Abstract
Primary restoration of the mandibular continuity remains the standard of care for defects, and yet several constraints preclude this objective. Interim reconstructions with plate and nonvascular bone grafts have high failure rates. The secondary reconstruction, when becomes inevitable, remains a formidable task. This retrospective study evaluates various issues to address secondary reconstruction. Twenty-one patients following mandibulectomy presented with various complications between 2012 and 2016 were included in the study. The profile of primary reconstruction includes reconstruction plate ( n = 9), reconstruction plate with rib graft ( n = 3), soft tissue only reconstruction ( n = 4), free fibula ( n = 2), inadequate growth of reconstructed free fibula during adolescence ( n = 1), nonvascular bone graft alone ( n = 1), and no reconstruction ( n = 1). All had problems or complications related to unsatisfactory primary reconstruction such as plate fracture, recurrent infection, plate exposure, deformity, malocclusion, and failed fibula reconstruction. All were reconstructed with osteocutaneous free fibula flap with repair of soft-tissue loss. All flaps survived and had satisfactory outcome functionally and aesthetically. Dental rehabilitation was done in four patients. One flap was reexplored for thrombosis and salvaged. The challenges in secondary reconstruction include difficulty in recreating true defects, extensive fibrosis and loss of planes, unanticipated soft-tissue and skeletal defects, reestablishing the contour and occlusion, insufficient bone strength, dearth of suitable recipient vessels, nonpliable skin, tissue contraction to accommodate new mandible, need of additional flap for defect closure, and postirradiation effects. Notwithstanding them, the reasonable successful outcome can be attainable.
Keywords: mandible reconstruction, salvage reconstruction, free fibula, microvascular reconstruction
Primary reconstruction stands as the paradigm for osseous defects of the mandible. 1 However, in developing or resource-constrained countries, primary mandibular reconstruction is not always achieved due to various limitations such as patient factors, lack of expertise, and lack of resources. Instead, interim procedures to maintain mandibular continuity and stability are employed. 2 Considering the biomechanics of the mandible that has tensile, compression, and torsion forces, a continuity defect rehabilitation using only plate (without bone) is doomed to failure. When reconstruction plate is used alone, the complication rate exceeds 50% with one-tenth of them resulting in fracture, and at least 30% requiring premature removal. 3 4 The failed reconstruction results in poor oral cavity function and deleterious effect on general well-being of the patient. For a reconstructive surgeon, it is a formidable task to meet the ideals of reconstruction working in a compromised situation. The reconstructive attempts are met with higher complications, 5 and any further failure will be devastating to the patient. The literature is sparse on the information and guidelines to clinicians who deal with this difficult problem. We present a series of patients who presented with complications, necessitating definitive reconstruction, and have made an attempt to address the challenges involved in that.
Aims and Objectives
This article aims to evaluate the multitude of factors involved in the secondary reconstruction of the mandible, the problems associated with inadequate primary reconstruction, technical difficulties and the outcome.
Patients and Methods
In this retrospective study, 21 patients, who underwent secondary reconstruction of the mandible between January 2012 and December 2016, were included. This retrospective review was done with the approval of the Institutional Ethical Board. All patients had a primary mandibular resection for either benign or malignant conditions elsewhere and presented with complications associated with it.
Routine evaluation included orthopantomogram (OPG) and three-dimensional (3D) computed tomographic (CT) scans. Any residual or local recurrence was ruled out before considering reconstruction. Patients were optimized with corrections of anemia, hypoproteinemia, and comorbidities. All patients were reconstructed with free osteocutaneous fibula flap.
Results
Sixteen male and five female patients who underwent secondary mandibular reconstruction during the study period were evaluated ( Table 1 ). The average age was 42 years (17–68 years). The primary disease affecting the lower jaw was squamous cell carcinoma ( n = 13), benign ameloblastoma ( n = 7), and adenoid cystic carcinoma ( n = 1). The average duration of the presentation following the primary reconstruction was 17 months with a range of 3 to 60 months.
