Abstract
Blastocystis hominis is a common intestinal protozoan with global distribution. The prevalence of B. hominis is extremely diverse in different countries. Molecular studies show that there is a great deal of genetic variation in the Blastocystis parasite isolated from humans and animals. Therefore, this study was conducted to determine B. hominis genotypes in patients attending to health centers of Sari, Mazandaran Province, north of Iran. 420 fecal specimens were collected from individuals in health centers of Sari from April to December 2017. The samples were examined by direct methods, parasite test (formalin ether) and Giemsa staining. In order to determine the genotypes of Blastocystis parasite, all positive samples in direct methods were tested by PCR using SSU rDNA gene. Eventually, seven positive molecular samples were sequenced and recorded in the gene bank. The phylogenetic analysis was done by drawing a gene tree using the neighbor-joining procedure. B. hominis was detected in 60 stool specimens by microscopic method. All these 60 samples were positive by PCR and the bands 310 bp were observed. According to the phylogenetic tree, it was revealed that B. hominis isolates of Mazandaran Province are more similar to subtype ST3 (ST type) that reported in Khorramabad in Lorestan Province (West of Iran). The present study showed that, like most studies, ST3 is the dominant genotype of protozoan in Sari. Improvement of public health awareness, relative improvement in environmental health, urban wastewater treatment, proper clothing coverage and the use of gloves during dealing with livestock and the use of domestic water treatment devices can reduce the Blastocystis infection in this area.
Keywords: Blastocystis hominis, Prevalence, Genotype, Mazandaran, Iran
Introduction
Blastocystis hominis (B. hominis) is a common large intestinal protozoan that infects humans and many vertebrates animal. This microorganism have observed with various morphological forms in stool samples of about one billion people in the world (Ramírez et al. 2016). Clinical symptom in humans is followed by abdominal pain, irritable and inflammatory bowel syndrome, diarrhea, constipation, fatigue, skin itching and bloating. Asymptomatic infections, symptomatic acute infections and chronic symptomatic infection have been reported in the various groups of patients (Boreham and Stenzel 1993). This parasite observed in various morphological forms such as vacuole, granulate, amoeboid and cysts in wet smear and culture medium. This mysterious and unique parasite has led to a reduction in the recognition of its sensitivity and its microscopic features. By recognizing different Blastocystis species, a more complete evaluation of the diagnosis and treatment of infection can be made; considering that this agent is unknown in infections (Boreham and Stenzel 1993; Tan and Suresh 2006).
According to a recent research by molecular methods based on the SSU rDNA (small subunit ribosomal DNA) gene, B. hominis has 17 subtypes. ST1-ST4 forms isolated from more than 90% of human cases. It has been suggested that genetically different genotypes (subtypes) may be associated with the pathogenic potential of B. hominis (Yan et al. 2006; Whipps et al. 2010). For this reason, studies on human Blastocystis have focused on genotype analysis. Nowadays, its genotype is usually examined by molecular methods based on the replication of the small subunit rRNA (Rene et al. 2009; Tan et al. 2013). PCR can be utilized to determine the strain, species and pathogenic potential of B. hominis isolates. Ribosomal rRNA gene analysis has been used repeatedly for finding taxonomic and phylogenetic relations among the isolates of this organism. Particularly, the small subunit ribosomal DNA has been increasingly used for comparing the genetic spin of the organism, because it is a quick and relatively simple method (Yan et al. 2006; Rene et al. 2009). Considering the interest of the scientific and medical communities to epidemiology and importance of this disease for the World Health Organization’s, sequencing of the complete genome of a single-breed type of protozoa has great importance for preventing of such diseases. (Yoshikawa et al. 2004; Tan and Suresh 2006; Rene et al. 2009).
Since a few studies have been conducted about Blastosystis species in Iran, this study was conducted to identify B. hominis genotypes using PCR molecular method in patients referring to health centers in Sari city, north of Iran.
Materials and methods
Collection of stool samples
420 fecal specimens were collected from individuals attending to health centers of Sari from April to December 2017. A questionnaire form was also provided to individuals who study variables such as demographic questions and risk factors associated with blastocystosis disease, were included.
