Abstract
Barrett’s oesophagus is a condition which predisposes towards development of oesophageal adenocarcinoma, a highly lethal tumour which has been increasing in incidence in the Western world over the past three decades. There have been tremendous advances in the field of Barrett’s oesophagus, not only in diagnostic modalities, but also in therapeutic strategies available to treat this premalignant disease. In this review, we discuss the past, present and future of Barrett’s oesophagus. We describe the historical and newevolving diagnostic criteria of Barrett’s oesophagus, while also comparing and contrasting the British Society of Gastroenterology guidelines, American College of Gastroenterology guidelines and International Consensus (BOBCAT) for Barrett’s oesophagus. Advances in endoscopic modalities such as confocal and volumetric laser endomicroscopy, and a non-endoscopic sampling device, the Cytosponge, are described which could aid in identification of Barrett’s oesophagus. With regards to therapy we review the evidence for the utility of endoscopic mucosal resection and radiofrequency ablation when coupled with better characterization of dysplasia. These endoscopic advances have transformed the management of Barrett’s oesophagus from a primarily surgical disease into an endoscopically managed condition.
Keywords: Barrett’s oesophagus, diagnosis, management, Cytosponge, Trefoil Factor 3, TP53, endoscopy, radiofrequency ablation, endoscopic mucosal resection, dysplasia, low grade dysplasia, high grade dysplasia, oesophageal adenocarcinoma, confocal laser endomicroscopy, volumetric laser endomicroscopy, cryotherapy, chemoprevention
Introduction
Population studies have suggested that up to 1.6% of Europeans have Barrett’s oesophagus (BO), a condition in which the native squamous epithelial lining of the distal oesophagus undergoes metaplastic change to a columnar epithelium due to chronic damage caused by gastro-oesophageal reflux disease (GORD) (1, 2). Barrett’s oesophagus and its predisposing condition, GORD is a major risk factor for the development of oesophageal adenocarcinoma (OAC), a highly malignant cancer which has been increasing in the Western population over the past three decades (3–6).
Ever since the relationship between BO and OAC was established in the 1970s, there has been a rapid increase in research activity in the field of BO particularly in its diagnosis and management. The common goal among investigators is to curb the progression of this precancerous condition before incurable malignancy sets in (7–9). However, with advancing knowledge has come misconception and controversy particularly with regards to the definition and the diagnostic criteria of BO. Even today there remains no universally adopted definition of BO among authorities in this field.
In this review, we describe the past, present and future of BO. We further explore the evolving definition and diagnostic criteria of BO and try to understand where there is consensus and which areas still require resolution. In addition, we describe developments in therapeutic modalities and how this has the potential to impact on the mortality of OAC in the future.
Diagnosis of Barret’s Oesophagus
Historical perspective and evolution of the diagnostic criteria for Barrett’s oesophagus
BO bears its name from the pioneering British surgeon, Norman Barrett who in 1950 published his seminal paper–‘Chronic peptic ulcer of the oesophagus and ‘Oesophagitis” in which he described the columnar-lined oesophagus (10, 11). However, it was Wilder Tileston who first reported three cases of ‘peptic ulcer of the oesophagus’ in 1906wherein he described the histology of the ulcer and adjacent epithelium which resembled a gastric ulcer in columnar epithelium (12). Over the next four decades, disagreements regarding the distal oesophageal histology were prevalent, with some arguing that the ulcers in the distal oesophagus were not oesophageal, but gastric ulcers within an intrathoracic stomach in patients with congenital short oesophagus (13–16). In fact, this notion was supported by Barrett in his paper in 1950 (10).
In 1953, Allison and Johnstone published an influential report rejecting Barrett’s hypothesis, and suggesting that the tubular structure within the distal thorax could not be stomach since it: 1) lacked an outer peritoneal lining; 2) had musculature identical to oesophagus; 3) consisted of columnar epithelium interspersed with squamous islands; 4) lacked mucosal oxyntic cells; and 5) had mucosal glands typical of the oesophagus (17). Subsequent reassessment of these ‘gastric’ ulcers by Barrett led him to acknowledge his prior misjudgement, and he published a revised report in 1957, redefining this tubular structure as ‘lower oesophagus lined by columnar epithelium’ (18).
Between 1960 to the mid-1970s, there were varying histological descriptions of the columnar subtypes in the distal oesophagus including junctional (gastric cardiac epithelium), gastric-fundal, and intestinal epithelium with goblet cells (19–21). This histologic conundrum was clarified in 1976 by Paull et al, who performed biopsies on 11 patients with a columnar-lined distal oesophagus and elucidated the presence of a histologic spectrum which from most proximal to distal comprised: columnar epithelial containing villi and goblet cells (now known as intestinal metaplasia, IM and sometimes referred to as Specialised Intestinal Metaplasia); followed by junctional epithelium; and finally, atrophic gastric fundal epithelium with chief and parietal cells (22).
In the 1980sit was established that GORD and the presence of a hiatal hernia were risk factors for BO and it grew to be appreciated that these could distort the anatomic landmarks of the GOJ during endoscopy making a precise diagnosis difficult (23, 24). To avoid error, diagnostic criteria for BO were established by Skinner et al who proposed that a (25). By the mid-1980s, the association between BO and OAC was well established (7–9) and it became clear that IM had a mosaic distribution with strong predisposition to dysplasia which led to IM becoming the defining feature for BO (26, 27).
In the mid-1990s, Spechler et al challenged the conventional practice of only performing biopsies on BO ≥3cm because he demonstrated that 18% of patients with endoscopically apparent BO measuring less than 3 cm still contained IM (28). Furthermore, there were reports of OAC developing from BO <3 cm (29, 30). These results, coupled with the categorization of BO into short (≤3 cm) and long segments (≥3 cm) have proved essential in shaping the diagnostic criteria for BO over the years (31).
Current Diagnostic Criteria for Barrett’s Oesophagus
The quality of endoscopic images has improved significantly with the advent of high resolution endoscopes making it easier to discern the landmarks. Today, a diagnosis of BO requires endoscopic visualization of columnar epithelium ≥1 cm above the gastro-oesophageal junction (GOJ) in addition to histological confirmation of columnar metaplasia (32).
Endoscopic Diagnosis of Barrett’s Oesophagus
Endoscopy remains the gold standard to diagnose BO. During endoscopy, three important landmarks need to be recognized: 1) the GOJ, 2) the diaphragmatic pinch and 3) the squamo-columnar junction (SCJ). The GOJ signals the end of the oesophagus and the start of the stomach and is best identified as the most proximal margin of the gastric folds (33). The diaphragmatic pinch is the point at which the diaphragmatic crura constricts or ‘pinches’ the oesophagus and is an important landmark to denote the presence of a hiatal hernia. The SCJ is the transitional point between stratified squamous and columnar epithelial of the stomach. Visually, squamous epithelial has a pale glossy colour while columnar epithelial adopts a darker reddish appearance due to its increased vasculature. In normal oesophagus, the GOJ and SCJ coincides. However, when the SCJ lies ≥1 cm above the GOJ at the level of its most proximal extension, then this suggests the presence of BO.
