, and infections. A better understanding of how goblet cell quantities or proportions in the airway epithelium are regulated may provide novel therapeutic targets to mitigate GCMH in these devastating diseases. We identify canonical SMAD signaling as the principal pathway restricting goblet cell differentiation in human airway epithelium. Differentiated goblet cells express low levels of phosphorylated SMAD. Accordingly, inhibition of SMAD signaling markedly amplifies GCMH induced by mucous mediators. In contrast, SMAD signaling activation impedes goblet cell generation and accelerates the resolution of preexisting GCMH. SMAD signaling inhibition can override the suppressive effects imposed by a GABAergic receptor inhibitor, suggesting the GABAergic pathway likely operates through inhibition of SMAD signaling in regulating mucous differentiation. Collectively, our data demonstrate that SMAD signaling plays a determining role in mucous cell differentiation, and thus raise the possibility that dysregulation of this pathway contributes to respiratory pathophysiology during airway inflammation and pulmonary diseases. In addition, our study also highlights the potential for SMAD modulation as a therapeutic target in mitigating GCMH.
Keywords: SMAD signaling, goblet cell, human airway epithelium, GABAergic pathway, airway diseases
Goblet cell metaplasia and hyperplasia (GCMH) and overproduction of mucus features in many human respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), primary ciliary dyskinesia, allergic bronchopulmonary aspergillosis, and infections. Overproduction of mucus impairs mucociliary clearance, causing retention of pathogens, allergens, and toxic particles. Inflammatory mediators released from goblet cells may act in an autocrine and paracrine manner to further enhance inflammation in diseases and complex repair processes contributing to persistent airway remodeling (1). The progression of airway diseases and declining lung function are often associated with accumulation of inflammatory mucus and airflow obstruction (2). Modulating goblet cell differentiation and function may be a useful approach in the prevention and treatment of airway diseases. Current treatments available for regulating mucus hypersecretion have limited efficacy and are mainly related to dampening inflammatory responses and facilitating mucus expulsion, highlighting the need for an effective and specific treatment directly targeting GMCH.
IL-13 is one of the major T helper type 2 cytokines implicated in the pathogenesis of asthma. The IL-13–STAT6 pathway is a cardinal signaling pathway that induces mucus production and goblet cell hyperplasia in airway epithelial cells (3, 4). In addition, several other signaling pathways have been identified as important regulators of goblet cell differentiation, such as the NOTCH signaling pathway (5–8) and the epidermal growth factor cascade (9–14). Recent studies have linked the γ-aminobutyric acid (GABA)-ergic system to asthma and mucin production (15–17). In this context, GABA secreted from pulmonary neuroendocrine cells acts on airway epithelial cells through the subtype A GABA receptor (GABAAR) (15–17). The action of this autocrine–paracrine GABAergic system in airway epithelial cells is essential to induce goblet cell hyperplasia in allergen-challenged mice and humans affected by asthma (15–17). These results suggest the possibility that targeting the GABAergic system may be a useful approach for modulating mucus secretion in airway diseases (15–18).
In this study, we identify the bone morphogenetic proteins (BMPs) and transforming growth factor (TGF)-β superfamily canonical SMAD signaling pathway as critical in controlling mucous cell differentiation. BMP/TGF-β/SMAD signaling is exceptionally versatile, and has been implicated in orchestrating multiple biological functions, including organ development, tissue homeostasis, and injury repair in numerous studies. Previously, we reported that BMP and TGF-β signaling balances stem cell proliferation and differentiation in airway epithelium and in epithelia at large (19). Specifically, we observed that the degree of SMAD signaling activation is associated with epithelial maturity. In addition, suppression of BMP and TGF-β signaling activity was essential for maintaining p63+ basal cell stemness by impeding epithelial differentiation (19). Dual SMAD signaling inhibition can be deployed through in vitro cell culture to achieve prolonged expansion of murine and human cells, while maintaining their ability to differentiate into functional tissues (19). Here, we demonstrate that, although mucin-secreting goblet cells are postmitotic differentiated cells, SMAD signaling activity is largely suppressed. SMAD signaling inhibition markedly amplified GCMH induced by inflammatory mediators, IL-13 and IL-17A. In comparison, SMAD signaling activation restricts the development of GCMH, facilitating its resolution. Furthermore, we demonstrate that inhibitory effects on goblet cell generation imposed by GABAergic system inhibitors can be overcome by SMAD signaling inhibition, suggesting a functional relationship of these two pathways. Together, our data demonstrate an essential role of the SMAD signaling pathway in regulating mucous cell fate determination, and suggest that targeting the SMAD pathway may lead to new therapeutic strategies for the management of airway diseases.
Methods
An expanded methods section describing human airway basal stem cell culture, human tissue sectioning and staining, mucocilliary differentiation of tissues at air–liquid interface (ALI), ALI culture immunofluorescence and analysis, microscopic imaging and quantification, and statistical analysis is available in the data supplement.
