Abstract
Mast cell leukemia (MCL) is a highly fatal malignancy characterized by devastating expansion of immature mast cells in various organs. Although considered a stem cell disease, little is known about MCL-propagating neoplastic stem cells. We here describe that leukemic stem cells (LSCs) in MCL reside within a CD34+/CD38− fraction of the clone. Whereas highly purified CD34+/CD38− cells engrafted NSGhSCF mice with fully manifesting MCL, no MCL was produced by CD34+/CD38+ progenitors or the bulk of KIT+/CD34− mast cells. CD34+/CD38− MCL cells invariably expressed CD13 and CD133, and often also IL-1RAP, but did not express CD25, CD26 or CLL-1. CD34+/CD38− MCL cells also displayed several surface targets, including CD33, which was homogenously expressed on MCL LSC in all cases, as well as the D816V mutant form of KIT. Whereas CD34+/CD38− cells were resistant against single drugs, exposure to combinations of CD33-targeting and KIT-targeting drugs resulted in LSC-depletion and markedly reduced engraftment in NSGhSCF mice. Together, MCL LSCs are CD34+/CD38− cells that express distinct profiles of markers and target antigens. Characterization of MCL LSCs should facilitate their purification and should support the development of LSC-eradicating curative treatment approaches in this fatal type of leukemia.
Introduction
Mast cell leukemia (MCL) is a rare and highly fatal variant of systemic mastocytosis (SM).1–3 The disease is characterized by invasive growth and leukemic expansion of neoplastic mast cells (MC) in various internal organs and a poor prognosis. In most patients, the malignant clone expands rapidly, and even when treated with poly-chemotherapy or targeted drugs, the median survival in these patients is below 12 months.1,2,4–7 In a small subset of patients, hematopoietic stem cell transplantation (SCT) results in disease eradication and cure.8
So far, only little is known about the biology and origin of MCL. The phenotype of neoplastic MC in MCL suggests a close relationship to other variants of SM.9–12 In most patients, MC display CD13, CD33 and KIT as well as CD25.9–11,13 Moreover, MCL cells usually express gain-of-function mutations in KIT.14–17 In most patients, the D816V mutation is found, although other KIT mutations may also be detected.16,17 In a few MCL patients, no KIT mutations are found.14,15 However, MCL cells may exhibit additional molecular defects. Indeed, several different molecular lesions, including RAS-, TET2-, SRSF2-, ASXL1- and RUNX1- mutations, have been detected in patients with advanced SM, including MCL.18–21
The concept of leukemic stem cells (LSC) was established to explain cellular hierarchies and clonal architectures in leukemias and to improve treatment-strategies by eliminating disease-propagating cells.22–28 The leukemia-initiating and propagating ability of LSC can usually be demonstrated in highly immuno-deficient mice, such as non-obese diabetic severe combined immunodeficiency (NOD/SCID) mice lacking an IL-2 receptor-gamma chain (NSG). In acute myeloid leukemia (AML), NSG mouse-engrafting LSC usually reside within a CD34+ compartment of the clone.29 Depending on the AML-type and mouse strain employed, AML LSC may be detected in CD34+/CD38− and/or in CD34+/CD38+ sub-fractions.29 In the blast phase of chronic myeloid leukemia (CML), CD34+ LSC also display CD38,30 whereas in chronic phase CML, LSC reside primarily in a CD34+/CD38− cell-compartment,23–25 thereby resembling the phenotype of normal hematopoietic stem cells (HSC). However, in contrast to normal bone marrow (BM) HSC, CML LSC aberrantly express CD25, CD26 and IL-1RAP.31,32 Aberrantly expressed surface markers on CD34+/CD38− AML LSC include CD25 and CLL-1.33,34
In patients with aggressive systemic mastocytosis (ASM) and MCL, the malignant clone consists almost entirely of MC that express KIT but do not express CD34.1–3,6 Although CD34+ cells are also detectable in these patients, their frequency in the peripheral blood (PB) and BM is extremely low unless the patient is suffering from an associated non-MC-lineage leukemia such as AML.35–38 So far, the origin, frequency and phenotype of MCL LSC remain unknown.
In this study, we were able to define the phenotype, target expression profile and functional properties of MCL-initiating and -propagating LSC. Of a total of 63 patients with SM screened for the presence of putative LSC, 6 with advanced SM/MCL were found to be suitable for xenotransplantation experiments. Since MC development requires membrane-bound stem cell factor (SCF) as important niche-molecule, we employed NSG mice expressing human membrane-bound SCF (NSGhSCF)39,40 in our engraftment studies.
Materials and Methods
Patients
The patients’ characteristics are shown in Table S1A. From April 2011 to December 2016, 63 patients with SM (29 female, 34 male; median age: 63 years, range: 24-90) were included. Patients were diagnosed at the Medical University of Vienna (Austria) or the University Hospital of Mannheim (Germany). Diagnoses were established according to published criteria and the classification of the World Health Organization (WHO).3 Most patients were freshly diagnosed at the time of sampling. In 20 patients, previous anti-neoplastic therapy had been administered (Table S1B). Treatment consisted of anti-neoplastic drugs in advanced SM and anti-mediator type drugs, including histamine receptor blockers in all patients. Patients with MCL and other SM subtypes received cytoreductive agents and/or KIT-targeting drugs according to available guidelines.3,6,14 In 2 patients treated with midostaurin (100-200 mg/day), 5 patients receiving 3-6 cycles of cladribine (0.1-0.13 mg/kg/day over 7 days per cycle), 1 patient receiving fludarabine (30 mg/m2 per day; days 1-5) and cytarabine (2.000 mg/m2 per day; days 1-5), and 1 patient receiving fludarabine (30 mg/m2 per day; days 1-5), cytarabine (2.000 mg/m2 per day; days 1-5), and gemtuzumab-ozogamicin (2 injections of 5 mg on days 1 and 7), BM samples were obtained both before and during or after therapy. An overview of patient-derived samples and their use in individual experiments is provided in Table S1C. Control BM samples obtained from healthy donors were purchased from Lonza. All studies were approved by the ethics committees of the Medical University of Vienna (Austria) and University of Mannheim, Heidelberg (Germany) and conducted in accordance with the declaration of Helsinki. Informed consent was obtained in each case before BM or blood samples were analyzed.
Animal models
For xenotransplantation experiments, NOD-SCID-IL-2Rγ-/- (NSG) mice expressing a 220 amino acid isoform of the human membrane-bound SCF (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg[PGK1-KITLG*220]441Daw/SzJ) (NSGhSCF) as well as NSG mice without human SCF were used. Experiments were performed on adult (8-12 weeks) animals. Littermates of the same sex were randomly assigned to experimental groups. Animals were purchased from The Jackson Laboratory (Bar Harbor, ME, USA, stock #017830 and #005557) and housed in individual ventilated cages to maintain pathogen-free conditions. Animal studies were approved by the ethics committee of the Medical University of Vienna and the University of Veterinary Medicine Vienna, and carried out in accordance with guidelines for animal care and protection and protocols approved by Austrian law (GZ 66.009/0040-II/10b/2009).