Histological Diagnosis of Barrett’s Oesophagus
Histologic criteria for BO still remain a contentious issue. The recent American College of Gastroenterology (ACG) requires biopsies confirming IM as a pre-requisite to diagnose BO (34, 35). However, the British Society of Gastroenterology (BSG) guideline stipulates that in the context of visible columnar epithelium with biopsy confirmation, IM is not a pre-requisite and hence gastric metaplasia is also regarded as a type of BO (32) (Table 1). The recent International Benign Barrett’s and Cancer Taskforce (BOBCAT) consensus defines BO as presence of columnar epithelial but stipulates that it should be clearly stated whether IM is present above the GOJ (36). The BSG and ACG difference hinges on the differential risk of malignant transformation between columnar epithelium with and without IM. The emphasis on IM as a defining feature of BO is based on increasing number of studies that have demonstrated a stronger association between IM and OAC than non-IM. For example, a study of 8,522 patients with BO reported that the risk for malignant progression of IM was greater compared to gastric metaplasia (0.38%/year vs 0.07%/year, hazard ratio, HR=3.54, 95% CI= 2.09–6.00, P<0.01) (37). Chandrasoma et al then showed that among 214 patients with columnar oesophagus who had biopsies taken with strict adherence to Seattle protocol, IM was noted in all patients who had dysplasia or OAC, while none of the patients with cardiac-type epithelium alone displayed dysplastic features or OAC (38). More recently, detailed genomic analysis comparing IM and non-IM epithelium in 45 patients with BO reported a higher frequency of mutations in cancer-associated genes such as CDKN2A, WWOX, c-MYC and GATA6 in IM (39).
Table 1. Comparison between BSG guidelines, ACG guidelines and BOBCAT consensus in the diagnosis of Barrett’s oesophagus.
| Diagnostic feature | British Society of Gastroenterology, 2014 (32) | American College of Gastroenterology, 2016 (34) | International Consensus (BOBCAT), 2015 (36) |
|---|---|---|---|
| Definition | Endoscopically visible metaplastic columnar epithelial ≥1cm above the GOJ plus biopsy confirmation of columnar metaplasia | Endoscopically visible metaplastic columnar epithelial ≥1cm above the GOJ plus biopsy confirmation of IM | Endoscopically visible metaplastic columnar epithelial ≥1cm above the GOJ, and pathologist should clearly state whether IM is present on biopsies above the GOJ |
| Endoscopic landmark for localizing GOJ | Proximal extent of the gastric folds | Proximal extent of the gastric folds | Proximal extent of the gastric folds |
| Reporting the extent of Barrett’s oesophagus | Usage of Prague C&M criteria | Usage of Prague C&M criteria | Usage of Prague C&M criteria |
| Biopsy Protocol for suspected Barrett’s oesophagus | Random 4 quadrant biopsies every 2cm plus biopsy of visible lesions | Random 4 biopsies every 2cm or 8 random biopsies to maximize IM yield. For suspected short segment BO where 8 biopsies is unattainable, a minimum of 4 biopsies/cm circumferential extent and 1 biopsy/cm Barrett’s tongue is recommended | Random 4 quadrant biopsies every 2cm plus biopsy of visible lesions |
| Normal Z line or Z line <1cm from GOJ | Routine biopsy not recommended | Routine biopsy not recommended | Not discussed |
| Confirmation of Dysplasia | Cases of suspected dysplasia need to be confirmed by a second GI pathologist | Cases of suspected dysplasia need to be confirmed by a second GI pathologist | Cases of suspected dysplasia need to be confirmed by a second GI pathologist |
| Use of p53 biomarker to aid dysplasia diagnosis | Should be be considered as adjunct to current diagnostic tools in the diagnosis of dysplasia | Not recommended | Not recommended for routine use, but can be considered as adjunct to aid diagnosis if done in specialist centers |
BSG, British Society of Gastroenterology; ACG, American College of Gastroenterology; BOBCAT; Benign Barrett’s and Cancer Taskforce, GOJ, Gastro-oesophageal junction; C&M, circumferential and maximum; IM, Intestinal Metaplasia; GI, gastrointestinal
However, other studies have not corroborated such findings. A retrospective analysis of 688 patients reported no significant difference in cancer risk of IM versus non-IM (40). However, this study did not provide details regarding endoscopic findings, and whether those without IM went on to develop IM during follow-up. In another study, Takubo et al examined the mucosa adjacent to 141 cases of OAC resected endoscopically and found >70% of OAC were lying adjacent to non-IM columnar epithelial, indicating that non-IM epithelial could also harbour features for malignancy (41). However, given that IM has a non-uniform distribution in BO, the extent of the pathological examination is critical and it is also possible that these cancers could have originated from the stomach.
The BSG guidelines argues for a broader diagnostic criteria to encompass columnar metaplasia with or without IM because IM detection is prone to sampling error and because understanding of the cellular and molecular basis for malignant risk continues to evolve (42). The Seattle protocol which incorporates 4-quadrant biopsies every 1-2cm provides a rigorous and reliable method for obtaining adequate biopsies for BO diagnosis; however, this protocol is not strictly adhered to in clinical practice. A Dutch study showed that adherence to the Seattle protocol was 79% for BO up to 5cm, but decreased to 30% for BO lengths 10-15cm (43). Similar findings were reported in a large study of 2,245 patients where only 51% of BO diagnosis adhered to the protocol (44). In a comparative study designed to determine the optimal number of oesophageal biopsies for IM, investigators showed that the diagnostic yield for IM increases with the number of biopsies (45). When the number of biopsies increased from 4, to 8 and to ≥16, diagnostic accuracy for IM increased from 34.7% to 67.9% and to 100%, respectively (45). These conclusions have led to the latest ACG recommendation of obtaining at least 8 random biopsies on suspected Barrett’s column during index endoscopy to maximize diagnostic yield (34). While obtaining ≥16 biopsies would achieve 100% accuracy, this would not only be time consuming, but might also increase the risk of post-biopsy haemorrhage as well as the increased cost of processing biopsy.
In practical terms, the three definitions are consistent. A European consensus statement is currently being written and agrees with the statements defined here, however, similar to the ACG, it requires IM for diagnosis (personal communication). Whilst the BSG guideline includes all histologically confirmed metaplasia in the definition, the clinical follow-up for any individual patient is determined by the risk of cancer progression and aside from dysplasia two of the strongest risk factors are segment length (46, 47) and presence of IM. A recent study by Pohl et al showed that the annual risk of cancer progression of long (≥3cm), short (≥1 to ≤3cm) and ultra-short (≤1cm) BO varied considerably, with the risk being 0.22%, 0.03% and 0.01% respectively (46). Hence, for patients with short segments (<3cm) without IM, it is recommended that the procedure is repeated to ensure that there is indeed columnar mucosa within the oesophagus (and that this has not been mistaken for a hiatus hernia) and to repeat biopsies for IM. In patients with a short segment of gastric-type epithelium the risks of surveillance are thought to outweigh the benefits in view of the low risk for cancer progression (46). All three guidelines support the use of the proximal gastric folds as the landmark for delineating the GOJ. Similarly, all three guidelines endorse the application of the Prague C&M criteria (grading system for BO according to its circumferential extent (C value, in cm), and the maximum length of BO tongues (M value, in cm)) when reporting BO length since it has good inter-observer reliability (r =0.72) for BO≥1 cm (48).
Confirmation of Dysplasia
Dysplasia is a biomarker for cancer risk in BO and is graded according to the Vienna classification (49). However, there remains substantial inter-observer variability with regards to the grading of dysplasia between pathologists. In a Dutch study where two gastrointestinal (GI) pathologists retrospectively reviewed 293 BO specimens with a prior diagnosis of low–grade dysplasia (LGD), only 27% had ‘true’ LGD, while the remaining 73% were downgraded to non-dysplasia (ND) or indefinite-for-dysplasia (IND) (50). Following histologic review, patients with confirmed LGD were shown to have a higher risk for cancer progression (9.1%/patient-year) compared to those who were downgraded to ND (0.6%/patient-year) and IND (0.9%/patient-year) (50). Recently, a study comparing pathologist from the United States and Europe showed poor inter-observer agreement when diagnosing LGD (k=0.11, 95% CI 0.004-0.15) (51). As the grading of dysplasia invariably dictates management strategies, unsurprisingly all three guidelines require that the diagnosis of dysplasia is confirmed by two GI pathologists (32, 34, 36).