Results
BMP/TGF-β/SMAD Signaling Is Suppressed in Human Airway Epithelial Goblet Cells
We previously reported that the BMP/TGF-β/SMAD signaling pathway is critical in regulating normal architecture of multiple epithelial organs (19). In human airway epithelium, BMP and TGF-β signaling is suppressed in p63+ immature basal cells, but is activated in luminal differentiated cells, including FOXJ1+ ciliated cells and CC10+ secretory cells (19). Mucin-secreting goblet cells are one of the major cell types in human conducting airway epithelium. Because goblet cells are postmitotic-differentiated cells, we predicted that SMAD signaling would be highly activated in these cells, as we had previously observed in ciliated epithelial cells (19). To evaluate this hypothesis, we imaged BMP/TGF-β/SMAD signaling pathway activation by the costaining of phosphorylated (p) SMAD1/5/8 (p-SMAD1/5/8) and p-SMAD2/3 with lineage markers on human bronchial epithelium. Cell lineage markers stained included the goblet cell marker, mucin 5AC (MUC5AC), the ciliated cell marker, FOXJ1, and the basal cell marker, p63. Consistent with prior results (19), we found FOXJ1+ ciliated cells were strongly positive for p-SMADs and p63+ basal cells were weakly positive for p-SMADs. Contrary to our initial hypothesis, p-SMAD1/5/8 and p-SMAD2/3 staining was low in MUC5AC+ cells (Figures 1A and 1B). To test whether this pattern of p-SMAD expression would also be seen in tissue grown in culture, we examined p-SMAD1/5/8 and p-SMAD2/3 staining patterns on human airway epithelium generated from primary p63+ airway basal stem cells at ALI culture (19) (Figure 1C). Consistent with the findings from sectioned human bronchus, staining of cultured human airway epithelium demonstrated that p-SMAD staining was weak in immature CK5+ basal cells, strongly positive in FOXJ1+ luminal ciliated cells, and moderately positive in CC10+ luminal club cells. Similar to the tissue sections, MUC5AC+ luminal goblet cells had weak costaining for p-SMADs, despite their terminally differentiated state (Figure 1C).
Figure 1.
SMAD signaling activity is suppressed in differentiated goblet cells in human airway epithelium. (A and B) Costaining of phosphorylated (p)-SMAD1/5/8 (A) and p-SMAD2/3 (B) with goblet cell marker (MUC5AC), ciliated cell marker (FOXJ1), and basal cell marker (p63) in human bronchial epithelium. The nucleus is stained by DAPI. Scale bars: 10 μm. Insets show a fourfold-enlarged portion of the staining image to highlight finer detail. (C) Costaining of p-SMAD1/5/8 and p-SMAD2/3 (mixed antibodies) with goblet cell marker (MUC5AC), club cell marker (CCSP), ciliated cell marker (FOXJ1), and basal cell marker (CK5) in human airway epithelium differentiated on air–liquid interface (ALI). The nucleus is stained by DAPI. Scale bars: 10 μm. The dotted lines outline the shape of club cells or goblet cells. The staining shown was performed from bronchial sections of one representative donor. Staining has been replicated in n = 4 donors and similar results were observed.
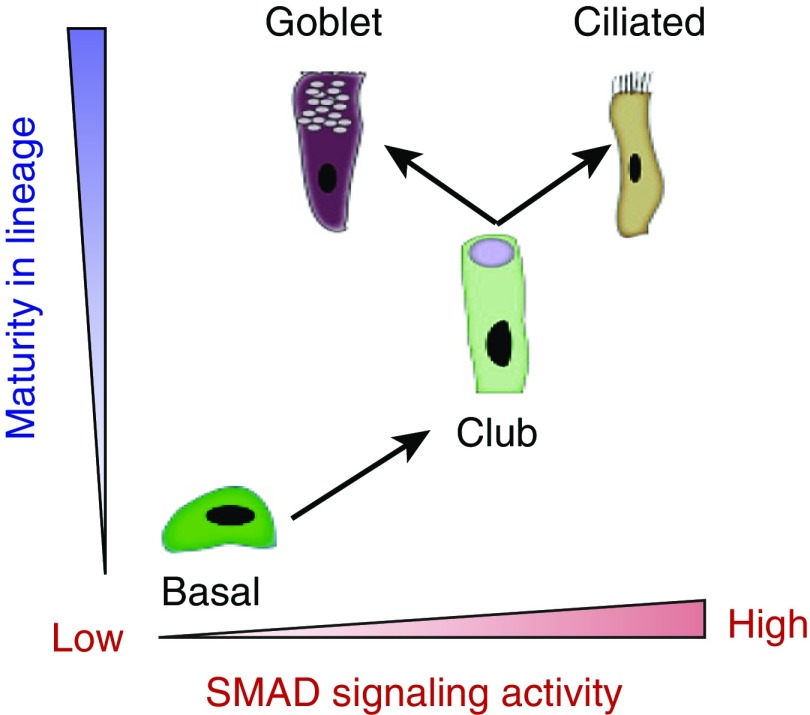
These findings suggested a model whereby SMAD signaling plays distinct roles in goblet cells and nonmucous luminal cells of the airway (Figure 2). In this model, SMAD signaling is activated in nonmucous airway luminal cells, such as ciliated and club cells, with SMAD signaling activity increasing in parallel with cell maturity and differentiation. In contrast, SMAD signaling activity in MUC5AC+ goblet cells is suppressed, and thus may play a distinct role via regulating mucin production. Consistent with this proposed model, SMAD binding sites have been previously described in the promoter regions of MUC5AC as well SPDEF (SAM pointed domain containing ETS transcription factor), a transcription factor that contributes to goblet cell differentiation (20, 21). Despite these observations, the contribution of SMAD signaling to goblet cell differentiation in primary human airway epithelium had not been previously defined.
Figure 2.