Xenotransplantation assay
A total of six patients (patient #1, #3, #5, #7, #10, and #22; patient sample details are shown in Tables S1A-C) were found eligible for xentrotransplantation experiments based on their disease variant (advanced SM/MCL), time point of sample acquisition, and the numbers of CD34+ cells that could be purified by cell sorting. In this subset of patients, sub-populations of primary BM MNC (i.e. CD45+; CD45+/KIT+; CD45+/KIT+/CD34−; CD45+/CD34+, CD45+/CD34+/CD38+; CD45+/CD34+/CD38−; CD45+/CD38+; CD45+/CD38−; see Figure 1A and Figure S1) were purified by FACS-sorting. Sorted cells were washed, resuspended in 0.15 ml RPMI medium containing 10% fetal calf serum (FCS), and injected into the lateral tail vein of NSGhSCF mice. In each experiment, a total number of 0.3-3 x 105 cells per mouse (3-5 mice per experimental group) were injected. Twenty-four hours prior to injection, mice were sub-lethally irradiated (2.4 Gy). After injection, mice were inspected daily and sacrificed when they showed disease symptoms or after 35 weeks. BM cells were obtained from flushed femurs, tibias, and humeri. Engrafted MCL cells were detected in BM samples by multicolor flow cytometry using mAb against CD45 and KIT. In pilot experiments, we found that engrafted cells invariably express KIT. In most patients, the engrafted MCL cells also expressed CD45, but in one patient with MCL, MC expressed only very low amounts of CD45. Therefore, MCL-repopulation was measured by determining the percentage of human KIT+ cells in mouse BM samples by flow cytometry. In a separate set of experiments, cells were treated in vitro for 1 h with GO (5 μg/ml), midostaurin (1 μM), alemtuzumab (500 μg/ml), or combinations of these drugs prior to injection. Afterwards, drugs were removed by centrifugation before cells were injected.
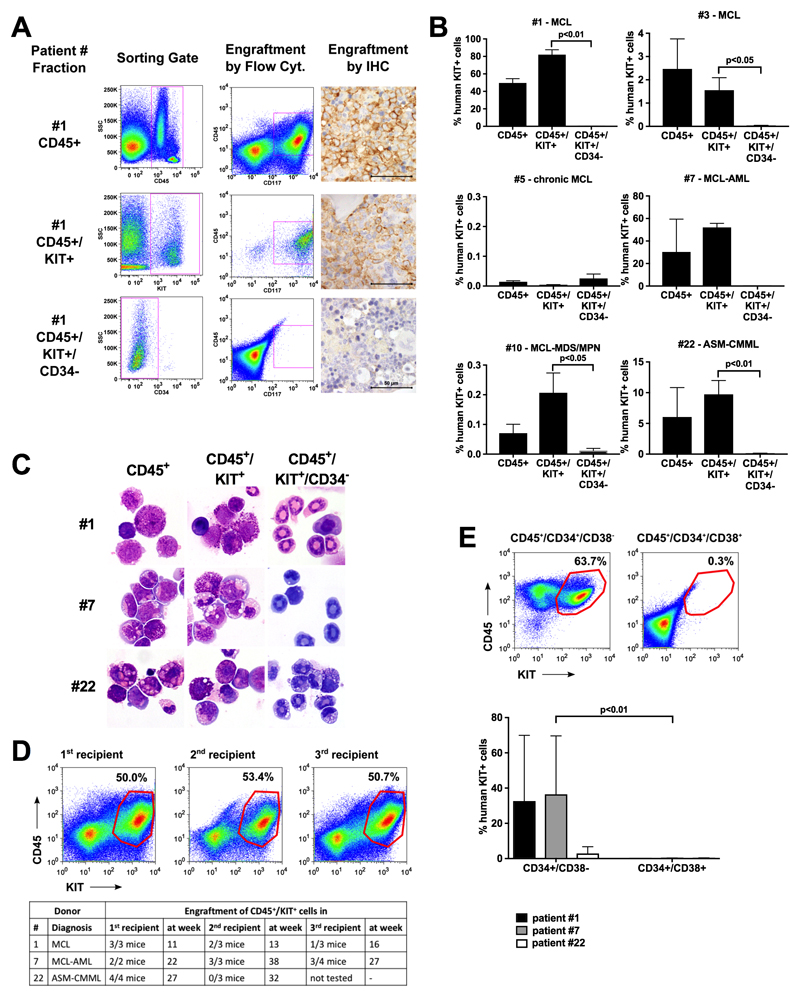
Figure 1. NSGhSCF-repopulating MCL LSC reside in a KIT+/CD34+ compartment of the clone whereas the huge bulk of neoplastic KIT+/CD34− MCs have no MCL-propagating capacity.
(A) Sorting-strategy to obtain MCL subfractions in patient #1 (left column) and their engraftment in NSGhSCF mice after 11 weeks (middle and right columns). The bulk of CD45+ cells (top row panels) and KIT+ fractions (containing CD34+ cells; middle row panels) produced engraftment in NSGhSCF mice, whereas the KIT+ pure mast cell fractions, depleted of CD34+ cells, did not produce engraftment (bottom row panels). The right columns show immunohistochemical demonstration of MCL-formation in the BM of NSGhSCF mice using an antibody against KIT. The scale bar represents 50 μm. (B) Summary of engraftment results in 6 patients. In each case, 3-4 mice per group were injected with the indicated cell fractions. Results show the percentage of human (hu) KIT+ cells in mouse BM samples and are expressed as mean±S.D. from all mice in each group. Sample details are summarized in Tables S1A-C. (C) Giemsa-stained cytospin-preparations of engrafted MCL cells (left and middle columns) or lack of engraftment (i.e. murine neutrophilic granulocytes, right column) in mouse BM samples in patients #1, #7 and #22. (D) Engraftment in secondary and tertiary recipient-mice after injection of bulk CD45+ cells (initial sample) and mouse BM cells (secondary/tertiary transfer). The upper panel shows engraftment-examples in primary, secondary and tertiary recipient mice in patient #1, and the lower panel shows a table summarizing engraftment results. (E) Engraftment of CD34+ sub-populations in NSGhSCF mice. The upper panel shows an example of engrafted CD45+/KIT+ cells from patient #7 in the BM of mice and the lower panel provides a summary of engraftment results in the three donors tested (n=3-5 mice per cohort and patient).
In vitro drug incubation experiments
Drug effects on cell viability were examined by flow cytometry. In these experiments, primary MNC from selected patients with MCL or ASM (patient #1, #3, #7, #8, #10, #15, #18, #24, #26; patient sample details are shown in Tables S1A-C) or ROSAKIT D816V cells were treated with GO (0.5-50 ng/ml), midostaurin (0.01-1 μM) or a combination of both drugs (GO, 50 ng/ml and midostaurin, 1 μM) for 48 hours. Viability and apoptosis of drug-exposed cells were determined by staining with Annexin-V and PI (ROSAKIT D816V) or Annexin-V and 4’,6-diamidino-2-phenylindole (DAPI; primary MNC). In the case of primary MNC, cells were stained for CD34, CD38, and CD45 to detect CD34+/CD38− cells as shown in Figure S1. All experiments were performed in triplicates.