Histologic confirmation of dysplasia thus remains the only acceptable predictor for cancer progression; however, it is prone to sampling bias and high inter-observer variability. Alternative biomarkers, in particular p53 expression has emerged as a possible adjunct to improve risk stratification of BO. Sikkema et al showed that p53 protein overexpression was a more powerful predictor of progression to high-grade dysplasia (HGD) orOAC irrespective of histology (HR 6.5; 95% CI 2.5-17.1) compared to a diagnosis of LGD (HR 3.6; 95% CI 1.6-8.1) (52). More recently, an analysis of >12,000 biopsies from 635 patients with BO showed that aberrant p53 expression (p53 overexpression or loss of p53 expression which can occur with a truncating mutation of the p53 gene) was associated with increased cancer risk, and furthermore the risk was higher for BO with loss of p53 expression (adjusted relative risk, RRa 14.0, 95% CI 5.3-37.2) compared to BO with p53 overexpression (RRa 5.6, 95% CI 3.1-10.3) (53). Furthermore, immunohistochemistry for p53 detection had good inter-observer reliability (53). Therefore, although p53 staining has not yet reach mainstream clinical use, its use could allow more accurate risk stratification of BO into higher risk groups who will require more intensive surveillance.
Future Diagnostic Strategies for Barrett’s Oesophagus
Technologies for diagnosing BO have advanced over the years with a number of technologies aimed to better characterize dysplasia in secondary care (confocal and volumetric laser endomicroscopy). However, whether these modalities actually increase dysplasia detection has not been proven. Since the majority of Barrett’s is undiagnosed technologies have alse been developed for diagnosing BO more readily in primary care have also emerged (tethered capsule endomicroscopy, transnasal endoscopy and Cytosponge).
Confocal Laser Endomicroscopy
Confocal Laser Endomicroscopy (CLE) is a powerful imaging modality that combines endoscopy and microscopy to obtain high resolution and magnified images of the GI mucosa. CLE comes in the form of a probe-based system (pCLE) where a probe is passed through a port within the endoscope. An endoscope-based CLE (eCLE) previously existed, however, this system is no longer available on the market. CLE is based on the principle of tissue illumination by a blue laser (488nm), with detection of fluorescence reflecting off tissues aided by the application of fluorescein which is excited by the laser (54, 55). CLE can achieve subcellular resolution up to 250μm depth with 500-1000x magnification (56). This permits in-vivo tissue evaluation at endoscopy and can effectively distinguish non-dysplastic, dysplastic and neoplastic epithelium (57, 58).
Trials for pCLE have been promising in which addition of pCLE to WLE and narrow band imaging (NBI, an endoscopic technology that uses light of shorter wavelength to allow better visualization of mucosal abnormalities and vascular patterns associated with dysplasia) (59) have reported increased sensitivity of detection of neoplasia from 45.0% to 75.8% (p=0.01) (60). Further, the use of autofluorescence imaging (AFI, a technology that detects abnormal tissue architecture by exploiting fluorescence off oesophageal mucosa) with pCLE to detect any grade of dysplasia in real-time (‘optical biopsy’) has been shown to have a sensitivity of 96.4% sensitivity compared to 57.1% sensitivity for AFI with NBI (61). While CLE promise the interpretation requires specialist training and expertise in interpretation therefore its use is currently restricted to academic hospitals. Current work is underway to define criteria for diagnosing dysplasia that could be adopted across studies.
Volumetric Laser Endomicroscopy
Optical coherence tomography (OCT) relies on the principle of backscattering of light to produce high resolution images. A new generation OCT, Volumetric Laser Endomicroscopy (VLE) is an emerging technology that incorporates a rotating optical laser probe centered within a transparent balloon. A laser (wavelength 1350nm) emanating from the probe in a helical fashion, with an automated pullback, circumferentially scans 6cm of the distal oesophagus up to 3mm depth to produce cross-sectional images of the oesophagus up to the submucosal layer (62). A case series of 6 patients with long segment BO who underwent both WLE with NBI at index endoscopy, followed-up by VLE with targeted biopsies within 6 months showed that VLE led to upstaging of disease status, allowing these patient to qualify for ablative therapy (63). The requirement for a gold standard for these studies can lead to difficulty in determining which is superior.
A tethered-capsule endomicroscopy device has also been designed which utilises optical frequency domain imaging technology to generate 3D, microscopic images of the oesophageal wall at 30μm lateral, and 7μm axial resolution (64). The capsule is swallowed and then withdrawn upon reaching the stomach. During transit, cross-sectional images of the oesophagus are acquired and the images are reconstructed to produce a 3D representation of the entire oesophagus (64). A feasibility study on 7 healthy volunteers and 6 volunteers with BO showed that this procedure is safe whilst also producing high quality subsurface images that are easily missed on WLE (64).
This technology opens up new avenues for BO imaging as it not only can be used as a screening modality, but could also detect architectural abnormalities of mucosa and submucosa which could indicate dysplasia (64). The ease of performing the procedure with minimal training required, coupled with the ability of the capsule to be disinfected and reused might make it cost-effective and feasible as a screening tool in primary care. Although promising, larger studies assessing the accuracy of VLE imaging and histopathologic correlation are necessary prior to adopting this technology into routine practice.
Transnasal Endoscopy
Transnasal endoscopy (TNE) has emerged as a possible alternative to transoral endoscopy for diagnosing BO. Major endoscope companies (Fujinon, Pentax, Olympus, Vision Sciences) have produced ‘ultrathin’ endoscopes with a slimmer diameter (5-6mm) than the standard endoscope, whilst also containing a working channel (up to 2mm diameter) which allows for biopsies. A portable and disposable transnasal endoscope (E.G. Scan™, IntroMedic, Seoul, South Korea) has also been developed and when compared to traditional endoscopy, was shown to have reasonable level of agreement of detecting BO (k=0.617, 95% CI 0.378-0.860)(65). More recently, a transnasal endoscope with a disposable sheath (TNE-5000 with Endosheath, Vision Science, NY, USA) has been developed which protects the scope from contact with body fluids and circumvents the need for decontamination. In a pilot crossover RCT, Endosheath technology had a 100% sensitivity and specificity for obtaining an endoscopic diagnosis of BO, and a 66.7% and 100% sensitivity and specificity respectively, for obtaining a histologic diagnosis of BO when compared to trans-oral endoscopy (66). The advantages of TNE includes: 1) better patient tolerance and acceptability, 2) better safety profile (no need for sedation) and 3) suitability for use in primary care (E.G. Scan™) (66, 67). It could also be cost-effective as it can be performed by technicians after sufficient training and does not require post-procedural vital sign monitoring (67). Despite its many advantages, limitations of TNE include failure of intubation (due to narrow nasal canal) and epistaxis (up to 5%) (67). Although transoral endoscopy remains the standard for upper GI endoscopy, the many advantages of TNE is a promising tool for BO screening in primary care. It is not recommended for surveillance as the field of view, image quality and size of biopsies are not optimal for detection of dysplasia.
Cytosponge and Trefoil Factor-3
The Cytosponge is a cell sampling device that comprises a small compressed mesh within a gelatin capsule (Figure 1) (68). The capsule is swallowed and disintegrates upon reaching the stomach to release a 3cm-diameter spherical mesh that is withdraw by pulling the string which then samples the entire length of the oesophagus, collecting up to one-million cells (42). Immunohistochemistry for Trefoil Factor-3 (TFF3), a protein which is over-expressed in BO, is then performed on paraffin-embedded cytologic specimens as an objective diagnostic biomarker which is scored as positive or negative (69).
Figure 1.
