Abstract
Objective
To estimate the prevalence of anxiety disorders in pregnant and postpartum women and identify predictors accounting for variability across estimates.
Data Sources
An electronic search of PsycINFO and PubMed was conducted from inception until July 2016, without date or language restrictions, and supplemented by articles referenced in the obtained sources. A Boolean search phrase utilized a combination of keywords related to pregnancy, postpartum, prevalence, and specific anxiety disorders.
Study Selection
Articles reporting the prevalence of one or more of eight common anxiety disorders in pregnant or postpartum women were included. A total of 2,613 records were retrieved, with 26 studies ultimately included.
Data Extraction
Anxiety disorder prevalence and potential predictor variables (e.g., parity) were extracted from each study. A Bayesian multivariate modeling approach estimated the prevalence and between-study heterogeneity of each disorder and the prevalence of having one or more disorder.
Results
Individual disorder prevalence estimates ranged from 1.1% for PTSD to 4.8% for specific phobia, with the prevalence of having at least one or more disorder estimated to be 20.7% [16.7% to 25.4%]. Substantial between-study heterogeneity was observed suggesting that “true” prevalence varies broadly across samples. There was evidence of a small (3.1%) tendency for pregnant women to be more susceptible to anxiety disorders than postpartum women.
Conclusions
Peripartum anxiety disorders are more prevalent than previously thought, with 1 in 5 women in a typical sample meeting diagnostic criteria for at least one disorder. These findings highlight the need for anxiety screening, education and referral in obstetrics and gynecology settings.
Approximately 30% of adults suffer from an anxiety disorder (AD) at some point in their lives,1 with evidence suggesting these disorders are 2-to-3 times more common than mood, impulse-control, or substance-abuse disorders over a twelve-month period.2 This represents a major public health concern because anxiety leads to significant impairments in social, emotional and physical functioning,3 causing a high level of health care service utilization.4–7 In addition to direct public health costs, ADs are associated with substantial indirect costs related to functional impairment (e.g., diminished work capacity, unemployment).8,9 Women are at particular risk as they are significantly more likely (1.2 to 6.8 times) to suffer from an AD than are men.1,10
Maternal anxiety and fetal/infant development
Screening and treatment of peripartum ADs is especially important given the potential short- and long-term effects of anxiety on offspring. Maternal prenatal anxiety has been associated with adverse pregnancy outcomes such as miscarriage, pre-eclampsia, pre-term delivery, and low birth weight,11–17 with particularly strong evidence for increased risk of preterm birth and low birth weight.18 Prenatally anxious women have been found to interact less skillfully and communicate less with their infants.19 Maternal anxiety has also been associated with impaired adaptability including negative behavioral responses to novelty, negative mood, and soothing difficulty in offspring.20,21 Finally, mothers with ADs are more likely to have children who are behaviourally inhibited and insecurely attached.22
Longitudinal studies of mother-child pairs demonstrate a higher rate of ADs in children of mothers with an AD compared to children of mothers without an AD.23 Children of mothers in the top 15% for symptoms of antenatal anxiety have been shown to have twice the risk for ADHD at ages 4 and 7.24,25 Finally, adolescents of mothers with high levels of anxiety during pregnancy have also shown deficits in cognitive control linked to the orbitofrontal cortex.26
Prenatal anxiety has also been identified as a very strong predictor of postpartum depression, even when controlling for prenatal depression levels.27–29 Antenatal depression has also been significantly associated with preterm birth and low birth weight, with higher risk among women from lower socioeconomic status and developing countries.30 Risk factors identified for both perinatal mood and anxiety include ethnic minority status, low socioeconomic status, poor educational attainment, poor quality partner relationships, history of poor mental health, adverse circumstances around the pregnancy and birth, history of abuse/domestic violence, adverse life events, high perceived stress, being single, and unplanned or unwanted pregnancy.31–34 Given the above, knowing the prevalence of perinatal ADs is important in helping to determine the scope of the problem and supporting the recommendation for routine perinatal anxiety screening, education, and referral to treatment in healthcare settings.35
Prior to the publication of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5),36 the core ADs were: obsessive-compulsive disorder (OCD), panic disorder (PD), agoraphobia (AG), generalized anxiety disorder (GAD), social phobia (SP), specific phobia, posttraumatic stress disorder (PTSD), and acute stress disorder (ASD).37 In the DSM-5, OCD, PTSD and ASD have been moved into their own sections (Obsessive-Compulsive and Related Disorders, and Trauma- and Stressor-Related Disorders, respectively),36 despite widespread agreement that anxiety is a core feature of these problems (e.g., shared pathology and treatment response).38 Since the DSM-5 changes are quite recent, there are very few prevalence studies based on DSM-5 criteria. To remain in line with the bulk of the published literature, we have operationalized total AD prevalence as meaning the probability of having one or more of the common DSM-IV ADs.
Although not included in the DSM, it is important to note that pregnancy anxiety or pregnancy-related anxiety (PrA) has been identified in the literature as a distinct clinical phenomenon, in that worries are tied directly to pregnancy, childbirth, and the maternal role.39–41 Similar to DSM defined anxiety disorders, there is a correlation with adverse obstetric and child development outcomes that persists after controlling for medical and obstetric risk factors.42 Factor analysis of PrA reveals two distinct factors: concerns about the child’s health and concerns about the birth.41 Concerns about the child’s health predicted infant birth weight independently of GAD, with both factors showing only modest associations with clinical measures of generalized anxiety (SCID).41 Thus, it is possible that pregnancy specific worries contribute to a portion of the symptoms experienced by women diagnosed with DSM defined disorders.
The prevalence of anxiety disorders during the perinatal period
With an established emphasis on screening and treatment of perinatal depression, it is only recently that research has shined a spotlight on perinatal anxiety disorders and their frequency.43 Several reliable prevalence estimates from well-designed studies of maternal perinatal AD now exist as a result of utilizing (a) gold standard assessment procedures (i.e., diagnostic interviews by trained interviewers), and (b) representative or unselected samples. Studies that use selected samples of pregnant or postpartum women (e.g., women experiencing a medically high-risk pregnancy or whose infant was stillborn) fail to provide accurate estimates of perinatal anxiety. Similarly, questionnaire-based assessments of mental health conditions significantly over-estimate prevalence and incidence rates.44
Unfortunately, studies using representative samples with gold standard assessment procedures still vary considerably in their reported prevalence estimates. For instance, studies in which four or more DSM-IV ADs were assessed have reported estimates ranging from 5.1%45 to 37.5%.46 Recent meta-analyses have themselves produced estimates ranging from 8.5%47 to 15.2%48 prenatally, and 9.9% postnatally,48 although they have been based on 10 or fewer studies in each case. Importantly, current meta-analyses on this topic have aggregated prevalence estimates that were incompatible due to variation in: (a) the number of disorders assessed, and (b) the subset of ADs included. For example, Austin et al.49 defined the probability of having an AD as being diagnosed with GAD, SP, PD, or agoraphobia whereas Navarro et al.50 defined this same quantity as being diagnosed with GAD, PD with or without agoraphobia, agoraphobia, SP, OCD, PTSD, or non-specified anxiety. Nonetheless, these estimates were pooled together.47 Statistical simulations suggest that estimating the prevalence of a disorder category – such as ADs – by combining studies that differ with respect to the disorders measured requires special consideration to avoid catastrophically underestimating the true prevalence.51
We address this concern by modelling the individual ADs using a modern Bayesian multivariate approach. Specifically, the current analysis estimates the probability of having one or more AD by combining individual disorder prevalences and simulating data from a large, typical sample to estimate the probability of having one or more of those disorders. We also estimate co-morbidities across disorders using individual patient data, where available. This modelling approach has been shown to outperform other means of estimating the prevalence of a disorder category and permits us to make probabilistic statements about other facets of the data (e.g., comorbidity) not possible using traditional approaches (for details and code, see Fawcett et al.).51 Furthermore, the current approach is not limited to studies that include multiple AD prevalences, allowing for additional studies to be included measuring only one AD. Anticipating heterogeneity, we also explore potential moderators. Given the potential for ADs to have serious negative consequences for both mother and child, ascertaining the prevalence of ADs among pregnant and postpartum women may help to raise awareness of this important issue.
Method
Literature Search
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.52 We conducted a search of the online resources PsycINFO and PubMed, using the Boolean search phrase: ((“perinatal” OR “prenatal” OR “antenatal” OR “pregnancy” OR “pregnant” OR “postnatal” OR “postpartum” OR “childbirth” OR “birth”) AND (“prevalence” OR “epidemiology” OR “incidence”) AND (“anxiety disorder” OR “anxiety disorders” OR “Panic Disorder” OR “Agoraphobia” OR “Obsessive Compulsive Disorder” OR “Obsessive-Compulsive Disorder” OR “Generalized Anxiety Disorder” OR “Social Phobia” OR “Social Anxiety Disorder” OR “Specific Phobia” OR “Phobic Disorder” OR “Posttraumatic Stress Disorder” OR “Post-traumatic Stress Disorder” OR “Anxiety Not Otherwise Specified” OR “Anxiety NOS”)). The search was conducted until July 2016 without date or language restrictions and was supplemented by articles referenced in the obtained sources. Additional articles were identified using references from review articles and meta-analyses, as well as correspondence with experts in the field.
