Abstract
Azetidines are four-membered nitrogen-containing heterocycles that hold great promise in current medicinal chemistry due to their desirable pharmacokinetic effects. However, a lack of efficient synthetic methods to access functionalized azetidines has hampered their incorporation into pharmaceutical lead structures. As a [2+2] cycloaddition reaction between imines and alkenes, the aza Paternò-Büchi reaction arguably represents the most direct approach to functionalized azetidines. Hampered by competing reaction paths accessible upon photochemical excitation of the substrates, the current synthetic utility of these transformations is greatly restricted. We herein report the development of a visible light-enabled aza Paternò-Büchi reaction that surmounts existing limitations and represents a mild solution for the direct formation of functionalized azetidines from imine and alkene containing precursors.
Subject terms: Drug discovery and development, Photocatalysis, Synthetic chemistry methodology
The Aza Paternò-Büchi reaction is arguably among the most direct approaches to functionalized azetidines, which are common medicinal scaffolds. Here, the authors report a mild and selective visible light-enabled intramolecular aza Paternò-Büchi reaction yielding bicyclic azetidines in high yields and diastereoselectivity.
Introduction
Nitrogen-containing heterocycles are essential structural components for drug design and are currently incorporated in more than 59% of all pharmaceuticals approved by the US Food and Drug Administration (FDA)1. The majority of these contain five- and six-membered pyrrolidine and pyridine derivatives, regardless of the fact that smaller heterocycles, such as the four-membered azetidines (1), are known to display superior physicochemical properties and increased bioavailability, as well as metabolic stability2–6. Despite these desirable characteristics, azetidines remain underutilized in current medicinal chemistry, which is a direct result of a lack of efficient synthetic methods for their construction7–9. The most important strategy for the synthesis of saturated nitrogen-containing heterocycles relies on unimolecular cyclization reactions via nucleophilic substitution (4, Fig. 1a)10. While this approach results in the efficient formation of three-, five-, and six-membered heterocycles, it often fails to yield the four-membered azetidines (11). In general, four-membered rings are considered the hardest of all to form11. The reason for this lack of reactivity lies in the preferred conformation of the acyclic precursors (Fig. 1b). Specifically, the formation of three-membered rings (9) is favorable as 8b represents both the preferred as well as reactive conformation for cyclization. In comparison, formation of the four-membered azetidines is hampered as it requires access to conformation 10b, which is higher in energy due to unfavorable eclipsing interactions12. Thus, strategies that proceed upon strain release of azabicyclobutanes (5) as three-membered ring analogs were developed as viable alternatives for the synthesis of azetidines13–18. In addition, orthogonal strategies for azetidine synthesis were developed that rely on the reduction of more readily accessible β-lactam precursors (6)10,19,20. Arguably, aza Paternò–Büchi reactions (7) represent the most efficient and direct strategy towards azetidines. Analogous to the Paternò–Büchi reaction21, in which an excited state carbonyl undergoes a [2+2] cycloaddition with an alkene, aza Paternò–Büchi reactions rely on imines and alkenes. However, the development of these transformations has met challenges associated with the decreased photoreactivity of imine precursors22,23. In particular, the excited state of imines (13) is known to undergo facile and preferential radiationless decay upon rotation about the C = N π-bond (12b)24–27. This results in dissipation of electronic energy and a lack of reactivity in [2+2] cycloadditions with alkenes (14)28–42 (Fig. 1c). Hence, successful reports of aza Paternò–Büchi reactions are rare and limited to rigid imine- and alkene-containing systems predisposed for cycloaddition in addition to the stringent requirement of high energy ultraviolet (UV) light43–51. Consequently, the development of a mild and general reaction protocol for aza Paternò–Büchi reactions relying on visible light52–57 would be highly desirable.
Fig. 1.
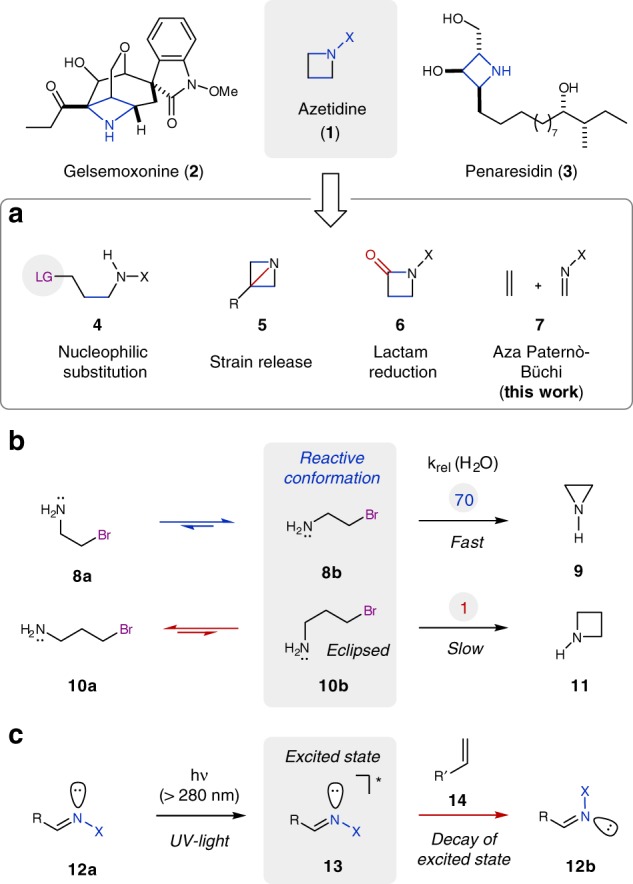
Previous strategies towards azetidines and this approach. a Select synthetic strategies. b Challenges in nucleophilic substitution reactions. c Challenges in aza Paternò–Büchi reactions
Here, we report the development of a visible light-mediated aza Paternò–Büchi reaction, which enables the synthesis of highly functionalized azetidines from readily available imine and alkene-containing precursors. Notably, this strategy is characterized by its mild conditions, operational simplicity, and scalability. The accessible azetidine products are readily converted into more valuable azetidine building blocks.