Table 1. Profile and summary of all patients.
| No. | Age/Sex | Primary disease | Mandible defect and site | Primary reconstruction | Outcome of primary reconstruction | Duration of presentation (mo) | Outcome of secondary reconstruction/remarks |
|---|---|---|---|---|---|---|---|
| 1 | 32/F | SCCA | Left segment | Nasolabial flap only | Malocclusion, scar and contour deformity | 12 | Successful Dental rehabilitation |
| 2 | 23/F | Benign adamantinoma | Left segment | Reconstruction plate + free rib graft | Infection, loss of rib graft, sinus formation | 9 | Successful Developed Frey's syndrome |
| 3 | 34/M | Benign | Right segment | Recon plate with free rib graft | Infected sinus | 24 | Successful |
| 4 | 42/M | SCCA | Right segment | Free fibula partial loss | Loss of projection, contour loss, sinus, oral incompetence | 13 | Successful Dental rehabilitation |
| 5 | 62/M | Adenocystic ca. with osteoradionecrosis | Right segment | PMMC soft-tissue reconstruction only | Persistent sinus, osteomyelitis | 18 | Successful Plate infection, removed after 6 mo |
| 6 | 56/M | SCCA with ORN | Left segment | Plating | Discharging sinus with exposed plate | 20 | Successful |
| 7 | 22/ F | Ameloblastoma Condyle |
Left condyle and ramus | Nonvascular rib bone graft | Contour deformity, cross bite, chin deviation | 16 | Successful |
| 8 | 32/F | Benign Adamantinoma | Right segment with central segment | Reconstruction plate | Recon., plate fracture, contour defect, malocclusion | 22 | Successful |
| 9 | 21/M | Benign ameloblastoma | Central segment | Previous free fibula 5 y ago | Retrognathia | 60 | Successful Dental rehabilitation done |
| 10 | 17/M | Benign | Central segment | Reconstruction plate | Orocutaneous fistula | 6 | Successful |
| 11 | 66/M | SCCA | Central segment | Free fibula | Retrognathia, incompetence | 3 | Successful Exposed intraoral implant, removed after 6 mo |
| 12 | 63/M | SCCA | Left segment | Reconstruction plate | Plate infection, sinus | 11 | Successful |
| 13 | 46/M | SCCA/ORN | Right Segment | No reconstruction | Deformity/malocclusion | 30 | Successful |
| 14 | 58/M | SCCA | Left segment | Reconstruction plate | Deformity with plate fracture | 26 | Successful Skin necrosis, plate exposure, local flap cover, plate removal |
| 15 | 36/F | Ameloblastoma | Central | Reconstruction plate + rib graft | Loss of graft and fistula | 10 | Successful Dental rehabilitation |
| 16 | 39/M | SCCA | Right lateral | Reconstruction plate | Plate exposure | 24 | Successful |
| 17 | 30/M | SCCA | Right segment | PMMC | Trismus, contour deformity, malocclusion | 11 | Successful 2nd free flap to improve contour |
| 18 | 36/M | SCCA | Central | Reconstruction plate | Intraoral exposure of plate, fistula | 6 | Successful |
| 19 | 66/M | SCCA | Central | Reconstruction plate | Exposure and fistula | 12 | Successful |
| 20 | 68/M | SCCA | Central | Reconstruction plate | Infected plate with sinus | 16 | Successful |
| 21 | 33/M | SCCA | Left lateral | PMMC | Contour deformity, malocclusion | 8 | Successful |
Abbreviations: F, female; M, male; ORN, osteoradionecrosis; PMMC, pectoralis myocutaneous flap; SCCA, squamous cell carcinoma.
The primary reconstructions performed elsewhere following the resection surgery include reconstruction plate ( n = 9), reconstruction plate with rib graft ( n = 3), soft-tissue only reconstruction ( n = 4), free fibula ( n = 3), nonvascular bone graft alone ( n = 1), and no reconstruction ( n = 1). All had problems or complications related to unsatisfactory primary reconstruction ( Table 2 ). The patients with a reconstruction plate with or without rib grafts ( n = 11) had presented with infected sinus, plate exposure, or plate fracture. Four patients with free fibula reconstruction had either primary failure ( n = 3) or inadequate growth ( n = 1). Loss of contour and malocclusion with functional problems were seen in patients without any reconstruction. In addition, two patients presented with osteoradionecrosis.
Table 2. Primary reconstruction: presentation.
| Type of primary reconstruction | Presentation |
|---|---|
| Reconstruction plate alone (9) | Infection with sinus (6) |
| Reconstruction plate with nonvascular bone grafts (3) | Exposed plate (4) Plate fracture with deformity (2) |
| Soft-tissue only reconstruction (4) | Deformity |
| No reconstruction (1) | Malocclusion |
| Nonvascular bone graft (1) | Trismus |
| Vascularized free fibula (3) | Failed flap with deformity |
Note: Number in brackets = number of patients.