Statistics
Details on statistical evaluations are found in the supplementary material.
Data sharing statement
Gene array data are available as GEO record GSE103223. For other data, please contact the corresponding author.
Results
NSGhSCF-repopulating MCL LSC reside in a KIT+/CD34+ compartment of the clone whereas the bulk of neoplastic KIT+/CD34− MC have no MCL-propagating capacity
Highly enriched sub-populations of BM cells were obtained from patients with advanced SM/MCL (Table S1) by flow-sorting and were injected into NSGhSCF mice to define their MCL-initiating and propagating capacity. The gating strategy to delineate between CD45+/KITint/CD34+ stem cells and CD45+/KIT+/CD34− MCs is shown in Figure S1. Whereas the bulk of KIT+/CD34− MC did not produce a MCL-like disease in NSGhSCF mice (<1% engraftment), the CD45+/KIT+ fractions containing a small population of CD34+ cells (<1% of all leukocytes) engrafted and produced overt SM/MCL in NSGhSCF mice after 11-31 weeks (Figure 1A-C; Table 1; Figure S2). Although CD34-containing cells produced MCL in most NSGhSCF mice, the time to leukemic engraftment and engraftment-levels varied from donor to donor (Figure 1B). In one patient with hyper-acute MCL,17 leukemic engraftment was detected after 11 weeks, whereas in the other patients with MCL and those with ASM, engraftment was detected after 22-31 weeks (Table 1). In one patient with chronic MCL41 no engraftment was detectable after 35 weeks. MCL engraftment was confirmed by morphologic and phenotypic studies (Figure 1A, C, S1 and S2) and KIT mutation analysis (Table S2). Unexpectedly, however, KIT D816V was not detected in all engrafting recipient mice even if the morphology suggested fully manifesting MCL. The type of engrafting cells also varied, depending on the variant of ASM/MCL. In patients with pure ASM or pure MCL, most NSGhSCF-engrafting cells were MC or metachromatic blasts, whereas in MCL-AML, engrafting cells were MC and AML blasts (Figure 1C; Table 1 and S2). Highly purified CD34+ cells also produced MCL-like engraftment (Table S3). In limiting dilution experiments performed using isolated CD34+ cells, the calculated frequency of NSGhSCF-repopulating LSC within the CD34+ fractions ranged between 0.019% and 0.478%, and the calculated frequency of LSC in the total bulk of leukemic cells ranged between 0.0003% and 0.0045% (Table S3). Engrafted MCL cells produced MCL in secondary and tertiary recipient mice in 2/3 donors (Figure 1D). These data formally establish that MCL-initiating and -propagating LSC reside in a CD34+ subset of the malignant clone.
Table 1. Engraftment results in patients with ASM and MCL.
| Patient | Diagnosis | Cell Fraction | Cell number injected | engraftment in n/n mice | at week | % huKIT+ cells in BM | engrafted cell types |
|---|---|---|---|---|---|---|---|
| #1 | Acute MCL | CD45+ | 1.3 x 105 | 3/3 | 11 | 49.60±08.60 | MC |
| CD45+/KIT+ | 1.3 x 105 | 3/3 | 11 | 82.03±09.64 | MC | ||
| CD45+/KIT+/CD34- | 1.3 x 105 | 0/3 | 11 | 00.19±00.17 | - | ||
| #3 | Acute MCL | CD45+ | 1.25 x 105 | 4/4 | 31 | 02.47±02.58 | MC |
| CD45+/KIT+ | 0.5 x 105 | 4/4 | 31 | 01.55±01.07 | MC | ||
| CD45+/KIT+/CD34- | 0.5 x 105 | 0/4 | 31 | 00.04±00.01 | - | ||
| #5 | Chronic MCL | CD45+ | 1.2 x 105 | 0/3 | 35 | 00.01±00.01 | - |
| #7 | MCL-AML | CD45+ | 2.0 x 105 | 2/3 | 22 | 30.28±50.51 | blasts, MC |
| CD45+/KIT+ | 2.0 x 105 | 2/2 | 22 | 52.10±05.09 | blasts, MC | ||
| CD45+/KIT+/CD34- | 1.9 x 105 | 0/1 | 22 | 00.22 | - | ||
| #10 | MCL-MDS/MPN | CD45+ | 2.5 x 105 | 2/4 | 29 | 00.07±06.0 | MC |
| CD45+/KIT+ | 0.55 x 105 | 3/4 | 29 | 00.20±00.13 | MC | ||
| CD45+/KIT+/CD34- | 0.3 x 105 | 0/4 | 29 | 00.01±00.02 | - | ||
| #22 | ASM-CMML | CD45+ | 2.5 x 105 | 3/4 | 27 | 06.04±09.58 | MC |
| CD45+/KIT+ | 3.0 x 105 | 4/4 | 27 | 09.73±04.50 | MC | ||
| CD45+/KIT+/CD34- | 3.0 x 105 | 0/4 | 27 | 00.14±00.02 | - |
Highly enriched sub-fractions of primary neoplastic cells were obtained from patients with acute or chronic mast cell leukemia (MCL), MCL associated with AML (MCL-AML), MCL associated with MDS/MPN, or ASM-CMML. Purified (FACS-sorted) cell fractions were injected into the tail vein of NSGhSCF mice. Technical details are described in the text. After 11-27 weeks, mice were sacrificed and their BM cells were analyzed for the percentage of human KIT+ cells (MC and blasts) by flow cytometry. Engraftment levels were determined by measuring the percentage of CD117+ cells relative to all nucleated BM cells in each mouse group. Results are expressed as mean±SD of all mice per group. Detailed engraftment results for each animal are provided in Table S2. Abbreviations: MC, mast cells; ASM, aggressive systemic mastocytosis; MCL, mast cell leukemia; BM, bone marrow; AML, acute myeloid leukemia; MDS/MPN, myelodysplastic/myelo-proliferative overlap neoplasm; CMML, chronic myelomonocytic leukemia.