Parasitology detection procedure
Initially, the stool samples were condensed by parasite test condensation method and then one drop of condensed stool was taken and after adding a drop of Logel to it, observed by light microscope with × 40 and × 100 magnifications. Then, the positive samples were used in the next step for molecular techniques.
DNA extraction and conducting PCR method
DNA extraction from the stool sample was performed by the kit from BIONEER Company and completely according to its instructions. Then, at first, for PCR, the amount of 12.5 μl of Master Mix 1 X, 0.1 mM of Forward b11400 primer SSU rDNA gene (5′-GGA ATC CTA TTA GAG GGA CAC TAT ACA T-3′), 0.1 mM of Reverse b11710 primer SSU rDNA gene (5′-TTA CTA AAA TCC AAA GTG TTC ATC GGA C-3′) and 100 ng of DNA template were added to the microtubes with 0.2 μm diameter, and totally the final volume became 25 μl. Finally, after adding the solutions, they were placed, inside the ThermoCycler machine. A negative control of sterile distilled water and a positive control of the DNA of Blastocystis parasite also used.
Genotyping and phylogenetic analysis
The preparing of sequences were done using Chromas version 2.33 software (http://www.technelysium.com.au/chromas.html). Multalin program (Corpet 1988) was applied to compare and align the nucleotide sequences with each other.
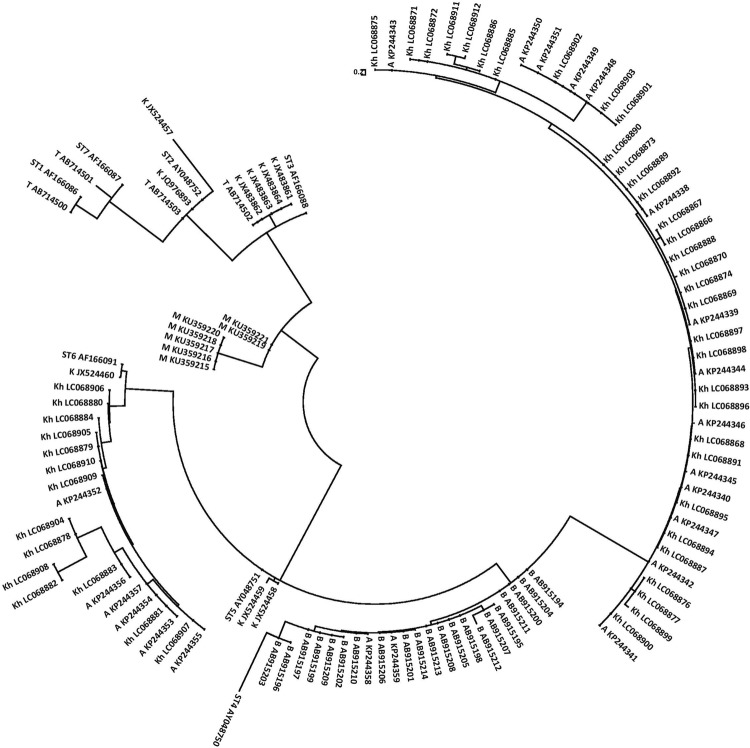
Phylogenetic analysis was performed with the construction of gene tree using the neighbor-joining (NJ) method in molecular evolutionary genetics analysis (MEGA) version4.0 software (Tamura et al. 2007). The bootstrap method with 1000 replicates was also used to assess the reliability of the gene tree. The nucleotide sequences were deposited in the GenBank database under accession numbers KU359215-KU359221 (Fig. 1).
Fig. 1.
Phylogenetic tree (phylogram) of 18S ribosomal RNA gene nucleotide sequences of seven isolates of B. hominis of Mazandaran Province (M), northern Iran, along with 103 isolates of B. hominis from Tehran (T), Baghmalek (B), Khoramabad (K), Ahvaz (A) and Khozestan (Kh), as well as Sub Types (ST) 1-7 (ST1-6) B. hominis registered at the GenBank. The tree was constructed by using the neighbor-joining method (NJ) with Tamura 3-parameter genetic distance model in molecular evolutionary genetics analysis (MEGA) version 4.0 software (Tamura et al. 2007). The accession number of each isolate has been writhed along with its name
Results
The prevalence of B. hominis was 14.29%. Totally, 60 fecal specimens that were microscopically tested or become positive by direct methods, Giemsa staining or parasite testing, and were investigated by PCR method for amplifying the SSU rDNA gene marker. All 60 samples were positive by PCR method and a pair band of 310 bp was observed, which was confirming to the microscopic method. Samples of positive PCR products were analyzed for molecular analysis just because of determining sequences and recording gene and also drawing phylogenetic charts. By studying phylogenetic tree, it was revealed that B. hominis isolates of Mazandaran province have more similar with isolated that reported from Khorramabad in Lorestan Province (West of Iran) (sub types ST3 and ST2 B. hominis) (Fig. 2).