Study inclusion criteria
Articles that reported the prevalence of one or more of eight common ADs (panic disorder, agoraphobia, obsessive-compulsive disorder, generalized anxiety disorder, social phobia, specific phobia, posttraumatic stress disorder, and anxiety not otherwise specified) in pregnant or postpartum women (up to 12 months) were included. Substance-induced anxiety disorder, anxiety disorder due to a general medical condition, and acute stress disorder were not examined in the present study due to the fact that estimates of these disorders are rarely included in anxiety prevalence studies. Inclusion in the current meta-analysis also required the use of a structured diagnostic interview to diagnose ADs prospectively according to DSM or ICD criteria, a minimum age requirement of 16 years or older, and the use of a sample representative of the greater pregnant and postpartum population at large. Studies were not considered representative of the population at large if they focused on sub-populations of pregnant or postpartum women (e.g., still birth/infant loss, women with specific medical problems, infertility, substance abuse).
Studies were excluded for the following reasons: 1) failure to use a diagnostic interview, or full diagnostic criteria not assessed, 2) retrospective studies or chart reviews, 3) falling below the minimum age requirement, 4) postpartum studies beyond the first year, 5) qualitative studies/case report/case series, 6) review articles only, 7) non-representative samples (including sub-populations, intervention studies, women who were excluded on the basis of receiving treatment for a mental health condition [e.g., antidepressants], and studies that oversampled for high-risk women [e.g., women experiencing intimate partner violence]), 8) the same sample and measures reported in another source, 9) lifetime only rather than current prevalence assessed, 10) comorbid anxiety reported in a sample of women with diagnosed depression or who scored above a cut-off score for depression according to a self-report questionnaire, 11) prevalence was not assessed, 12) insufficient information was presented to compute prevalence estimates, 13) only incidence reported, and 14) only childbirth-related PTSD reported.
The current modelling approach required prevalence estimates for each individual disorder measured (see below). Therefore, studies that reported only the total prevalence of multiple ADs without the individual prevalence estimates were excluded, but only in instances when this information could not be obtained through correspondence with the authors. Studies that included samples of both pregnant and postpartum women53,54 were included if the samples were independent of one another. Specifically, for longitudinal studies where the same sample of women were prospectively followed from pregnancy to postpartum, the samples were not independent and therefore only one estimate was used from either pregnancy or postpartum. For the few studies that reported prevalence estimates across both pregnancy and postpartum in the same sample,55,56 we used the postpartum estimate as there were fewer postpartum studies compared to pregnancy studies. There were also a small number of studies where women were assessed at multiple time points throughout the postpartum period.56,57 As only one-time point could be used, we used data from the first diagnostic interview in the postpartum period, as this represented the time point that was closest to the median for the other postpartum studies (12 weeks).
Data Extraction
The first author screened each article by title and abstract, retrieving articles that met inclusion criteria. The third author independently screened one third of the articles from the electronic search. Disagreements over study inclusion were resolved through discussion between the first and second author. The first author extracted the following data from each article: author name, year of publication, sample size, group (pregnant, postpartum), total AD prevalence, the number of ADs assessed, the individual prevalence of each AD measured, structured diagnostic interview used (e.g., MINI, SCID, DIS), diagnostic criteria used (ICD-10, DSM-IV), country/region the study was conducted in, average gestational week or average postpartum week, average age, proportion married or cohabitating, proportion primiparous, average education of the sample, and medically-based exclusion criteria (e.g., severe medical problems in the mother, fetal malformation, pregnancy complications). The second and third author extracted the time frame of the diagnostic assessment (in weeks), including corresponding with authors to ascertain this information when needed. Finally, the World Bank Classifications for Income were used to classify each study country into low, lower-middle, upper-middle, and high-income categories.
Quality Ratings
To assess the quality of studies included in the current meta-analysis, the first author scored each study using a 10-point scale that was created based on key methodological criteria outlined in the literature.58–61 Key factors assessed included description of the study setting, eligibility criteria, sampling method, response rate, demographic characteristics, information on completers vs. non-completers, time frame of the assessment, qualifications of diagnostic interviewers, reporting of AD prevalence estimates, and discussion of study limitations/potential biases. Quality ratings were then reported categorically for each study, corresponding to low (0-3), moderate (4-6), or high (7-10) quality scores. The exact questions and scoring information can be viewed in eAppendix 1.
Data Analysis Approach
Past meta-analyses in this area applied a univariate approach to prevalence estimates reflecting different combinations of disorders. This risks underestimating the prevalence of having an AD. For this reason, our analyses employed a Bayesian multivariate model, discussed in detail elsewhere,51 to describe the data actually reported. This approach models the prevalence of each individual disorder, estimates the correlations amongst the disorders, uses the resulting information to produce an unbiased estimate of the probability of having one or more disorder in a typical sample, and also produces prediction intervals reflecting variation in the “true” underlying prevalence estimates across the distribution of included samples. To accomplish this, the correlations between the individual anxiety disorders (representing comorbidity) were estimated using all available individual patient data.45,46,55,62–65 By estimating the correlations amongst disorders, we were also able to provide a meta-analytic summary of the comorbidities between disorders. Finally, study-level prognostic factors were explored by allowing the prevalence of each disorder to depend on one predictor variable at a time.
Our modelling approach follows the procedure laid out in Fawcett et al.,51 including our model specification and priors. Parameters are reported as the posterior median along with a 95% Highest Density Interval (HDI)66 within which we are 95% certain the true parameter value lies after accounting for our model assumptions and prior knowledge. Predictors were examined individually, each in its own model; although it would be preferable to analyze all predictors concurrently, in a single model, this was not possible due to variation in the information reported across studies.
Results
Description of Studies
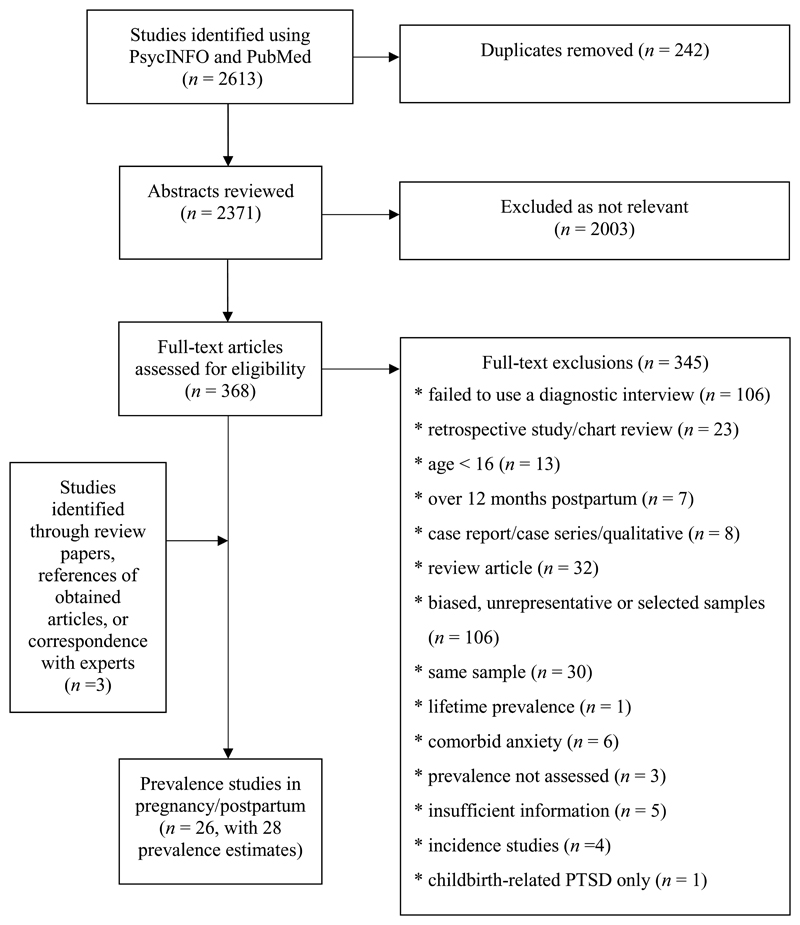
Of the 2,613 studies initially identified, 26 studies were included (see Figure 1), including a total of 28 prevalence estimates. The prevalence studies resulted in 19 estimates during pregnancy and 9 estimates during the postpartum period. Study characteristics are summarized in Table 1.
Figure 1.
Meta-analysis inclusion flowchart.
Table 1.