All patients had successful outcomes following secondary reconstruction with free fibula flap in terms of the flap survival and amelioration of the presenting complaints. Contralateral neck vessels were chosen for anastomosis in five patients. Reexploration in one patient for arterial thrombosis was successful. Secondary procedures included removal of exposed plate in three, which was done 6 months following the reconstruction. One patient had skin necrosis over the chin, which was debrided and closed with advancing contralateral skin. Unsatisfactory contour was seen in one patient following secondary reconstruction in the areas of cheek and mandibular region. Adiposofascial anterolateral thigh flap was utilized to augment the soft tissue and satisfactory correction was achieved (patient 11, Table 1 ). Dental rehabilitation with implant was performed in four patients ( Table 3 ).
Table 3. Secondary reconstruction: outcome.
| Complications and problems | Additional procedures and remarks |
|---|---|
| Skin necrosis (1) | Local flap cover |
| Plate exposure (3) | Plate removal after 6 mo |
| Insufficient contour following secondary reconstruction with free fibula (1) | Second free flap (adiposofascial anterolateral thigh flap) for facial contour restoration |
| Non availability of ipsilateral recipient vessels (5) | Contralateral neck for anastomosis |
| Arterial thrombosis (1) | Successful reexploration |
| Dental rehabilitation | Performed in four patients with implant |
Illustrations of Cases
Case 1
A 32-year-old woman (patient 8, Table 1 ) presented with a deformity, involving loss of contour of the right jaw and deviated chin to the left ( Fig. 1a ). She had undergone right segmental resection of the mandible for a benign adamantinoma and reconstruction with a reconstruction plate 20 months earlier. The OPG showed fractured right reconstruction plate ( Fig. 1b ). The previous incision was used for the approach and the fractured plate was replaced with a fresh reconstruction plate, and free fibula osteocutaneous flap was used for the reconstruction ( Fig. 1c ). The small skin paddle was used for intraoral lining and monitoring purpose. Donor defect was closed primarily. The patient had satisfactory outcomes ( Fig. 1d ).
Fig. 1.
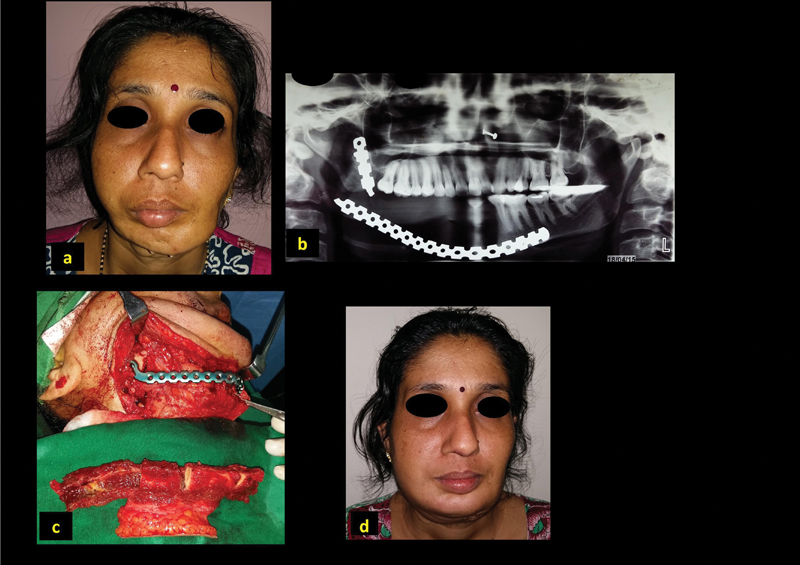
A 32-year-old patient with reconstruction plate fracture and deformity ( a , b ), reconstruction using free fibula ( c ) and postoperative outcome ( d ).
Case 2
A 21-year-old man (patient 9, Table 1 ) presented with retruded chin, who previously underwent resection of the central segment of the mandible for a benign adamantinoma and reconstruction using microvascular fibula flap 5 years ago ( Fig. 2a , b ). The CT scan evaluation showed a thin bone bridging the symphysis of the mandible ( Fig. 2c ). There was no growth of this bone graft during the growth phase of the mandible in the past 5 years. The chin was exposed with visor flap, and resection of the previous bone graft, replacing with fresh free fibula flap, was done ( Fig. 2d , e ). Class I occlusion was achieved, skin paddle was used to line intraoral defect, and anastomosis was performed to the neck vessels. The patient had a successful outcome with dental rehabilitation 1 year following reconstruction ( Fig. 2f, g ).