Results
Reaction optimization of aza Paternò–Büchi reaction
At the outset of our studies, we envisioned an orthogonal approach for aza Paternò–Büchi reactions that relies on selective activation of the alkene functionality to avoid excitation of the imine and associated competing reaction paths (Fig. 2). Triplet energies of alkenes58 (e.g., styrenes, dienes) are known to be lower than those of functionalized imines59–62, and could thus engage selectively in an energy transfer process with a suitable excited photocatalyst to reach the triplet state of the alkene. Subsequent [2+2] cycloaddition with the C = N double bond of the imine moiety would then lead to the formation of the desired azetidine product. Importantly, activation of the photocatalyst could be achieved by irradiation with visible light and thus forgo excitation of the imine and associated decay pathways.
Fig. 2.
Reaction design of the aza Paternò–Büchi reaction. Direct irradiation leads to unproductive excitation of the oxime moiety, while selective activation of the alkene via triplet energy transfer enables the [2 + 2] photocycloaddition
Our initial investigations into the development of a mild protocol for an aza Paternò–Büchi reaction centered on oximes such as 15 due to their facile synthesis and superior stability towards hydrolysis compared to imines. Consistent with literature reports, irradiation of oxime 15 with UV light resulted in E/Z isomerization of the oxime and only trace amounts of azetidine 16 as the desired aza Paternò–Büchi product63 (entry 1, Table 1). Conversion of 15 with catalytic amounts of xanthone as photosensitizer and UV light following conditions previously reported by Sivaguru and co-workers51 resulted in 43% yield albeit complete conversion of the substrate (entry 2, Table 1). We postulated that substrate decomposition is a direct consequence of the high redox properties of xanthone upon irradiation with UV light64. Following our initial hypothesis that selective alkene activation to its corresponding triplet state could lead to a mild reaction protocol for aza Paternò–Büchi reactions, we next evaluated a variety of photocatalysts in combination with lower energy visible light (40 W blue light-emitting diode (LED) light at 427 nm). When oxime 15 was irradiated with visible light in the presence of catalytic amounts of [Ru(bpy)3](PF6)2, no formation of azetidine 16 was observed (entry 3, Table 1). Nevertheless, when Ir(ppy)3 was used as photocatalyst under otherwise identical conditions, the desired aza Paternò–Büchi product 16 was formed in 39% yield (entry 4, Table 1). Reaction of oxime 15 with 2.5 mol% of Ir[dF(CF3)ppy]2(dtbbpy)PF6 (17•PF6) resulted in the formation of azetidine 16 in increased yields of 97% and >20:1 diastereomeric ratios (d.r.) (entry 5, Table 1). The potency of this photocatalyst was previously established by Yoon group65,66 in the [2+2] cycloaddition between alkenes for the formation of functionalized cyclobutanes. Further reaction optimization identified THF as optimal solvent resulting in 98% yield of azetidine 16 with catalyst loadings of only 0.5 mol% 17•PF6 (entry 15, Table 1). Finally, control reactions revealed that both light and photocatalyst were necessary for the [2 + 2] photocycloaddition to proceed (entries 16–17, Table 1)67–71.
Table 1.
Reaction optimization

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Wavelength (nm) | Solvent | Concentration (M) | Yield (%)a |
| 1b | – | 365 | CH2Cl2 | 0.01 | 6 |
| 2c | Xanthone (30) | 365 | MeCN | 0.01 | 43 |
| 3 | [Ru(bpy)3](PF6)2 (2.5) | 427 | THF | 0.01 | – |
| 4 | Ir(ppy)3 (2.5) | 427 | THF | 0.01 | 39 |
| 5 | 17•PF6 (2.5) | 427 | THF | 0.01 | 97 |
| 6 | 17•PF6 (2.5) | 427 | CH2Cl2 | 0.025 | 72 |
| 7 | 17•PF6 (2.5) | 427 | MeOH | 0.025 | 87 |
| 8 | 17•PF6 (2.5) | 427 | EtOAc | 0.025 | 87 |
| 9 | 17•PF6 (2.5) | 427 | acetone | 0.025 | 86 |
| 10 | 17•PF6 (2.5) | 427 | MeCN | 0.025 | 88 |
| 11 | 17•PF6 (2.5) | 427 | THF | 0.025 | 93 |
| 12 | 17•PF6 (2.5) | 427 | THF | 0.05 | 88 |
| 13 | 17•PF6 (2.5) | 427 | THF | 0.10 | 90 |
| 14 | 17•PF6 (1.0) | 427 | THF | 0.01 | 96 |
| 15 | 17•PF6 (0.5) | 427 | THF | 0.01 | 98 |
| 16 | – | 427 | THF | 0.01 | – |
| 17d | 17•PF6 (0.5) | – | THF | 0.01 | – |
Conditions: Reactions performed on 0.1 mmol scale under irradiation with a blue LED light (427 nm) for 0.5 h at ambient temperature (fan cooling)
aYield determined by quantitative 1H NMR analysis from the crude mixture using an internal standard
bFor 24 h
cFor 12 h
dRun in the dark 
Substrate scope of aza Paternò–Büchi reaction