Model of differential SMAD signaling activity in human respiratory epithelial cells. In a simplified model of human airway epithelium, basal cells give rise to club cells, which subsequently differentiate into ciliated cells or goblet cells, depending on environmental signals. SMAD signaling is suppressed in immature basal cells. During the differentiation of basal cells into club cells and ciliated cells, SMAD signaling increases in parallel with increasing maturity. During the differentiation of goblet cells from club cells, SMAD signaling is decreased. This model proposes that SMAD signaling changes in club cells drive the determination of whether club cells will give rise to ciliated cells or goblet cells.
SMAD Signaling Inhibition Amplifies GCMH Induced by Inflammatory Mediators
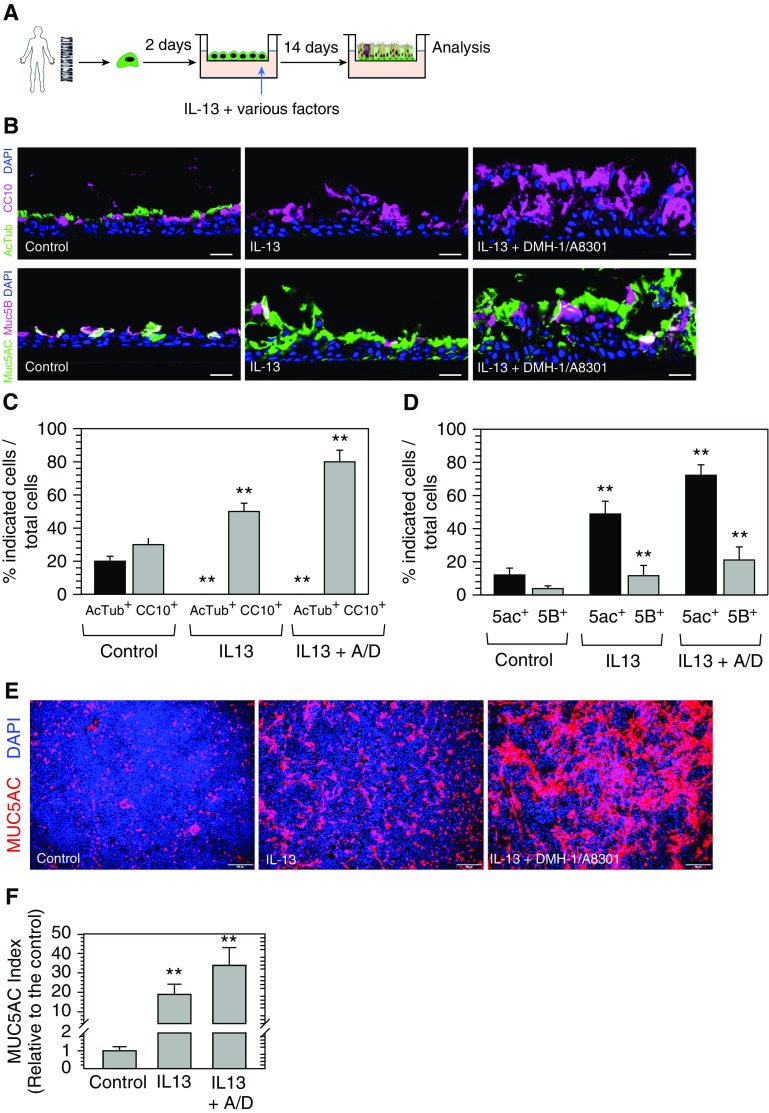
Because p-SMAD staining was low in MUC5AC+ cells, we hypothesized that inhibition of SMAD signaling can increase goblet cell production. To test this hypothesis, we generated differentiated airway epithelium from human airway basal cells collected from healthy donors (n = 4). After 16 days of ALI differentiation, various small molecules and/or cytokines were added to the basolateral compartment of the transwell chamber for 4 days of treatment before analysis by immunofluorescence (Figure 3A). Exposure to DMH-1 (a BMP-SMAD pathway inhibitor) or/and A-8301 (a TGF-β/SMAD pathway inhibitor) marginally increased the goblet cell number, and did not result in significant GCMH (Figures 3B and 3C; see also Figure E1 in the data supplement). In the presence of IL-13, a significant increase in MUC5AC+ staining was observed in airway epithelial cells (Figures 3B and 3C). In addition to increases in MUC5AC+ cells, IL-13 treatment also increased CC10+ cells, MUC5AC+ cells, and CC10+/MUC5AC+ cells (Figure E2). Cotreatment with IL-13 and SMAD signaling inhibitors (DMH-1 and A-8301) provided a further significant increase in MUC5AC staining (Figures 3B and 3C and Figure E2). In contrast, the forced activation of SMAD signaling by BMP4 or TGF-β treatment resulted in a striking deficit in MUC5AC+ staining, both with and without IL-13 stimulation (Figures 3B and 3C and Figure E1).
Figure 3.