We next asked whether NSGhSCF-engrafting MCL LSC reside within a particular sub-fraction of CD34+ cells. To address this critical question, ASM- or MCL-derived BM fractions containing CD34+/CD38+ and CD34+/CD38− cells were purified by cell-sorting and were then injected separately into NSGhSCF mice (n = 0.5–2.5 × 105 cells per mouse; 4-5 mice per cohort per patient). In these experiments, only the CD38− subset of CD34+ cells engrafted NSGhSCF mice, whereas no leukemic engraftment was seen in mice injected with CD34+/CD38+ cells (Figure 1E), suggesting that MCL LSC strictly reside within a CD34+/CD38− compartment of the clone similar to LSCs in chronic phase CML.25,31,32
Evaluation of surface marker- and target expression profiles in MCL CD34+/CD38− cells
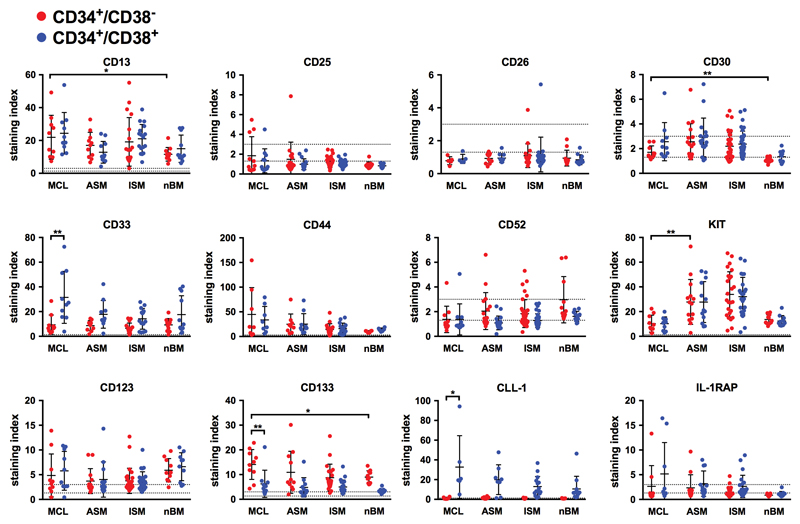
As assessed by gene array profiling, CD34+ cells obtained from patients with MCL expressed several functionally relevant markers and target antigens. Some of these antigens, like CD123, IFNγR1, CD44, IFNαR2, or IL-1RAP, were expressed at higher levels in CD34+ MCL cells compared to CD34+ BM cells from healthy BM (Figure S3; Table S4). In a next step, CD34+/CD38− and CD34+/CD38+ cells were analyzed separately by flow cytometry. Although most surface antigens, including CD13, CD33, CD44, CD54, CD117 (KIT), CD123 and CD133 were expressed on both, CD34+/CD38− and CD34+/CD38+ cells, differences in expression levels were found (Table 2). CD34+/CD38− MCL cells displayed higher levels of CD133 than CD34+/CD38+ progenitor cells (Figure 2, Table 2). CD34+/CD38+ MCL cells, in turn, displayed higher levels of CD33 and CLL-1 (CD371) compared to CD34+/CD38− MCL cells (Figure 2, Table 2). Compared to normal BM HSC, MCL CD34+/CD38− cells expressed higher levels of CD13, CD30 and CD133. Moreover, in a subset of patients with advanced SM, CD34+/CD38− cells expressed IL-1RAP (2/9 patients with MCL, 5/12 patients with ASM) and CD52 (3/11 patients with MCL, 10/17 patients with ASM) (Figure 2; Table S5). The only marker that was found to be downregulated on the surface of MCL CD34+/CD38− cells (compared to HSC and ASM CD34+/CD38− cells) appeared to be KIT, although it was still expressed at clearly detectable levels (Figure 2). Otherwise, CD34+/CD38− cells in ASM and MCL exhibited an almost identical phenotype (Table 3). CD25 and CD26, both of which are expressed on CML LSC,31 were not detectable on CD34+/CD38− cells in ASM or MCL (Figure 2; Table 3, S5, and S6). To test whether previously administered anti-neoplastic therapy (analyzed in 20 of the 63 patients) had an influence on the phenotype of CD34+/CD38− cells, we analyzed surface marker expression on CD34+/CD38− and CD34+/CD38+ cells in BM samples obtained from untreated and previously treated patients separately. However, no differences in surface marker expression between these two cohorts were observed (Figure S4) and the results obtained in the two subsets of patients were also very similar to the results obtained by analyzing surface marker expression on CD34+/CD38− and CD34+/CD38+ cells in all patients together (Figure 2). A summary of surface markers expressed on CD34+/CD38− cells in MCL, ASM, indolent systemic mastocytosis (ISM) and normal BM is shown in Table 3, and a comparison of phenotype of CD34+/CD38− cells in MCL with the phenotype of CD34+/CD38− cells in other human leukemias, determined with the same antibody-panel and methodology by our group,31,42 is provided in Table S6.
Table 2. Cell surface antigen profile of neoplastic CD34+/CD38−stem cells in patients with MCL* and comparison to CD34+/CD38+ progenitor cells and neoplastic mast cells.
| Antigen | CD | Expression of Cell Surface Antigens on | ||
|---|---|---|---|---|
| Stem cells (CD34+/CD38-) | Progenitor cells (CD34+/CD38+) | Mast cells (CD34-/CD117+) | ||
| LFA-2 | CD2 | - | - | +/- |
| Amino-P-N | CD13 | ++ | ++ | + |
| FAL-3 | CD15 | - | - | - |
| IL-2RA | CD25 | - | - | + |
| DPPIV | CD26 | - | - | - |
| Ki-1 | CD30 | +/- | +/- | + |
| Siglec-3 | CD33 | + | ++ | ++ |
| Leukosialin | CD43 | ++ | ++ | + |
| Pgp-1 | CD44 | ++ | ++ | ++ |
| VNR | CD51/CD61 | - | - | +/- |
| Campath-1 | CD52 | +/- | - | +/- |
| NCAM | CD56 | - | - | - |
| Thy-1 | CD90 | - | - | - |
| KIT | CD117 | + | ++ | ++ |
| IL-3RA | CD123 | + | + | +/- |
| IL-9R | CD129 | - | - | - |
| AC133 | CD133 | ++ | + | - |
| CXCR4 | CD184 | + | + | - |
| CLL-1 | CD371 | - | ++ | + |
| IL-1RAP | n.c. | - | +/- | +/- |
The table shows a summary of all multicolor flow cytometry staining results obtained in all patients with MCL (n=11). *Detailed results obtained in each patient are shown in Table S5. Scoring system: median fluorescence intensity (MFI) values for each cell population were calculated and scored according to the following system: -, MFI <1.3; +/-, MFI 1.31-3.00; +, MFI 3.01-9.99; ++, MFI >10; the values refer to the median values (of MFI) obtained from all MCL donors examined.
Abbreviations: LFA-2, lymphocyte function-associated antigen-2; Amino-P-N, aminopeptidase N; FAL-3, 3-fucosyl-N-acetyl-lactosamine; IL-2RA, interleukin-2 receptor alpha chain; DPPIV, dipeptidylpeptidase IV; Siglec-3, sialic acid-binding immunoglobulin-type lectin 3; Pgp-1, phagocytic glycoprotein-1; VNR, vitronectin receptor; NCAM, neural cell adhesion molecule; Thy-1, thymocyte differentiation antigen-1; IL-3RA, interleukin-3 receptor alpha chain; CXCR4, C-X-C chemokine receptor type 4; IL-9R, interleukin-9 receptor; IL-1RAP, interleukin-1 receptor accessory protein; CLL-1, C-type lectin-like molecule-1; CD, cluster of differentiation; n.c., not clustered; MFI, median fluorescence intensity.
Figure 2. Evaluation of surface marker- and target expression profiles in MCL CD34+/CD38− and CD34+/CD38+ cells.