With optimized reaction conditions established, we next evaluated different oximes and hydrazones for their ability to undergo the [2 + 2] cycloaddition (Table 2). It is important to note that all substrates were prepared and used as mixtures of E/Z oxime or hydrazone isomers. However, the observed diastereoselectivity of the azetidine products formed was found to be independent of the E/Z ratio of the substrate. O-Benzyl oxime 18a converted smoothly to 19a in 96% yield and very good diastereoselectivity of >20:1 d.r., providing similar results as the O-methyl oxime 15 (entries 1–2, Table 2a). Notably, the reaction can be performed on gram scale at slightly higher concentration with no significant decrease in yield. Free oxime 18b and N-Boc hydrazone 18c were reacted under the optimized conditions, and the corresponding azetidines 19b and 19c were isolated in 54 and 62% yield, respectively (entries 3–4, Table 2a). Additionally, the structure of 19c was subsequently verified by X-ray analysis (Table 2b). Interestingly, no reaction was observed with N,N-dimethyl hydrazone 18d and only unreacted starting material was recovered from the reaction mixture (entry 5, Table 2a). While other substituted imines (e.g., N-tosyl imines) successfully underwent the aza Paternò–Büchi reaction, the poor stability towards hydrolysis of the corresponding substrates prohibited sufficient purification and led to reproducibility issues.
Table 2.
Evaluation of oxime and hydrazone substrates

We subsequently investigated the scope of the aza Paternò–Büchi reaction with a particular emphasis on functional group tolerance and the ability to rapidly construct functionalized azetidines (Fig. 3). The optimized conditions proved efficient for a variety of electronically diverse styrenes including both electron-rich and electron-deficient systems, affording the corresponding azetidines in excellent yields and diastereoselectivities (20–24). Furthermore, different substitution patterns on the styrene moiety as well as the substrate backbone including esters and sulfonamides were well tolerated, providing a set of densely substituted azetidines (25–29). While the developed transformation could be readily conducted under ambient atmosphere in short reaction times of 30 min, substrates bearing increased steric constraints were found to require extended reaction times (30–36). It was beneficial to conduct these transformations in degassed solvent to minimize undesired reactivity with atmospheric oxygen to ultimately obtain the desired azetidine products in high yields of up to 98%. Specifically, oximes derived from methyl and phenyl ketones were converted to the corresponding azetidines 30 and 31 in excellent yield. The reaction is also amenable to heteroaromatic ketones—2-pyridyl azetidine 32 was afforded in 74% yield and excellent diastereoselectivity. Additionally, azetidine 33 bearing a lactone tether was formed in 60% (75% brsm) after irradiation for 70 h at elevated temperatures. Importantly, substrates containing internal esters are often found less reactive in many ring-closing transformations, considering that the reaction can only proceed through the less favored s-trans ester conformation72. Furthermore, cyclic tetrasubstituted styrenes are compatible with the reaction conditions, providing tricyclic azetidine 34 in 98% yield. We also evaluated the feasibility of substrates to form azetidines fused to six-membered rings upon [2 + 2] cycloaddition. The corresponding substrates were found to be significantly less reactive; nevertheless, chromane 35 and cyclohexane 36 were obtained after irradiation for 72 h in 42 and 93% yield, respectively. Based on the fact that many pharmaceutical or agrochemical products contain cyclic oxime or hydrazone motifs, we were interested in evaluating whether the aza Paternò–Büchi reaction protocol developed herein would allow for their late-stage modification. Cyclic oxime 38 derived from the herbicide safener isoxadifen ethyl (37) provided highly functionalized azetidine 39 in 87% yield, thus enabling late-stage modification of an industrially important oxime.
Fig. 3.
Scope of the [2 + 2] cycloaddition reaction. Reactions were performed with 0.25 mmol substrate (mixture E/Z oxime isomers) and 0.5–1.0 mol% 17•PF6 in THF (0.01 M) at ambient temperature (fan cooling) under blue LED irradiation (427 nm) unless noted; diastereomeric ratios (d.r.) were determined by 1H NMR from the crude reaction mixture; isolated yields refer to the mixture of diastereomers (major diastereomer is given); aon 500 mg scale; bunder N2 atmosphere; crun at 82 °C
Subsequent investigations focused on the compatibility of dienes as alkene equivalent with the developed procedure for visible light-mediated aza Paternò–Büchi reactions (Fig. 4)66. After reacting the corresponding diene 40 under the optimized conditions, strained bicycle 41 was isolated as the product of the reaction in 39% yield. Similarly, azetidine 43 was obtained in 99% yield. Notably, no products resulting from competing [4 + 2] cycloadditions were observed in either one of these reactions.
Fig. 4.

