Abstract
The current study was designed to evaluate the in vivo fasciolicidal activity of Moringa (M.) oleifera leaf aqueous extract oral administration as well as its antibacterial activity against Clostridium (C.) novyi in sheep naturally co-infected with fascioliasis and C. novyi. Sheep naturally infected with fascioliasis were divided into 3 groups, heavily infected treated group, lightly infected treated group and mixed infection control (non-treated) group. Treatment groups were orally administered M. oleifera leaves aqueous extract at a dose of 150 mg/kg every 48 h for 21 days. Animal body weights, fecal egg count, serum levels of anti-Fasciola IgG, cytokines (IL-2, IL-17, IL-10), and bacterial count of C. novyi were evaluated. The results showed that treatment with M. oleifera improved the body weight gain and decreased fecal egg count in lightly and heavily infected groups compared to the nontreated group with 100% reduction in egg count in lightly infected sheep. Furthermore, the treatment with M. oleifera significantly reduced the serum levels of IgG, IL-2, and IL-17. Interestingly, elevated levels of IL-10 were recorded in both heavily and lightly infected sheep. The treatment with Moringa extract significantly decreased the fecal bacterial count of C. novyi in both heavily and lightly infected groups. In conclusion, this study highlights the potential beneficial effects of M. oleifera leaf against Fasciola (F.) gigantica and C. novyi.
Keywords: Moringa oleifera, Anthelmintic, Antimicrobial, Liver flukes, Cytokines, Th17, Immunoglobulins
Introduction
Fascioliasis, a parasitic infection caused by Fasciola (F.) hepatica and F. gigantica, is considered an important disease of ruminants causing significant weight loss, increased rates of liver condemnation and animal mortalities (Mas-Coma et al. 2014; Nyindo and Lukambagire 2015). Moreover, Fasciola species have zoonotic importance, affecting approximately 50 million people worldwide, and are listed as a foodborne infection by the World Health Organization (2015).Clostridium (C.) species are normal bacterial inhabitants found in the gastrointestinal tract of ruminants. Migration of Fasciola species to the liver and the subsequent necrosis and damage to the hepatic parenchyma facilitate the transmission and anaerobic medium required for systemic clostridial infection (Benavides et al. 2015; Cullen and Stalker 2015; Redford et al. 2017). Infection with Clostridium novyi type B causes “black disease” in sheep. It is associated with necrotic hepatitis due to migration of immature liver fluke that release toxins destroying the animal liver and causing sudden death (Uzal et al. 2016). The synergistic interaction between the two infections alters the infection duration, transmission risks, and clinical symptoms (Cox 2001; Vaumourin et al. 2015). Infection with F. gigantica is able to elicit Th2 immune response and downregulated Th1 and Th17- type inflammatory immune responses, leading to increased secretion of IFN-γ, Il-2, IL-6, IL-12, IL-17, and IL-1β cytokines in order to support the parasite’s existence within the infected host (Pleasance et al. 2011; Zhang et al. 2017). The down regulation of protective Th1 responses results in an increase in the host’s susceptibility to many bacterial infections (Cerf-Bensussan and Gaboriau-Routhiau 2010).
The use of anthelmintics and antibiotics in food animals is fundamental to animal health and to the agricultural economy as well. Drugs used in food-animals and drug residues in food products do increase the health risk in persons who consume products from treated animals (Hao et al. 2014). Moreover, during the last decade, there has been an increasing report in antibiotic resistance to different clostridial infections in animals and humans (Gholamiandehkordi et al. 2009; Bannam et al. 2011; Zidaric et al. 2012). Similarly, multiple reports confirmed anthelmintic resistance of Fasciola species in sheep, cattle and humans (Ortiz et al. 2013; Brockwell et al. 2014; Kelley et al. 2016). Medicinal plants have been widely utilized, either as a single drug or in combination with synthetic drugs to aid in control/treatment of various bacterial and parasitic infections in animals and human. These plants provide valuable natural products and drugs for development of medicines against various disorders and diseases (Pan et al. 2013; Bahmani et al. 2014).