Expression of cell surface antigens on CD34+/CD38− (●) and CD34+/CD38+ cells (●) obtained from the BM of patients with mast cell leukemia (MCL, n=11), aggressive systemic mastocytosis (ASM, n=17) and indolent systemic mastocytosis (ISM, n=35) was determined by multi-color flow cytometry (see also Figure S1, Table S5). Sample details are summarized in Tables S1A-C. CD34+/CD38− and CD34+/CD38+ cells obtained from healthy/reactive BM (nBM, n=11) served as controls. Results are expressed as dot blots and indicate the staining index (=median fluorescence intensitymarker / median fluorescence intensityisotype control) for each individual patient (dots) as well as the mean expression levels (bars) for each marker. Dotted horizontal lines represent cut-off values for negativity (staining index <1.0) and positivity (staining index >1.3). Samples located between these lines were considered weakly positive (staining index 1.0 – 2.99). * p<0.05; ** p<0.01 (student’s t-test with Bonferroni correction).
Table 3. Phenotype of CD34+/CD38−stem cells in various categories of systemic mastocytosis (SM) and comparison to normal bone marrow (BM) stem cells.
| Antigen | CD | Expression of Antigens on CD34+/CD38− stem cells in | |||
|---|---|---|---|---|---|
| MCL | ASM | ISM | normal BM | ||
| LFA-2 | CD2 | - | - | - | +/- |
| Amino-P-N | CD13 | ++ | ++ | ++ | ++ |
| FAL-3 | CD15 | - | - | - | +/- |
| IL-2RA | CD25 | - | - | - | - |
| DPPIV | CD26 | - | - | - | - |
| Ki-1 | CD30 | +/- | +/- | +/- | - |
| Siglec-3 | CD33 | + | + | + | + |
| Leukosialin | CD43 | ++ | ++ | ++ | ++ |
| Pgp-1 | CD44 | ++ | ++ | ++ | ++ |
| VNR | CD51/CD61 | - | - | - | +/- |
| Campath-1 | CD52 | +/- | +/- | +/- | +/- |
| NCAM | CD56 | - | +/- | +/- | - |
| Thy-1 | CD90 | - | - | - | +/- |
| KIT | CD117 | + | ++ | ++ | ++ |
| IL-3RA | CD123 | + | + | + | + |
| IL-9R | CD129 | - | - | - | - |
| AC133 | CD133 | ++ | + | + | + |
| CXCR4 | CD184 | + | + | +/- | - |
| CLL-1 | CD371 | - | - | - | - |
| IL-1RAP | n.c. | - | - | - | - |
The table shows a summary of all multicolor flow cytometry staining results obtained in all patients in each SM group or normal BM. Scoring system: median fluorescence intensity values were calculated and scored according to the following scoring system: -, MFI <1.3; +/-, MFI 1.31-3.00; +, MFI 3.01-9.99; ++, MFI >10; results refer to the median MFI values obtained in all donors examined in each group.
Abbreviations: BM, bone marrow; LFA-2, lymphocyte function-associated antigen-2; Amino-P-N, aminopeptidase N; FAL-3, 3-fucosyl-N-acetyl-lactosamine; IL-2RA, interleukin-2 receptor alpha chain; DPPIV, dipeptidylpeptidase IV; Siglec-3, sialic acid-binding immunoglobulin-type lectin 3; Pgp-1, phagocytic glycoprotein-1; VNR, vitronectin receptor; NCAM, neural cell adhesion molecule; Thy-1, thymocyte differentiation antigen-1; IL-3RA, interleukin-3 receptor alpha chain; CXCR4, C-X-C chemokine receptor type 4; IL-9R, interleukin-9 receptor; IL-1RAP, interleukin-1 receptor accessory protein; CLL-1, C-type lectin-like molecule-1; CD, cluster of differentiation; n.c., not clustered; MFI, median fluorescence intensity.
Combinations of targeted drugs may overcome drug resistance in MCL CD34+/CD38− cells
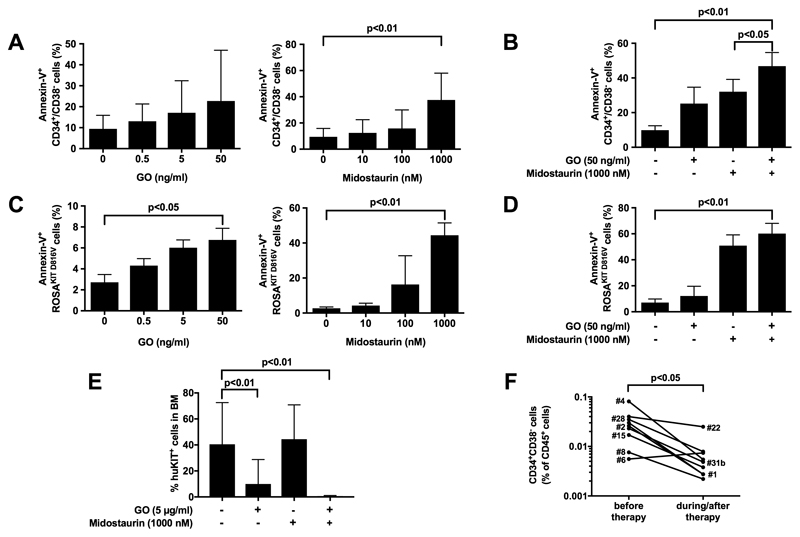
Subsequent experiments were conducted to validate therapeutically relevant targets using primary MCL CD34+/CD38− cells and the ROSAKIT D816V cell line. ROSAKIT D816V cells express KIT and several surface target antigens, including CD33 and CD52, and can engraft NSG mice.43 In vitro incubation of primary MCL CD34+/CD38− cells with the CD33-targeting drug gemtuzumab-ozogamicin (GO) or the KIT D816V-targeting drug midostaurin did not result in apoptosis unless rather high drug concentrations were applied, suggesting resistance (Figure 3A). However, when combined, GO and midostaurin were found to produce modest, but statistically significant cooperative apoptosis-inducing effects on MCL CD34+/CD38− cells (Figure 3B). In addition, both drugs were found to induce growth inhibition and apoptosis in ROSAKIT D816V cells (Figure 3C, 3D, and S5). Additionally, we were able to show that exposure to GO for 1 hour interferes with engraftment of primary MCL CD34+/CD38− cells and ROSAKIT D816V cells in NSGhSCF mice, and that combined treatment with midostaurin augments antibody-mediated depletion of NSGhSCF-engrafting cells (Figure 3E and S6A). Shortly after drug incubation (before injection into NSG mice) these cells were fully viable (Figure S6B). Exposure of ROSAKIT D816V cells or primary CD52+ CD34+/CD38− cells to the CD52-targeting drug alemtuzumab for 1 hour resulted in cell lysis in vitro (Figure S7A). Confirming results obtained in AML,42 the ROSAKIT D816V-killing effect of alemtuzumab in vitro was strictly dependent on the presence of complement (Figure S7A). No alemtuzumab effects were seen in the same samples in CD34+/CD38+ cells that were found to lack CD52 (Figure S7B). In addition, short-term incubation of ROSAKIT D816V cells or primary CD52+ MCL CD34+/CD38− cells with alemtuzumab (500 μg/ml) also reduced long-term engraftment in NSGhSCF mice (Figure S8A and S8B). Together, CD33 and, less frequently CD52, are clinically relevant targets on MCL CD34+/CD38− cells and drug-responsiveness may be most likely dependent on target-expression.