Study characteristics
| First Author | Year | Group | Measure | Criteria | Average Weeks Gesttaioni/Postpartum | Country | N | # Anxiety Disorders Assessed | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|
| Zar65 | 2002 | Preg. | ADIS-R | DSM-IV | 32 | Sweden | 453 | 7a | Moderate |
| Andersson67 | 2003 | Preg. | PRIME-MD | DSM-IV | 16 | Sweden | 1556 | 5 | Moderate |
| Sutter-Dallay29 | 2004 | Preg. | MINI | DSM-IV | 32 | France | 497 | 6 | Moderate |
| Felice68 | 2007 | Preg. | CIS-R | ICD-10 | 18.6 | Matla | 229 | 5 | High |
| Rogal69 | 2007 | Preg. | MINI | DSM-IV | - | USA | 1100 | 1 | Moderate |
| Uguz70 | 2007 | Preg. | SCID | DSM-IV | 35.08 | Turkey | 434 | 1 | High |
| Borri71 | 2008 | Preg. | SCID-I | DSM-IV | 13.5 | Italy | 1066 | 8b | Moderate |
| Guler72 | 2008 | Preg. | SCID | DSM-IV | 35.3 | Turkey | 512 | 1 | High |
| Mota54 | 2008 | Preg. | AUDADIS-IV | DSM-IV | - | USA | 451 | 4 | Moderate |
| Seng73 | 2009 | Preg. | NWS-PTSD | DSM-IV | 4 | USA | 1581 | 1 | Moderate |
| Chaudron46 | 2010 | Preg. | SCID | DSM-IV | 32.63 | USA | 24 | 5 | Moderate |
| Fisher53 | 2010 | Preg. | SCID | DSM-IV | 28+ | Viet Nam | 199 | 2 | Moderate |
| Uguz74 | 2010 | Preg. | SCID-I | DSM-IV | 23.26 | Turkey | 309 | 6 | Moderate |
| Matthey64c | 2011 | Preg. | MINI | DSM-IV | 13.4 | Australia | 171 | 6 | Moderate |
| Giardinelli75 | 2012 | Preg. | SCID-I | DSM-IV | 30 | Italy | 590 | 7 | Moderate |
| Fadzil62c | 2013 | Preg. | MINI | DSM-IV | 26.82 | Malaysia | 175 | 6 | Moderate |
| Kim76 | 2014 | Preg. | SCID | DSM-IV | - | USA | 745 | 1 | High |
| Marchesi77 | 2014 | Preg. | PRIME-MD | DSM-IV | - | Italy | 299 | 2 | High |
| Usuda45c | 2016 | Preg. | MINI | DSM-IV | 17.26 | Japan | 177 | 6 | High |
| Wenzel78 | 2003 | Post. | SCID | DSM-IVd | 8 | USA | 68 | 1 | Moderate |
| Wenzel79 | 2005 | Post. | SCID-NP | DSM-IVd | 8.7 | USA | 147 | 5e | High |
| Mota54 | 2008 | Post. | AUDADIS-IV | DSM-IV | - | USA | 1061 | 4 | Moderate |
| Kersting57 | 2009 | Post. | SCID | DSM-IV | 2 | Germany | 65 | 4 | Moderate |
| Fisher53 | 2010 | Post. | SCID | DSM-IV | 4-8 | Viet Nam | 165 | 2 | Moderate |
| Fisher63c | 2010 | Post. | CIDI | DSM-IVd | 27.6 | Australia | 196 | 5 | Moderate |
| Martini56 | 2013 | Post. | CIDI | DSM-IV | 8 | Germany | 281 | 7f | Moderate |
| Prenoveau80 | 2013 | Post. | SCID | DSM-IV | 12 | UK | 2202 | 1 | Moderate |
| Fairbrother55 | 2016 | Post. | SCID | DSM-IVd | 13 | Canada | 310 | 8 | High |
Eight disorders were assessed in this study, but extreme fear of childbirth was not included
Ten disorders were assessed in this study, but substance-induced anxiety disorder and anxiety disorder due to a general medical condition were excluded
These authors provided us with raw data. Prevalence estimates from Matthey and Ross-Hamid64 were calculated from raw data inclusive of 8 additional subjects beyond those reported in their article.
The 6-month duration criterion for generalized anxiety disorder was waived
Six disorders were assessed in this study, but PTSD was excluded as it was only childbirth related PTSD
Eight disorders were assessed, but Phobia Not Otherwise Specified was excluded
Abbreviations: CIS-R = Clinical Interview Schedule-Revised, ICD-10 = International Classification of Diseases, Tenth Revision, SCID-I = the Structured Clinical Interview for DSM-IV, ADIS-R = Anxiety Disorder Interview Schedule-Revised, MINI = Mini International Neuropsychiatric Interview, PRIME-MD = Primary Care Evaluation of Mental Disorders, AUDADIS-IV = Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV version, CIDI = Composite International Diagnostic Interview, SCID-NP = Nonpatient SCID, NWS-PTSD = the National Women's Study PTSD Module, DIS = Diagnostic Interview Schedule, Preg. = Pregnant, Post. = Postpartum.
Quality Ratings
Overall quality ratings ranged from 4 to 9 (M = 6.3, SD = 1.04). Of the 26 included studies, 18 (69.2%) were classified as moderate quality (scores between 4 and 6) and 8 (30.8%) were classified as high quality (scores between 7 and 10; see Table 1). There was no difference in mean quality ratings between pregnant (M = 6.42, SD = 0.69) and postpartum (M = 5.89, SD = 1.45) samples, t(26) = 1.33, p = .20. Furthermore, there was no statistically significant difference in prevalence rates between studies classified as moderate versus high quality for studies reporting a total any anxiety disorder prevalence, t(18) = 0.95, p = .35.
Of the 10 methodological criteria that were scored for each study, the following four were the least likely to be met overall: sampling method, confidence intervals, non-responders, and response rate. Specifically, of the 26 studies included, only 11.5% of studies used random samples, 19.2% reported confidence intervals or standard errors with anxiety prevalence estimates, 30.8% reported information about people who completed the study versus those who refused, and 50% reported an adequate response rate for their study (70% or higher). The biggest differences between moderate and high-quality studies were in regard to non-responders, confidence intervals, and setting of the study. Whereas information on non-responders was reported by 62.5% of high-quality studies, it was reported in only 16.6% of moderate quality studies. Confidence intervals were reported by 37.5% of high quality versus 11.1% of moderate quality studies, and the setting of the study was clearly described by 100% of high-quality studies versus 77.8% of moderate quality studies.
Individual Disorder Estimates
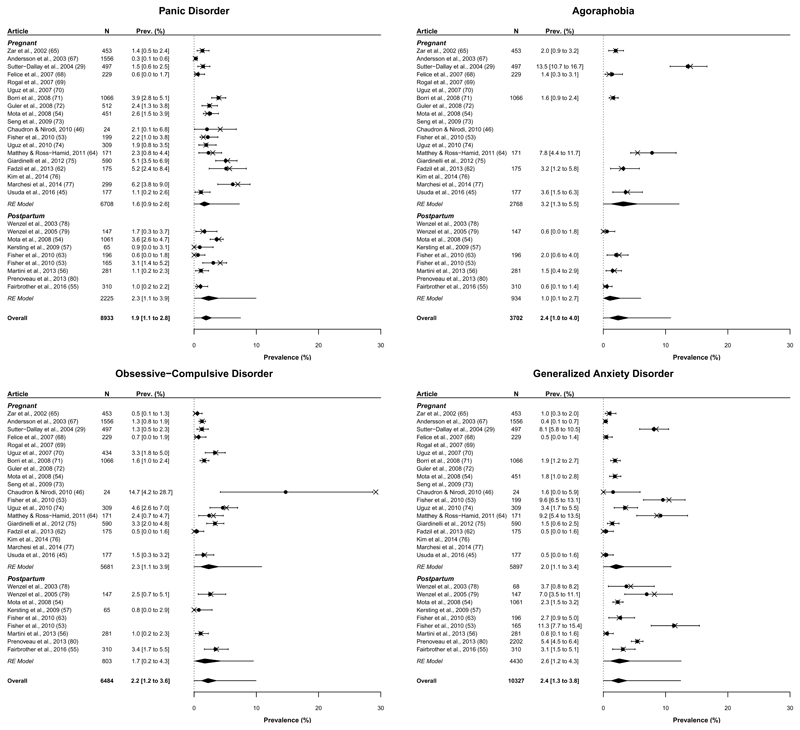
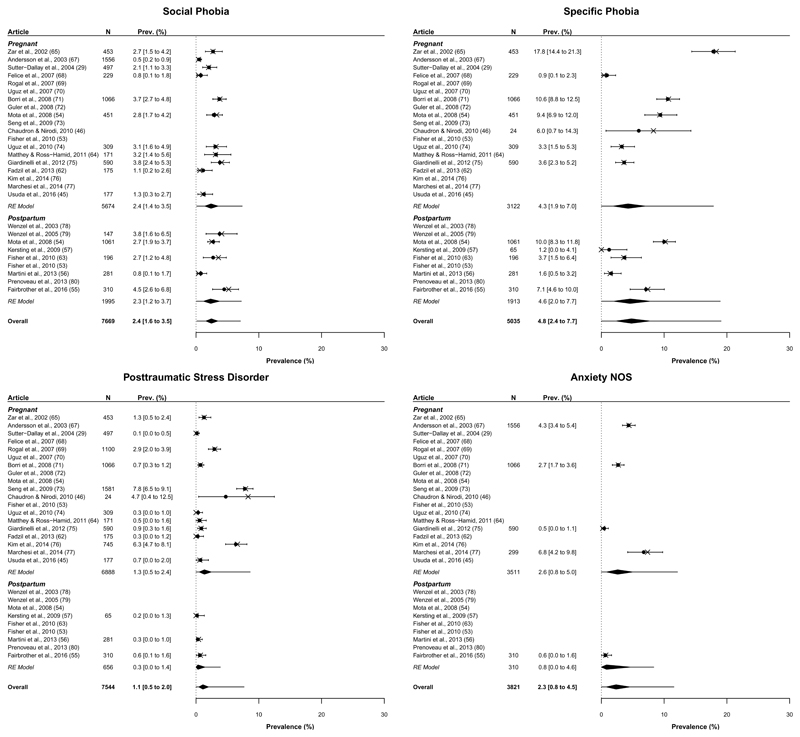
The individual prevalence estimates and prediction intervals for each disorder are presented in Figures 2 and 3; further details pertaining to the model parameters are available upon request. Estimates range from 1.1% for PTSD to 4.8% for Specific Phobia with the remaining estimates falling between 1.9% and 2.4%. Figures 2 and 3 also show that prevalence varies widely across studies – so estimates must be interpreted cautiously. This heterogeneity is captured by the prediction intervals, which represent the credible range of “true” prevalence estimates one might expect within a “new” population similar to those included in this analysis.