Moringa oleifera, commonly known as Drumstick, have many active components such as alkaloids, tannins, flavonoids, saponins and triterpenoids (Vats and Gupta 2017), which showed potent anthelmintic activity and displayed a potential antibacterial activity against Gram negative and Gram-positive bacteria (El-Kholy et al. 2018). Aqueous extract of M. oleifera has been shown a potent immunomodulatory effect that can modulate the activation of B cells and stimulate production of IgM, IgA and IgG level after administration (Ojeka et al. 2018), and T cells where it will result for induction of IL 10 production (Fard et al. 2015; Tan et al. 2015) and inhibit production of TNFα, IL-6, IL-8 (Kooltheat et al. 2014) and IL-2 (Sashidhara et al. 2009). Studies on humans and animals revealed that M. oleifera is considered safe for consumption (Stohs and Hartman 2015). The ovicidal activity for Moringa extracts on Fasciola eggs in vitro was previously reported by Hegazi et al. (2018).
When these findings taking together we should warrant further consideration of M. olifiera as an immunomodulatory for immune disorders induced by fascioliasis. Therefore, we hypothesized that the oral administration of M. oleifera aqueous extract can provide protection against F. gigantica and C. novyi by regulating the immune responses. To test this hypothesis, The present study was designed to evaluate the oral administration effect of M. olifiera aqueous extract on the body weight, fecal egg count, IgG, cytokines (IL-2, IL-17, IL-10), and the bacterial load of C. novyi in sheep naturally co-infected with F. gigantica and C. novyi.
Materials and methods
Ethical approval
All experimental procedures were performed in accordance with the institutional guidelines of the National Research Centre’s Animal Research Committee under protocol number 16/219.
Animals and ethical standard
A number of 30 reared sheep, aged 6–12 months, were examined in this study. The animals were determined for infection with F. gigantica using egg detection in fecal samples by sedimentation technique according to the method of Happich and Boray (1969). The examined animals were raised in the River Nile’s basin and fed regularly on clover grasses and a concentrate mix. The infected animals were classified based on the severity of fascioliasis according to Attallah et al. (2013). The animals were further classified to a lightly infection treated group, a heavily infection treated group, a mixed lightly/heavily infection control non treated group and healthy, non-infected, sheep as a negative control.
Preparation of M. oleifera leaf aqueous extract and animal treatment
Moringa leaves powder was a courtesy from Dr. Aboelfetoh Abdalla’s research group at the National Research Centre. 150 g of dried M. oleifera powder was soaked in a 1 liter distilled water and incubated at 4° C on a shaking incubator for 24 h then filtered using a filter paper into a clean conical flask. The M. oleifera extract was orally administrated to the lightly and heavily infection treated groups every 48 h for 21 days at a dose of 150 mg/kg body weight (Almanzor et al. 2014).
Body weight, fecal and blood sampling
All sheep were weighed on day 0 and re-weighed on day 21. Fecal samples were collected aseptically using rectal digital palpation from each animal on days 0, 3, 5, 7, 9, 11, 13, 15, 17, 19, and 21. The degree of body condition was scored 0–5 according to Russel et al. (1969) at the zero days of treatment and 21 day post treatment (dpt). A portion of each fecal sample was saved for Fasciola egg count per gram using the sedimentation technique. The remaining portion of the egg sample was saved for bacteriological examination to estimate C. novyi colony forming unit per gram (CFU/g). Blood samples were obtained via Jugular venipuncture at different treatment intervals. Serum was separated and stored at − 20 °C until further analyses.
F. gigantica egg count and morphology
Briefly, 1 g of fecal sample was added to 45 ml of distilled water in a graduated cylinder and thoroughly mixed using a glass rod. The solution was filtrated using a sieve and allowed to sediment. F. gigantica egg count was then calculated and reported as eggs per gram (Happich and Boray 1969).
Detection of F. gigantica specific antibodies
Antigen preparation
Adult F. gigantica flukes were obtained from infected livers of water buffaloes freshly slaughtered at El Monib local abattoir, Giza Governorate and washed several times with distilled water. The crude worm antigen was prepared according to the method previously described (Abdel-Rahman et al. 2016). Adult flukes were homogenized in 0.2 M Tris–Hcl buffer, pH 7.4, with a glass homogenizer at 4 °C. The homogenate was then centrifuged at 14,000 round per minute (rpm) for 30 min at 4 °C, then the supernatant was collected and assayed for protein content using Lowery method (Lowry et al. 1951). The supernatant was aliquoted and stored at − 20 °C.