The Cytosponge expanded (left) and encapsulated (right). Reproduced with permission from Kadri et al. (68)
The BEST-1 feasibility study evaluated the use of Cytosponge-TFF3 to diagnose BO and showed that it was applicable in primary care and although not the primary outcome had a promising specificity of 93.8% and sensitivity of 73.3% for detecting BO ≥1 cm. For segments ≥2cm, the specificity and sensitivity were 93.5% and 90.0%, respectively (68). The subsequent BEST-2 study which enrolled 1,110 participants in a case-control design to enable assessment of sensitivity and specificity, reported 79.9% sensitivity for Cytosponge-TFF3 to detect BO increasing to 87.2% for BO circumferential segment ≥3 cm (70). The specificity for BO was 92.4% (70). These figures are comparable to the current colorectal cancer screening programme using faecal occult blood test (FOBT) which has a sensitivity and specificity range of 6.2%–83.3% and 65%–99% respectively (71).
The BEST-3 study will begin recruitment in early 2017 with the aim of comparing the use of the Cytosponge-TFF3 with standard care (lifestyle advice, acid-suppressing medications and, Helicobacter Pylori eradication) against standard care alone in patients with reflux disease in the primary care setting. This study is designed to assess whether Cytosponge-TFF3 could lead to increased detection of BO in primary care and to evaluate the health economics of this approach. It is hoped that this study will provide pivotal information regarding the development of a comprehensive and cost-effective screening programme for BO.
Therapy for Barrett’s Oesophagus
Treatments for BO have evolved considerably over the past twenty years and have altered the clinical rationale for detection of Barrett’s. Traditionally, oesophagectomy was the only option for high-grade dysplasia and carcinoma; however, with advancing technology, endoscopic therapy has become the mainstay treatment for BO. We begin by describing the brief history of oesophagectomy, followed by discussion on the current (surveillance, radiofrequency ablation and endoscopic resection) and future (cryotherapy and chemoprevention) management strategies for BO.
Historical treatment for Barrett’s Oesophagus- Oesophagectomy
Oesophagectomy still remains the only definitive therapy for invasive OAC. Although surgical outcomes have improved over the years, oesophagectomy still remains a challenging procedure as patients often have multiple existing co-morbidities and so it is not without risks. The transthoracic oesophagectomy (Ivor Lewis oesophagectomy, ILO) is considered the gold standard procedure and was first performed by Ivor Lewis in 1944 on a patient who had OAC of the distal oesophagus (72). He performed a laparotomy to mobilize the stomach and a left-sided thoracotomy for resecting the oesophagus (72). Although there are variations to the standard oesophagectomy including a transhiatal approach, the Ivor Lewis procedure is often preferable as it permits better visualization of abdominal contents and allows for wide margins of lymph node dissection (73).
With the success of laparoscopic surgery during the late 1980s, Watson et al reported two cases of minimally invasive oesophagectomy (MIO) by utilising laparoscopic means for gastric mobilization, followed by a thoracoscopic approach for oesophageal resection and anastomosis, achieving excellent results with shorter hospital stay and convalescence (74). A recent phase 3 RCT (MIRO trial) comparing open ILO to MIO reported favourable short-term outcomes for MIO with lower post-operative morbidity (37 vs 67, p=0.0001) and pulmonary complications (18 vs 31, p=0.037), but no difference in 30-day mortality between groups (75). More recently, robotic-ILO is gaining popularity since it provides magnified images and better freedom of movement via wristed motions compared to laparoscopic approach (76). However, more studies are needed to assess the safety and outcomes of robotic-ILO compared to open or MIO.
Current management for Barrett’s oesophagus
Surveillance and Endoscopic therapy
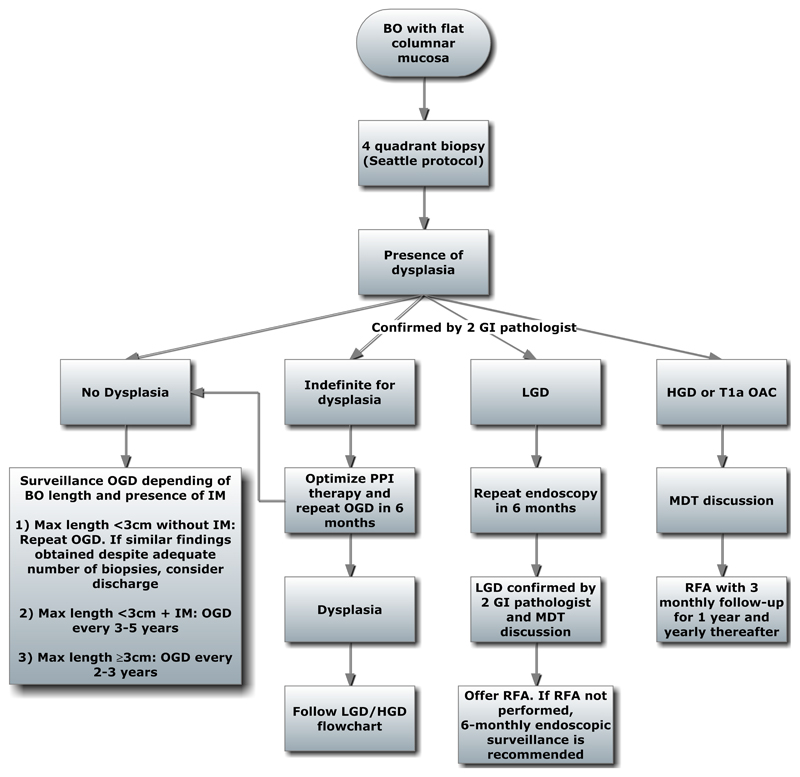
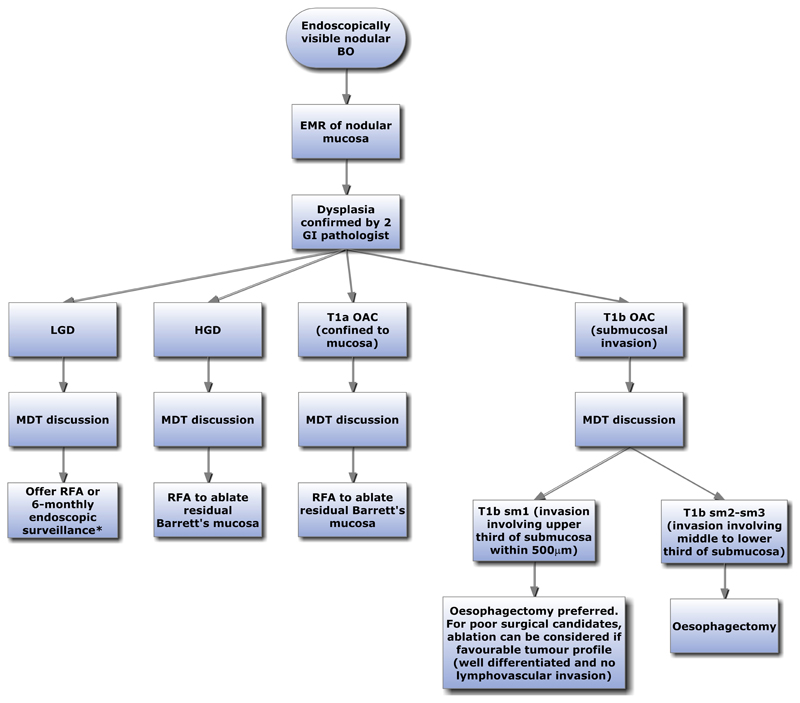
Algorithms for the management of flat and nodular BO, with or without dysplasia are shown (Figure 3 and 4). For non-dysplastic BO, both the ACG and BSG recommend endoscopic surveillance (Table 2). However, the BOBCAT consensus does not recommend surveillance, but if surveillance is undertaken, suggests that it should be targeted at high risk patients stratified according to patient demographics, BO length, frequency and severity of symptoms (Table 2). However, there is currently no clinically adopted algorithm for a risk stratification approach due to paucity of data and this is an area which requires further research. A recent paper describes a risk-stratification panel comprising clinical factors (age, waist-to-hip ratio and BO segment length) and molecular biomarkers applied to a Cytopsonge sample (glandular atypia, Aurora Kinase A, and p53). A risk stratification calculator can then be used to risk stratify BO with dysplasia (77). Such approaches are promising and as more data become available, future surveillance programme will likely be based upon risk stratification using biomarkers.