Fig. 2.
Comparison and alignment of 18S ribosomal RNA gene nucleotide sequences of seven isolates of B. hominis of Mazandaran Province, northern Iran, using Multalin software (Corpet 1988). The dash (–) in the sequences represents the removed nucleotide
Details of demographic data, risk factors and the prevalence of B. hominis are summarized in Table 1. Some risk factors significantly associated with prevalence B. hominis including; sex (P = 0.0002), residence area (P = 0.007), consumed water (P < 0.0001), job (P = 0.002) and contact with domestic animals (P < 0.0001). There was no significant association between the prevalence of B. hominis and clinical symptoms (P > 0.05).
Table 1.
Frequency of B. hominis in Sari city by demographic data
| Variables | No. of examined | No. of positive (%) | P value |
|---|---|---|---|
| Age | |||
| < 10 | 7 | 1 (14.2) | 0.03 |
| 11–20 | 14 | 1 (7.1) | |
| 21–30 | 105 | 11 (10.4) | |
| 31–40 | 84 | 18 (21.4) | |
| 41–50 | 98 | 20 (20.4) | |
| ≥ 50 | 112 | 9 (8.1) | |
| Sex | |||
| Male | 245 | 48 (19.5) | 0.0002 |
| Female | 175 | 12 (6.8) | |
| Residence | |||
| Rural | 280 | 31 (11.1) | 0.007 |
| Urban | 140 | 29 (20.7) | |
| Consumed water | |||
| Healthy water | 84 | 27 (47.3) | |
| Unhealthy water | 336 | 33 (9.8) | P < 0.0001 |
| Job | |||
| Student | 14 | 2 (14.2) | |
| Private business | 252 | 33 (13.1) | 0.002 |
| Housewife | 70 | 5 (7.1) | |
| Government employee | 42 | 5 (11.9) | |
| Agriculture | 42 | 15 (35.7) | |
| Clinical symptoms | |||
| Yes | 42 | 8 (19.1) | 0.35 |
| No | 378 | 52 (13.7) | |
| Contact with domestic animals | |||
| Yes | 84 | 28 (33.3) | P < 0.0001 |
| No | 336 | 32 (9.5) | |
Discussion
According to scientific knowledge, the pathogenesis of B. hominis in humans is still in doubt (Kaneda et al. 2001). Some clinical studies have suggested, B. hominis as a causative agent of intestinal diseases including diarrhea, irritable bowel syndrome and ulcerative colitis. Moreover, recently it has been suggested a potential association between irritable bowel syndrome and B. hominis infection (MacPherson and MacQueen 1994; Suresh and Smith 2004; Poirier et al. 2012; Khademvatan et al. 2017; Cifre et al. 2018). There are some evidences which suggesting that, people with immunodeficiency, especially those with AIDS, are more prone to suffer from diarrhea associated with B. hominis. Also, there are reports of an outbreak of Blastocystis among cancer patients (Tan and Suresh 2006). On the other hand, in some studies, its pathogenicity has not been yet proven, and this parasite has been seen in many healthy individuals without any specific symptoms. A wide variety of genetic variations of Blastocystis have been described among human and animal, suggesting that certain genotypes may cause pathogenesis (Patino et al. 2008).
Prevalence of Blastocystis sp. around the world can vary from up to 10% in developed countries to 50–60% in developing countries (Mohamed et al. 2017). Also, prevalence of B. hominis has been reported to reach an average of 20% in Europe continent and to exceed 50% in numerous developing countries as in Africa continent (Greige et al. 2018). So far, the highest prevalence of B. hominis has been reported in Senegal (El Safadi et al. 2014). This high variance may be due to misdetection and misreporting in some clinical laboratories (Heydari-Hengami et al. 2018). The high prevalence of B. hominis in developing countries is usually related to consumption of contaminated water or food and poor hygiene practices (Belleza et al. 2015).