F. gigantica specific IgG assay
The assay was utilized to evaluate the total IgG antibody response of sheep after treatment using M. oleifera leaf aqueous extract at different time points in treated and control groups. The assay was performed according to the method previously described (Oldham 1983), and the optimal dilution of serum and concentration of the antigen were determined using checkerboard titration. The cutoff point of optical density values was determined as described by Almazán et al. (2001).
Quantification of cytokines in sheep sera
Sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure serum cytokine levels. Cytokine concentrations for sheep IL-10, IL-2 and IL-17 determined with commercially available reagents and ELISA kits purchased from Bioneovan Co., China and following the manufacturer’s instructions.
Determination of C. novyi levels (bacterial count CFU/g)
One gram from each fecal sample was homogenized in 9 ml of Phosphate Buffer Saline (1 × PBS). 10 fold serial dilutions of the homogenates in 1 × PBS was cultured in duplicates on neomycin blood agar using 100 µl of the diluted specimen for each plate. The inoculated plates were incubated at 37 °C and 5% CO2 for 24–48 h then the bacterial colonies were counted using colony counter according to the predefined criteria as described before (Black 1996). The colonies of C. novyi were confirmed microscopically and biochemically.
Statistical analysis
Data are expressed as mean ± standard deviation of the mean (SD). Statistical analyses were performed using two-way ANOVA followed by a post hoc test when appropriate. The result were considered significant at the level p < 0.05. Statistics were computed using GraphPad Prism software (version 6; GraphPad Software, Inc, La Jolla, CA, USA).
Results
The effects of M. oleifera aqueous extract oral administration on the body weight of sheep naturally infected with F. gigantica
The heavy infected treatment group showed a significant weight loss compared to the light and mixed infected groups before initiation of the treatment with M. oleifera aqueous extract (Day 0). Oral treatment with M. oleifera resulted in a significant weight gain at day 21 in the lightly infection treated group compared to heavily infected treated and mixed infection control groups (Fig. 1). The treatment with M. olifera improved the body weight of heavily infected sheep by 3.9 ± 0.1 and 4.5 ± 0.5 in treated lightly infected ones.
Fig. 1.
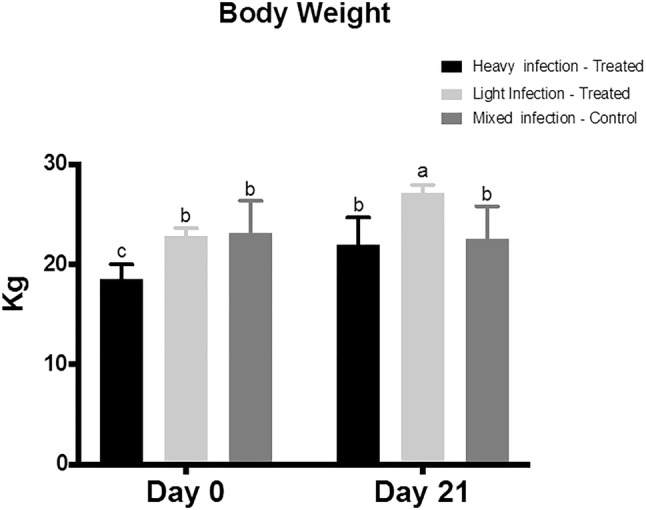
The effect of Moringa (M.) oleifera aqueous extract oral adminstration on the body weight of sheep naturally infected with Fasciola (F.) gigantica. The Sheep (6–12 months age) were classified based on the severity of fascioliasis into three groups; lightly infected treated group, heavily infected treated group, and mixed infected control group. The lightly and heavily infected groups were treated with M. oleifera aqueous extract for 21 days. The body weight of sheep in the three groups was recorded at 0 and 21 days. Two-way ANOVA was performed followed by multiple comparison tests. Data represented in mean ± standard deviation (SD). Different letters denote significance (p < 0.05)
The effects of M. oleifera aqueous extract oral administration on the fecal egg count and morphology
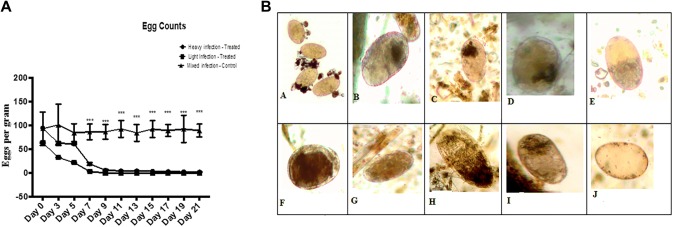
The lightly and heavily infected treated groups showed a significant lower EPG across treatment time compared to the mixed infection control groups. Significant differences were noted between the lightly and heavly infected groups at all-time points. The treatment with M. oleifera aqueous extract resulted in a significant decrease in EPG in the lightly infected treated group with complete resolution of the infection (Fig. 2a). However, in heavily infected group the mean egg count was significantly (p ≤ 0.001) decreased with 99.2% reduction after 21 dpt. Furthermore, the treatment with M. oleifera aqueous extract resulted in a degeneration of Fasciola eggs and accumulated dark embryo after the first dose of treatment at the end of experiment the egg showed empty without cells or embryo (Fig. 2b) This observation illustrates that treatment using Moringa extract have an obivious effect on egg viability and development.