Figure 3.
Algorithm for management of Barrett’s oesophagus with flat mucosa (non-nodular).
BO; Barrett’s oesophagus, GI; Gastrointestinal, PPI; Proton Pump inhibitor, OGD; Oesophagogastroduodenoscopy, IM; Intestinal metaplasia, LGD; Low grade dysplasia, HGD; High grade dysplasia, RFA: Radiofrequency ablation, MDT; Multidisciplinary team
Figure 4.
Algorithm for management of Barrett’s oesophagus with endoscopically visible nodular lesion.
*Data for management of LGD with nodular lesion treated with EMR is limited. However, a similar management strategy to that of flat LGD can be considered.
BO; Barrett’s oesophagus, EMR; Endoscopic mucosal resection, GI; Gastrointestinal, LGD; Low grade dysplasia, RFA; Radiofrequency ablation, HGD; High grade dysplasia, MDT; Multidisciplinary team, OAC; Oesophageal adenocarcinoma, sm; submucosa
Table 2. Comparison between BSG, ACG and BOBCAT consensus on the management of Barrett’s oesophagus.
| Degree of dysplasia at index endoscopy | British Society of Gastroenterology (BSG) (32) | American College of Gastroenterology (ACG) (34) | International consensus (BOBCAT) (36) | |
|---|---|---|---|---|
| No Dysplasia |
-BO length <3cm without IM: Repeat OGD and if IM absent, consider discharge -BO length <3cm with IM: OGD in 3-5 years -BO length ≥3cm: OGD every 2-3 years |
-Repeat OGD with biopsy in 3-5 years |
-Routine surveillance not recommended, but if undertaken, it should be targeted at high risk patients of which risk stratifications is based on age, sex, BO length, central obesity, duration, frequency and severity of symptoms, smoking status (influence of IM on surveillance is unclear) -No surveillance if life expectancy <5 years |
|
| Indefinite for dysplasia | Confirmation by 2 GI pathologists. Optimize PPI, and repeat OGD in 6 months | Confirmation by 2 GI pathologists. Optimize PPI and repeat OGD (interval not specified) | Confirmation by 2 GI pathologists. Optimize PPI, and repeat OGD within 12 months | |
| LGD | Flat mucosa | Optimize PPI and repeat OGD in 6 months. If repeat OGD confirms LGD (by 2 GI pathologist) offer RFA. If RFA not undertaken, then 6-monthly surveillance is recommended | Optimize PPI and repeat OGD (interval not specified). If repeat OGD confirms LGD (by 2 GI pathologist) offer RFA. If RFA not undertaken, then yearly surveillance | Optimize PPI and repeat OGD in 6-12 months. If repeat OGD confirms LGD (by 2 GI pathologist) offer RFA. |
| Nodular | EMR to obtain optimal histopathological staging. If LGD confirmed, offer RFA of remaining BO or 6 monthly surveillance$ | EMR to obtain optimal histopathological staging. If LGD confirmed, offer RFA of remaining BO or 6 monthly surveillance* | EMR to obtain optimal histopathological staging. If HGD or IMC present, offer RFA of remaining BO | |
| HGD or T1a stage (IMC) | Flat mucosa | Confirmation by 2nd GI pathologist. If HGD confirmed, then RFA | Confirmation by 2nd GI pathologist. If HGD confirmed, then RFA | Confirmation by 2nd GI pathologist. If HGD confirmed, then RFA (97) |
| Nodular | EMR of nodule. If histopathological confirmation of HGD/IMC, then RFA of remaining BO epithelium | EMR of nodule. If histopathological confirmation of HGD/IMC, then RFA of remaining BO epithelium | EMR of nodule. If histopathological confirmation of HGD/IMC, then RFA of remaining BO epithelium (97) | |
| OAC | T1b sm1 | Oesophagectomy is preferred. If poor surgical candidates, EMR + RFA can be considered if low risk tumour profile | Oesophagectomy is preferred. If poor surgical candidates, EMR + RFA can be considered if low risk tumour profile | Oesophagectomy is preferred. If poor surgical candidates, EMR + RFA can be considered if low risk tumour profile (97) |
| ≥T1b sm2 | Oesophagectomy | Oesophagectomy | Oesophagectomy | |
There is limited data and no optimal recommendation regarding the management of LGD diagnosed on EMR specimens of nodular BO. However, a similar management strategy to that of flat LGD should be considered.
The ACG recommendation for LGD diagnosed on nodular EMR specimens of nodular BO is based on expert opinion only due to the paucity of data surrounding this clinical entity
LGD; Low grade dysplasia, HGD; High grade dysplasia, IMC; Intramucosal carcinoma, OAC; oesophageal adenocarcinoma OGD; oesophagogastroduodenoscopy, BO; Barrett’s oesophagus, RFA; Radiofrequency ablation, GI; Gastrointestinal
BO is considered indefinite for dysplasia when pathologists are unable to accurately delineate dysplastic features from inflammatory atypia (78). For such cases, PPI optimization with repeat OGD in 6 months is recommended (32). If no dysplasia is found on follow-up, then surveillance should follow non-dysplastic BO.
As discussed earlier cases of LGD should be confirmed by a second GI pathologist with a repeat endoscopy in 6 months. Additionally, the ACG recommends aggressive PPI for LGD followed by repeat endoscopy in 6 months since PPI may lead to downgrading of dysplastic status (79). If repeat endoscopy confirms LGD, ablative therapy should be offered. Radiofrequency ablation (RFA) is the ablative therapy of choice and is performed via a balloon catheter containing a bipolar electrode array which delivers thermal energy onto targeted tissue. RFA can be delivered by circumferential ablation (Halo360 system) or focal ablation (Halo90 system). A RCT comparing RFA versus sham-therapy for dysplasia showed that RFA was associated with complete eradication of dysplasia in 90.5% versus 22.7% in the sham-controlled group (p<0.001) (80). RFA was also associated with lower rate of dysplastic progression (3.6% vs 16.3%, p=0.03) and cancer development (1.2% vs 9.3%, p=0.045) than the control group with a good safety profile (80). More recently, a RCT which compared RFA versus surveillance for LGD showed that RFA led to 25% reduction in risk of progression to HGD/IMC (81). Results from these trials have led to the BSG, ACG and BOBCAT consensus recommending RFA for treating LGD (32, 34, 36).
For HGD or IMC, nodular lesions should be removed with endoscopic mucosal resection (EMR) followed by RFA of remaining BO (32) (Figure 4). RFA post-EMR is recommended as the risk of developing metachronous neoplasia within 5 years after EMR is 14.5% (82). A recent study also reported favourable outcomes for eliminating residual BO using a modified Argon Plasma Coagulation (APC, an ablative technique using ionized argon gas) system (83). Patients with residual BO ≥1cm post-EMR for early neoplasia were treated with Hybrid-APC (fluid injection into submucosa before ablation) and achieved histological remission of BO in 78% (39/50) of cases (83).
For neoplasia staged as T1b (invasion into submucosa), oesophagectomy is preferred since up to 22% of submucosal tumour will inherently have regional lymph node metastases (84). However, poor surgical candidates with stage T1b sm1 tumour (invasion of submucosa but confined to upper 3rd submucosal layer within 500 μm) but with low risk tumour profile (well differentiated tumour without lymphovascular invasion); endoscopic therapy can be offered as an alternative (85, 86). The current BSG recommendations for HGD, T1a and T1b tumours are similar to that of the ACG and BOBCAT consensus (34, 36).