Fig. 2.
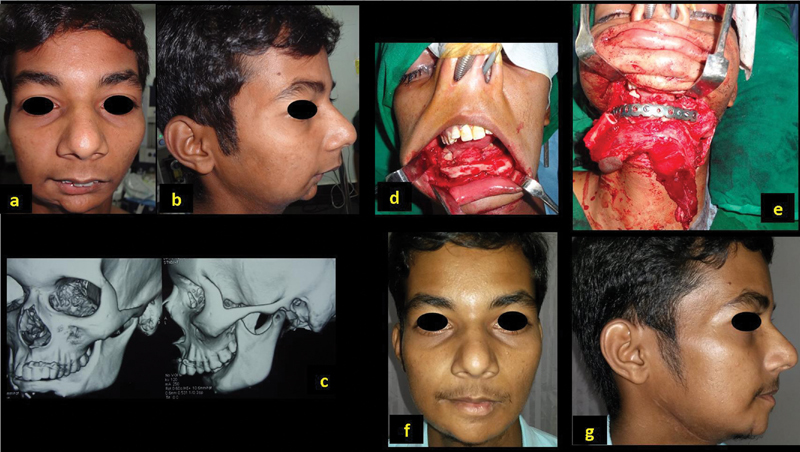
A 21-year-old man with previous reconstruction of symphysis with free fibula for benign lesion ( a , b ); CT scan showing the deformity with previous fibula ( c ). Reconstruction with osteocutaneous fibula ( d , e ). Postoperative outcome with dental rehabilitation ( f , g ).
Case 3
A 66-year-old man (patient 19, Table 1 ) presented with exposed implant following central segmental resection, reconstruction, and external radiation for malignancy ( Fig. 3a ). Reconstruction plate alone was used during the primary surgery ( Fig. 3b ). The exposed plate was removed and free fibula flap was used to restore the mandibular central segment ( Fig. 3c ) with successful outcome ( Fig. 3d ).
Fig. 3.
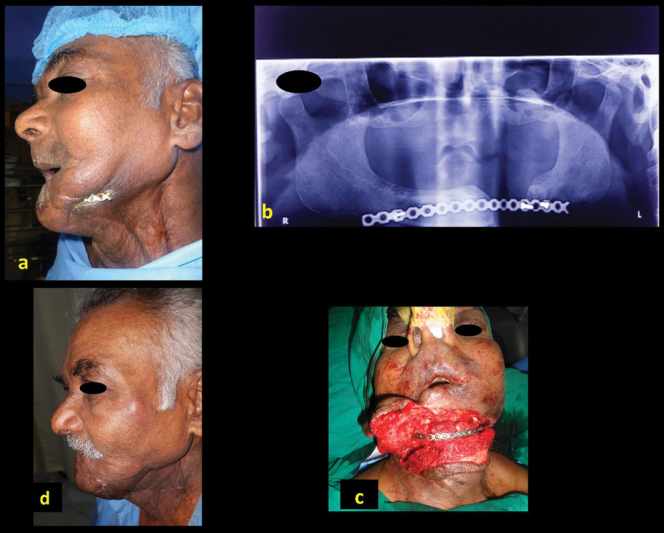
A 66-year-old man, with repeated abscess in the left neck with sinus (a), previous reconstruction with only reconstruction plate ( b ), reconstruction with free fibula ( c ), showing postoperative outcome ( d ).
Case 4
A 63-year-old man (patient 12, Table 1 ) presented with sinus and recurrent episodes of acute infection and abscess in the left neck ( Fig. 4a , b ). He underwent composite segmental mandibulectomy for left lower alveolar squamous cell carcinoma. The mandible defect was bridged with only a reconstruction plate ( Fig. 4c , d ). He was treated with removal of old plate and reconstruction with free osteocutaneous fibula flap ( Fig. 4e–g ). He had a successful outcome with restoring the function and aesthetics ( Fig. 4h–k ).
Fig. 4.