Fig. 2.
a The effect of M. oleifera aqueous extract oral adminstrationon the fecal egg count of sheep naturally infected with F. gigantica. Eggs per gram (EPG) were counted using fecal sedimentation technique in the three groups; lightly infected treated group, heavily infected treated group, and mixed infected control group. Data represented in mean ± standard deviation (SD). *Denotes significance (p < 0.05) between the three groups. Two-way ANOVA followed by multiple comparisons test was applied. b Photomicrograph to show the ovicidal activity of M. oleifera aqueous extracts treatment on sheep. A Normal F. gigantica egg. B, C, D, E, F, G, H, I showed degenerated Fasciola egg and accumulated dark embryo at 3, 5, 7, 9, 11, 13, 15, 17 dpt respectively. J Showed empty F. gigantica egg at 21 dpt (×100)
The effects of M. oleifera aqueous extract oral administration on the fecal bacterial load of C. novyi
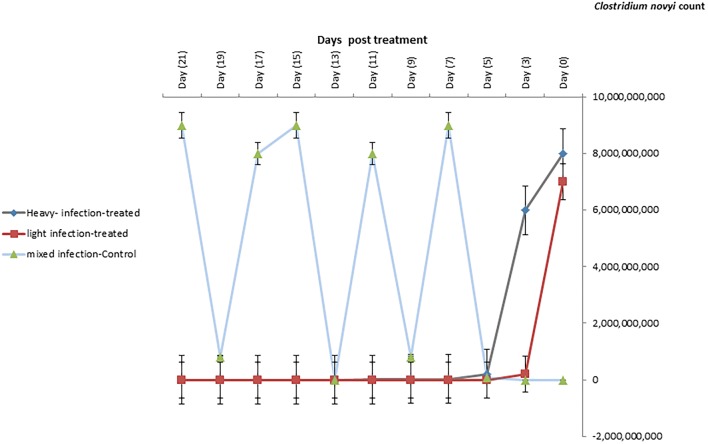
The C. novyi was numerous short, thick, straight; round-ended, gram positive rod occurs singly or in short chains under a microscope. On blood agar, the pure culture was small, irregular, whitish pale colored, finely granular and surrounded by a typical zone of β-hemolysis. In cooked meat medium, organism produced turbidity and gas with pinkish discoloration of meat particles. Biochemical characterization showed that all isolates fermented dextrose, lactose, sucrose, maltose and produced acid and gas but did not ferment mannitol. They were negative catalase, oxidase, Indole, Voges–Proskauer, and methyl red. The bacterial load of C. novyi was reduced significantly during the treatment with M. oleifera extract in both lightly infected and heavily infected groups compared to untreated control group till reach 2 × 104 after 21 day post treatment (dpt) (Fig. 3).
Fig. 3.
The bacterial counts of C. novyi in heavily, lightly, and mixed infected sheep with F. gigantica and treated with M. oleifera leaves. Data represented in mean ± standard deviation (SD). *Denotes significance (p < 0.05) between the three groups. Two-way ANOVA followed by multiple comparisons test was applied
The effects of M. oleifera aqueous extract oral administration on serum IgG expression
The levels of IgG showed a decrease in the lightly infected group compared to the heavily and mixed infection groups. Significant differences were noted between the lightly and heavily infected groups at most of the time points. The treatment with M. oleifera aqueous extract resulted in a gradual significant decrease in IgG in the lightly infected treated group with complete resolution of the infection (Fig. 4).