Aza Paternò–Büchi reaction utilizing dienes. Reactions were performed with 0.25 mmol substrate; diastereomeric ratios (d.r.) were determined by 1H NMR from the crude reaction mixture; isolated yields refer to the mixture of diastereomers (major diastereomer is given)
Mechanistic investigations
Ensuing efforts focused on gaining additional insights into the controlling features of the visible light-mediated aza Paternò–Büchi reaction. A Stern–Volmer quenching study unambiguously showed that the styrene moiety is necessary for productive quenching of the photocatalyst, while the corresponding oxime 45 lacking a styrene moiety does not result in significant quenching of 17•PF6 (Fig. 5a). We postulate that a photoredox process is unlikely under the optimized reaction conditions as the excited state redox potentials of 17•PF6 (E1/2III*/II = +1.21 V vs. SCE; E1/2IV/III* = –0.89 V vs. SCE)73 are not sufficient for an effective oxidation or reduction of substrate 15 (see Supplementary Methods for additional details). To further confirm this hypothesis, a series of control experiments was conducted, which imply that a triplet energy transfer mechanism from the photocatalyst to the styrene moiety of the substrate is operative. Styrenes possess a triplet energy (ET) of ~60 kcal mol−1, which suggest that 17•PF6 (ET = 62 kcal mol−1) is capable of sensitizing substrate 1558. In comparison, the efficiency of this transformation significantly decreases with photocatalysts that have a triplet energy below 60 kcal mol−1. While fac-Ir(ppy)3 (ET = 58 kcal mol−1) is still able to mediate product formation, albeit less efficiently, Ru(bpy)32+ (ET = 49 kcal mol−1) was found incapable of catalyzing the desired aza Paternò–Büchi reaction (Fig. 5d)74. Consistent with this hypothesis, substrates bearing terminal alkenes with significantly higher triplet energy (~76–84 kcal mol−1)58 were not found to undergo the desired transformation, but instead resulted in exclusive isolation of starting material (see Supplementary Methods for additional details). Additionally, the aza Paternò–Büchi reaction developed herein was found to be stereoconvergent, as both (E)- and (Z)-15 gave identical results under standard conditions (Fig. 5b). The interconversion of both styrene isomers upon photosensitization is fast and occurs at a rate similar to product formation (see Supplementary Fig. 4). Interestingly, monitoring the oxime isomer ratio of 15 over the course of the reaction revealed that oxime E/Z scrambling occurs at low conversion (Fig. 5c). In particular, within the first 2 min of the reaction an increase in (Z)-oxime concentration was observed along with a change in E/Z ratio from 1.6:1 to 1:1. This observation cannot be accounted for based on the faster conversion of (E)-oxime to azetidine 16 in comparison to (Z)-oxime. In contrast, no change in E/Z ratio was observed for compound 45 lacking the styrene moiety. Based on the results obtained in these investigations, we propose a reaction mechanism that relies on a styrene triplet manifold (intermediate I, Fig. 5). Efficient triplet energy transfer from photoexcited 17•PF6 accessible upon irradiation with visible light results in a triplet styrene (intermediate I) that undergoes subsequent reversible C–C bond formation to result in a 1,4-biradical (intermediate II). This intermediate allows for free rotation around the C–N bond that ultimately leads to the observed E/Z scrambling of the oxime after ring-opening (intermediate I) and relaxation back to the ground state. Alternatively, intermediate II can undergo intersystem crossing (ISC) (intermediate III), and deliver the azetidine product (16) after the final C–N bond-forming step. Importantly, the biradical nature of the involved triplet intermediates leads to complete loss of stereoinformation, which results in the formation of the same diastereomer independent of the oxime or alkene isomer ratios of the starting material.
Fig. 5.
Mechanistic investigations of the title reaction. a Stern–Volmer quenching studies. b Stereoconvergence of the aza Paternò–Büchi reaction. c Oxime E/Z isomerization during the course of the reaction. d Correlation of triplet energy and reaction efficiency (see Supplementary Methods for details). ET = triplet energy; ET = energy transfer; ISC = intersystem crossing
Synthetic applications
The developed [2+2] cycloaddition protocol enables rapid access to highly functionalized azetidines under mild conditions that can function as versatile building blocks to undergo further diversification (Fig. 6). The azetidine N–O bond can be readily cleaved with zinc metal under acidic conditions providing free azetidine 46 in 87% yield. To demonstrate the utility of the 2-phenylazetidine motif accessible by this method, azetidine 27 was converted to the corresponding N-Ts azetidine 47 (70% yield over 2 steps), which is amenable to phenyl ring oxidation utilizing RuCl3/H5IO6 to provide carboxylic acid 48 in 38% yield. Notably, 48 represents a previously inaccessible analog of the non-proteinogenic amino acid azetidine-2-carboxylic acid (Aze). Finally, the lactone tether of azetidine 33 is readily cleaved with LiAlH4 to provide 49 in 84% yield, resembling the product of a net intermolecular [2 + 2] cycloaddition reaction.
Fig. 6.