Figure 2.
Prevalence (%) of panic disorder, agoraphobia, obsessive-compulsive disorder and generalized anxiety disorder. X’s represent the observed prevalence estimate reported by the corresponding study. Circles and numerical prevalence values represent the shrunken estimates (with 95% highest density interval) for each study as estimated from the model including group (pregnant, postpartum) as a predictor; entries without a circle or numerical prevalence value reflect studies for which that disorder was not measured. Diamonds depict the aggregate estimate for each group and overall. Error bars surrounding each diamond represent the 95% prediction interval.
Figure 3.
Prevalence (%) of social phobia, specific phobia, posttraumatic stress disorder and anxiety not otherwise specified. X’s represent the observed prevalence estimate reported by the corresponding study. Circles and numerical prevalence values represent the shrunken estimates (with 95% highest density interval) for each study as estimated from the model including group (pregnant, postpartum) as a predictor; entries without a circle or numerical prevalence value reflect studies for which that disorder was not measured. Diamonds depict the aggregate estimate for each group and overall. Error bars surrounding each diamond represent the 95% prediction interval.
Overall Prevalence Estimate
The prevalence of having at least one AD during pregnancy or the postpartum period is estimated to be 20.7%, HDI95% [16.7% to 25.4%], with a trend towards greater prevalence in pregnancy versus the postpartum period (see Predictors section below). We attribute these estimates being higher than previous meta-analytic estimates to the fact that the present model correctly accounts for the number of disorders reported by each of the included studies whereas past studies have combined estimates based on varying combinations of disorders (for supporting simulations, see Fawcett et al.).51 * In addition, 1 in 20 (5.5%) women met criteria for at least two ADs. See Supplementary Table 1 (in eAppendix 1) for the probability of having 1+, 2+, 3+, or 4+ anxiety disorders and associated prediction intervals.
Predictors
Having established the presence of substantial heterogeneity in our prevalence estimates, we next sought to explore potential sources of that variability through predictors. Prior to analysis, several variables were found to be unsuitable as predictors due to minimal variation across studies (percent of the sample that was married or cohabitating, diagnostic criteria, country income) and were excluded from further consideration; we return to this issue in the discussion. Of the included predictors, the comparison between pregnant and postpartum samples was of particular theoretical interest and constituted our primary predictor analysis. Although there was a trend towards pregnant women being at greater risk, 21.1%, HDI95% [16.7% to 25.8%], than postpartum women, 18.0%, HDI95% [13.1% to 24.3%], the difference was small and failed to exclude 0 as a credible value, 3.1%, [-2.6% to 8.5%]. Even so, current evidence suggests that we are 86% certain that this difference is positive, providing preliminary – if tentative – support for the conclusion that pregnant women are at greater risk.*
Of the remaining predictors, region was the only other finding of note. Due to the sparsity of the disorders measured across different locations, our analysis of region compared North America to all other regions. There was a trend in this analysis suggesting a higher prevalence estimate for North American samples, 26.9%, HDI95% [18.1% to 37.5%], compared to other samples, 18.5%, HDI95% [14.6% to 23.1%], resulting in a difference of 8.4%, HDI95% [-0.7% to 18.8%]. This effect was driven by differences in the prevalence of OCD, 3.2%, HDI95% [-0.3% to 8.6%], Social Phobia, 1.5%, HDI95% [-0.8% to 4.5%], and PTSD, 2.4%, HDI95% [0.2% to 4.9%] between North America and elsewhere. Statistics pertaining to other predictors are available from the first author upon request, but none credibly predicted overall prevalence.
Comorbidity Analyses
Finally, we also estimated a comorbidity matrix reflecting the probability of having one anxiety disorder assuming a diagnosis of another anxiety disorder. These results are presented in Table 2. Rows reflect diagnoses that a given patient is assumed to have received, with the columns reflecting the probability of also having additional diagnoses. For example, someone with a diagnosis of panic disorder has a 1 in 6 chance of also having generalized anxiety disorder in a typical sample. Diagonal values reflect the probability of having any other disorder. The highest comorbidity was found for panic disorder (60.7% chance of having another diagnosis) and the lowest comorbidity was found for anxiety NOS (25.5% chance of having another diagnosis). Overall, the probability of having an additional AD given an initial diagnosis was quite high, at approximately 50% in most cases.
Table 2.
Estimated comorbidity (%) amongst disorders. Off-diagonal values reflect the probability of having the disorder listed in the column given the diagnosis in the row (e.g., if a patient has panic disorder the probability of also having OCD is 15.2%). Diagonal values (shaded) reflect instead the probability of having any other disorder given the diagnosis in the row (e.g., if a patient has panic disorder the probability of having at least one other disorder is 60.7%). 95% highest density intervals (HDI) are provided in rounded brackets and 95% prediction intervals are provided in square brackets.
| Potential Disorder | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Panic | Agora. | OCD | GAD | Soc. Phobia | Sp. Phobia | PTSD | Anxiety NOS |
| Panic | 60.7 (43.2 to 76.1) [34.6 to 95.3] |
22.4 (9.6 to 38.3) [0.7 to 63.9] |
15.2 (4.3 to 29.7) [0.0 to 48.5] |
15.9 (5.1 to 29.8) [0.0 to 50.9] |
10.6 (3.0 to 21.4) [0.3 to 28.8] |
24.1 (8.6 to 42.9) [0.0 to 60.5] |
6.5 (0.7 to 15.8) [0.0 to 29.1] |
3.2 (0.0 to 18.1) [0.0 to 25.1] |
| Agoraphobia | 16.9 (8.2 to 28.1) [0.7 to 53.4] |
51.5 (36.9 to 66.4) [27.9 to 93.4] |
12.5 (3.6 to 24.0) [0.0 to 46.6] |
14.6 (5.7 to 26.0) [0.0 to 43.6] |
14.8 (6.3 to 25.1) [1.4 to 46.4] |
10.9 (2.7 to 22.6) [0.0 to 37.1] |
6.2 (1.0 to 14.6) [0.0 to 34.5] |
5.1 (0.0 to 21.2) [0.0 to 29.4] |
| OCD | 12.5 (4.1 to 24.3) [0.2 to 41.6] |
13.5 (3.6 to 27.2) [0.0 to 49.4] |
50.3 (34.7 to 66.0) [52.2 to 87.2] |
15.7 (5.1 to 28.6) [0.0 to 46.3] |
11.6 (3.4 to 22.1) [0.6 to 30.7] |
15.7 (4.6 to 29.9) [0.0 to 48.9] |
7.2 (0.8 to 16.6) [0.0 to 27.3] |
3.0 (0.0 to 15.1) [0.0 to 24.6] |
| GAD | 10.9 (3.1 to 20.7) [0.0 to 39.1] |
13.3 (4.4 to 24.7) [0.0 to 41.2] |
13.3 (4.1 to 25.4) [0.0 to 42.4] |
54.5 (39.6 to 69.5) [30.8 to 90.6] |
14.6 (6.5 to 24.8) [1.9 to 35.7] |
23.4 (9.7 to 40.4) [0.4 to 64.0] |
5.6 (0.6 to 14.0) [0.0 to 28.1] |
3.5 (0.0 to 15.1) [0.0 to 22.7] |
| Soc. Phobia | 9.4 (2.5 to 19.0) [0.0 to 25.9] |
17.3 (7.2 to 30.5) [0.1 to 51.4] |
12.8 (3.7 to 24.5) [0.0 to 34.2] |
18.9 (8.4 to 31.4) [0.0 to 43.8] |
56.9 (42.4 to 71.2) [30.6 to 87.7] |
18.2 (8.1 to 30.9) [0.0 to 42.1] |
5.5 (0.5 to 13.8) [0.0 to 22.3] |
9.0 (0.3 to 25.4) [0.0 to 41.1] |
| Sp. Phobia | 9.6 (3.3 to 17.1) [0.1 to 29.6] |
5.7 (1.3 to 12.2) [0.0 to 22.1] |
7.6 (2.4 to 15.3) [0.0 to 27.2] |
13.5 (6.3 to 22.8) [0.0 to 44.1] |
8.2 (3.7 to 13.8) [0.7 to 19.0] |
38.7 (27.7 to 50.8) [15.6 to 74.0] |
8.9 (2.9 to 16.8) [0.0 to 34.7] |
2.8 (0.0 to 11.2) [0.0 to 15.4] |
| PTSD | 8.4 (0.9 to 20.5) [0.0 to 36.9] |
10.6 (1.5 to 25.4) [0.0 to 51.9] |
11.4 (1.3 to 26.4) [0.0 to 40.1] |
10.6 (1.2 to 24.7) [0.0 to 46.6] |
7.9 (0.9 to 19.8) [0.0 to 29.7] |
29.0 (10.7 to 51.0) [0.9 to 76.1] |
53.9 (33.6 to 74.4) [27.9 to 96.1] |
3.1 (0.0 to 17.5) [0.0 to 26.6] |
| Anxiety NOS | 2.4 (0.0 to 13.0) [0.0 to 19.5] |
5.1 (0.0 to 20.4) [0.0 to 29.2] |
2.8 (0.0 to 13.6) [0.0 to 22.0] |
3.8 (0.0 to 15.2) [0.0 to 24.0] |
7.5 (0.1 to 20.9) [0.0 to 35.8] |
5.2 (0.0 to 20.0) [0.0 to 27.8] |
1.8 (0.0 to 10.1) [0.0 to 15.2] |
25.5 (5.9 to 50.3) [1.4 to 66.2] |
Discussion
The current study is the first meta-analysis to estimate the probability of having at least 1 of 8 common ADs across pregnancy and the postpartum period while correctly accounting for variation in the disorders reported by the individual estimates. Results suggest that ADs are more prevalent in these populations than previously thought, with approximately 1 in 5 (20.7%) women meeting diagnostic criteria for at least one AD and 1 in 20 (5.5%) women meeting criteria for at least two ADs. These estimates are based on studies that employed structured diagnostic interviews and are representative of community samples. Although the overall prevalence rate was 20.7% for at least one AD, the prediction interval ranged from 7.5% to 38.8% – reflecting a high degree of variation across populations. One major goal moving forward in this area should be identifying sources of heterogeneity.
