Pulmonary arterial hypertension (PAH) is a rare but life-threatening disorder with an estimated prevalence of 10–50 cases per million (1). Although the pathobiology of PAH is complex and incompletely understood, a common feature of all manifestations of PAH is an abnormal increase in pulmonary vascular resistance as a result of a reduction in the cross-sectional area of pulmonary arteries and arterioles (2). This process of pulmonary vascular remodeling is driven by a combination of processes, including impaired vasorelaxation, excessive thrombosis, excessive inflammation, and dysregulated proliferative/obstructive remodeling of the pulmonary vessels (3). Endothelial-to-mesenchymal transition (EndMT), a process by which endothelial cells differentiate and acquire phenotypic characteristics of mesenchymal/smooth muscle cells, is believed to play a critical role in the initiation and progression of pathologic remodeling of the pulmonary vasculature (4). Although many therapies aimed at vasodilating the pulmonary arteries are available, no current therapies have shown benefit in the prevention or regression of vascular remodeling, perhaps because the underlying mechanisms are poorly understood, and there are no therapies that directly target the mechanisms underlying vascular remodeling in PAH.
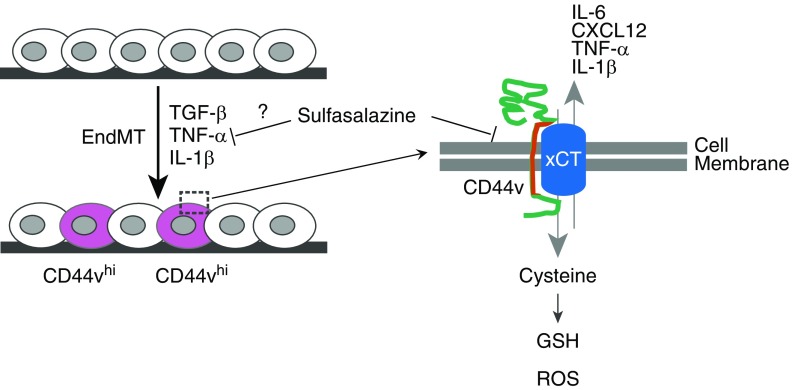
The study by Isobe and colleagues (pp. 367–379) in this issue of the Journal identifies a potential contributor to and mediator of EndMT in pulmonary hypertension (5). Based on their central theme that pulmonary vascular remodeling mechanisms share pathobiologic features with cancer, they identified CD44, a receptor for hyaluronic acid that is implicated in the progression and metastatic potential of multiple malignancies, as a possible therapeutic target in EndMT (Figure 1). Furthermore, the authors demonstrate that splicing variants of CD44, termed CD44v, were more frequently expressed in plexiform lesions and neointimal hyperplasia in lungs from patients with idiopathic PAH compared with control lung tissue. The CD44v+ cells also expressed markers of endothelial cells (von Willebrand factor) and smooth muscle cells (α-smooth muscle actin), suggesting that the CD44v+ cells had undergone EndMT. They confirmed this finding in vitro in human pulmonary vascular endothelial cells that were subjected to conditions of EndMT (transforming growth factor β, TNF-α, and IL-1β). Notably, after EndMT induction, ∼10% of the cells expressed CD44v. CD44v+ cells also showed increased transcription of the proinflammatory cytokines TNF-α, IL1-β, IL-6, and CXCL12. The authors demonstrated that CD44v+ cells may have a survival advantage via CD44-mediated stabilization of a cystine-glutamate antiporter, xCT, a cell-surface receptor that is known to promote antioxidant glutathione synthesis via cystine import and thus to enhance the scavenging of reactive oxygen species. Although the authors suggest that this may promote cell survival and limit apoptosis, the functional link between xCT-mediated glutathione synthesis and downstream functional changes resulting in EndMT and dysregulation require further study. Finally, using a well-established model of chronic hypoxia and vascular endothelial growth factor inhibitor–induced pulmonary hypertension (Sugen + hypoxia), the authors demonstrate that treatment with sulfasalazine, which functions in part by inhibiting xCT-mediated cystine transport, prevented and partially reversed pulmonary hypertension by reducing vascular remodeling in vivo.
Figure 1.
Effect of a CD44 variant (CD44v) on cellular function in pulmonary arterial hypertension, and possible effects of sulfasalazine. EndMT = endothelial-to-mesenchymal transition; GSH = glutathione; ROS = reactive oxygen species; TGF-β = transforming growth factor-β; xCT = cytosine-glutamate transporter.
It is interesting that only ∼10% of the cells, when subjected to conditions of EndMT, expressed CD44v. Although experimental knockdown of xCT, the proposed target of CD44v, did not alter EndMT gene transcription in endothelial cells, the proportion of CD44v+ cells was decreased when they were pretreated with siRNA targeting xCT. As such, CD44v+ cells may have similarities to cancer stem cells in that they may promote an EndMT-permissive microenvironment in pulmonary arteries. The authors also show that sulfasalazine treatment is effective in preventing and partially reversing pulmonary vascular remodeling in the Sugen + hypoxia model of pulmonary hypertension. Although it is mechanistically attractive that sulfasalazine mediates its effect by directly targeting CD44v+ cells in vivo, it is unclear whether sulfasalazine had any off-target effects on other cells and organ systems that may have contributed to reduced vascular remodeling (6). CD44 is known to be expressed on a number of immune cells (7), and the contribution of inflammation to the pathogenesis of pulmonary hypertension is well accepted, even if incompletely understood (8). Additionally, sulfasalazine has been shown to interfere with the production of leukotrienes and prostaglandins, potent mediators of vasoconstriction and vasorelaxation (9). To determine the therapeutic potential of sulfasalazine in PAH, it will be important to understand its mechanism of action in vivo; however, the present studies cannot differentiate whether sulfasalazine’s antiinflammatory properties or its inhibition of xCT mediate its beneficial effects.