Figure 3. Combinations of targeted drugs may overcome drug resistance in MCL CD34+/CD38− cells.
(A-D) Primary leukemic cells obtained from patients with MCL (n=5) or ASM (n=4) (A, B) or ROSAKIT D816V cells (C, D) were incubated in the absence or presence of GO (A, C, left panel) or midostaurin (A, C, right panel) or a combination of both drugs (B, D) at 37°C for 48 hours. Bars represent the percentage of Annexin-V+ cells (A, B: gated for CD34+/CD38− cells) after drug exposure and are expressed as mean±S.D. of all experiments. (E) Primary MCL cells (patients #1 and #7) were incubated in control medium or GO (5 μg/ml), midostaurin (1 μM) or a combination of both drugs at 37°C for 1 hour. Then, cells were injected into NSGhSCF mice. Bars show the percentage of huKIT+ cells found in the murine BM after 10 weeks. (F) The percentage of CD34+CD38− cells among all CD45+ cells was determined in BM samples of 2 patients treated with midostaurin (100-200 mg/day), 5 receiving cladribine (0.1-0.13 mg/kg/day over 7 days per cycle), 1 patient receiving fludarabine (30 mg/m2 per day, days 1-5) and cytarabine (2.000 mg/m2 per day, days 1-5), and 1 patient receiving fludarabine (30 mg/m2 per day, days 1-5), cytarabine (2.000 mg/m2 per day, days 1-5), and gemtuzumab-ozogamicin (2 injections of 5 mg on day 1 and day 7). BM cells were examined before therapy and during/after therapy (median follow-up: 7.86 months, range: 1.6-33.3 months). Sample details are summarized in Table S1B.
Chemotherapy is reducing but not eliminating CD34+/CD38− cells in patients with ASM/MCL
We assessed the percentage of CD34+CD38− cells in the BM of 5 patients receiving cladribine, 2 patients receiving midostaurin, 1 patient receiving fludarabine and cytarabine, and 1 patient receiving fludarabine, cytarabine, and gemtuzumab-ozogamicin, before and during therapy (Table S1B). The median duration between initial and follow-up samples was 7.86 months (range 1.6-33.3 months). We found that the fraction of CD34+CD38- cells decreased during therapy in eight of nine patients (overall/all patients - before therapy: 0.029±0.022% vs post-therapy: 0.007±0.007% of all CD45+ cells) but these putative stem cells were still detectable (Figure 3F). Therefore, apparently, single-drug treatment did not result in a complete eradication of CD34+CD38- cells, suggesting stem cell resistance.
Discussion
In most human leukemias, including AML and CML, disease-initiating and leukemia-propagating LSC are considered to reside within a CD34+ fraction of the malignant clone.22–26,28–30 However, in certain types of AML, LSC may also be CD34-negative.44 In the present study, MCL-initiating and propagating LSC were found to be CD34+ cells, whereas CD34-depleted cell populations containing the bulk of KIT+ MC did not produce leukemic engraftment in NSGhSCF mice. Based on the aggressiveness of the disease and very low numbers of CD34+ cells in these patients (<0.1% of cells) this clear-cut result was somehow unexpected. However, the data were reproducible and seen in all patients tested, independent of the subtype of disease. The long-term-propagating capacity (LSC-function) of these cells was confirmed by demonstrating engraftment in secondary and tertiary recipient mice.
In various disease-models, aggressiveness may correlate with engraftment-rates and the time to engraftment in NSG mice. Likewise, in most patients with AML, LSC engraft NSG mice relatively quickly with an overt leukemia, whereas in CML, engraftment of LSC takes much longer, often up to 12 months.22,24–26,29–32 In the present study, corresponding results were obtained. In fact, in a patient with rapidly progressing (hyper-acute) MCL,17 rapid engraftment in NSGhSCF mice (11 weeks) was seen, whereas in other patients with MCL or ASM, it took a longer time-period (up to 33 weeks) until overt leukemia developed. Finally, in a patient with chronic MCL, a newly defined MCL entity with very slow or no progression,41 no measurable engraftment was seen after 35 weeks.
Based on the assumption that SCF is an important homing molecule for normal and neoplastic MC, most engraftment experiments were performed using NSG mice expressing membrane-bound human SCF (NSGhSCF).40 Our phenotypic studies also confirmed that leukemic stem- and progenitor cells invariably express the SCF receptor, KIT. However, in control experiments, we found that MCL CD34+/CD38− cells can also engraft in normal NSG mice (Figure S9). This observation confirms that murine SCF may be capable of replacing human SCF as a homing and growth-triggering cytokine. Alternatively, MCL LSC can grow and undergo homing in vivo independent of SCF. In this regard it is noteworthy, that neoplastic cells expressed KIT-activating mutations in almost all patients examined and that the levels of surface KIT were lower in MCL CD34+/CD38− cells compared to HSC. All in all, the NSG background apparently provides a sufficient environment for engraftment of CD34+/CD38− cells in ASM and acute MCL. For other types of SM and chronic MCL this may not be the case. Whether in these less aggressive SM-variants, other mouse strains, for example NSG mice expressing multiple human cytokines, provide a more permissive environment, is currently under investigation.
An interesting observation was that KIT codon 816 mutations were not detected in all engrafted MCL cells in all mice even when overt MCL was detected in the mouse BM and even when the mutant was identified in other mice in the same experiment. One explanation would be that KIT mutant-negative sub-clones can expand in NSGhSCF mice and sometimes even possess a developmental advantage over KIT-mutated sub-clones, a phenomenon that has been described also in MCL patients.45 In line with this hypothesis, one of our patients examined (#22) indeed developed a KIT D816V-negative AML in the follow up. Alternatively, normal MC also developed (from residual normal stem cells) because of expression of human SCF in these mice. However, both the morphology and the leukemic infiltration-pattern argued against the presence of normal MC in these transplants.
Recent data suggest that AML and CML LSC express several surface molecules in an aberrant manner. These markers include IL-1RAP, CD25 and CD26 in CML,31,32 and CD25, CD96 and CLL-1 in AML.33,34 We asked whether one or more of these markers are expressed on MCL CD34+/CD38− cells. Indeed, we found that both, the CD34+/CD38− and CD34+/CD38+ cells, express IL-1RAP in a subset of patients. By contrast, we were unable to detect CD25, CD26 or CLL-1 on CD34+/CD38− MCL cells. Confirming our previous data46 CD34+/CD38− MCL cells also expressed CD123, the alpha-chain of the IL-3 receptor.