Inhibition of bone morphogenetic protein (BMP)/transforming growth factor (TGF)-β/SMAD signaling amplifies IL-13– and IL-17A–induced goblet cell metaplasia and hyperplasia (GCMH) in human airway epithelium. (A) Human airway basal cells derived from healthy donors (n = 4) were cultured on ALI for 16 days to establish differentiated airway epithelium. IL-13 (20 ng/ml) alone or together with BMP4 (100 ng/ml), TGF-β (10 ng/ml), DMH-1 (1 μM), or A-8301 (1 μM) were added to the ALI medium in the basolateral chamber and ALIs were cultured for another 4 days. (B) ALI membranes were fixed and stained for MUC5AC. Representative images shown here were from one representative experiment. Scale bars: 100 μm. (C) Quantification of MUC5AC production in ALI membranes generated from the experiment shown in B. MUC5AC index was scored using ImageJ software (National Institutes of Health) based on the total area of MUC5AC immunopositivity out of the imaging area normalized to the control without IL-13 stimulation (mean ± SD; n = 3 independent imaging fields; **P ≤ 0.001 and ***P ≤ 0.0001). (D) Immunoblot analysis of FOXA3 and SAM pointed domain containing ETS transcription factor (SPDEF) of ALI cultures generated as described in B. β-actin was used as loading control. (E) Human airway basal cells from donors with asthma (n = 2), cystic fibrosis (CF) (n = 3), and chronic obstructive pulmonary disease (COPD; n = 2) were cultured on ALI for 16 days and then treated with IL-13 (20 ng/ml), IL-13 with BMP (100 ng/ml) and TGF-β (10 ng/ml), or IL-13 with DMH-1 (1 μM) and A8301 (1 μM) for 4 days. After treatment, ALI membranes were fixed and stained for MUC5AC. Representative images shown here were from one representative experiment on representative donors. Scale bars: 100 μm. (F) Quantification of MUC5AC in the experiment described in E. A total of three independent areas were imaged for each sample and quantified (mean ± SD; n = 3 independent imaging fields; **P ≤ 0.001 and ***P ≤ 0.0001) (G) Human airway basal cells derived from a healthy donor and a donor with asthma were cultured on ALI for 16 days and then treated with IL-17A (50 ng/ml), IL-17A with BMP (100 ng/ml) and TGF-β (10 ng/ml), or IL-17A with DMH-1 (1 μM) and A8301 (1 μM) for 4 days. ALI membranes were fixed and stained with MUC5AC. Representative images shown here are from one representative experiment on representative donors. Scale bars: 100 μm. (H) Quantification of MUC5AC in the experiment described in G. MUC5AC index was scored as described previously here. A total of three independent areas were imaged and quantified (mean ± SD; n = 3 independent imaging fields; ***P ≤ 0.0001 and **P ≤ 0.001). DMH-1 = a BMP-SMAD pathway inhibitor.
FOXA3 and SPDEF are two transcription factors that are critical for mediating mucus production and goblet cell differentiation (22–24). SMADs bind to the promoter region of SPDEF (20), whereas FOXA3 is a downstream target of SPDEF (23). We hypothesized that SMAD inhibition may, in part, regulate GCMH by affecting expression levels of both SPDEF and FOXA3. To test this hypothesis, expression levels of FOXA3 and SPDEF were measured by immunoblot in cultured epithelium with either BMP4/TGF-β/SMAD activation or inhibition (Figure 3D). IL-13 treatment alone led to an increase in protein levels of both FOXA3 and SPDEF. SMAD signaling inhibition with A-8301 and DMH-1 further increased levels of FOXA3 and SPDEF in response to IL-13 treatment. On the contrary, forced activation of SMAD signaling with BMP4/TGF-β abolished the effect of IL-13 treatment on FOXA3 and SPDEF protein levels (Figure 3D).
To determine whether the effect of BMP/TGF-β/SMAD signaling on GCMH is unique to healthy donors or is a generalizable feature of airway basal stem cells, we next examined IL-13–induced GCMH in airway epithelium derived from multiple independent donors. Airway epithelium was generated from healthy donors (n = 4), as well as donors with asthma (n = 2), CF (n = 3), and active smokers with COPD (n = 2) (Figures 3E and 3F). A similar response to SMAD pathway modulation was seen in all samples, such that SMAD signaling activation reduced MUC5AC+ staining and SMAD signaling inhibition increased the relative abundance of MUC5AC+ staining. In addition to IL-13, IL-17A is capable of inducing mucous cell differentiation (5). Our results suggested that inhibition of BMP/TGF-β/SMAD signaling could amplify IL-17A–induced goblet cell differentiation (Figures 3G and 3H).
In the experiments described previously here, we treated fully established airway epithelium with pro–goblet cell mediators (IL-13 and IL-17A) for 4 days; thus, goblet cells are likely derived from their immediate precursors—club cells (25, 26). To test whether the SMAD signaling pathway influences goblet cell differentiation from early committed airway basal cells or immature club cells (27, 28), we started IL-13 treatment at the second day of ALI initiation (Day 2) and continued to expose the differentiating ALI with IL-13 for 2 weeks (Figure 4A). Without IL-13, major cell types of airway epithelium, including AcTub+ ciliated cells, CC10+ secretory cells, as well as MUC5AC+ and MUC5B+ goblet cells, could be detected in established ALI cultures (Figures 4B–4E). IL-13 treatment resulted in GCMH with some of these goblet cells highly expressing CC10. No AcTub+ ciliated cells were detected by immunofluorescence in these samples (Figures 4B–4E). This finding suggested that early IL-13 treatment switched the differentiation fate choice of early committed airway basal cells or immature club progenitors toward goblet cells and away from ciliated cells (Figures 4B–4E). In addition, cotreatment of early ALI samples with IL-13 and SMAD signaling inhibitors (A-8301 and DMH-1) led to increased MUC5AC and MUC5B immunofluorescence positivity compared with control samples or samples treated only with IL-13 (Figures 4B–4F). Treatment of basal cells with BMP4 and TGF-β quickly induces spontaneous differentiation in basal cells and cell detachment from the membranes, leading to abolished differentiation (19) (data not shown). In summary, we concluded that SMAD signaling plays an essential role in modulating goblet cell differentiation, as induced by pro–goblet cell mediators in both physiologic and diseased states.