The current prevalence estimate for having at least one AD during pregnancy or the postpartum period (20.7%) is 1.5 to 2.5 times larger than similar meta-analytic estimates for pregnant or postpartum women.47,48 The prevalence rate found in the current study is consistent with 12-month prevalence rates found for ADs in national samples (18.1%)2 – or moderator based univariate meta-analytic estimates51 – but is considerably higher than Goodman et al.’s 47 estimate of 8.5% in postpartum samples or Dennis et al.’s 48 estimate of 15.2% and 9.9% in pregnant or postpartum samples, respectively. The “any anxiety disorder” estimates from previous meta-analyses were based on 6 postpartum estimates,47 or 9 prenatal and 9 postnatal assessments,48 whereas 28 prevalence estimates across pregnancy and the postpartum period contributed to the current prevalence estimate.
Our study also contributes significantly to the estimation of the prevalence of individual ADs. Whereas Dennis et al.48 only report the prevalence rate for one individual disorder (GAD), the prevalence rates for some of the disorders in Goodman et al.’s47 study were based on the availability of only two estimates (e.g., agoraphobia, specific phobia, and anxiety disorder NOS). Thus, the current study includes significantly more individual AD estimates, ranging from 5 estimates (anxiety NOS) to 22 estimates (panic disorder). Knowing which individual ADs are most prevalent also informs clinicians and researchers where help is most needed. In the only other meta-analysis to provide prevalence estimates for the individual ADs, Goodman et al.47 found that the most common disorders in the postpartum period were GAD, OCD, and panic disorder. In comparison, the current study found specific phobia, GAD, and social phobia to be the most prevalent perinatal disorders.
When examining potential predictors of anxiety disorder prevalence, tentative support was found for the conclusion that pregnant women are at greater risk than postpartum women. Although this is consistent with a previous meta-analysis,48 the current estimate shows a smaller disparity between the two prevalence rates (3.1% versus 5.3%) meaning that postpartum rates may be higher than previously expected. Of the remaining predictors we examined, a trend was found for region suggesting higher anxiety prevalence for North American samples versus elsewhere. This is consistent with a systematic review and meta-regression of the global prevalence of anxiety disorders,81 where the risk for anxiety was found to be 20-50% lower in all cultures compared with Euro/Anglo cultures. The fact that no other predictors were credible is perhaps unsurprising given the fact that our prevalence estimate was derived from individual disorder estimates based – at times – on few studies and within which we observed considerable heterogeneity. Further data are required before strong conclusions may be drawn concerning predictors in this literature.
Strengths and Limitations
One strength of the current analysis is that our overall prevalence estimates account for variation amongst the individual ADs. Previous attempts have simply combined studies reporting total AD estimates, despite variation in the ADs composing these estimates. However, the current modelling approach is therefore dependent on individual AD estimates, which are not always reported in the context of the same published sample (e.g., due to practical, financial or other considerations). For instance, only two of the studies included in the meta-analysis reported all 8 ADs.55,71 Whereas panic disorder was measured most consistently across studies, agoraphobia and anxiety NOS were the two disorders measured most infrequently. Perhaps part of the reason that anxiety NOS is measured infrequently is the variability in how it is defined across studies. Future studies can help reduce heterogeneity by clearly defining the diagnostic categories and measuring as many disorders as possible.
Our study was also limited by its need for individual patient data. Although few studies currently report individual patient data, several authors include tables describing each patient and their assigned diagnoses, which is sufficient to recreate the data.46,65 As a note to the field, if more researchers presented data tables such as those highlighted above, it would allow for more complex statistical models and as a result, more meaningful conclusions.
The ability of our statistical approach to estimate comorbidity across disorders is also a unique strength. To our knowledge this is the first study to meta-analytically aggregate comorbidity across ADs in pregnancy and the postpartum period. Our estimates suggest that if a pregnant or postpartum woman is diagnosed with an AD, there is an approximate 50% chance she will be diagnosed with an additional AD. Our estimate is consistent with the literature outside of the perinatal period, including an adolescent community sample where 41% of participants had more than one AD,82 or a clinical sample where 43% of patients had at least one additional AD diagnosis.83 Furthermore, our model allows clinicians to identify which disorders are most comorbid. For instance, our findings from Table 2 predict that the highest comorbidities amongst disorders are between PTSD, panic disorder, and GAD with specific phobia. Consistent with these findings, Brown et al.83 found that the diagnoses associated with the highest risk of comorbid ADs were GAD and panic disorder with agoraphobia. Given the estimate that 5.5% of pregnant or postpartum women meet criteria for more than one AD, screening for multiple disorders and differential diagnosis is essential.
Several factors impeded our ability to identify sources of heterogeneity across AD prevalence studies in pregnancy and the postpartum period. For one, we suspect our predictor analyses were underpowered because many studies did not report the information necessary to permit inclusion in a given model. Standardized reporting of basic demographic information such as age, parity, income and education would increase the power of such analyses and allow researchers to identify factors that explain substantial variability in prevalence estimates. When studies do report such information, it is often done so inconsistently. For instance, variation in how education level was reported across studies made it difficult to merge these categories. Similarly, ethnicity was not coded as a predictor because ethnic composition was rarely reported, with only 3 studies (11.5%) reporting the percentage of the sample that identified as African-American. One solution is for researchers to include additional demographic and study design information in an appendix or online supplement, or to be more responsive through email to requests for additional study details. Full reporting of demographic variables would allow future meta-analyses the power to better examine demographic risk factors.
Clinical Implications
Given the attention to screening for depression during pregnancy and the postpartum period over the last decade, it is important that screening for anxiety disorders also take place, which is intuitive based on the well-established comorbidity of the two types of disorders as well as the data presented in this systematic review. Depression and anxiety are thought to share a common diathesis, and when considering lifetime diagnoses, comorbidity rates are as high as 76%.84 With the high prevalence for ADs in our model, and the prediction interval extending as high as 39%, our data corroborate the strong need and call for routine prenatal and postnatal anxiety screening in healthcare settings.35 Although women are increasingly screened for postpartum depression using measures such as the Edinburgh Postnatal Depression Scale (EPDS),85 ADs receive significantly less clinical focus and media attention. However, research suggests that anxiety is likely more prevalent than depression during pregnancy and the postpartum period.79 For example, Lee and colleagues86 found that anxiety was more prevalent than depression in antenatal assessments (54% versus 37% respectively). Likewise, Reck et al.87 used DSM-IV criteria to examine over 1,000 postpartum women across a three-month period and found that ADs were more common than depressive disorders (11.1% versus 6.1%, respectively).