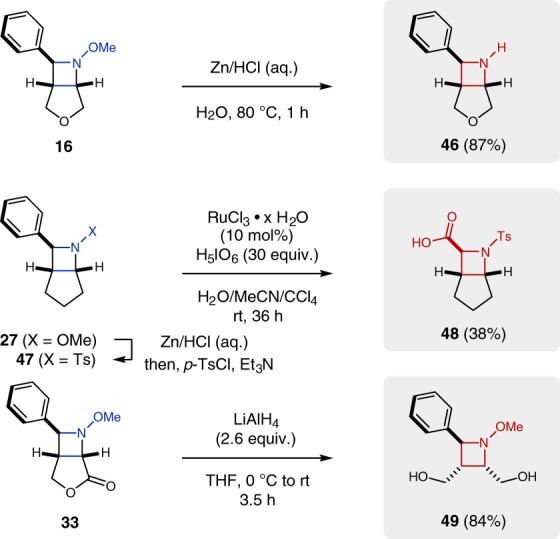
Synthetic modifications of azetidine products. The accessed azetidines can be converted to the corresponding unprotected azetidine (46), azetidine-2-carboxylic acid (48), or monocyclic azetidine (49)
Discussion
We herein report the development of a visible light-mediated aza Paternò–Büchi reaction between alkene and oxime moieties that results in the direct formation of functionalized azetidines, with yields of up to 99% and >20:1 d.r. The approach described relies on the selective activation of the alkene functionality upon energy transfer from a suitable photocatalyst to its corresponding triplet state. As a result, the aza Paternò–Büchi reaction developed herein overcomes previous challenges associated with the excitation of functionalized imines and resulting undesired competing reaction paths. We expect that this strategy will provide a new platform for the facile synthesis of azetidines and will enable further advancements in developing new enabling [2 + 2] cycloadditions involving carbon–nitrogen double bonds.
Methods
Representative procedure
A test tube was charged with 15 (0.25 mmol), 17•PF6 (0.5 mol%), and tetrahydrofuran (25 mL), sealed with a rubber septum and placed in front of a 40 W PR160-427 nm Kessil light (5 cm distance; 100% intensity). Upon completion as judged by thin layer chromatography analysis, the reaction mixture was concentrated in vacuum. The diastereomeric ratio was determined by 1H NMR (proton nuclear magnetic resonance) analysis from the crude reaction mixture, before purification by flash column chromatography (10–20% EtOAc/hexanes) to afford pure 16.
Supplementary information
Acknowledgements
We thank Dr. Jeff W. Kampf for X-ray crystallographic studies. C.S.S. thanks the Alfred P. Sloan Foundation, the David and Lucile Packard Foundation, and the Camille and Henry Dreyfus Foundation for fellowships. M.R.B. is grateful for a Peter A.S. Smith Endowment Award for Research.
Author contributions
M.R.B., A.D.R., and C.S.S. designed the experiments. M.R.B. and A.D.R. conducted and analyzed the experiments described in this report. M.R.B., A.D.R., and C.S.S. prepared this manuscript for publication.
Data availability
Experimental data as well as 1H and 13C NMR spectra for all new compounds prepared in the course of these studies are provided in the supplementary information of this manuscript. The X-ray crystallographic coordinates for compound 19c have been deposited at the Cambridge Crystallographic Data Center (CCDC) with the accession code 1873931 (10.5517/ccdc.csd.cc20wzd9). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. All other data including synthetic procedures are available in the supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13072-x.
References
- 1.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 2.Kerns, E. H. & Di, L. Drug-Like Properties: Concepts, Structure Design and Methods 1st edn 137–168 (Academic Press, 2008).
- 3.St. Jean DJ, Fotsch C. Mitigating heterocycle metabolism in drug discovery. J. Med. Chem. 2012;55:6002–6020. doi: 10.1021/jm300343m. [DOI] [PubMed] [Google Scholar]
- 4.Shu Y-Z, Johnson BM, Yang TJ. Role of biotransformation studies in minimizing metabolism-related liabilities in drug discovery. AAPS J. 2008;10:178–192. doi: 10.1208/s12248-008-9016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovering F, Bikker J, Humblet C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 6.Lovering F. Escape from Flatland 2: complexity and promiscuity. Med. Chem. Commun. 2013;4:515–519. doi: 10.1039/c2md20347b. [DOI] [Google Scholar]
- 7.Antermite D, Degennaro L, Luisi R. Recent advances in the chemistry of metallated azetidines. Org. Biomol. Chem. 2017;15:34–50. doi: 10.1039/C6OB01665K. [DOI] [PubMed] [Google Scholar]
- 8.Brandi A, Cicchi S, Cordero FM. Novel syntheses of azetidines and azetidinones. Chem. Rev. 2008;108:3988–4035. doi: 10.1021/cr800325e. [DOI] [PubMed] [Google Scholar]
- 9.Cromwell NH, Phillips B. The azetidines. Recent Synth. Dev. Chem. Rev. 1979;79:331–358. [Google Scholar]
- 10.Yoda, H., Takahashi, M. & Sengoku, T. in Heterocycles in Natural Product Synthesis 1st edn (eds Majumdar, K. C. & Chattopadhyay, S. K.) (Wiley-VCH, 2011).
- 11.Clayden, C., Greeves, N. & Warren, S. Organic Chemistry 2nd edn 805–807 (Oxford Univ. Press, 2012).
- 12.Warren, S. & Wyatt, P. Organic Synthesis: The Disconnection Approach 2nd edn 217–219 (Wiley, 2008).