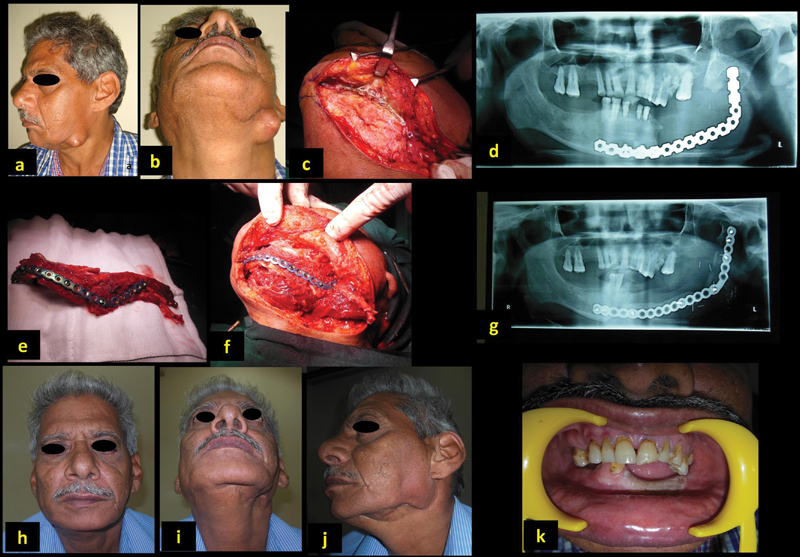
A 66-year-old man with exposed plate with previous plate reconstruction ( a–d ). Reconstruction with free fibula ( e–g ). Satisfactory outcome post-op ( h–k ).
Case 5
A 42-year-old man (patient 4, Table 1 ) presented with discharging sinus of the right lower jaw with deformity following resection and reconstruction for lower alveolar carcinoma ( Fig. 5a , b ). The defect from angle to angle was reconstructed with free fibula in a different hospital. The distal segments of fibula did not survive, resulting in infection and sinus ( Fig. 5c ). The infected plate was removed in the first sitting, while the reconstruction of the defect was planned. On exploration of the right neck, the defect was seen on the right lateral segment ( Fig. 5d ). The proximal end of the reconstructed bone had united well. The defect was released, and reconstruction plate was fixed, keeping the chin (the “ V ”-shaped previous reconstruction) in the center ( Fig. 5e ). The 5-cm defect was reconstructed with free fibula osteocutaneous flap ( Fig. 1f–h ). The patient had satisfactory outcome ( Fig. 5i–k ).
Fig. 5.
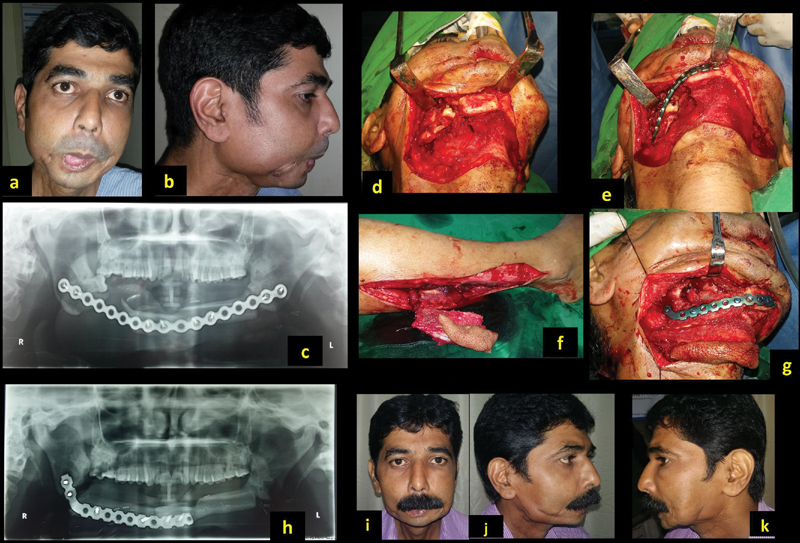
A 42-year-old man with discharging sinus and infected plate ( a , b ). He previously underwent mandible reconstruction with partial loss ( c ). The defect was recreated and stabilized with reconstruction plate ( d , e ), and reconstruction with free fibula ( f , g ). Post-op pictures after 8 months ( h–k ).