In line with these observations, the American College of Obstetricians and Gynecologists (ACOG) recommend that clinicians screen patients for both depression and anxiety symptoms at least once during the perinatal period with a standardized, validated tool.88 Although there are few anxiety screening measures validated within perinatal populations, the Perinatal Anxiety Screening Scale (PASS)89 was recently developed and uses a cut-off score to identify women at risk for problematic anxiety, and the three-item Anxiety Subscale of the EPDS (known as the EPDS-3A) is also validated for anxiety screening in this population.90 Research measuring the prevalence of PrA is also becoming more common, including a recent revision of the Pregnancy Related Anxieties Questionnaire (PRAQ-R).91
Before perinatal anxiety screening programs can be implemented universally, proper mental health education and training is required for health care professionals. The unique features of anxiety and related disorders when they present during pregnancy and the postpartum are not well known and may prevent accurate and timely diagnosis. Health care providers who work with pregnant and postpartum women would benefit from education regarding the special features of perinatal anxiety disorders (e.g., obsessions of infant-related harm in obsessive compulsive disorder and women’s motivation to conceal the occurrence of this ideation),92 as well as the symptom overlap with normal postpartum experiences (e.g., fatigue, difficulty sleeping). Further, the fact that these conditions are more common than depression and may not be disclosed by women unless asked, should also be taught to health care professionals who care for this vulnerable population. Staff who administer screening measures should have specific training on how to use these tools and identify women at risk (e.g., through use of validated cut-off scores). The development of a consistent response protocol is needed to facilitate appropriate consultation and treatment referrals when needed, including emergency referrals due to psychosis, and suicidal or homicidal ideation.35,93 Maternal health education is also a requirement of an effective mental health program. For instance, it is important that mothers are provided with sufficient and effective education about the meaning of screening results, including understanding the difference between normal levels of anxiety, being “at-risk” for an anxiety disorder, and receiving a formal psychiatric diagnosis from a licensed professional.35,93
Given the significant role that ADs play in women’s perinatal mental health and the potential for adverse outcomes for both mothers and infants, evidence-based treatment for perinatal anxiety disorders is essential. However, research evaluating potential evidence-based treatments for perinatal anxiety is limited, with systematic reviews identifying a reliance on case reports and case series (83%),94 with only 5 studies identified in which psychological interventions in the perinatal period are evaluated.95 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), the medications most commonly used to treat anxiety disorders,96 are also the classes of medication which appear to be safest in pregnancy and the postpartum.97,98 That said, there are a number of safety concerns related to their use in pregnant and breastfeeding women.98–100 For instance, Furu and colleagues101 found a 30% increase in the prevalence of cardiovascular defects after maternal exposure to paroxetine or fluoxetine. According to a recent systematic review, sertraline and paroxetine showed the best neonatal safety profile of all SSRIs/SNRIs examined during breastfeeding and are recommended as the first line choice for antidepressants in nursing women,102 whereas fluoxetine shows greater transfer into human milk.103 Aside from the safety concerns, and the fact that some women may be unable or prefer not to take medications during pregnancy or breastfeeding (hence, require an alternative to pharmacotherapy), perinatal women have been found to largely prefer non-pharmacological approaches to the treatment of AD.104
In a recent systematic review, cognitive-behavioural therapy (CBT) is recommended as a first line treatment for pregnant and breastfeeding women with anxiety disorders,94 with no known contraindications of CBT in pregnancy.105,106 Outside of the perinatal period CBT has also been shown to be the first line psychological treatment for anxiety disorders.107–109 Further, randomized controlled trials comparing pharmacological and psychological interventions for ADs indicate that CBT is both safe and generally equal or superior to pharmacological approaches.105–109 Because CBT is time-consuming and expensive, it has not been broadly publicly funded. Yet access to CBT is critical for pregnant and postpartum women due to the potential negative effects of AD medication on the developing fetus and nursing infant.101,103 CBT is increasingly being offered in online settings and is therefore becoming more readily available to both remote and low-income populations. Self-administered, online CBT delivered with therapist support (as little as 15-minutes of therapist support a week), has been shown to be as equally effective as face to face treatment.110 Now that impact can be maximized via these more accessible options, the value of providing effective and accurate perinatal AD screening dramatically increases.
Future Research
Future research should examine whether ADs are more common in pregnant and postpartum women compared to the general population. For instance, pregnant and postpartum women were found to be 1.5 to 2 times more likely to experience OCD compared to the general population,111 suggesting that pregnancy and the postpartum period may be an especially vulnerable period for the development of OCD. Intrusive thoughts surrounding accidental or intentional harm to the fetus or infant are common in the clinical presentation of perinatal OCD,92 but should be distinguished from postpartum psychosis. For instance, a case study describes a woman named Sara who avoided bathing and being alone with her son due to obsessive thoughts and images of drowning him.112 In perinatal OCD aggressive thoughts are ego-dystonic and are perceived as extremely distressing to the mother. Whereas women with OCD are not at increased risk of harming their infants, immediate intervention is critical for women with postpartum psychosis as judgment and reality testing are impaired.113 In one sample of women with postpartum psychosis, 35% were admitted to hospital with safety concerns related to severe behavioural disturbance, acting on delusions, or incorrect handling of the infant.114 Although postpartum psychosis is rare, women with a personal or family history of bipolar disorder are at increased risk as it often is conceptualized as an episode of bipolar disorder with psychotic features.115
Outside of the research on OCD, there are currently no other AD for which there is evidence of an increased risk of onset and/or exacerbation in the perinatal period. Large scale studies including both a sample of pregnant and/or postpartum women and a matched comparison group of women in the general population are severely lacking, and would help to more fully determine whether the period surrounding childbirth is a risk factor for the development or exacerbation of ADs.
The majority of studies included in the current meta-analysis were classified as moderate quality. Several recommendations are shared in the hopes of encouraging higher quality studies with perinatal populations. For instance, research in this area would benefit from less reliance on convenience or consecutive sampling and greater use of random sampling. Confidence intervals or standard errors should be presented with anxiety prevalence estimates in order to measure precision of the estimate, as an imprecise point prevalence estimate is not a good representation of the true prevalence value. Researchers should also try to include information about participants who completed the study versus non-completers and explore whether they differ in any meaningful way. Finally, with only half of the current studies reporting an adequate response rate (70% or higher), this should be a target for increasing study quality in perinatal samples. With these considerations in mind, future meta-analyses will be better poised to use a standardized quality rating system (e.g., The Newcastle-Ottawa Scale),116 which can be challenging without uniformity in research designs.
In conclusion, the current meta-analysis finds that ADs in pregnancy and the postpartum period are more prevalent than previously thought (1 in 5 women). There was substantial between-study heterogeneity suggesting that the “true” prevalence rate varies broadly across samples. Large-scale longitudinal studies are needed including the following: multiple AD measurement, sufficient detail reported to recreate the data, and enough demographic and methodological information to readily access potential moderating variables. Further work is needed to determine which variables are contributing heterogeneity to the AD estimates. Given the personal and economic burden of both full and subthreshold ADs, as well as potential short and long-term consequences for child development, proper screening and treatment of antenatal and postnatal ADs is crucial. It is time that perinatal distress no longer be synonymous only with depression.
Supplementary Material
Clinical Points.
This is the first meta-analysis to correctly estimate the probability of having at least 1 of 8 common anxiety disorders across pregnancy and the postpartum period, with previous research combining prevalence estimates that were incompatible due to differences in the number of individual anxiety disorders assessed.
Anxiety disorders in pregnancy and the postpartum period are more prevalent than previously thought, with 1 in 5 (20.7%) women meeting diagnostic criteria for at least one anxiety disorder and 1 in 20 (5.5%) meeting criteria for at least two anxiety disorders.
Given the attention to screening for depression during pregnancy and the postpartum period over the last decade, it is now time to spotlight the strong need for routine perinatal anxiety disorder screening.
Funding/support
This research received no direct financial support from any granting agency. Ian White is supported by the Medical Research Council Unit Programme MC_UU_12023/21.
Footnotes
In support of this assertion, a supplementary meta-analysis was conducted using the univariate approach described by Goodman et al.47 or Dennis et al.48 applied to the any disorder prevalence estimates reported by the available samples. This model produced an estimate of 9.9%, CI95% [7.3% to 13.4%]. While more comparable to the prevalence estimate reported by those authors, it is our view that this underestimates the true prevalence of peripartum ADs. To further support this claim, we re-conducted the same analysis including only the samples for which at least 4 disorders were measured. If we are correct that aggregating studies that differ in respect to the disorders measured, this new model should produce a higher estimate. Supporting our hypothesis, this model produced an estimate of 14.1%, CI95% [10.8% to 18.2%], reflecting the fact that studies measuring more disorders tend to produce higher estimates. Finally, we refit the model to the original sample of estimates, including the number of disorders measured as a moderator – and predicting the prevalence of a hypothetical study in which all 8 disorders were measured. This produced an estimate of 20.6%, CI95% [14.1% to 29.0%], which is closer to our own – only with broader confidence intervals. Simulations conducted by Fawcett et al.,51 predict precisely this pattern of results. They further demonstrated that of these univariate models only the moderator approach produces an unbiased estimate, and even then, the estimate produced by that model is more variable and less efficient than the one reported in-text. Overall, these models support the notion that previous meta-analyses have underestimated the prevalence of peripartum ADs.
Prevalence estimates for individual disorders broken down by peripartum group are provided in Figures 2 and 3. No single disorder prevalence varied credibly between groups with differences of -0.7%, HDI95% [-2.0% to 0.4%], 2.1%, HDI95% [-0.5% to 4.9%], 0.6%, HDI95% [-2.4% to 3.1%], -0.5%, HDI95% [-1.9% to 0.6%], 0.1%, HDI95% [-1.3% to 1.3%], -0.3%, HDI95% [-2.2% to 1.5%], 1.0%, HDI95% [-0.6% to 2.4%], and 1.6%, HDI95% [-2.7% to 5.1%] for panic, agoraphobia, OCD, GAD, social phobia, specific phobia, PTSD and anxiety NOS, respectively. As detailed by these figures, there was a trend towards greater disorder prevalence in pregnant than postpartum populations across 5 of the 8 disorders – contributing to the apparent difference reported.