Figure 4.
Inhibition of BMP/TGF-β/SMAD signaling amplifies GCMH from early committed progenitor cells. (A) Human airway basal cells derived from a healthy donor were seeded on ALI. At Day 2, cells were cultured in control ALI medium alone, or ALI medium with IL-13 (20 ng/ml), or ALI medium with IL-13 (20 ng/ml) with A8301 (1 μM)/DMH-1 (1 μM) for another 14 days. ALI membranes were fixed to examine various airway epithelial cell markers. (B) Staining of AcTub (ciliated cells), CCSP (club cells), and MUC5AC and MUC5B (goblet cells) on ALI trans-sections. Scale bars: 10 μm. (C and D) Quantification of AcTub+ cells and CCSP+ cells (C) and MUC5AC+ cells and MUC5B+ cells (D) out of the total cells (total DAPI number) based on staining described in B. A total of three independent areas were imaged and quantified (mean ± SD; n = 3 independent imaging fields; **P ≤ 0.001). (E) Whole-mount staining of MUC5AC on ALI membranes. Scale bars: 100 μm. (F) Quantification of MUC5AC production in the experiment described in E. MUC5AC index was scored using ImageJ software based on the total area of MUC5AC immunopositivity out of the imaging area and calculated relative to the control without IL-13 stimulation. A total of three independent areas were imaged and quantified (mean ± SD; n = 3 independent imaging fields; **P ≤ 0.001). A/D = A8301 + DMH-1.
Activation of SMAD Signaling Facilitates the Resolution of GCMH
The ability of SMAD signaling activation to restrict GCMH in human airway epithelium prompted us to hypothesize that SMAD activation may promote the resolution of IL-13–driven GCMH. To test this, we first induced GCMH by treating ALI cultures with IL-13 for 4 days. IL-13 was removed and ALI cultures were further cultured for an additional 7 days to monitor resolution of preexisting IL-13–induced GCMH (Figure 5A). In the control conditions (no SMAD pathway modulators), MUC5AC+ staining remained elevated in the epithelium 1 week after removal of IL-13 (Figures 5B and 5C). Conversely, treatment with BMP4 and TGF-β led to a rapid decrease in MUC5AC+ staining with a substantial decrease in MUC5AC positivity by 1 day after treatment. Given the rapidity of goblet cell reduction in the presence of BMP/TGF-β, we speculate that this effect may be due to the induction of cell death in preexisting goblet cells. Indeed, apoptosis has been suggested to play a role in the resolution of allergen-induced mucous cell metaplasia and hyperplasia (29, 30). ROCK (Rho kinases) has been implicated in the regulation of cell death and survival (31, 32). Consistent with this hypothesis, addition of a ROCK inhibitor (Y-27632) delayed BMP/TGF-β–induced resolution of GCMH driven by IL-13 (Figures 5B and 5C).
Figure 5.
Activation of SMAD signaling facilitates the resolution of GCMH. (A and B) Human airway basal cells derived from a representative healthy donor were cultured on ALI for 16 days and treated with IL-13 (20 ng/ml) for 4 days to induce GCMH. ALI cultures were then rinsed gently and cultured further in various conditions (ALI medium only; ALI medium + BMP4 [100 ng/ml] + TGF-β [10 ng/ml]; or ALI medium + BMP4 [100 ng/ml] + TGF-β [10 ng/ml] + Y-27632 ROCK inhibitor [10 μM]) for another 7 days. ALI membranes were fixed and stained with MUC5AC antibody. Scale bars: 100 μm. (C) Quantification of MUC5AC production in the experiment described in A and B. MUC5AC index was scored based on the total area of MUC5AC immunopositivity out of the imaging area and calculated relative to Day 0 (the day when IL-13 stimulation was stopped and washed off). A total of three independent areas were imaged and quantified (mean ± SD; n = 3 independent imaging fields; **P ≤ 0.001). ROCKi = ROCK inhibitor.