In this study, the prevalence rate of B. hominis was 14.3%. Moreover, the prevalence of B. hominis was reported 17.26% in Tabriz city by Fallah et al. (Fallah et al. 2014). In another study conducted in Mashhad by Mokhtari et al. (2010), the prevalence of B. hominis was reported 36.8% (Mokhtari 2010). Also in couple of studies that conducted by Akhlaghi and Meamar in Tehran, the prevalence of B. hominis have been reported 5.6% and 12.8%, respectively (Meamar et al. 2007; Akhlaghi et al. 2009). According to the results of various studies, it is observed that the prevalence of B. hominis is not the same in different regions. This may be due to the weather conditions, public health and food and varies cultural habits. Demographic factors such as gender, age, place of residence, level of education, type of occupation and epidemiological factors such as geographical conditions, also have a clear and obvious influence on the severity of B. hominis infection (Horiki et al. 1997; Abe et al. 2002; Yan et al. 2006; Mokhtari 2010; Anvari et al. 2018, 2019).
Our statistical analysis showed that there is a significant association between prevalence of B. hominis and sex (P = 0.0002), so that it is more prevalent in men than women. The reason for this presumably is due to more pollution related to agricultural activities, more spending time outside the home and more activities in the farming and livestock sector of men (AbuOdeh et al. 2016).
In the current study, the highest prevalence of B. hominis was observed in the age group of 30-40 years old with the prevalence of 21.4%. There was a significant difference between the age and the prevalence of B. hominis (P = 0.03). No clear reason has been identified for this issue yet; however, it may be due to longer exposure to the risk factors and transmission routes (Pandey et al. 2015).
In this study, statistical analysis showed that prevalence of B. hominis decreases with increasing educational level, so that the highest percentage of positive cases was observed in the subjects with elementary degree (23.8%) and the lowest percentage of positive cases was observed in those who had university education (9. 1%) (P < 0.0001). This could mean that those with university education have more information about infectious diseases as well as their transmission and prevention (Villegas-Gómez et al. 2016).
Our analysis showed that the infection rate in ranchers (38.29%) and farmers (28.57%) was higher than other occupations, which is consistent with the results of the study that conducted by Shahdoust et al. (Shahdoust et al. 2016). There was a significant difference between occupation and prevalence of Blastocystis parasite (P = 0.0012). This can be due to the nature of their occupation, which is directly related to the soil and animals, so they are more likely to be infected (Horiki et al. 1997; Oliveira-Arbex et al. 2018).
The result showed that, people living in villages were more exposed to Blastocystis infection (20.71%). Also, there was a significant difference between living in villages and the presence of parasites (P = 0.0078), which can be due to a lack of hygiene in these areas, food habits and occupation of farming and animal husbandry which spend more time with water and livestock (Wang et al. 2018).
In our study, the highest prevalence of B. hominis was observed in people who used healthy water (47.34%) and the lowest percentage of people using unhealthy water (9.82%). Furthermore, there was a significant difference between consumed water and the prevalence of B. hominis. (P = 0.0001). This difference may be due to the lack of safe drinking water, the sanitation system and the lack of sanitation facilities in rural areas compared with urban areas (Lee et al. 2012).
In this study, the highest perevalnce of B. hominis detected in individuals that close to the livestock (33.33%), which could indicate the importance of the zoonotic aspect of this parasite (Abe et al. 2002).
Recently, B. hominis isolates were classified in 17 types (STs) based on genetic phylogenetic analysis. So far, nine types of Blastocystis have been identified in human, while some of these types are limited to separated isolates from mammals or birds or a specific group of animals (Yoshikawa et al. 2016). Genotypes of ST1-8 are common between humans and animal hosts. ST9 is only observed in humans, also ST10-17 are seen in human hosts. ST3 is the most common genotype until now and ST4 has been reported only in Europe (Stenzel et al. 1997; Yan et al. 2006; Moosavi et al. 2012; Yoshikawa et al. 2016). Currently, molecular studies have shown that ST3 type is common, while there are a few studies that show that ST1 and ST4 are more likely to infect humans than ST3. Most of the animals which ST3-type were reported from them were domestic and had close contact to humans. These evidences suggest that ST3 genotype of B. hominis can easily infect humans, while other types are sporadic and often found in animal hosts (Tan and Suresh 2006).