Potential conflicts of interest: The authors report no potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fifer SK, Mathias SD, Patrick DL, et al. Untreated anxiety among adult primary care patients in a health maintenance organization. Arch Gen Psychiatry. 1994;51(9):740–750. doi: 10.1001/archpsyc.1994.03950090072010. [DOI] [PubMed] [Google Scholar]
- 4.Demers M. Frequent users of ambulatory health care in Quebec: the case of doctor-shoppers. CMAJ. 1995;153(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier L, Lesage AD, Toupin J, et al. Telephone surveys as an alternative for estimating prevalence of mental disorders and service utilization: a Montreal catchment area study. Can J Psychiatry. 1997;42(7):737–743. doi: 10.1177/070674379704200706. [DOI] [PubMed] [Google Scholar]
- 6.McCusker J, Boulenger JP, Boyer R, et al. Use of health services for anxiety disorders: a multisite study in Quebec. Can J Psychiatry. 1997;42(7):730–736. doi: 10.1177/070674379704200705. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM, Shapiro CM, Kennedy SH. Differentiating DSM-IV anxiety and depressive disorders in the general population: comorbidity and treatment consequences. Can J Psychiatry. 2000;45(2):166–172. doi: 10.1177/070674370004500207. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60(7):427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 9.Leon AC, Portera L, Weissman MM. The social costs of anxiety disorders. Br J Psychiatry. 1995;166(7):19–22. [PubMed] [Google Scholar]
- 10.Somers JM, Goldner EM, Waraich P, et al. Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry. 2006;51(2):100–113. doi: 10.1177/070674370605100206. [DOI] [PubMed] [Google Scholar]
- 11.Huizink AC, Mulder EJH, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull. 2004;130(1):115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- 12.Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev. 2002;26(4):457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 13.Mulder EJH, Robles de Medina PG, Huizink AC, et al. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;70(1–2):3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- 14.Schneider ML, Moore CF, Kraemer GW, et al. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27(1–2):285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 15.Wadhwa PD, Glynn L, Hobel CJ, et al. Behavioral perinatology: biobehavioral processes in human fetal development. Regul Pept. 2002;108(2–3):149–157. doi: 10.1016/s0167-0115(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 16.Qiu C, Williams MA, Calderon-Margalit R, et al. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am J Hypertens. 2009;22(4):397–402. doi: 10.1038/ajh.2008.366. [DOI] [PubMed] [Google Scholar]
- 17.Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95(4):487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- 18.Ding XX, Wu YL, Xu SJ, et al. Maternal anxiety during pregnancy and adverse birth outcomes: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2014;159:103–110. doi: 10.1016/j.jad.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Field T, Hernandez-Reif M, Vera Y, et al. Anxiety and anger effects on depressed mother-infant spontaneous and imitative interactions. Infant Behavior & Development. 2005;28(1):1–9. [Google Scholar]
- 20.Coplan RJ, O'Neil K, Arbeau KA. Maternal anxiety during and after pregnancy and infant temperament at three months of age. J Prenat Perinat Psychol Health. 2005;19(3):199–215. [Google Scholar]
- 21.Davis E, Snidman N, Wadhwa P, et al. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6(3):319–331. [Google Scholar]
- 22.Manassis K, Bradley S, Goldberg S, et al. Behavioral-inhibition, attachment and anxiety in children in mothers with anxiety disorders. Can J Psychiatry. 1995;40(2):87–92. doi: 10.1177/070674379504000206. [DOI] [PubMed] [Google Scholar]
- 23.Schreier A, Wittchen HU, Höfler M, et al. Anxiety disorders in mothers and their children: prospective longitudinal community study. Br J Psychiatry. 2008;192(4):308–309. doi: 10.1192/bjp.bp.106.033589. 2008. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor TG, Heron J, Glover V, et al. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1470–1477. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor TG, Heron J, Golding J, et al. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44(7):1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- 26.Mennes M, Stiers P, Lagae L, et al. Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci Biobehav Rev. 2006;30(8):1078–1086. doi: 10.1016/j.neubiorev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Matthey S, Barnett B, Howie P, et al. Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? J Affect Disord. 2003;74(2):139–147. doi: 10.1016/s0165-0327(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 28.Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, et al. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. Eur Psychiatry. 2004;19(8):459–463. doi: 10.1016/j.eurpsy.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson AM, Benzies KM, Cairns SL, et al. Who is distressed? A comparison of psychosocial stress in pregnancy across seven ethnicities. BMC Pregnancy Childbirth. 2016;16(1):215. doi: 10.1186/s12884-016-1015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furtado M, Chow CH, Owais S, et al. Risk factors of new onset anxiety and anxiety exacerbation in the perinatal period: a systematic review and meta-analysis. J Affect Disord. 2018;238:626–635. doi: 10.1016/j.jad.2018.05.073. [DOI] [PubMed] [Google Scholar]
- 33.Leach LS, Poyser C, Fairweather-Schmidt K. Maternal perinatal anxiety: a review of prevalence and correlates. Clinical Psychologist. 2017;21(1):4–19. [Google Scholar]
- 34.Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62–77. doi: 10.1016/j.jad.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Accortt EE, Wong MS. It is time for routine screening for perinatal mood and anxiety disorders in obstetrics and gynecology settings. Obstet Gynecol Surv. 2017;72(9):553–568. doi: 10.1097/OGX.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 36.American psychiatric association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 37.American psychiatric association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 38.American psychiatric association. Highlights of changes from DSM-IV-TR to DSM-5. 2013 http://dx.doi.org/10.1176/appi.books.9780890425596.changes.
- 39.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 40.Guardino CM, Dunkel Schetter C. Understanding pregnancy anxiety concepts, correlates, and consequences. Zero to Three. 2014;34(4):12–21. [Google Scholar]
- 41.Blackmore ER, Gustafsson H, Gilchrist M, et al. Pregnancy-related anxiety: evidence of distinct clinical significance from a prospective longitudinal study. J Affect Disord. 2016;197:251–258. doi: 10.1016/j.jad.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roesch SC, Schetter CD, Woo G, et al. Modeling the types and timing of stress in pregnancy. Anxiety Stress Coping. 2004;17(1):87–102. [Google Scholar]
- 43.Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry. 2006;67(8):1285–1298. doi: 10.4088/jcp.v67n0818. [DOI] [PubMed] [Google Scholar]
- 44.Verreault N, Da Costa D, Marchand A, et al. PTSD following childbirth: a prospective study of incidence and risk factors in Canadian women. J Psychosom Res. 2012;73(4):257–263. doi: 10.1016/j.jpsychores.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Usuda K, Nishi D, Makino M, et al. Prevalence and related factors of common mental disorders during pregnancy in Japan: a cross-sectional study. Biopsychosoc Med. 2016;10(1):17. doi: 10.1186/s13030-016-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudron LH, Nirodi N. The obsessive–compulsive spectrum in the perinatal period: a prospective pilot study. Arch Womens Ment Health. 2010;13(5):403–410. doi: 10.1007/s00737-010-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman JH, Watson GR, Stubbs B. Anxiety disorders in postpartum women: a systematic review and meta-analysis. J Affect Disord. 2016;203:292–331. doi: 10.1016/j.jad.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- 49.Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395–401. doi: 10.1007/s00737-010-0153-7. [DOI] [PubMed] [Google Scholar]
- 50.Navarro P, García-Esteve L, Ascaso C, et al. Non-psychotic psychiatric disorders after childbirth: prevalence and comorbidity in a community sample. J Affect Disord. 2008;109(1):171–176. doi: 10.1016/j.jad.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Fawcett JM, Fairbrother N, Fawcett EJ, et al. A Bayesian multivariate approach to estimating the prevalence of a superordinate category of disorders. Int J Methods Psychiatr Res. 2018;27(4):e1742. doi: 10.1002/mpr.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher J, Tran T, Kriitmaa K, et al. Common perinatal mental disorders in northern Viet Nam: community prevalence and health care use. Bull World Health Organ. 2010;88(10):737–745. doi: 10.2471/BLT.09.067066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mota N, Cox BJ, Enns MW, et al. The relationship between mental disorders, quality of life, and pregnancy: findings from a nationally representative sample. J Affect Disord. 2008;109(3):300–304. doi: 10.1016/j.jad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148–155. doi: 10.1016/j.jad.2015.12.082. [DOI] [PubMed] [Google Scholar]
- 56.Martini J, Wittich J, Petzoldt J, et al. Maternal anxiety disorders prior to conception, psychopathology during pregnancy and early infants’ development: a prospective-longitudinal study. Arch Womens Ment Health. 2013;16(6):549–560. doi: 10.1007/s00737-013-0376-5. [DOI] [PubMed] [Google Scholar]
- 57.Kersting A, Kroker K, Steinhard J, et al. Psychological impact on women after second and third trimester termination of pregnancy due to fetal anomalies versus women after preterm birth—a 14-month follow up study. Arch Womens Ment Health. 2009;12(4):193–201. doi: 10.1007/s00737-009-0063-8. [DOI] [PubMed] [Google Scholar]
- 58.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giannakopoulos NN, Rammelsberg P, Eberhard L, et al. A new instrument for assessing the quality of studies on prevalence. Clin Oral Investig. 2012;16(3):781–788. doi: 10.1007/s00784-011-0557-4. [DOI] [PubMed] [Google Scholar]