The GABAerigic Pathway Modulates Goblet Cell Differentiation through SMAD Inhibition
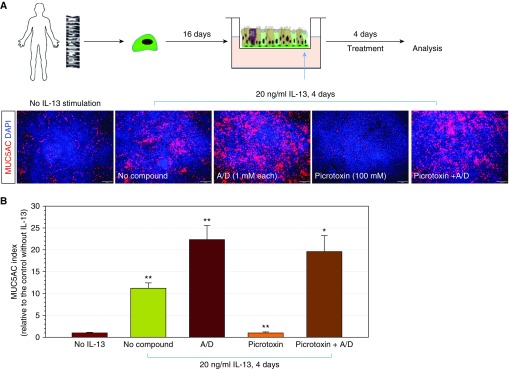
Multiple studies have implicated an essential role for the GABAAR cascade in airway GCMH and mucus overproduction (15–18). However, the mechanism by which GABAergic signaling impacts IL-13–induced mucus production and mucous cell generation is not well characterized. We noted that, despite our routinely used ALI culture medium containing a high concentration of GABA, BMP/TGF-β treatment still effectively blocked GCMH induced by IL-13 (Figures 3 and 4). These results suggested that the coactivation of the GABAergic and IL-13 pathways failed to induce GCMH in the context of high SMAD signaling activity. We hypothesized that SMAD signaling operates downstream of the GABAergic pathway. To test this hypothesis, we investigated the combined effects of a GABAAR inhibitor (picrotoxin) and SMAD pathway inhibitors on goblet cell production in ALI cultures (Figures 6A and 6B). As before, treatment with A8301 and DMH-1 increased IL-13–induced GCMH. Treatment with picrotoxin suppressed IL-13–induced GCMH. When ALI cultures where treated simultaneously with picrotoxin and SMAD inhibitors, MUC5AC+ staining returned to levels seen in SMAD inhibitor treatment alone. This finding suggested that SMAD signaling inhibition could dominate over the inhibitory effect of picrotoxin in inducing goblet cell production (Figures 6A and 6B). These data support a model where the GABAergic system in airway epithelium exerts its effects via inhibition of SMAD signaling in the regulation of GCMH. Taken together, these findings suggest that SMAD activation is a key restrictor to limit GCMH triggered by multiple signals, including IL-13, IL-17A, and the GABAergic system.
Figure 6.
SMAD signaling inhibition overrides the suppressive effect of γ-aminobutyric acid (GABA) receptor inhibitor in goblet cell production. (A) Human airway basal cells derived from a representative healthy donor were cultured on ALI for 16 days and treated with IL-13 (20 ng/ml) for 4 days to induce goblet cell hyperplasia. SMAD signaling inhibitors (1 μM A8301 + 1 μM DMH-1) and GABAergic system receptor (100 μM picrotoxin), or in combination, were included in ALI culture to examine their effects on goblet cell generation. ALI membranes were fixed and stained with MUC5AC antibody. Scale bars: 100 μm. (B) Quantification of MUC5AC production in the experiment shown in A. MUC5AC index was calculated based on the total area of MUC5AC positivity out of total imaging area. The score was relative to the control (without IL-13 stimulation; mean ± SD; n = 3 independent imaging fields; *P ≤ 0.01 and **P ≤ 0.001).
Discussion
GCMH and mucus hypersecretion are pathophysiological features of asthma, CF, allergic bronchopulmonary aspergillosis, and COPD. Not only does increased mucus production in the airways lead to impaired resistance to infection and decreased lung function via airway obstruction, but it is also associated with increased morbidity and mortality. Novel therapeutic strategies to prevent or reverse GCMH may help alleviate exacerbation of these diseases. Currently, the mechanisms underlying expansion and reduction of goblet cell mass in airways remain poorly understood. Recently, the GABAergic system has been implicated in goblet cell hyperplasia in an asthmatic setting (15–18). In this study, we demonstrated that canonical TGF-β and BMP signaling play a critical role in the development of GCMH in human airway epithelium. Previous studies demonstrated that conserved SMAD binding sites are present in the promotor region of MUC5AC, and disruption of the SMAD4 binding site in this promoter region could decrease gene transcription in a reporter cell line (21). It has been noted that SMAD binding sites are also present in the promotor regions of SPDEF (20), suggesting that SMAD inhibition likely promotes GCMH via an impact on a number of genes. Consistent with this hypothesis, we demonstrate that SMAD inhibition promotes IL-13–driven increases in SPDEF and FOXA3, a downstream target of SPDEF. Extended loss of SMAD signaling not only stimulates the differentiation of goblet cells from club cells or committed basal cells, but also prevents the resolution of GCMH, leading to excessive mucous secretion. Our data suggest a model where the GABAergic pathway operates through canonical BMP/TGF-β/SMAD signaling to regulate goblet cell hyperplasia. Similar to receptors of the GABAergic system, SMAD receptors are located on the apical membrane (33–35). It may be possible to target the SMAD signaling pathways with inhaled medications to limit or reverse goblet cell hyperplasia, while minimizing off-target effects.
The potential for targeting the SMAD signaling pathway to modulate GCMH may be complicated by the complex nature of TGF-β signaling in the lung. In our experimental model, forced activation of SMAD signaling by BMP/TGF-β prevents IL-13–induced GCMH. TGF-β signaling pathways are thought to play important roles in the regulation of inflammatory responses. For example, some studies have suggested that TGF-β has potent antiinflammatory activity, thus increased production and activation of TGF-β was linked to increased susceptibility to opportunistic infection (36, 37). In allergic asthmatic animal models, TGF-β is proinflammatory, as seen by attenuated inflammatory responses and suppressed monocyte/macrophage recruitment into the lung with TGF-β inhibition (38, 39). Furthermore, TGF-β drives airway and lung tissue remodeling in a number of diseases (40). The multifaceted physiological and pathogenic functions of BMP/TGF-β/SMAD signaling add complexity to the search for novel therapeutic opportunities to activate or reduce SMAD signaling for treatment. Thus, we must consider interactions between mucosal epithelia, immune cells, and fibrotic complications.