In some studies, subtype 3 has been associated with pathogenesis such as acute intestinal problems. Also, some studies in Malaysia, Singapore, and the United States have reported subtype 3 as the dominant subtype in patients with chronic intestinal problems (Sukthana 2001; Yoshikawa et al. 2004; Tan and Suresh 2006). In Iran, several molecular studies have been done on B. hominis. In a study conducted by Sardarian et al. on the genetic diversity of 41 isolates of B. hominis showed that subtype 1 was dominated by 56.1% and then subtype 2 and 3 were followed by 22% and 7.3% respectively. They also reported a 14.6% prevalence of mixed infections of subtypes 1 and 3(Sardarian et al. 2012). In another study by Mousavi et al. in Tehran genotypic variation of 100 positive samples for B. hominis by PCR method was investigated. Finally subtypes 1, 2, 3, and 5 were identified. Prevalence of subtype 3 as a dominant subtype (53%), subtype 1 (48%), subtype 5 (33%) and subtype 2 (7%) were reported. Also, the high amount of mixed infections (33%) with three different subtypes reported (Moosavi et al. 2012).
Conclusion
The prevalence of B. hominis in Sari city can be interpreted in the way of transmission directly and through contaminated water and food. Because, most of the water consumed by the city is supplied through springs and rivers that are likely to be contaminated. On the other hand, due to the livestock position of the province, observance of sanitary measures such as proper clothing and wearing gloves during dealing with livestock, seems necessary for prevention, and due to its potential pathogenicity it should be considered.
Acknowledgements
This research is a part of the first author’s MSc thesis. The authors thank all colleagues working in Toxoplasmosis Research Centre (TRC) at Mazandaran University of Medical Sciences.
Author’s contribution
ShG, MF, HZH and AM provided the research proposal. DS, DA, SG collected the samples and performed laboratory works. ShG, MF and DA supervised the project. ShG was a scientific and lab diagnostic advisor to the project. SAH analysed the data. The manuscript was written by DS, finally revised by ShG. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for the present study was duly obtained from and approved by Research committee of Mazandaran University of Medical Sciences.
Informed consent
Informed consent was obtained from all individual participants included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe N, Nagoshi M, Takami K, et al. A survey of Blastocystis sp. in livestock, pets, and zoo animals in Japan. Vet Parasitol. 2002;106:203–212. doi: 10.1016/S0304-4017(02)00050-X. [DOI] [PubMed] [Google Scholar]
- AbuOdeh R, Ezzedine S, Samie A, et al. Prevalence and subtype distribution of Blastocystis in healthy individuals in Sharjah, United Arab Emirates. Infect Genet Evol. 2016;37:158–162. doi: 10.1016/j.meegid.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Akhlaghi L, Shamseddin J, Meamar AR, et al. Frequency of intestinal parasites in Tehran. Iran J Parasitol. 2009;4:44–47. [Google Scholar]
- Anvari D, Saadati D, Nabavi R, Alipour Eskandani M. Epidemiology and molecular prevalence of Toxoplasma gondii in Cattle Slaughtered in Zahedan and Zabol Districts, South East of Iran. Iran J Parasitol. 2018;13:114–119. [PMC free article] [PubMed] [Google Scholar]
- Anvari D, Sharif M, Sarvi S, et al. Seroprevalence of Toxoplasma gondii infection in cancer patients: a systematic review and meta-analysis. Microb Pathog. 2019;129:30–42. doi: 10.1016/J.MICPATH.2019.01.040. [DOI] [PubMed] [Google Scholar]
- Belleza MLB, Cadacio JLC, Borja MP, et al. Epidemiologic study of Blastocystis infection in an urban community in the Philippines. J Environ Public Health. 2015;2015:1–7. doi: 10.1155/2015/894297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham PFL, Stenzel DJ. Blastocystis in humans and animals: morphology, biology, and epizootiology. Adv Parasitol. 1993;32:1–70. doi: 10.1016/S0065-308X(08)60206-7. [DOI] [PubMed] [Google Scholar]