Discussion
Primary mandible reconstruction remains a standard of care 6 and missing out this opportunity or inadequate primary reconstruction poses considerable hardships for patients. They suffer with poor oral cavity functions, deformities, poor health, psychosocial problems, and substantial financial burden. Functional problems include oral incompetence, malocclusion, trismus due to tissue contraction, difficulties in speech, mastication, deglutition, and disfigurement. Reconstruction of these defects secondarily poses a formidable challenge and many of these are not correctable to the full extent. There are qualitative and irreversible changes in the tissues due to fibrosis, tissue contraction, and postradiation fibrosis. 7
Several obstacles and challenges emerge when considering reconstructing these defects. From this study, we list out the problems and attempt to address them. They include (1) recreating the primary defect, (2) loss of tissue plane, (3) choice of suitable recipient vessels, (4) soft-tissue contraction, (5) measuring a true bone gap, (6) absence of condyle, (7) reestablishing contour and occlusion, (8) nonpliable skin, (9) neck defect closure, and (10) measure of success ( Table 4 ).
Table 4. Key points in salvage reconstruction.
| Anticipated problem | Remarks |
|---|---|
| 1. Recreating the primary defect | Not a familiar procedure to the reconstructive surgeon, additional help may be sought. Plan and outcome is based on obtaining true defect |
| 2. Loss of tissue plane | Results from surgery and radiation. Extreme caution is exercised to avoid injury to underlying vessels |
| 3. Soft-tissue contraction | Seen when no or soft tissue alone was used without splinting the mandible. The defect needs more tissue than anticipated |
| 4. Measuring a true bone gap | Mandible requires trimming on edges and avoiding previous fixation points |
| 5. Choice of suitable recipient vessels | Should be the first step and often contralateral neck is explored. Planning the orientation of the flap is based on site of anastomosis and pedicle length |
| 6. Absence of condyle | Soft-tissue pocket at glenoid helps support ascending part of neomandible. Anchoring may be used |
| 7. Re establishing contour and occlusion | Use of previous reconstruction plate or CT-guided 3D printing templates |
| 8. Non pliable skin and hypovascularity | Watch for skin necrosis at the most distal ends and chin. Additional skin or secondary closure is option |
| 9. Neck defect closure | Loose approximation avoids pressure and allows drainage |
| 10. Measure of success | Goals are similar to primary but in reality limited to survival of the flap and amelioration of symptoms |
Recreating the primary defect: Definitive reconstruction with osseous free flaps is ideal, but there is reluctance, and not always preferred when it comes to secondary reconstruction. 8 During primary reconstruction, a clear measurable defect is created by the ablative surgeon. The burden of recreating the defects in secondary surgery rests with the reconstructive surgeon. This is not only a daunting task but often beyond their tailored skills and comfort levels even for an accomplished surgeon. Obtaining a true defect may not be possible owing to extensive tissue contractions. Failure to recreate original 3D defects itself could be deterrent to choose appropriate reconstruction.
Extensive fibrosis and loss of tissue plane: This is particularly encountered following radical neck dissection with further external radiation therapy. Following neck dissection and removal of investing neck fascia, the vessels are directly under the skin flap with thin atrophied platysma intervening between skin and vessels. Dissection in this plane potentially results in injury to thin-walled veins. Further, radiation causes hypovascularity and induces fibrosis which is visible as dense, thin, and pale tissue planes.
-
Finding suitable recipient vessels is a paramount task and should be the first step of the procedure without committing to the defect. Frequently during the neck dissection for the primary disease, the tributaries of the internal jugular veins (IJVs) are either not preserved or the vein itself is removed. In the absence of both internal and external jugular vein, we explored the contralateral neck. Should that becomes necessary, the orientation of the flap is kept such a way that the exit end of pedicle is nearest to the recipient vessels in the contralateral neck.
The choice of recipient artery is also limited. The facial artery is often ligated and thrombosed. However, we find adequate length of the artery is available deep under the digastric muscle. This is accessed by dividing the digastric tendon and lifting the muscle to uncover the vessel. The available vessel length is generally healthy, well preserved, and protected under the digastric muscle from previous surgery and radiation. The other reliable vessel is the superior thyroid artery. The venous anastomosis could be end to end to the IJV tributary or end to side to IJV. We prefer to explore and dissect recipient vessels as a first step before proceeding, as often the contralateral neck is utilized. In this series, five patients underwent anastomosis in the contralateral neck. Digital mapping of good caliber vessels is possible 9 and reliability of patency needs to be ascertained.
The soft-tissue release required is often much more than the anticipated volume to achieve adequate mouth opening and occlusion. The defect thus created is larger, needing inclusion of good amount of soft tissue with the fibula. Typically, the defects include floor of mouth following release of lingually shifted mandible. The intraoral defect often includes cheek, gingivo-buccal sulcus, and the floor of mouth. Extraorally, though the closure is achieved in most cases, additional skin may be needed to close incisions in the neck or chin. Reconstruction of these 3D defects needs careful planning. Large skin paddle either single or bipaddle preserving perforators is necessary. Additionally, flexor halluces longus muscle is useful in obliterating the floor defects and in augmenting soft-tissue loss to improvise contour around the mandible.