Future therapeutic options for Barrett’s oesophagus
EMR and RFA combination have proven to be a highly effective treatment for dysplastic BO; however, cryotherapy is a new technology which is being evaluated. Here, we also discuss the possible role of chemoprevention in BO.
Cryotherapy
Cryotherapy involves the use of a cryogen, usually liquid nitrogen or cold carbon dioxide (CO2) to induce tissue damage. The CryoSpray Ablation device (CSA Medical) allows endoscopic delivery of liquid nitrogen while the Polar Wand (GI Supply) and Coldplay cryoballoon system (C2 Therapeutics, Figure 2) delivers cold CO2. Both systems causes freezing and thawing of Barrett’s mucosa, resulting in apoptosis and subsequent sloughing of dead epithelial followed by regrowth of neo-squamous epithelial (87). Early data for cryotherapy has demonstrated it to be safe and effective, achieving up to 87% eradication of all forms of dysplasia (97% for HGD), and 3% stricture rate which were easily treated with balloon dilation (88). More recently Canto et al also showed high success rate for eradication of HGD with CO2 therapy, achieving 94% eradication at 1 year follow-up, with better success for treatment naïve patients than as rescue therapy for those treated unsuccessfully with other forms of ablative therapy (100% vs 91% respectively) (89). Despite promising results for cryotherapy, larger trials with direct comparison to RFA are necessary prior to adopting this procedure into clinical practice.
Figure 2.
Cryotherapy using the Coldplay cryoballon system (C2 Therapeutics)
Role of Chemoprevention
In BO, prolonged gastric acid reflux can lead to DNA strand breaks, oxidative damaged and increased cellular proliferation, processes which could promote carcinogenesis (90–92). A recent prospective cohort study which investigated 540 patients with known BO with a median 5.2 years follow-up showed that proton-pump inhibitors (PPI), but not Histamine-2 receptor antagonist, was associated with a 79% decreased cancer risk (HR=0.21, 95% CI 0.07-0.66) (93). More recently, a meta-analysis of 7 studies with >2800 patients showed that PPI was associated with 71% reduction in cancer risk (adjusted OR 0.29; 95% CI 0.12-0.79) (94). Interestingly however, a population based study of 9,883 Danish patients with a median 10.2-year follow-up showed no protective effect of PPI on the incidence of HGD or OAC (95). In fact, this study showed that longer-term use of PPI was associated with higher risk of HGD orOAC (95). As the role of PPI as a chemopreventive agent is not well substantiated, established guidelines has only recommended PPI to be used for symptomatic control only (32, 34, 36).
There has been some indirect evidence that aspirin or other non-steroidal anti-inflammatory (NSAIDs) has a chemopreventive role. A pooled analysis of observational studies showed a 40% reduction in cancer risk among patients taking aspirin or other NSAIDS (96). However, even if there is a true reduction, its use may be offset by the potential for an increased risk of GI or intracranial haemorrhage. Current societal guidelines do not recommend routine use of aspirin/NSAIDs as chemoprevention for OAC due to its potential side effects and the lack of level-1 evidence. However, the AspECT trial, a RCT designed and powered to assess the benefits of high or low-dose PPI with or without aspirin in reducing risk of OAC in BO has recently completed recruitment, and the results of this study are awaited.
Conclusion
There have been significant advances in the field of BO not only in diagnosis, but also in the different endoscopic imaging and therapeutic modalities for BO. Although minor variations between the BSG, ACG and BOBCAT statements exist, these societal recommendation do achieve consensus in many domains, such as the reporting of BO (Prague C&M criteria) and IM, endoscopic landmarks, diagnosis and grading of dysplasia, and treatment strategies for dysplastic Barrett’s. Currently, surveillance forms the mainstay of BO management with surveillance intervals varying depending on the grade of dysplasia. However, the future of surveillance in BO is gradually migrating towards risk stratifying those at higher risk for cancer progression based on risk factors and biomarkers in order to prioritise those patients with highest risk for cancer with endoscopic therapy. Finally, in order to diagnose more BO and have any chance of reducing the population mortality from OAC a better strategy for diagnosis in primary care is required. The development of novel tests such such as the Cytosponge-TFF3 test, capsule-tethered VLE, and transnasal endoscopy are an important step toward achieving this goal.
References
- 1.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling–Sternevald E, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129(6):1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57(10):1354–9. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiology Biomarkers & Prevention. 2010;19(6):1468–70. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 4.Thrift AP, Whiteman D. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Annals of oncology. 2012;23(12):3155–62. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 5.Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103(11):2694–9. doi: 10.1111/j.1572-0241.2008.02191.x. [DOI] [PubMed] [Google Scholar]
- 6.Derakhshan MH, Arnold M, Brewster DH, Going JJ, Mitchell DR, Forman D, et al. Worldwide Inverse Association between Gastric Cancer and Esophageal Adenocarcinoma Suggesting a Common Environmental Factor Exerting Opposing Effects. Am J Gastroenterol. 2016;111(2):228–39. doi: 10.1038/ajg.2015.405. [DOI] [PubMed] [Google Scholar]
- 7.Adler R. The lower esophagus lined by columnar epithelium. Its association with hiatal hernia, ulcer, stricture, and tumor. The Journal of thoracic and cardiovascular surgery. 1963;45:13–34. [PubMed] [Google Scholar]
- 8.Hawe A, Payne WS, Weiland LH, Fontana RS. Adenocarcinoma in the columnar epithelial lined lower (Barrett) oesophagus. Thorax. 1973;28(4):511–4. doi: 10.1136/thx.28.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haggitt RC, Tryzelaar J, Ellis FH, Colcher H. Adenocarcinoma complicating columnar epithelium-lined (Barrett’s) esophagus. American journal of clinical pathology. 1978;70(1):1–5. doi: 10.1093/ajcp/70.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Barrett N. Chronic peptic ulcerz of the œophagus and ‘œsophagitis’. British Journal of Surgery. 1950;38(150):175–82. doi: 10.1002/bjs.18003815005. [DOI] [PubMed] [Google Scholar]
- 11.Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology. 2010;138(3):854–69. doi: 10.1053/j.gastro.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tileston W. PEPTIC ULCER OF THE OESOPHAGUS. The American Journal of the Medical Sciences. 1906;132(2):240–65. [Google Scholar]
- 13.Stewart M, Hartfall S. Chronic peptic ulcer of the oesophagus. The Journal of Pathology and Bacteriology. 1929;32(1):9–14. [Google Scholar]
- 14.Lyall A. Chronic peptic ulcer of the oesophagus: a report of eight cases. British Journal of Surgery. 1937;24(95):534–47. [Google Scholar]
- 15.Findlay L, Kelly AB. Congenital shortening of the oesophagus and the thoracic stomach resulting therefrom. Proceedings of the Royal Society of Medicine. 1931;24(11):1561. [PMC free article] [PubMed] [Google Scholar]
- 16.Dick R, Hurst A. Chronic peptic ulcer of the oesophagus and its association with congenitally short oesophagus and diaphragmatic hernia. QJM. 1942;11(2):105–20. [Google Scholar]
- 17.Allison P, Johnstone A. The oesophagus lined with gastric mucous membrane. Thorax. 1953;8(2):87–101. doi: 10.1136/thx.8.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett N. THE LOWER ESOPHAGUS LINED BY COLUMNAR EPITHELIl’? II. Surgery. 1957 [PubMed] [Google Scholar]
- 19.Hayward J. The lower end of the oesophagus. Thorax. 1961;16(1):36–41. doi: 10.1136/thx.16.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrams L, Heath D. Lower oesophagus lined with intestinal and gastric epithelia. Thorax. 1965;20(1):66–72. doi: 10.1136/thx.20.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trier JS. Morphology of the epithelium of the distal esophagus in patients with midesophageal peptic strictures. Gastroenterology. 1970;58(4):444–61. [PubMed] [Google Scholar]