- Cifre S, Gozalbo M, Ortiz V, et al. Blastocystis subtypes and their association with Irritable Bowel Syndrome. Med Hypotheses. 2018;116:4–9. doi: 10.1016/j.mehy.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucl Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Safadi D, Gaayeb L, Meloni D, et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect Dis. 2014;14:1–11. doi: 10.1186/1471-2334-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah E, Mahami L, Mahami-Oskouei M, Safaiyan AR. Prevalence of Blastosystis hominis infection in Tabriz in 2009-2010. Urmia Med J. 2014;25:1027–3727. [Google Scholar]
- Greige S, El Safadi D, Bécu N, et al. Prevalence and subtype distribution of Blastocystis sp. isolates from poultry in Lebanon and evidence of zoonotic potential. Parasit Vectors. 2018;11:389. doi: 10.1186/s13071-018-2975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari-Hengami M, Hamedi Y, Najafi-Asl M, Sharifi-Sarasiabi K. Prevalence of intestinal parasites in food handlers of Bandar Abbas, Southern Iran. Iran J Public Health. 2018;47:111. [PMC free article] [PubMed] [Google Scholar]
- Horiki N, Maruyama M, Fujita Y, et al. Epidemiologic survey of Blastocystis hominis infection in Japan. Am J Trop Med Hyg. 1997;56:370–374. doi: 10.4269/ajtmh.1997.56.370. [DOI] [PubMed] [Google Scholar]
- Kaneda Y, Horiki N, Cheng X, et al. Ribodemes of Blastocystis hominis isolated in Japan. Am J Trop Med Hyg. 2001;65:393–396. doi: 10.4269/ajtmh.2001.65.393. [DOI] [PubMed] [Google Scholar]
- Khademvatan S, Masjedizadeh R, Rahim F, et al. Blastocystis and irritable bowel syndrome: frequency and subtypes from Iranian patients. Parasitol Int. 2017;66:142–145. doi: 10.1016/j.parint.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Lee LI, Chye TT, Karmacharya BM, Govind SK. Blastocystis sp.: waterborne zoonotic organism, a possibility? Parasites and Vectors. 2012;5:130. doi: 10.1186/1756-3305-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson DW, MacQueen WM. Morphological diversity of Blastocystis hominis in sodium acetate-acetic acid-formalin-preserved stool samples stained with iron hematoxylin. J Clin Microbiol. 1994;32:267–268. doi: 10.1128/jcm.32.1.267-268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meamar AR, Rezaian M, Mohraz M, et al. A comparative analysis of intestinal parasitic infections between HIV +/AIDS patients and non-HIV infected individuals. Iran J Parasitol. 2007;2:1–6. [Google Scholar]
- Mohamed AM, Ahmed MA, Ahmed SA, et al. Predominance and association risk of Blastocystis hominis subtype i in colorectal cancer: a case control study. Infect Agent Cancer. 2017;12:21. doi: 10.1186/s13027-017-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari M (2010) Prevalence of Blastocystis homonis infection in Gayem hospital, Iran. In: 7th National 2nd Reg Congr Parasitol Parasit Dis Tehran,Iran
- Moosavi A, Haghighi A, Mojarad EN, et al. Genetic variability of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Iran. Parasitol Res. 2012;111:2311–2315. doi: 10.1007/s00436-012-3085-5. [DOI] [PubMed] [Google Scholar]
- Oliveira-Arbex AP, David ÉB, Guimarães S. Blastocystis genetic diversity among children of low-income daycare center in Southeastern Brazil. Infect Genet Evol. 2018;57:59–63. doi: 10.1016/j.meegid.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Pandey PK, Verma P, Marathe N, et al. Prevalence and subtype analysis of Blastocystis in healthy Indian individuals. Infect Genet Evol. 2015;31:296–299. doi: 10.1016/j.meegid.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Patino WD, Cavuoti D, Banerjee SK, et al. Cytologic diagnosis of Blastocystis hominis in peritoneal fluid. Acta Cytol. 2008;52:718–720. doi: 10.1159/000325628. [DOI] [PubMed] [Google Scholar]