Conflicting results regarding the change of TGF-β level in airway inflammation and GCMH have been reported (41). For example, no obvious change or elevated levels of TGF-β was detected in lung BAL fluid and whole-lung tissues in ovalbumin (OVA)-sensitized mice (42–44). Similarly, patients with asthma express increased TGF-β1 in BAL fluid (45). However, careful histologic examination of lung sections revealed that TGF-β1 and activin A protein expression in airway epithelium were lost in OVA-sensitized mice as well as in patients with asthma (46). Furthermore, reduced expression of TGF-β1 and loss of activin A leads to increased mucus secretion in response to OVA sensitization and IL-13 stimulation. In accordance with this finding, genetic deletion of TGF-β signaling in murine conjunctival epithelium induces goblet cell hyperplasia (20). BMPs are known to be critical for lung development (47), yet their role during inflammatory responses in the adult lung remains unclear. It was reported that BMP ligand expression did not differ between asthmatic and control airways at baseline; however, BMP receptor expression is downregulated in the asthmatic airway (48). These data support the possibility that downregulation of BMP-mediated signaling may contribute to asthma pathophysiology. Together, these observations suggest that previous controversy surrounding the function of TGF superfamily in allergic diseases (41) can be ascribed to the complexity of regional and differential regulation of TGF-β1, BMP4, and SMAD signaling activity. Although these conflicting results are difficult to reconcile, we speculate that TGF-β1 is differentially regulated between the airway epithelium and the alveolar tissues of the lung. The observation of elevated TGF-β1 levels in BAL may be more reflective of profibrinogenic TGF-β1 production in the alveolar tissues. Meanwhile, the downregulation of TGF-β1 in airway epithelium is associated with GCMH in airways diseases.
The current study has several limitations that should be identified. First, a pharmacological approach was primarily used for this study. Although the small molecule inhibitors used have been previously reported for their role in specific signaling pathway inhibition (19), the possibility remains that off-target effects could be contributing to the findings reported here. Future studies using gene editing approaches to disrupt BMP/TGF-β/SMAD signaling will be helpful in confirming the role of SMAD in mucous cell differentiation. In addition, the immunofluorescence techniques used to monitor GCMH do not readily allow for a clear distinction to be drawn between processes of goblet hyperplasia and metaplasia. Previous models have shown that allergic stimulation can promote both GCMH (25, 26). As such, we have described the process observed here as GCMH.
Although upstream receptors and ligands of the BMP/TGF-β pathways are shared, their downstream biological outcomes modulated through SMAD pathways can be varied. We show that these pathways control basal cell stemness and proliferation (19) and govern mucous cell fate determination in human airway epithelial tissue in this study. This difference may enable these two important pathways to control both the stem cell pool and GCMH in response to variable degrees of physiological and pathogenic stimuli with more specificity and stringency. This may be achieved by partnering with distinct transcription factors and cross-talking with other signaling pathways, resulting in cell-state–specific modulation. Indeed, in COPD and other airway diseases, basal cell hyperplasia and goblet cell hyperplasia can be observed in adjacent locations (49), suggesting that the SMAD signaling pathway is elegantly positioned to enable the airway to respond to environmental insults by modulating basal cell proliferation, tissue regeneration, and mucous secretion. Further investigation is needed to identify underlying molecular mechanisms by which this pathway is regulated in different cells and physiological contexts.
Supplementary Material
Acknowledgments
Acknowlegement
The authors thank the laboratory of Dr. Bryan Hurley (The Mucosal Immunology and Biology Research Center, Massachusetts General Hospital, Boston, MA) for constructive criticisms, comments, valuable discussion, and support. In addition, they thank Dr. Rebecca Ward (Division of Infectious Diseases, Department of Medicine, Massachusetts General Hospital, Boston, MA) for her comments and editing of the manuscript.
Footnotes
Supported by Cystic Fibrosis Foundation Research grant MOU16G0, the Massachusetts General Hospital Stem Cell Sundry Fund (A.L.), and the Mucosal Immunology and Biology Research Center Institution Fund, and in part by the Ruth L. Kirschstein National Research Service Award T32 HL116275 from the National Institutes of Health/National Heart, Lung, and Blood Institute (M.B.F).
Author Contributions: M.B.F., M.W., A.L., and H.M. designed the project, developed the experimental pipeline, conducted experiments, and wrote the manuscript.