MCL CD34+/CD38− cells also expressed several surface-targets, including Siglec-3 (CD33) and Campath-1 (CD52). In those patients in whom CD34+/CD38− cells expressed CD33 and/or CD52, targeted antibody-constructs directed against these antigens, namely GO (anti-CD33) and alemtuzumab (anti-CD52) induced apoptosis or lysis in CD34+/CD38− cells. However, relatively high drug concentrations were required to obtain these effects suggesting resistance. We therefore combined drugs directed against mutant KIT (midostaurin), CD33 (GO) and CD52 (alemtuzumab). Indeed, when applying any two of these agents in combination, modest but statistically significant cooperative apoptosis-inducing or cell-lytic effects on MCL CD34+/CD38− cells were seen. In addition, these drugs suppressed engraftment of MCL CD34+/CD38− cells in NSGhSCF mice. Whereas most experiments were carried out using primary MCL CD34+/CD38− cells, we also employed the recently established MCL-like cell line ROSAKIT D816V, known to express both CD33 and CD52.43 As in primary MCL, both GO and alemtuzumab were found to block in vitro growth and viability of ROSA cells and their in vivo engraftment in NSGhSCF mice. These data suggest that the concept of targeting LSC is applicable to MCL. In line with this notion, treatment of a patient with advanced SM with GO induced major anti-neoplastic effects.47 On the other hand, however, some of these targets, like CD52, are not all expressed on CD34+/CD38− cells in all MCL patients, and even in patients in whom most CD34+/CD38− cells display CD33 and/or CD52, smaller (quiescent) LSC sub-clones may lack these cell surface targets. Indeed, in patients in whom CD34+/CD38− cells did not express CD33 or CD52, the respective targeted drugs were unable to induce cell death. Therefore, combinations of various targeted drugs, including the KIT D816V blocker midostaurin48 and antibodies directed against CD34+/CD38− cells, like GO or alemtuzumab may be a preferable strategy. Supporting this assumption, we were able to show that combinations of midostaurin and GO cooperate in inhibiting growth of MCL CD34+/CD38− cells and ROSAKIT D816V cells in vitro. Moreover, these drug combinations were found to inhibit engraftment of ROSAKIT D816V cells in NSGhSCF mice. Finally, we were able to show that midostaurin exerts suppressive effects on CD34+ cells in vivo in patients with ASM/MCL. However, as expected, no complete eradication of CD34+ stem and progenitor cells was produced by midostaurin. An advantage of applying antibody-type drugs may be that these drugs also kill slowly cycling or even quiescent LSC. However, not all antibody-druggable targets were found to be expressed on MCL CD34+/CD38− cells. Likewise, CD52 was only expressed on MCL CD34+/CD38− cells in a smaller subset of patients. The cell surface target Ki-1 (CD30) that has recently been identified on neoplastic MC in SM,49,50 was only slightly expressed on MCL CD34+/CD38− cells. By contrast, although expression levels varied from donor to donor, CD33 was found to be expressed on MCL CD34+/CD38− cells in all patients tested, suggesting that this target may be a reasonable candidate to consider for immunotherapies employing GO, novel targeted antibodies or a CAR-T cell-approach.
In summary, we have identified a disease-initiating and -propagating neoplastic stem cell in MCL. These cells reside within a small fraction of CD34+/CD38− cells in the malignant clone. Moreover, we established the phenotype and target expression profiles of MCL CD34+/CD38− cells and provide evidence that targeting LSC with specific antibody-based drugs or small molecules may lead to LSC-depletion. Our data may have theoretical and clinical implications and could assist in the development of novel LSC-eradicating treatments in this fatal disease.
Supplementary Material
Supplementary information is available at Leukemia’s website.
Acknowledgement
We would like to thank Tina Bernthaler, Mathias Schneeweiss, Karin Bauer, Niklas Müller and Sabine Cerny-Reiterer for skillful technical assistance. This study was supported by the Austrian Science Fund (FWF), SFB grants F4701 and F4704, and a Research Grant of the Medical University of Vienna, Austria. Cell sorting experiments were performed with support from the Core Facility Flow Cytometry, Medical University of Vienna.
Footnotes
Author Contributions
G.E. and P.V. planned the study; G.E., I.S., A.K., D.B., K.B., G.S., and C.W. performed the experiments; G.E., G.H., M.B., M.W., T.R., and P.V. analyzed data; J.S., M.J., M.W., W.R.S., T.R., A.R., M.A., and P.V. provided research materials; G.E., G.H., M.W., M.A., A.R., and P.V. wrote the manuscript.
Conflict of Interest
P.V. received a research grant from Novartis, from Blueprint, and from Deciphera, and received honoraria from Novartis, Celgene, Pfizer, and Deciphera. P.V. and A.R. served as a Consultant in the global Novartis trial examining the effects of midostaurin in advanced SM. W.R.S. received honoraria from Novartis and Celgene. M.A. received honoraria from Deciphera, Novartis and Roche Diagnostics, and a research grant from Agensys Inc, Blueprint Medicines and Deciphera. A.R. received a research grant from Novartis, honoraria from Novartis and BMS, and served in advisory boards organized by Deciphera, Blueprint, and Baxalta/Shire. G.H. received honoraria from Novartis.