- Poirier P, Wawrzyniak I, Vivarès CP, et al. New insights into Blastocystis spp: a potential link with irritable bowel syndrome. PLoS Pathog. 2012;8:1002545. doi: 10.1371/journal.ppat.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez JD, Sánchez A, Hernández C, et al. Geographic distribution of human Blastocystis subtypes in South America. Infect Genet Evol. 2016;41:32–35. doi: 10.1016/j.meegid.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Rene BA, Stensvold CR, Badsberg JH, Nielsen HV. Subtype analysis of Blastocystis isolates from Blastocystis cyst excreting patients. Am J Trop Med Hyg. 2009;80:588–592. doi: 10.4269/ajtmh.2009.80.588. [DOI] [PubMed] [Google Scholar]
- Sardarian K, Hajilooi M, Maghsood A, et al. A study of the genetic variability of Blastocystis hominis Isolates in Hamadan, West of Iran. Jundishapur J Microbiol. 2012;6:11–15. doi: 10.5812/jjm.4171. [DOI] [Google Scholar]
- Shahdoust S, Niyyati M, Haghighi A, et al. Prevalence of intestinal parasites in referred individuals to the medical centers of Tonekabon city, Mazandaran province. Gastroenterol Hepatol from Bed to Bench. 2016;9(Suppl1):S75. [PMC free article] [PubMed] [Google Scholar]
- Stenzel DJ, Lee MG, Boreham PFL. Morphological differences in Blastocystis cysts–an indication of different species? Parasitol Res. 1997;83:452–457. doi: 10.1007/s004360050279. [DOI] [PubMed] [Google Scholar]
- Sukthana Y. Is Blastocystis hominis a human pathogenic protozoan. J Trop Med Parasitol. 2001;24:16–22. [Google Scholar]
- Suresh K, Smith H. Comparison of methods for detecting Blastocystis hominis. Eur J Clin Microbiol Infect Dis. 2004;23:509–511. doi: 10.1007/s10096-004-1123-7. [DOI] [PubMed] [Google Scholar]
- Tamura Koichiro, Dudley Joel, Masatoshi Nei SK. MEGA4: molecular evolutionary genetics analysis (MEGA) Software Version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tan TC, Suresh KG. Amoeboid form of Blastocystis hominis—a detailed ultrastructural insight. Parasitol Res. 2006;99:737–742. doi: 10.1007/s00436-006-0214-z. [DOI] [PubMed] [Google Scholar]
- Tan TC, Tan PC, Sharma R, et al. Genetic diversity of caprine Blastocystis from Peninsular Malaysia. Parasitol Res. 2013;112:85–89. doi: 10.1007/s00436-012-3107-3. [DOI] [PubMed] [Google Scholar]
- Villegas-Gómez I, Martínez-Hernández F, Urrea-Quezada A, et al. Comparison of the genetic variability of Blastocystis subtypes between human carriers from two contrasting climatic regions of México. Infect Genet Evol. 2016;44:334–340. doi: 10.1016/j.meegid.2016.07.036. [DOI] [PubMed] [Google Scholar]
- Wang J, Gong B, Yang F, et al. Subtype distribution and genetic characterizations of Blastocystis in pigs, cattle, sheep and goats in northeastern China’s Heilongjiang Province. Infect Genet Evol. 2018;57:171–176. doi: 10.1016/j.meegid.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Boorom K, Bermudez LE, Kent ML. Molecular characterization of Blastocystis species in Oregon identifies multiple subtypes. Parasitol Res. 2010;106:827–832. doi: 10.1007/s00436-010-1739-8. [DOI] [PubMed] [Google Scholar]
- Yan Y, Su S, Lai R, et al. Genetic variability of Blastocystis hominis isolates in China. Parasitol Res. 2006;99:597–601. doi: 10.1007/s00436-006-0186-z. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Abe N, Wu Z. PCR-based identification of zoonotic isolates of Blastocystis from mammals and birds. Microbiology. 2004;150:1147–1151. doi: 10.1099/mic.0.26899-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Tokoro M, Nagamoto T, et al. Molecular survey of Blastocystis sp. from humans and associated animals in an Indonesian community with poor hygiene. Parasitol Int. 2016;65:780–784. doi: 10.1016/j.parint.2016.03.010. [DOI] [PubMed] [Google Scholar]