- 22.Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett's esophagus. The New England journal of medicine. 1976;295(9):476–80. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 23.Naef A, Savary M, Ozzello L. Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett's esophagus with 12 adenocarcinomas. The Journal of thoracic and cardiovascular surgery. 1975;70(5):826–35. [PubMed] [Google Scholar]
- 24.Borrie J, Goldwater L. Columnar cell-lined esophagus: assessment of etiology and treatment. A 22 year experience. The Journal of thoracic and cardiovascular surgery. 1976;71(6):825–34. [PubMed] [Google Scholar]
- 25.Skinner DB, Walther BC, Riddell RH, Schmidt H, Iascone C, DeMeester TR. Barrett's esophagus. Comparison of benign and malignant cases. Annals of surgery. 1983;198(4):554. doi: 10.1097/00000658-198310000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton SR, Smith RR. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett’s esophagus. American journal of clinical pathology. 1987;87(3):301–12. doi: 10.1093/ajcp/87.3.301. [DOI] [PubMed] [Google Scholar]
- 27.Reid BJ, Weinstein WM. Barrett's esophagus and adenocarcinoma. Annual review of medicine. 1987;38:477–92. doi: 10.1146/annurev.me.38.020187.002401. [DOI] [PubMed] [Google Scholar]
- 28.Spechler SJ, Zeroogian JM, Antonioli DA, Wang HH, Goyal RK. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994;344(8936):1533–6. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 29.Schnell TG, Sontag SJ, Chejfec G. Adenocarcinomas arising in tongues or short segments of Barrett's esophagus. Digestive diseases and sciences. 1992;37(1):137–43. doi: 10.1007/BF01308357. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Human pathology. 1988;19(8):942–8. doi: 10.1016/s0046-8177(88)80010-8. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Morales T, Sampliner R. Short segment Barrett's esophagus—the need for standardization of the definition and of endoscopic criteria. The American journal of gastroenterology. 1998;93(7):1033–6. doi: 10.1111/j.1572-0241.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 33.McClave SA, Boyce HW, Gottfried MR. Early diagnosis of columnar-lined esophagus: a new endoscopic diagnostic criterion. Gastrointestinal endoscopy. 1987;33(6):413–6. doi: 10.1016/s0016-5107(87)71676-9. [DOI] [PubMed] [Google Scholar]
- 34.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. quiz 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bas Weusten RB. Emanuel Coron, Mario Denis-Rebeiro, Jean-Marc Dumonceau, Jose-Miguel Esteban, Cesare Hassan, Oliver Pech, Alessandro Repici, Jacques Bergman, Massimilliano di Pietro: ESGE Position Statement on the endoscopic management of Barrett’s Oesophagus. European Society of Gastrointestinal Endoscopy. 2017 doi: 10.1055/s-0042-122140. (Pending Publication) [DOI] [PubMed] [Google Scholar]
- 36.Bennett C, Moayyedi P, Corley DA, DeCaestecker J, Falck-Ytter Y, Falk G, et al. BOB CAT: A Large-Scale Review and Delphi Consensus for Management of Barrett's Esophagus With No Dysplasia, Indefinite for, or Low-Grade Dysplasia. Am J Gastroenterol. 2015;110(5):662–82. doi: 10.1038/ajg.2015.55. quiz 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. Journal of the National Cancer Institute. 2011;103(13):1049–57. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandrasoma P, Wijetunge S, DeMeester S, Ma Y, Hagen J, Zamis L, et al. Columnar-lined esophagus without intestinal metaplasia has no proven risk of adenocarcinoma. The American journal of surgical pathology. 2012;36(1):1–7. doi: 10.1097/PAS.0b013e31822a5a2c. [DOI] [PubMed] [Google Scholar]
- 39.Bandla S, Peters JH, Ruff D, Chen SM, Li CY, Song K, et al. Comparison of cancer-associated genetic abnormalities in columnar-lined esophagus tissues with and without goblet cells. Ann Surg. 2014;260(1):72–80. doi: 10.1097/SLA.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelty CJ, Gough MD, Van Wyk Q, Stephenson TJ, Ackroyd R. Barrett's oesophagus: intestinal metaplasia is not essential for cancer risk. Scandinavian journal of gastroenterology. 2007;42(11):1271–4. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 41.Takubo K, Aida J, Naomoto Y, Sawabe M, Arai T, Shiraishi H, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Human pathology. 2009;40(1):65–74. doi: 10.1016/j.humpath.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald RC. Combining simple patient-oriented tests with state-of-the-art molecular diagnostics for early diagnosis of cancer. United European gastroenterology journal. 2015;3(3):226–9. doi: 10.1177/2050640615576677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curvers WL, Peters FP, Elzer B, Schaap AJ, Baak LC, van Oijen A, et al. Quality of Barrett's surveillance in The Netherlands: a standardized review of endoscopy and pathology reports. European journal of gastroenterology & hepatology. 2008;20(7):601–7. doi: 10.1097/MEG.0b013e3282f8295d. [DOI] [PubMed] [Google Scholar]
- 44.Abrams JA, Kapel RC, Lindberg GM, Saboorian MH, Genta RM, Neugut AI, et al. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(7):736–42. doi: 10.1016/j.cgh.2008.12.027. quiz 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison R, Perry I, Haddadin W, McDonald S, Bryan R, Abrams K, et al. Detection of intestinal metaplasia in Barrett's esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol. 2007;102(6):1154–61. doi: 10.1111/j.1572-0241.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 46.Pohl H, Pech O, Arash H, Stolte M, Manner H, May A, et al. Length of Barrett's oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut. 2016;65(2):196–201. doi: 10.1136/gutjnl-2015-309220. [DOI] [PubMed] [Google Scholar]
- 47.Anaparthy R, Gaddam S, Kanakadandi V, Alsop BR, Gupta N, Higbee AD, et al. Association between length of Barrett's esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(11):1430–6. doi: 10.1016/j.cgh.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131(5):1392–9. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 49.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47(2):251–5. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duits LC, Phoa KN, Curvers WL, Ten Kate FJ, Meijer GA, Seldenrijk CA, et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64(5):700–6. doi: 10.1136/gutjnl-2014-307278. [DOI] [PubMed] [Google Scholar]
- 51.Vennalaganti P, Kanakadandi V, Goldblum JR, Mathur SC, Patil DT, Offerhaus GJ, et al. Discordance Among Pathologists in the United States and Europe in Diagnosis of Low-Grade Dysplasia for Patients With Barrett's Esophagus. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Sikkema M, Kerkhof M, Steyerberg EW, Kusters JG, van Strien PM, Looman CW, et al. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett's esophagus: a case-control study. Am J Gastroenterol. 2009;104(11):2673–80. doi: 10.1038/ajg.2009.437. [DOI] [PubMed] [Google Scholar]
- 53.Kastelein F, Biermann K, Steyerberg EW, Verheij J, Kalisvaart M, Looijenga LH, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett's oesophagus. Gut. 2013;62(12):1676–83. doi: 10.1136/gutjnl-2012-303594. [DOI] [PubMed] [Google Scholar]
- 54.Aisenberg J. Gastrointestinal endoscopy nears "the molecular era". Gastrointestinal endoscopy. 2008;68(3):528–30. doi: 10.1016/j.gie.2008.03.1075. [DOI] [PubMed] [Google Scholar]
- 55.Kiesslich R, Neurath MF. Endoscopic confocal imaging. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2005;3(7 Suppl 1):S58–60. doi: 10.1016/s1542-3565(05)00252-1. [DOI] [PubMed] [Google Scholar]
- 56.De Palma GD. Confocal laser endomicroscopy in the "in vivo" histological diagnosis of the gastrointestinal tract. World journal of gastroenterology. 2009;15(46):5770–5. doi: 10.3748/wjg.15.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clinical Gastroenterology and Hepatology. 2006;4(8):979–87. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Leung KK, Maru D, Abraham S, Hofstetter WL, Mehran R, Anandasabapathy S. Optical EMR: confocal endomicroscopy-targeted EMR of focal high-grade dysplasia in Barrett's esophagus. Gastrointestinal endoscopy. 2009;69(1):170–2. doi: 10.1016/j.gie.2008.03.1068. [DOI] [PubMed] [Google Scholar]