References
- 1.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris M-O, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121:1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Li CY, Hoagland HC, Travis LB, Banks PM. Mast cell leukemia: report of a case and review of the literature. Mayo Clin Proc. 1986;61:957–966. doi: 10.1016/s0025-6196(12)62636-6. [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim K-H, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 5.Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–536. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 6.Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny H-P, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27:635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 7.Valentini CG, Rondoni M, Pogliani EM, Van Lint MT, Cattaneo C, Marbello L, et al. Mast cell leukemia: a report of ten cases. Ann Hematol. 2008;87:505–508. doi: 10.1007/s00277-007-0430-3. [DOI] [PubMed] [Google Scholar]
- 8.Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264–3274. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escribano L, Orfao A, Díaz-Agustin B, Villarrubia J, Cerveró C, López A, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998;91:2731–2736. [PubMed] [Google Scholar]
- 10.Escribano L, Díaz-Agustín B, Bellas C, Navalón R, Nuñez R, Sperr WR, et al. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk Res. 2001;25:563–570. doi: 10.1016/s0145-2126(01)00050-9. [DOI] [PubMed] [Google Scholar]
- 11.Sotlar K, Horny H-P, Simonitsch I, Krokowski M, Aichberger KJ, Mayerhofer M, et al. CD25 indicates the neoplastic phenotype of mast cells: a novel immunohistochemical marker for the diagnosis of systemic mastocytosis (SM) in routinely processed bone marrow biopsy specimens. Am J Surg Pathol. 2004;28:1319–1325. doi: 10.1097/01.pas.0000138181.89743.7b. [DOI] [PubMed] [Google Scholar]
- 12.Teodosio C, García-Montero AC, Jara-Acevedo M, Sánchez-Muñoz L, Alvarez-Twose I, Núñez R, et al. Mast cells from different molecular and prognostic subtypes of systemic mastocytosis display distinct immunophenotypes. J Allergy Clin Immunol. 2010;125:719–726, 726.e1-726.e4. doi: 10.1016/j.jaci.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Muñoz L, Teodosio C, Morgado JMT, Perbellini O, Mayado A, Alvarez-Twose I, et al. Flow cytometry in mastocytosis: utility as a diagnostic and prognostic tool. Immunol Allergy Clin North Am. 2014;34:297–313. doi: 10.1016/j.iac.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 15.Joris M, Georgin-Lavialle S, Chandesris M-O, Lhermitte L, Claisse J-F, Canioni D, et al. Mast Cell Leukaemia: c-KIT Mutations Are Not Always Positive. Case Rep Hematol. 2012;2012 doi: 10.1155/2012/517546. 517546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mital A, Piskorz A, Lewandowski K, Wasag B, Limon J, Hellmann A. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur J Haematol. 2011;86:531–535. doi: 10.1111/j.1600-0609.2011.01598.x. [DOI] [PubMed] [Google Scholar]
- 17.Valent P, Blatt K, Eisenwort G, Herrmann H, Cerny-Reiterer S, Thalhammer R, et al. FLAG-induced remission in a patient with acute mast cell leukemia (MCL) exhibiting t(7;10)(q22;q26) and KIT D816H. Leuk Res Rep. 2014;3:8–13. doi: 10.1016/j.lrr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damaj G, Joris M, Chandesris O, Hanssens K, Soucie E, Canioni D, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS ONE. 2014;9:e85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanssens K, Brenet F, Agopian J, Georgin-Lavialle S, Damaj G, Cabaret L, et al. SRSF2-p95 hotspot mutation is highly associated with advanced forms of mastocytosis and mutations in epigenetic regulator genes. Haematologica. 2014;99:830–835. doi: 10.3324/haematol.2013.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 21.Wilson TM, Maric I, Simakova O, Bai Y, Chan EC, Olivares N, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 23.Copland M. Chronic myelogenous leukemia stem cells: What’s new? Curr Hematol Malig Rep. 2009;4:66–73. doi: 10.1007/s11899-009-0010-9. [DOI] [PubMed] [Google Scholar]
- 24.Eisterer W, Jiang X, Christ O, Glimm H, Lee KH, Pang E, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19:435–441. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- 25.Kavalerchik E, Goff D, Jamieson CHM. Chronic myeloid leukemia stem cells. J Clin Oncol. 2008;26:2911–2915. doi: 10.1200/JCO.2008.17.5745. [DOI] [PubMed] [Google Scholar]
- 26.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 28.Valent P. Targeting of leukemia-initiating cells to develop curative drug therapies: straightforward but nontrivial concept. Curr Cancer Drug Targets. 2011;11:56–71. doi: 10.2174/156800911793743655. [DOI] [PubMed] [Google Scholar]
- 29.Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson CHM, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann H, Sadovnik I, Cerny-Reiterer S, Rülicke T, Stefanzl G, Willmann M, et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123:3951–3962. doi: 10.1182/blood-2013-10-536078. [DOI] [PubMed] [Google Scholar]
- 32.Järås M, Johnels P, Hansen N, Agerstam H, Tsapogas P, Rissler M, et al. Isolation and killing of candidate chronic myeloid leukemia stem cells by antibody targeting of IL-1 receptor accessory protein. Proc Natl Acad Sci USA. 2010;107:16280–16285. doi: 10.1073/pnas.1004408107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rhenen A, van Dongen GAMS, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 34.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, et al. Identification of Therapeutic Targets for Quiescent, Chemotherapy-Resistant Human Leukemia Stem Cells. Science Translational Medicine. 2010;2:17ra9–17ra9. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritsche-Polanz R, Fritz M, Huber A, Sotlar K, Sperr WR, Mannhalter C, et al. High frequency of concomitant mastocytosis in patients with acute myeloid leukemia exhibiting the transforming KIT mutation D816V. Mol Oncol. 2010;4:335–346. doi: 10.1016/j.molonc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgin-Lavialle S, Lhermitte L, Baude C, Barete S, Bruneau J, Launay J-M, et al. Blood CD34-c-Kit+ cell rate correlates with aggressive forms of systemic mastocytosis and behaves like a mast cell precursor. Blood. 2011;118:5246–5249. doi: 10.1182/blood-2011-02-335950. [DOI] [PubMed] [Google Scholar]
- 37.Nagai S, Ichikawa M, Takahashi T, Sato H, Yokota H, Oshima K, et al. The origin of neoplastic mast cells in systemic mastocytosis with AML1/ETO-positive acute myeloid leukemia. Exp Hematol. 2007;35:1747–1752. doi: 10.1016/j.exphem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Rottem M, Okada T, Goff JP, Metcalfe DD. Mast cells cultured from the peripheral blood of normal donors and patients with mastocytosis originate from a CD34+/Fc epsilon RI- cell population. Blood. 1994;84:2489–2496. [PubMed] [Google Scholar]
- 39.Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res. 2008;14:1926–1930. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- 40.Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, Hasegawa T, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–2777. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25:1691–1700. doi: 10.1093/annonc/mdu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blatt K, Herrmann H, Hoermann G, Willmann M, Cerny-Reiterer S, Sadovnik I, et al. Identification of campath-1 (CD52) as novel drug target in neoplastic stem cells in 5q-patients with MDS and AML. Clin Cancer Res. 2014;20:3589–3602. doi: 10.1158/1078-0432.CCR-13-2811. [DOI] [PubMed] [Google Scholar]
- 43.Saleh R, Wedeh G, Herrmann H, Bibi S, Cerny-Reiterer S, Sadovnik I, et al. A new human mast cell line expressing a functional IgE receptor converts to tumorigenic growth by KIT D816V transfection. Blood. 2014;124:111–120. doi: 10.1182/blood-2013-10-534685. [DOI] [PubMed] [Google Scholar]
- 44.Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(-) fraction. Blood. 2010;115:1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Födinger M, Fritsch G, Winkler K, Emminger W, Mitterbauer G, Gadner H, et al. Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood. 1994;84:2954–2959. [PubMed] [Google Scholar]
- 46.Florian S, Sonneck K, Hauswirth AW, Krauth M-T, Schernthaner G-H, Sperr WR, et al. Detection of molecular targets on the surface of CD34+/CD38-- stem cells in various myeloid malignancies. Leuk Lymphoma. 2006;47:207–222. doi: 10.1080/10428190500272507. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Twose I, Martínez-Barranco P, Gotlib J, García-Montero A, Morgado JM, Jara-Acevedo M, et al. Complete response to gemtuzumab ozogamicin in a patient with refractory mast cell leukemia. Leukemia. 2016;30:1753–1756. doi: 10.1038/leu.2016.30. [DOI] [PubMed] [Google Scholar]
- 48.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N Engl J Med. 2016;374:2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 49.Blatt K, Cerny-Reiterer S, Schwaab J, Sotlar K, Eisenwort G, Stefanzl G, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood. 2015;126:2832–2841. doi: 10.1182/blood-2015-03-637728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585–595. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.