Isobe and colleagues contribute to the interesting hypothesis that PAH represents a systemic disorder with a pathogenesis similar to that of cancer (10). A critical component of cancer biology is altered metabolism. Prior studies have demonstrated broad, multisystemic metabolic reprogramming in PAH as an important contributor to disease pathogenesis (11–15). Similar to what has been observed with cancer stem cells, pulmonary vascular changes in PAH are marked by significant shifts in oxidative phosphorylation toward a more glycolytic phenotype, as well as shifts in glutamate and glutamine utilization (16, 17). Consistent with known important metabolic mediators in PAH, Isobe and colleagues found a role for xCT-mediated glutamate transport in the pathogenesis of EndMT. Whether these metabolic shifts play a role in the initiation or progression of EndMT in PAH remains an unanswered question and a topic that is ripe for further investigation. Finally, given that EndMT has been implicated in the microvasculature of many organs beyond the lung (18), it would be intriguing to study whether sulfasalazine or a similar therapy could be beneficial for treating other PAH-related complications, such right ventricular failure. The findings from Isobe and colleagues demonstrate less adverse remodeling in the right ventricle of mice subjected to Sugen + hypoxia after sulfasalazine treatment. Whether this is primarily the result of a decreased afterload from less pulmonary vascular remodeling or it represents a systemic response to multiorgan EndMT changes is unclear and warrants further investigation. If they are truly effective in altering multiorgan dysfunction in PAH, sulfasalazine and other immunomodulatory therapies may also have a role in altering disease pathogenesis in other groups of patients with pulmonary hypertension who do not derive a benefit from known PAH-specific vasodilating therapies, i.e., patients with World Health Organization Group 2 pulmonary hypertension due to primary cardiac disease (19–21).
Regardless of these unresolved issues, Isobe and colleagues have identified a potentially important role for CD44 and xCT in the initiation and propagation of EndMT. Importantly, they highlight a key pathologic feature of PAH that deserves further study, and they propose an intriguing approach for reversing EndMT in PAH. If the population of cells identified by Isobe and colleagues is central to EndMT in pulmonary vessels, therapies aimed at CD44 or xCT may indeed be the silver bullet we have been looking for.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Prins KW, Thenappan T. World Health Organization group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin. 2016;34:363–374. doi: 10.1016/j.ccl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Lau EMT, Montani D, Jaïs X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 3.Hemnes AR, Humbert M. Pathobiology of pulmonary arterial hypertension: understanding the roads less travelled. Eur Respir Rev. 2017;26:170093. doi: 10.1183/16000617.0093-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 5.Isobe S, Kataoka M, Endo J, Moriyama H, Okazaki S, Tsuchihashi K, et al. Endothelial–mesenchymal transition drives expression of CD44 variant and xCT in pulmonary hypertension. Am J Respir Cell Mol Biol. 2019;61:367–379. doi: 10.1165/rcmb.2018-0231OC. [DOI] [PubMed] [Google Scholar]
- 6.Punchard NA, Greenfield SM, Thompson RPH. Mechanism of action of 5-arninosalicylic acid. Mediators Inflamm. 1992;1:151–165. doi: 10.1155/S0962935192000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson P, Arif AA, Lee-Sayer SSM, Dong Y. Hyaluronan and its interactions with immune cells in the healthy and inflamed lung. Front Immunol. 2018;9:2787. doi: 10.3389/fimmu.2018.02787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price LC, Wort SJ, Perros F, Dorfmüller P, Huertas A, Montani D, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield SM, Punchard NA, Teare JP, Thompson RP. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7:369–383. doi: 10.1111/j.1365-2036.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 10.Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ. 2017;7:285–299. doi: 10.1177/2045893217701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assad TR, Hemnes AR. Metabolic dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep. 2015;17:20. doi: 10.1007/s11906-014-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brittain EL, Talati M, Fessel JP, Zhu H, Penner N, Calcutt MW, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation. 2016;133:1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Austin ED, Talati M, Fessel JP, Farber-Eger EH, Brittain EL, et al. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur Respir J. 2017;50:1602337. doi: 10.1183/13993003.02337-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemnes AR, Luther JM, Rhodes CJ, Burgess JP, Carlson J, Fan R, et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight. 2019;4:123611. doi: 10.1172/jci.insight.123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talati MH, Brittain EL, Fessel JP, Penner N, Atkinson J, Funke M, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med. 2016;194:719–728. doi: 10.1164/rccm.201507-1444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Q, Chan SY. Mitochondrial and metabolic drivers of pulmonary vascular endothelial dysfunction in pulmonary hypertension. Adv Exp Med Biol. 2017;967:373–383. doi: 10.1007/978-3-319-63245-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S. Cancer stem cell metabolism and potential therapeutic targets. Front Oncol. 2018;8:203. doi: 10.3389/fonc.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong H, Lyu X, Wang Q, Hu M, Zhang X. Endothelial to mesenchymal transition in the cardiovascular system. Life Sci. 2017;184:95–102. doi: 10.1016/j.lfs.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–2536. doi: 10.1016/j.jacc.2016.09.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meoli DF, Su YR, Brittain EL, Robbins IM, Hemnes AR, Monahan K. The transpulmonary ratio of endothelin 1 is elevated in patients with preserved left ventricular ejection fraction and combined pre- and post-capillary pulmonary hypertension. Pulm Circ. 2018;8:2045893217745019. doi: 10.1177/2045893217745019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal V, Byrd BF, III, Brittain EL. Echocardiographic evaluation of diastolic function in the setting of pulmonary hypertension. Pulm Circ. 2019;9:2045894019826043. doi: 10.1177/2045894019826043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.