- 13.Funke W. Synthesis and properties of 1‐azabicyclo[1.1.0]butanes. Angew. Chem. Int. Ed. 1969;8:70–71. doi: 10.1002/anie.196900701. [DOI] [Google Scholar]
- 14.Funke. W. Über synthesen und reaktionen von 1‐aza‐bicyclo[1.1.0]butanen. Chem. Ber. 1969;102:3148–3159. doi: 10.1002/cber.19691020935. [DOI] [Google Scholar]
- 15.Gianatassio R, et al. Strain-release amination. Science. 2016;351:241–246. doi: 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopchuk JM, et al. Strain-release heteroatom functionalization: development, scope, and stereospecificity. J. Am. Chem. Soc. 2017;139:3209–3226. doi: 10.1021/jacs.6b13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianatassio R, Kadish D. Direct alkylation of 1-azabicyclo[1.1.0]butanes. Org. Lett. 2019;21:2060–2063. doi: 10.1021/acs.orglett.9b00321. [DOI] [PubMed] [Google Scholar]
- 18.Fawcett A, Murtaza A, Gregson CHU, Aggarwal VK. Strain-release-driven homologation of boronic esters: application to the modular synthesis of azetidines. J. Am. Chem. Soc. 2019;141:4573–4578. doi: 10.1021/jacs.9b01513. [DOI] [PubMed] [Google Scholar]
- 19.Pitts CR, Lectka T. Chemical synthesis of β-lactams: asymmetric catalysis and other recent advances. Chem. Rev. 2014;114:7930–7953. doi: 10.1021/cr4005549. [DOI] [PubMed] [Google Scholar]
- 20.Alcaide B, Almendros P, Aragoncillo C. β-Lactams: versatile building blocks for the stereoselective synthesis of non-β-lactam products. Chem. Rev. 2007;107:4437–4492. doi: 10.1021/cr0307300. [DOI] [PubMed] [Google Scholar]
- 21.Flores DM, Schmidt VA. Intermolecular 2+2 carbonyl-olefin photocycloadditions enabled by Cu(I)-Norbornene MLCT. J. Am. Chem. Soc. 2019;141:8741–8745. doi: 10.1021/jacs.9b03775. [DOI] [PubMed] [Google Scholar]
- 22.Padwa A. Photochemistry of the carbon-nitrogen double bond. Chem. Rev. 1977;77:37–68. doi: 10.1021/cr60305a004. [DOI] [Google Scholar]
- 23.Pratt AC. The photochemistry of imines. Chem. Soc. Rev. 1977;6:63–81. doi: 10.1039/cs9770600063. [DOI] [Google Scholar]
- 24.Stoermer R. Über die Umlagerung der stabilen stereoisomeren in labile durch ultraviolettes Licht (TOGGLE) Ber. Dtsch. Chem. Ges. 1911;44:637–668. doi: 10.1002/cber.19110440194. [DOI] [Google Scholar]
- 25.Amin JH, de Mayo P. The irradiation of aryl aldoximes. Tetrahedron Lett. 1963;4:1585–1589. doi: 10.1016/S0040-4039(01)90876-7. [DOI] [Google Scholar]
- 26.Anderson DG, Wettermark G. Photoinduced isomerizations in anils. J. Am. Chem. Soc. 1965;87:1433–1438. doi: 10.1021/ja01085a004. [DOI] [Google Scholar]
- 27.Stenberg VI, Barks PA, Bays D, Hammargren DD, Rao DV. Nitrogen photochemistry. Syn and anti isomers of semicarbazones. J. Org. Chem. 1968;33:4402–4406. doi: 10.1021/jo01276a020. [DOI] [Google Scholar]
- 28.Padwa A, Albrecht F. Photoisomerization about the carbon-nitrogen double bond of an oxime ether. J. Am. Chem. Soc. 1972;94:1000–1002. doi: 10.1021/ja00758a053. [DOI] [Google Scholar]
- 29.Rainoldi G, Faltracco M, Lo Presti L, Silvani A, Lesma G. Highly diastereoselective entry into chiral spirooxindole-based 4-methyleneazetidines via formal [2+2] annulation reaction. Chem. Commun. 2016;52:11575–11578. doi: 10.1039/C6CC05838H. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura I, Nemoto T, Yamamoto Y, de Meijere A. Thermally induced and silver‐salt‐catalyzed [2+2] cycloadditions of imines to (alkoxymethylene)cyclopropanes. Angew. Chem. Int. Ed. 2006;45:5176–5179. doi: 10.1002/anie.200600961. [DOI] [PubMed] [Google Scholar]
- 31.Akiyama T, Daidouji K, Fuchibe K. Cu(I)-catalyzed enantioselective [2+2] cycloaddition of 1-methoxyallenylsilane with α-imino ester: chiral synthesis of α,β-unsaturated acylsilanes. Org. Lett. 2003;5:3691–3693. doi: 10.1021/ol0353621. [DOI] [PubMed] [Google Scholar]
- 32.Uyehara T, et al. Lewis acid-promoted [2+2] azetidine annulation of N-acylaldimines with allyltriisopropylsilane. Chem. Lett. 1995;24:789–790. doi: 10.1246/cl.1995.789. [DOI] [Google Scholar]
- 33.Cabral J, Laszlo P, Montaufier MT. Schizoid reactivity of N-benzylidene aniline toward clay-catalyzed cycloadditions. Tetrahedron Lett. 1988;29:547–550. doi: 10.1016/S0040-4039(00)80146-X. [DOI] [Google Scholar]
- 34.Aben RWM, Smit R, Scheeren JW. High-pressure-promoted [2+2] cycloadditions of imines with electron-rich alkenes. A simple route to azetidines and β-amino carbonyl compounds. J. Org. Chem. 1987;52:365–370. doi: 10.1021/jo00379a009. [DOI] [Google Scholar]