The bony defect is often collapsed in the absence of reconstruction plate. The recreated defect and true bony gap is measured after keeping the mandible in occlusion. Importantly, it should be remembered that the true bony gap is calculated only after freshening the edges of the bone ends. Irregular ends are slivered with a saw for a smooth bleeding end. It may be necessary to resect old fixation points to get an adequate purchase for the new screw fixation.
Absence of condyle further complicates the reconstruction and fixation. If the glenoid fossa is well surrounded by the soft tissue, the upper end (ramus) of reconstructed mandible can occupy the temporomandibular joint (TMJ) space. For this, the width of the upper end of the vertical limb of contoured fibula requires narrowing to match the size of the condyle. This is kept projected at least 15 mm beyond the edge of the plate to resemble the condyle ( Fig. 6a–d ). This creates a pseudo joint which moves along with the contralateral TMJ. If there is instability to maintain the bony end in the glenoid fossa, the end of the bone is anchored to the soft tissue with nonabsorbable suture or rarely using stainless steel wire as suspension to the zygoma. It is important to limit the extent of plate much below the bony ends so that potential erosion into the glenoid fossa is avoided and removal of the plate, if necessary, is easier.
Reestablishing the precise contour of the mandible is a difficult task. The following are used for shaping the mandible: the previous plate, precontoured guiding plate, CT-guided 3D digital printing technology, and stereolithographic models. 9 10 In our patients, it was either previous plate or precontoured reconstruction plate based on the subjective assessment of the contralateral side while keeping the remaining jaw in occlusion. However, it is recommended to use objective means for accurate contouring whenever possible. The soft-tissue requirement and additional bone loss with freshening should be kept in mind during preoperative planning.
The skin of the neck generally is tougher and less pliable owing to contraction and fibrosis. Further redraping the additional volume of reconstructed mandible is difficult. This possibly results in a secondary defect which may require an additional skin paddle of the flap and skin grafting or may be left open for secondary healing or delayed suturing. Elevation of large poorly vascularized skin flaps is prone for devascularization and necrosis, particularly with tight closure. The most distal part of elevated lip-split incision is chin and remains vulnerable for necrosis.
Finally, as per our preference, neck skin closure is done with just few tacking sutures, avoiding compression on the pedicle and leaving 1- to 2-cm gap between sutures for adequate drainage. Since airtight closure is not done, no closed suction drain is utilized, instead a sterile absorbent pad is kept on the wound and changed frequently when soaked. Unlike in primary neck dissection, the potential space available for any fluid collection is extremely limited, and slightest collection under nonpliable skin causes pressure which is detrimental to the anastomosis. In addition, large hematoma can potentially compress the neck and upper airway due to pressure under closed space.
The measure of success in secondary reconstruction is limited to the flap survival and amelioration of the presenting problems. Though the goals of achieving near-perfect function and cosmesis remain as in the primary reconstruction, realistic approach is needed considering several limitations listed earlier. Despite technical difficulties to carry out microsurgical reconstruction, 8 the overall success of flap survival is similar to the primary reconstruction in our study. We encourage such an attempt, as good outcome can be anticipated by paying attention to several factors discussed above. However, it should also be kept in mind that failure of salvage reconstruction can be disastrous and life threatening for a patient who was “managing” without reconstruction, and should such situation is faced, a second salvage plan be kept as a “lifeboat.”
Fig. 6.