Fig. 4.
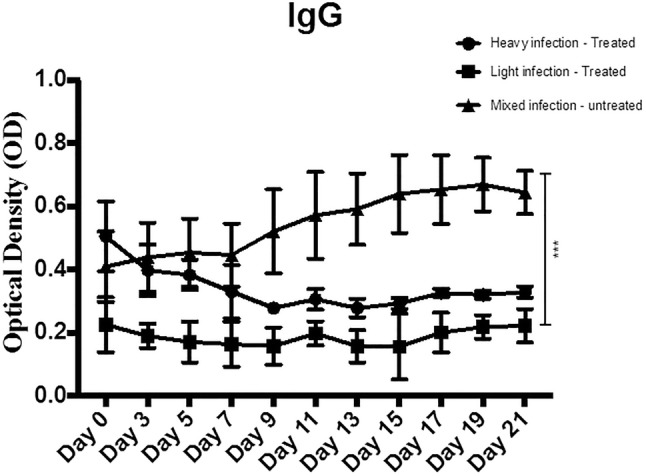
The effect of M. oleifera aqueous extract oral adminstration on the serum IgG expression in sheep naturally infected with F. gigantica. IgG levels were quantified using specific IgG assay in the three groups; lightly infected treated group, heavily infected treated group, and mixed infected control group. Data represented in mean ± standard deviation (SD). *Denotes significance (p < 0.05) between the three groups. Two-way ANOVA followed by multiple comparisons test was applied
The effects of M. oleifera aqueous extract oral administration on serum levels of IL-2, IL-17, and IL-10
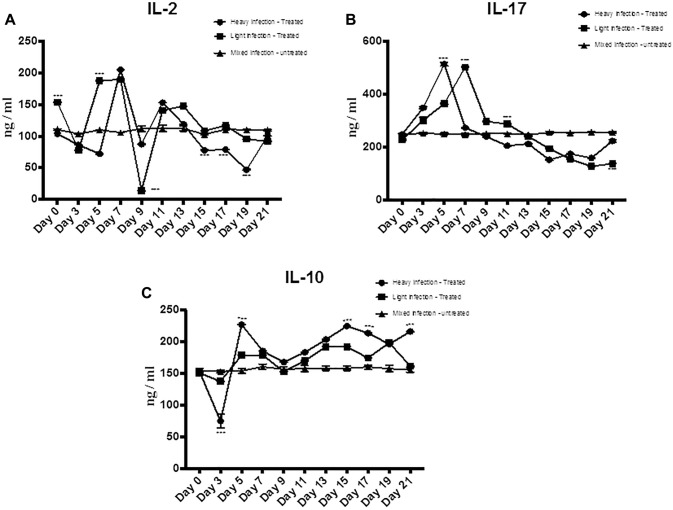
The treatment with M. oleifera aqueous extract resulted in a significant decrease in serum level of both IL-17 and IL-2 and increase in the level of IL-10 regulatory cytokine in the lightly infected treated group compared to the heavily infected and mixed infection groups (Fig. 5a–c). The temporal patterns of IL-2 and IL-17 showed an increase after treatment with M. oleifera aqueous extract then started to decrease at day 5 and continuous decrease until day 21 (Fig. 5a–c).
Fig. 5.
The effect of M. oleifera aqueous extract oral administration on the serum levels of IL-2, IL-17, and IL-10 in sheep naturally infected with F. gigantica. The serum levels of a IL-2, b IL-17, and c IL-10 were quantified using ELISA in the three groups; lightly infected treated group, heavily infected treated group, and mixed infected control group. Data represented in mean ± standard deviation (SD), *Denotes significance (p < 0.05) between the three groups. Two-way ANOVA followed by multiple comparisons test was applied
Discussion
The current study was designed to investigate the in vivo fasciolicidal and antibacterial effect of M. oleifera leaf aqueous extract oral administration in naturally infected sheep. The study showed a significant body weight gain in the infected sheep treated with M. oleifera. This result is anticipated as a reduction in parasites load would result in improved growth rates and body condition scores. Moreover, it is obvious that M. oleifera contained a considerable amount of protein, minerals and antioxidant that may have improved digestion and absorption resulting in increased body weight and host-resistance to the helminthes. These results are in agreement with Moyo et al. (2013) who reported an improved body condition score and a decrease in coccidian oocysts and helminthes load in goats after dietary supplementation with M. oleifera.