- 59.Goda K, Tajiri H, Ikegami M, Urashima M, Nakayoshi T, Kaise M. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett's adenocarcinoma. Gastrointestinal endoscopy. 2007;65(1):36–46. doi: 10.1016/j.gie.2006.03.938. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointestinal endoscopy. 2011;74(3):465–72. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.di Pietro M, Bird-Lieberman EL, Liu X, Nuckcheddy-Grant T, Bertani H, O'Donovan M, et al. Autofluorescence-Directed Confocal Endomicroscopy in Combination With a Three-Biomarker Panel Can Inform Management Decisions in Barrett's Esophagus. Am J Gastroenterol. 2015;110(11):1549–58. doi: 10.1038/ajg.2015.295. [DOI] [PubMed] [Google Scholar]
- 62.Trindade AJ, Smith MS, Pleskow DK. The new kid on the block for advanced imaging in Barrett’s esophagus: a review of volumetric laser endomicroscopy. Therapeutic advances in gastroenterology. 2016;9(3):408–16. doi: 10.1177/1756283X16639003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trindade AJ, George BJ, Berkowitz J, Sejpal DV, McKinley MJ. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett's esophagus. Endoscopy international open. 2016;4(3):E318–22. doi: 10.1055/s-0042-101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gora MJ, Sauk JS, Carruth RW, Gallagher KA, Suter MJ, Nishioka NS, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nature medicine. 2013;19(2):238–40. doi: 10.1038/nm.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aedo MR, Zavala-González MÁ, Meixueiro-Daza A, Remes-Troche JM. Accuracy of transnasal endoscopy with a disposable esophagoscope compared to conventional endoscopy. World journal of gastrointestinal endoscopy. 2014;6(4):128. doi: 10.4253/wjge.v6.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shariff MK, Varghese S, O'Donovan M, Abdullahi Z, Liu X, Fitzgerald RC, et al. Pilot randomized crossover study comparing the efficacy of transnasal disposable endosheath with standard endoscopy to detect Barrett's esophagus. Endoscopy. 2016;48(2):110–6. doi: 10.1055/s-0034-1393310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blevins CH, Iyer PG. Putting it Through the Nose: The Ins and Outs of Transnasal Endoscopy. Am J Gastroenterol. 2016;111(10):1371–3. doi: 10.1038/ajg.2016.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadri SR, Lao-Sirieix P, O’Donovan M, Debiram I, Das M, Blazeby JM, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. Bmj. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lao-Sirieix P, Boussioutas A, Kadri SR, O’Donovan M, Debiram I, Das M, et al. Non-endoscopic screening biomarkers for Barrett’s oesophagus: from microarray analysis to the clinic. Gut. 2009;58(11):1451–9. doi: 10.1136/gut.2009.180281. [DOI] [PubMed] [Google Scholar]
- 70.Ross-Innes CS, Debiram-Beecham I, O'Donovan M, Walker E, Varghese S, Lao-Sirieix P, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS medicine. 2015;12(1):e1001780. doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burch JA, Soares-Weiser K, John DJ, Duffy S, Smith S, Kleijnen J, et al. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. Journal of medical screening. 2007;14(3):132–7. doi: 10.1258/096914107782066220. [DOI] [PubMed] [Google Scholar]
- 72.Lewis I. Carcinoma of Lower End of Œsophagus. Radical Resection with Œsophagogastrostomy by a Left Transpleural Approach. Proceedings of the Royal Society of Medicine. 1945;38(9):482. [PMC free article] [PubMed] [Google Scholar]
- 73.Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World journal of gastroenterology. 2010;16(30):3804–10. doi: 10.3748/wjg.v16.i30.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watson D, Davies N, Jamieson G. Totally endoscopic Ivor Lewis esophagectomy. Surgical endoscopy. 1999;13(3):293–7. doi: 10.1007/s004649900969. [DOI] [PubMed] [Google Scholar]
- 75.Mariette C, Meunier B, Pezet D, Dalban C, Collet D, Thomas P-A, et al., editors. Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicenter, open-label, randomized phase III controlled trial, the MIRO trial. ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 76.Huang L, Onaitis M. Minimally invasive and robotic Ivor Lewis esophagectomy. Journal of thoracic disease. 2014;6(Suppl 3):S314. doi: 10.3978/j.issn.2072-1439.2014.04.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ross-Innes CS, Chettouh H, Achilleos A, Galeano-Dalmau N, Debiram-Beecham I, MacRae S, et al. Risk stratification of Barrett's oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. The Lancet Gastroenterology & Hepatology. 2016 doi: 10.1016/S2468-1253(16)30118-2. [DOI] [PubMed] [Google Scholar]
- 78.Odze RD. Barrett esophagus: histology and pathology for the clinician. Nature reviews Gastroenterology & hepatology. 2009;6(8):478–90. doi: 10.1038/nrgastro.2009.103. [DOI] [PubMed] [Google Scholar]
- 79.American Gastroenterological A. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140(3):1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 80.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. The New England journal of medicine. 2009;360(22):2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 81.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. Jama. 2014;311(12):1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 82.Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146(3):652–60.e1. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Manner H, May A, Kouti I, Pech O, Vieth M, Ell C. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc. 2016;30(4):1364–70. doi: 10.1007/s00464-015-4336-1. [DOI] [PubMed] [Google Scholar]
- 84.Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma: a retrospective review of esophagectomy specimens. Annals of surgery. 2011;253(2):271–8. doi: 10.1097/SLA.0b013e3181fbad42. [DOI] [PubMed] [Google Scholar]
- 85.Scholvinck D, Kunzli H, Meijer S, Seldenrijk K, van Berge Henegouwen M, Bergman J, et al. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc. 2016;30(9):4102–13. doi: 10.1007/s00464-016-5071-y. [DOI] [PubMed] [Google Scholar]
- 86.Manner H, Pech O, Heldmann Y, May A, Pauthner M, Lorenz D, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc. 2015;29(7):1888–96. doi: 10.1007/s00464-014-3881-3. [DOI] [PubMed] [Google Scholar]
- 87.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 88.Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71(4):680–5. doi: 10.1016/j.gie.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canto MI, Shin EJ, Khashab MA, Molena D, Okolo P, Montgomery E, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett's esophagus. Endoscopy. 2015;47(7):582–91. doi: 10.1055/s-0034-1391734. [DOI] [PubMed] [Google Scholar]
- 90.Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. 2007;56(6):763–71. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus. An ex vivo proliferation and differentiation model. The Journal of clinical investigation. 1996;98(9):2120–8. doi: 10.1172/JCI119018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clemons NJ, McColl KE, Fitzgerald RC. Nitric oxide and acid induce double-strand DNA breaks in Barrett's esophagus carcinogenesis via distinct mechanisms. Gastroenterology. 2007;133(4):1198–209. doi: 10.1053/j.gastro.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 93.Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clinical Gastroenterology and Hepatology. 2013;11(4):382–8. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 94.Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut. 2014;63(8):1229–37. doi: 10.1136/gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett's oesophagus: a nationwide study of 9883 patients. Alimentary pharmacology & therapeutics. 2014;39(9):984–91. doi: 10.1111/apt.12693. [DOI] [PubMed] [Google Scholar]
- 96.Liao LM, Vaughan TL, Corley DA, Cook MB, Casson AG, Kamangar F, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142(3):442–52.e5. doi: 10.1053/j.gastro.2011.11.019. quiz e22-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143(2):336–46. doi: 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]