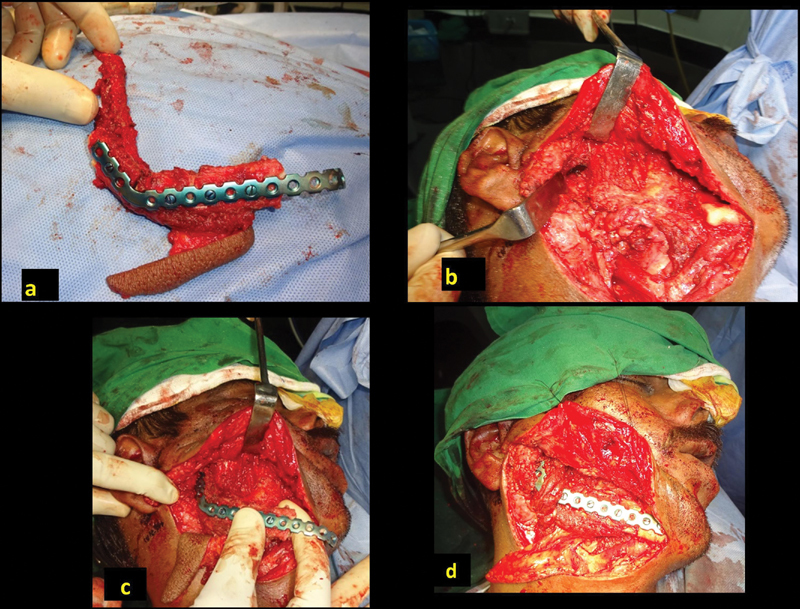
Reconstruction of condyle; the fibula beyond the plate ( a ). The glenoid fossa to accommodate new condyle, supported by surrounding soft tissue ( b ). Placement of the “condyle” into the fossa prior to fixation to the remaining mandible ( c ). Reconstruction with “condyle' in place ( d ).
Conclusion
Multitude of challenges in secondary reconstruction includes difficulty in recreating true defects, unanticipated soft-tissue defects, extensive fibrosis and loss of planes, lack of a guide to contour the mandible, insufficient bone strength at the margins to get good screw purchase, dearth of suitable recipient vessels, insufficient skin for adequate closure, and postirradiation effects. The challenges are many, yet successful outcome is achievable.
Funding Statement
Funding None.
Conflict of Interest None.
Note
Patient consent obtained for publication of photographs.
References
- 1.Hayden R E, Mullin D P, Patel A K. Reconstruction of the segmental mandibular defect: current state of the art. Curr Opin Otolaryngol Head Neck Surg. 2012;20(04):231–236. doi: 10.1097/MOO.0b013e328355d0f3. [DOI] [PubMed] [Google Scholar]
- 2.Ferretti C, Rikhotso E, Muthray E, Reyneke J. Interim reconstruction and space maintenance of mandibular continuity defects preceding definitive osseous reconstruction. Br J Oral Maxillofac Surg. 2013;51(04):319–325. doi: 10.1016/j.bjoms.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Freitag V, Hell B, Fischer H. Experience with AO reconstruction plates after partial mandibular resection involving its continuity. J Craniomaxillofac Surg. 1991;19(05):191–198. doi: 10.1016/s1010-5182(05)80546-3. [DOI] [PubMed] [Google Scholar]
- 4.Shibahara T, Noma H, Furuya Y, Takaki R. Fracture of mandibular reconstruction plates used after tumor resection. J Oral Maxillofac Surg. 2002;60(02):182–185. doi: 10.1053/joms.2002.29817. [DOI] [PubMed] [Google Scholar]
- 5.Iseli T A, Yelverton J C, Iseli C E, Carroll W R, Magnuson J S, Rosenthal E L. Functional outcomes following secondary free flap reconstruction of the head and neck. Laryngoscope. 2009;119(05):856–860. doi: 10.1002/lary.20200. [DOI] [PubMed] [Google Scholar]
- 6.Cannon T Y, Strub G M, Yawn R J, Day T A. Oromandibular reconstruction. Clin Anat. 2012;25(01):108–119. doi: 10.1002/ca.22019. [DOI] [PubMed] [Google Scholar]
- 7.Straub J M, New J, Hamilton C D, Lominska C, Shnayder Y, Thomas S M. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade W N, Lipa J E, Novak C B et al. Comparison of reconstructive procedures in primary versus secondary mandibular reconstruction. Head Neck. 2008;30(03):341–345. doi: 10.1002/hed.20705. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Zhang W B, Liu X J, Guo C B, Yu G Y, Peng X. A new procedure assisted by digital techniques for secondary mandibular reconstruction with free fibula flap. J Craniofac Surg. 2016;27(08):2009–2014. doi: 10.1097/SCS.0000000000003096. [DOI] [PubMed] [Google Scholar]
- 10.Ciocca L, Mazzoni S, Fantini M, Persiani F, Marchetti C, Scotti R. CAD/CAM guided secondary mandibular reconstruction of a discontinuity defect after ablative cancer surgery. J Craniomaxillofac Surg. 2012;40(08):e511–e515. doi: 10.1016/j.jcms.2012.03.015. [DOI] [PubMed] [Google Scholar]