- 60.Ross LE, Grigoriadis S, Mamisashvili L, et al. Quality assessment of observational studies in psychiatry: an example from perinatal psychiatric research. Int J Methods Psychiatr Res. 2011;20(4):224–234. doi: 10.1002/mpr.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Fadzil A, Balakrishnan K, Razali R, et al. Risk factors for depression and anxiety among pregnant women in Hospital Tuanku Bainun, Ipoh, Malaysia. Asia Pac Psychiatry. 2013;5(S1):7–13. doi: 10.1111/appy.12036. [DOI] [PubMed] [Google Scholar]
- 63.Fisher J, Wynter KH, Rowe HJ. Innovative psycho-educational program to prevent common postpartum mental disorders in primiparous women: a before and after controlled study. BMC Public Health. 2010;10(1):432. doi: 10.1186/1471-2458-10-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matthey S, Ross-Hamid C. The validity of DSM symptoms for depression and anxiety disorders during pregnancy. J Affect Disord. 2011;133(3):546–552. doi: 10.1016/j.jad.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Zar M, Wijma K, Wijma B. Relations between anxiety disorders and fear of childbirth during late pregnancy. Clin Psychol Psychother. 2002;9(2):122–130. [Google Scholar]
- 66.Kruschke J. Doing Bayesian data analysis: A tutorial with R, JAGS, and Stan. Academic Press; 2014. [Google Scholar]
- 67.Andersson L, Sundström-Poromaa I, Bixo M, et al. Point prevalence of psychiatric disorders during the second trimester of pregnancy: a population-based study. Am J Obstet Gynecol. 2003;189(1):148–154. doi: 10.1067/mob.2003.336. [DOI] [PubMed] [Google Scholar]
- 68.Felice E, Saliba J, Grech V, et al. Antenatal psychiatric morbidity in Maltese women. Gen Hosp Psychiatry. 2007;29(6):501–505. doi: 10.1016/j.genhosppsych.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Rogal SS, Poschman K, Belanger K, et al. Effects of posttraumatic stress disorder on pregnancy outcomes. J Affect Disord. 2007;102(1):137–143. doi: 10.1016/j.jad.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uguz F, Gezginc K, Zeytinci IE, et al. Obsessive-compulsive disorder in pregnant women during the third trimester of pregnancy. Compr Psychiatry. 2007;48(5):441–445. doi: 10.1016/j.comppsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Borri C, Mauri M, Oppo A, et al. Axis I psychopathology and functional impairment at the third month of pregnancy: results from the perinatal depression-research and screening unit (PND-ReScU) study. J Clin Psychiatry. 2008;69(10):1617–1624. doi: 10.4088/jcp.v69n1012. [DOI] [PubMed] [Google Scholar]
- 72.Guler O, Sahin FK, Emul HM, et al. The prevalence of panic disorder in pregnant women during the third trimester of pregnancy. Compr Psychiatry. 2008;49(2):154–158. doi: 10.1016/j.comppsych.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Seng JS, Low LM, Sperlich M, et al. Prevalence, trauma history, and risk for posttraumatic stress disorder among nulliparous women in maternity care. Obstet Gynecol. 2009;114(4):839–847. doi: 10.1097/AOG.0b013e3181b8f8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uguz F, Gezginc K, Kayhan F, et al. Is pregnancy associated with mood and anxiety disorders? A cross-sectional study. Gen Hosp Psychiatry. 2010;32(2):213–215. doi: 10.1016/j.genhosppsych.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15(1):21–30. doi: 10.1007/s00737-011-0249-8. [DOI] [PubMed] [Google Scholar]
- 76.Kim HG, Harrison PA, Godecker AL, et al. Posttraumatic stress disorder among women receiving prenatal care at three federally qualified health care centers. Matern Child Health J. 2014;18(5):1056–1065. doi: 10.1007/s10995-013-1333-7. [DOI] [PubMed] [Google Scholar]
- 77.Marchesi C, Ampollini P, Paraggio C, et al. Risk factors for panic disorder in pregnancy: a cohort study. J Affect Disord. 2014;156:134–138. doi: 10.1016/j.jad.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Wenzel A, Haugen EN, Jackson LC, et al. Prevalence of generalized anxiety at eight weeks postpartum. Arch Womens Ment Health. 2003;6(1):43–49. doi: 10.1007/s00737-002-0154-2. [DOI] [PubMed] [Google Scholar]
- 79.Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295–311. doi: 10.1016/j.janxdis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Prenoveau J, Craske M, Counsell N, et al. Postpartum GAD is a risk factor for postpartum MDD: the course and longitudinal relationships of postpartum GAD and MDD. Depress Anxiety. 2013;30(6):506–514. doi: 10.1002/da.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baxter AJ, Scott KM, Vos T, et al. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med. 2013;43(5):897–910. doi: 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- 82.Silva Júnior EA, Gomes CA. Psychiatric comorbidities among adolescents with and without anxiety disorders: a community study. J Bras Psiquiatr. 2015;64(3):181–186. [Google Scholar]
- 83.Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 84.Moses EB, Barlow DH. A new unified treatment approach for emotional disorders based on emotion science. Curr Dir Psychol Sci. 2006;15(3):146–150. [Google Scholar]
- 85.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 86.Lee AM, Lam SK, Lau SM, et al. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110(5):1102–1112. doi: 10.1097/01.AOG.0000287065.59491.70. [DOI] [PubMed] [Google Scholar]
- 87.Reck C, Struben K, Backenstrass M, et al. Prevalence, onset and comorbidity of postpartum anxiety and depressive disorders. Acta Psychiatr Scand. 2008;118(6):459–468. doi: 10.1111/j.1600-0447.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- 88.American college of obstetricians and gynecologists. Screening for perinatal depression. Committee Opinion No. 630. Obstet Gynecol. 2015;125:1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- 89.Somerville S, Dedman K, Hagan R, et al. The perinatal anxiety screening scale: development and preliminary validation. Arch Womens Ment Health. 2014;17(5):443–454. doi: 10.1007/s00737-014-0425-8. [DOI] [PubMed] [Google Scholar]
- 90.Matthey S, Fisher J, Rowe H. Using the Edinburgh Postnatal Depression Scale to screen for anxiety disorders: conceptual and methodological considerations. J Affect Disord. 2013;146(2):224–230. doi: 10.1016/j.jad.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Huizink AC, Mulder EJ, Robles de Medina PG, et al. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004;79:81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Misri S, Kendrick K. Obsessive-compulsive disorder in the perinatal period: a review of the literature. Current Psychiatry Reviews. 2007;3(4):265–270. [Google Scholar]
- 93.Kendig S, Keats JP, Hoffman MC, et al. Consensus bundle on maternal mental health: perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2017;46(2):272–281. doi: 10.1016/j.jogn.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: A systematic review. J Affect Disord. 2016;190:543–550. doi: 10.1016/j.jad.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Loughnan SA, Wallace M, Joubert AE, et al. A systematic review of psychological treatments for clinical anxiety during the perinatal period. Arch Womens Ment Health. 2018:1–10. doi: 10.1007/s00737-018-0812-7. [DOI] [PubMed] [Google Scholar]
- 96.Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive–compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- 97.Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf. 2005;14(12):823–827. doi: 10.1002/pds.1084. [DOI] [PubMed] [Google Scholar]
- 98.Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 99.Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 100.Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370(25):2397–2407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ. 2015;350:h1798. doi: 10.1136/bmj.h1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharm Clin. 2015;30(1):4–20. doi: 10.1002/hup.2451. [DOI] [PubMed] [Google Scholar]
- 103.Rowe H, Baker T, Hale TW. Maternal medication, drug use, and breastfeeding. Child Adolesc Psychiatr Clin N Am. 2015;24(1):1–20. doi: 10.1016/j.chc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Goodman JH, Guarino A, Chenausky K, et al. CALM pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17(5):373–387. doi: 10.1007/s00737-013-0402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bittner A, Peukert J, Zimmermann C, et al. Early intervention in pregnant women with elevated anxiety and depressive symptoms: efficacy of a cognitive-behavioral group program. J Perinat Neonatal Nurs. 2014;28(3):185–195. doi: 10.1097/JPN.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 106.Lemon EL, Vanderkruik R, Dimidjian S. Treatment of anxiety during pregnancy: room to grow. Arch Womens Ment Health. 2015;18(3):569–570. doi: 10.1007/s00737-015-0514-3. [DOI] [PubMed] [Google Scholar]
- 107.Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(Suppl5):34–41. [PubMed] [Google Scholar]
- 109.Stewart RE, Chambless DL. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4):595. doi: 10.1037/a0016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cuijpers P, Donker T, van Straten A, et al. Is guided self-help as effective as face-to-face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychol Med. 2010;40(12):1943–1957. doi: 10.1017/S0033291710000772. [DOI] [PubMed] [Google Scholar]
- 111.Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377–385. doi: 10.4088/JCP.12r07917. [DOI] [PubMed] [Google Scholar]
- 112.Christian LM, Storch EA. Cognitive behavioral treatment of postpartum onset: obsessive compulsive disorder with aggressive obsessions. Clinical Case Studies. 2009;8(1):72–83. [Google Scholar]
- 113.Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt) 2006;15(4):352–368. doi: 10.1089/jwh.2006.15.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348–353. doi: 10.1111/j.1471-0528.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 115.Sharma V, Smith A, Mazmanian D. Olanzapine in the prevention of postpartum psychosis and mood episodes in bipolar disorder. Bipolar Disord. 2006;8(4):400–404. doi: 10.1111/j.1399-5618.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 116.Wells GA, Shea B, O’Connell D, et al. Ottawa Hospital Research Institute; [Accessed January 13, 2019]. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Updated 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.