This article has a data supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0326OC on March 8, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tanabe T, Rubin BK. Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth fa2ctors. Chest. 2016;149:714–720. doi: 10.1378/chest.15-0947. [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 3.Parulekar AD, Kao CC, Diamant Z, Hanania NA. Targeting the interleukin-4 and interleukin-13 pathways in severe asthma: current knowledge and future needs. Curr Opin Pulm Med. 2018;24:50–55. doi: 10.1097/MCP.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 4.Rael EL, Lockey RF. Interleukin-13 signaling and its role in asthma. World Allergy Organ J. 2011;4:54–64. doi: 10.1097/WOX.0b013e31821188e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Reports. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lafkas D, Shelton A, Chiu C, de Leon Boenig G, Chen Y, Stawicki SS, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 2015;528:127–131. doi: 10.1038/nature15715. [DOI] [PubMed] [Google Scholar]
- 8.Tsao P-N, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, et al. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283:29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgel PR, Escudier E, Coste A, Dao-Pick T, Ueki IF, Takeyama K, et al. Relation of epidermal growth factor receptor expression to goblet cell hyperplasia in nasal polyps. J Allergy Clin Immunol. 2000;106:705–712. doi: 10.1067/mai.2000.109823. [DOI] [PubMed] [Google Scholar]
- 10.Casalino-Matsuda SM, Monzón ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581–591. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Shim JJ, Burgel P-R, Ueki IF, Dao-Pick T, Tam DC-W, et al. IL-13–induced Clara cell secretory protein expression in airway epithelium: role of EGFR signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2002;283:L67–L75. doi: 10.1152/ajplung.00404.2001. [DOI] [PubMed] [Google Scholar]
- 12.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414–L421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel PR, Takeyama K, et al. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol Lung Cell Mol Physiol. 2001;280:L134–L140. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- 14.Takeyama K, Tamaoki J, Kondo M, Isono K, Nagai A. Role of epidermal growth factor receptor in maintaining airway goblet cell hyperplasia in rats sensitized to allergen. Clin Exp Allergy. 2008;38:857–865. doi: 10.1111/j.1365-2222.2008.02951.x. [DOI] [PubMed] [Google Scholar]
- 15.Barrios J, Patel KR, Aven L, Achey R, Minns MS, Lee Y, et al. Early life allergen-induced mucus overproduction requires augmented neural stimulation of pulmonary neuroendocrine cell secretion. FASEB J. 2017;31:4117–4128. doi: 10.1096/fj.201700115R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360:pii:eaan8546. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang Y-Y, Wang S, Liu M, Hirota JA, Li J, Ju W, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 18.Shen M-L, Wang C-H, Lin C-H, Zhou N, Kao S-T, Wu DC. Luteolin attenuates airway mucus overproduction via inhibition of the GABAergic system. Sci Rep. 2016;6:32756. doi: 10.1038/srep32756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19:217–231. doi: 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCauley HA, Liu C-Y, Attia AC, Wikenheiser-Brokamp KA, Zhang Y, Whitsett JA, et al. TGFβ signaling inhibits goblet cell differentiation via SPDEF in conjunctival epithelium. Development. 2014;141:4628–4639. doi: 10.1242/dev.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young HWJ, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park K-S, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardo-Saganta A, Law BM, Gonzalez-Celeiro M, Vinarsky V, Rajagopal J. Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge. Am J Respir Cell Mol Biol. 2013;48:364–373. doi: 10.1165/rcmb.2012-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16:184–197. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo W-L, Shenoy SA, Li S, O’Beirne SL, Strulovici-Barel Y, Leopold PL, et al. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med. 2018;198:1375–1388. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesfaigzi Y, Fischer MJ, Daheshia M, Green FHY, De Sanctis GT, Wilder JA. Bax is crucial for IFN-gamma–induced resolution of allergen-induced mucus cell metaplasia. J Immunol. 2002;169:5919–5925. doi: 10.4049/jimmunol.169.10.5919. [DOI] [PubMed] [Google Scholar]
- 30.Tesfaigzi Y, Harris JF, Hotchkiss JA, Harkema JR. DNA synthesis and Bcl-2 expression during development of mucous cell metaplasia in airway epithelium of rats exposed to LPS. Am J Physiol Lung Cell Mol Physiol. 2004;286:L268–L274. doi: 10.1152/ajplung.00172.2003. [DOI] [PubMed] [Google Scholar]
- 31.Street CA, Bryan BA. Rho kinase proteins—pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011;31:3645–3657. [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007;55:61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldin C-H, Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol. 2016;8:a022053. doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol. 2013;2:47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- 35.Vander Ark A, Cao J, Li X. TGF-β receptors: in and beyond TGF-β signaling. Cell Signal. 2018;52:112–120. doi: 10.1016/j.cellsig.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690:24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 38.Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor β and severe asthma: a perfect storm. Respir Med. 2014;108:1409–1423. doi: 10.1016/j.rmed.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Bottoms SE, Howell JE, Reinhardt AK, Evans IC, McAnulty RJ. Tgf-β isoform specific regulation of airway inflammation and remodelling in a murine model of asthma. PLoS One. 2010;5:e9674. doi: 10.1371/journal.pone.0009674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng X-M, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 41.Bossé Y, Rola-Pleszczynski M. Controversy surrounding the increased expression of TGF beta 1 in asthma. Respir Res. 2007;8:66. doi: 10.1186/1465-9921-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JHT, Janssen-Heininger YMW, et al. Transforming growth factor-β1 suppresses airway hyperresponsiveness in allergic airway disease. Am J Respir Crit Care Med. 2007;176:974–982. doi: 10.1164/rccm.200702-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-β/activin-Smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol. 2001;25:60–68. doi: 10.1165/ajrcmb.25.1.4396. [DOI] [PubMed] [Google Scholar]
- 44.Rosendahl A, Pardali E, Speletas M, Ten Dijke P, Heldin C-H, Sideras P. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002;27:160–169. doi: 10.1165/ajrcmb.27.2.4779. [DOI] [PubMed] [Google Scholar]
- 45.Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, et al. Transforming growth factor-β 1 in asthma: measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997;156:642–647. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 46.Hardy CL, Rolland JM, O’Hehir RE. The immunoregulatory and fibrotic roles of activin A in allergic asthma. Clin Exp Allergy. 2015;45:1510–1522. doi: 10.1111/cea.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrisey EE, Hogan BLM. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kariyawasam HH, Xanthou G, Barkans J, Aizen M, Kay AB, Robinson DS. Basal expression of bone morphogenetic protein receptor is reduced in mild asthma. Am J Respir Crit Care Med. 2008;177:1074–1081. doi: 10.1164/rccm.200709-1376OC. [DOI] [PubMed] [Google Scholar]
- 49.Rock JR, Randell SH, Hogan BLM. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.