- 35.Behnke NE, Lovato K, Yousufuddin M, Kürti L. Titanium-mediated synthesis of spirocyclic NH-azetidines from oxime ethers. Angew. Chem. Int. Ed. 2019;58:1–6. doi: 10.1002/anie.201909151. [DOI] [PubMed] [Google Scholar]
- 36.Anderson LL. Applications of nitrones for the synthesis of heterocyclic compounds. Asian J. Org. Chem. 2019;5:9–30. doi: 10.1002/ajoc.201500211. [DOI] [Google Scholar]
- 37.Son J, Reidl TW, Kim KH, Wink DJ, Anderson LL. Generation and rearrangement of N,O-dialkenylhydroxylamines for the synthesis of 2-aminotetrahydrofurans. Angew. Chem. Int. Ed. 2018;57:6597–6600. doi: 10.1002/anie.201800908. [DOI] [PubMed] [Google Scholar]
- 38.Kroc MA, Prajapati A, Wink DJ, Anderson LL. Cascade synthesis of 3-functionalized indoles from nitrones and their conversion to cycloheptanone-fused indoles. J. Org. Chem. 2018;83:1085–1094. doi: 10.1021/acs.joc.7b02638. [DOI] [PubMed] [Google Scholar]
- 39.Reidl TW, Son J, Wink DJ, Anderson LL. Facile synthesis of azetidine nitrones and diastereoselective conversion into densely substituted azetidines. Angew. Chem. Int. Ed. 2017;56:11579–11583. doi: 10.1002/anie.201705681. [DOI] [PubMed] [Google Scholar]
- 40.Dowling MS, Fernando DP, Hou J, Liu B, Smith AC. Two scalable syntheses of (S)-2-methylazetidine. J. Org. Chem. 2016;81:3031–3036. doi: 10.1021/acs.joc.6b00149. [DOI] [PubMed] [Google Scholar]
- 41.Fritz SP, Moya JF, Unthank MG, McGarrigle EM, Aggarwal VK. An efficient synthesis of azetidines with (2-bromoethyl)sulfonium triflate. Synthesis. 2012;44:1584–1590. doi: 10.1055/s-0031-1290951. [DOI] [Google Scholar]
- 42.Andresini M, et al. Azetidine–borane complexes: synthesis, reactivity, and stereoselective functionalization. J. Org. Chem. 2018;83:10221–10230. doi: 10.1021/acs.joc.8b01441. [DOI] [PubMed] [Google Scholar]
- 43.Fischer G, Fritz H, Prinzbach H. An intramolecular imine/ene-photo-[2+2]-cycloaddition reaction. Tetrahedron Lett. 1968;27:1269–1272. doi: 10.1016/S0040-4039(00)84235-5. [DOI] [Google Scholar]
- 44.Marterer W, et al. The azo(Azoxy) functionality as a π2 component in photo [2+2] cycloadditions ‘syn’‐ and ‘anti’‐3,4‐diazatricyclo[4.2.2.22,5]dodeca‐3,7‐diene, syntheses, photolyses, X‐ray‐structure analysis, and PE spectra. Helv. Chim. Acta. 1988;71:1937–1965. doi: 10.1002/hlca.19880710813. [DOI] [Google Scholar]
- 45.Fischer G, et al. Proximate, syn‐periplanar, rigid imine(nitrone)/Ene‐, and diazene(diazeneoxy)/Ene systems: syntheses, homoconjugate reactivity and photochemistry. Eur. J. Org. Chem. 2000;2000:743–762. doi: 10.1002/(SICI)1099-0690(200003)2000:5<743::AID-EJOC743>3.0.CO;2-I. [DOI] [Google Scholar]
- 46.Howard KA, Koch TH. Photochemical reactivity of keto imino ethers. V. (2+2) Photocycloaddition to the carbon-nitrogen double bond of 3-ethoxyisoindolone. J. Am. Chem. Soc. 1975;97:7288–7298. doi: 10.1021/ja00858a015. [DOI] [Google Scholar]
- 47.Swenton JS, Hyatt JA. Photosensitized cycloadditions to 1,3-dimethyl-6-azauracil and 1,3-dimethyl-6-azathymine. Imine linkage unusually reactive toward photocycloaddition. J. Am. Chem. Soc. 1974;96:4879–4885. doi: 10.1021/ja00822a027. [DOI] [PubMed] [Google Scholar]
- 48.Nishio T, Omote Y. Photocycloaddition of quinoxalin-2-ones and benzoxazin-2-ones to aryl alkenes. J. Chem. Soc. Perk. Trans. 1987;1:2611–2615. doi: 10.1039/p19870002611. [DOI] [Google Scholar]
- 49.Nishio T. The (2+2) photocycloaddition of the carbon–nitrogen double bond of quinoxalin-2(1H)-ones to electron-deficient olefins. J. Org. Chem. 1984;49:827–832. doi: 10.1021/jo00179a015. [DOI] [Google Scholar]
- 50.Sakamoto R, Inada T, Sakurai S, Maruoka K. [2+2] Photocycloadditions between the carbon–nitrogen double bonds of imines and carbon–carbon double bonds. Org. Lett. 2016;18:6252–6255. doi: 10.1021/acs.orglett.6b03003. [DOI] [PubMed] [Google Scholar]
- 51.Kumarasamy E, Kandappa SK, Raghunathan R, Jockusch S, Sivaguru J. Realizing an aza Paternò–Büchi reaction. Angew. Chem. Int. Ed. 2017;56:7056–7061. doi: 10.1002/anie.201702273. [DOI] [PubMed] [Google Scholar]
- 52.Boyington AJ, Seath CP, Zearfoss AM, Xu Z, Jui NT. Catalytic strategy for the regioselective arylethylamine synthesis. J. Am. Chem. Soc. 2019;141:4147–4153. doi: 10.1021/jacs.9b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyington AJ, Riu M-L, Jui NT. Anti-Markovnikov hydroarylation of unactivated olefins using pyridyl radical intermediates. J. Am. Chem. Soc. 2017;139:6582–6585. doi: 10.1021/jacs.7b03262. [DOI] [PubMed] [Google Scholar]
- 54.Zhao K, et al. Catalytic ring expansions of cyclic alcohols enabled by proton-coupled electron transfer. J. Am. Chem. Soc. 2019;141:8752–8757. doi: 10.1021/jacs.9b03973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen ST, Zhu Q, Knowles RR. PCET-enabled olefin hydroamination reactions with N-alkyl amides. ACS Catal. 2019;9:4502–4507. doi: 10.1021/acscatal.9b00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kottisch V, Suppen MJ, Fors BP. Enhancing temporal control and enabling chain-end modification in photoregulated cationic polymerizations by using iridium-based catalysts. Angew. Chem. Int. Ed. 2018;57:8260–8264. doi: 10.1002/anie.201804111. [DOI] [PubMed] [Google Scholar]
- 57.Kottisch V, Michaudel Q, Fors BP. Photocontrolled Interconversion of cationic and radical polymerizations. J. Am. Chem. Soc. 2017;139:10665–10668. doi: 10.1021/jacs.7b06661. [DOI] [PubMed] [Google Scholar]
- 58.Ni T, Caldwell RA, Melton LA. The relaxed and spectroscopic energies of olefin triplets. J. Am. Chem. Soc. 1989;111:457–464. doi: 10.1021/ja00184a008. [DOI] [Google Scholar]
- 59.Padwa A, Bergmark W, Pashayan D. Mechanism of the photoreduction of N-alkylbenzylidenimines. J. Am. Chem. Soc. 1969;91:2653–2660. doi: 10.1021/ja01038a043. [DOI] [Google Scholar]
- 60.Padwa A, Koehn WP. Photochemical reduction in the N-acylketimine system. J. Org. Chem. 1975;40:1896–1902. doi: 10.1021/jo00901a005. [DOI] [Google Scholar]
- 61.Ohta H, Tokumaru K. Photochemistry of N-(4-dimethylaminobenzylidene)aniline. Bull. Chem. Soc. Jpn. 1975;48:1669–1670. doi: 10.1246/bcsj.48.1669. [DOI] [Google Scholar]
- 62.Yoshida M, Sakuragi H, Nishimura T, Ishikawa S, Tokumaru K. Nature of the excited triplet states in the photolysis of O-acyloximes. Chem. Lett. 1975;4:1125–1130. doi: 10.1246/cl.1975.1125. [DOI] [Google Scholar]
- 63.Roth, H. D. in PATAI’s Chemistry of Functional Groups (ed. Marek, I.) (Wiley, 2010).
- 64.Romero NA, Nicewicz DA. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 65.Lu Z, Yoon TP. Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 2012;51:10329–10332. doi: 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurtley AE, Lu Z, Yoon TP. [2+2] Cycloaddition of 1,3‐dienes by visible light photocatalysis. Angew. Chem. Int. Ed. 2014;53:8991–8994. doi: 10.1002/anie.201405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Q-Q, Zou Y-Q, Lu L-Q, Xiao W-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 2019;58:1586–1604. doi: 10.1002/anie.201803102. [DOI] [PubMed] [Google Scholar]
- 68.Zou Y-Q, et al. Visible light induced intermolecular [2+2]-cycloaddition reactions of 3-ylideneoxindoles through energy transfer pathway. Tetrahedron. 2012;68:6914–6919. doi: 10.1016/j.tet.2012.06.011. [DOI] [Google Scholar]
- 69.Alonso R, Bach T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew. Chem. Int. Ed. 2014;53:4368–4371. doi: 10.1002/anie.201310997. [DOI] [PubMed] [Google Scholar]
- 70.Huang X, et al. Direct visible-light-excited asymmetric Lewis acid catalysis of intermolecular [2+2] photocycloadditions. J. Am. Chem. Soc. 2017;139:9120–9123. doi: 10.1021/jacs.7b04363. [DOI] [PubMed] [Google Scholar]
- 71.Zhu M, Zheng C, Zhang X, You S-L. Synthesis of cyclobutane-fused angular tetracyclic spiroindolines via visible-light-promoted intramolecular dearomatization of indole derivatives. J. Am. Chem. Soc. 2019;141:2636–2644. doi: 10.1021/jacs.8b12965. [DOI] [PubMed] [Google Scholar]
- 72.Pawar DW, et al. E and Z conformations of esters, thiol esters, and amides. J. Am. Chem. Soc. 1998;120:2108–2112. doi: 10.1021/ja9723848. [DOI] [Google Scholar]
- 73.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strieth-Kalthoff F, James MJ, Teders M, Pitzer L, Glorius F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 2018;47:7190–7202. doi: 10.1039/C8CS00054A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental data as well as 1H and 13C NMR spectra for all new compounds prepared in the course of these studies are provided in the supplementary information of this manuscript. The X-ray crystallographic coordinates for compound 19c have been deposited at the Cambridge Crystallographic Data Center (CCDC) with the accession code 1873931 (10.5517/ccdc.csd.cc20wzd9). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. All other data including synthetic procedures are available in the supplementary information files.