The fecal egg count of the infected sheep treated with M. oleifera significantly decreased over the 21 days course of treatment in heavily infection with completely disappearance of eggs in lightly infection. Furthermore, the treatment resulted in egg morphological changes; as Fasciola eggs obtained from fecal samples showed marked degeneration and accumulated dark embryo. These results came in agreement with our previous research (Hegazi et al. 2018) which revealed the in vitro ovicidal activity of alcoholic and aqueous extract of M. oleifera leaf on Fasciola eggs. One explanations for this finding is that M. oleifera may induce toxic effect on F. gigantica worms, resulting in alternation in worm fecundity and distortion of their eggs similar to the effect reported on Haemonchus contortus (Cabardo Jr and Portugaliza 2017). This toxic effect may be related to the presence of saponins, tannins, and flavonoids in M. oleifera leaves (Fatima et al. 2014). Furthermore, M. oleifera treatment proved an immunomodulatory effect in rats (Jayanthi et al. 2015) leading to increasing the immune response which positively correlated with the reduction of worm number then reduction in egg shedding. However, the mechanism by which M. oleifera might function as fasciolicidal agent needs further investigation.
In the present study, M. oleifera leaf aqueous extract oral administration resulted in a significant decrease in IgG levels in highly infected sheep, from day 3 and continued until the end of the experiment. However, mean IgG titers remained significantly higher than the assay control negative sera obtained from healthy, non-infected sheep. Meanwhile, lightly infected treated sheep showed significant decreases in serum IgG level to levels equivalent to those of the assay negative control sera. This finding indicated that M. oleifera oral administration may lower the severity of the infection. Previous research reports concluded that M. oleifera is an immune boosting agent that can stimulate IgG, IgM and IgA production to fight invading organisms until neutralization (Abd-Elhakim et al. 2018). The resulted antibody may interact with critical sites in the surface membrane of the parasite. It is also possible that the anti fasciolicidal activity of M. oleifera aqueous extract may be related to tannins which can cause paralysis of the Fasciola adult worm (Hoste et al. 2006; Molan et al. 2000). Similarly, the presence of saponins is known to destabilize the cell membrane and cuticle collagen of the parasite (D’addabbo et al. 2011).
Cytokines are key regulators of immune inflammatory responses (Pleasance et al. 2011; Zhang et al. 2005). The beneficial effects of M. oleifera extract on the regulation of Th1/Th2/Th17 balance were evaluated (Sudha et al. 2010). Current results proved that both inflammatory Th1 cytokine; IL- 2 and Th17 cytokine; IL-17 in heavily and lightly infected sheep were suppressed by M. oleifera extract treatment with the lowest level at 21 dpt. This result running parallel with the previous reports which proved that M. oleifera downregulated the expression and production of IL-2 cytokine (Sashidhara et al. 2009), which inhibited the inflammatory reaction due to infection. On the other hand, the presented results demonstrated a significant gradual increase in anti-inflammatory cytokine IL 10 to reach to its highest level at the 21 dpt in both high and lightly infected sheep treated with M. oleifera extract. The present result comes in accord with the previous study of Fard et al. (2015) who proved that M. oleifera hydroethanolic bioactive leaves extract significantly modulate the production of IL- 10 from macrophage. However, the exact effect of M. oleifera on Th1/Th2 during fascioliasis remains obscured and needs further study.
The current results showed that the bacterial count of C. novyi at zero days of treatment was 7 × 109–9 × 109 and after treatment with Moringa extract the count were reduced in both heavly and lightly naturally infected sheep reached 2 × 104 after 21 dpt. M. oleifera was a potential source of the antimicrobial molecule against pathogenic Clostridium spp. The mechanism of reducing C. novyi count was due to direct contact between the M. oleifera and bacteria (El-Kholy et al. 2018). Moreover, M. oleifera downregulated the levels of IL-2 and IL-17 and up-regulated IL-10 expression resulting in a protective immune mechanism against C. novyi.
More studies are required to fully understand the immunological implications of using M. oliefera as target drug to control fascioliasis inflammation-based disorder. Further studies on understanding the effect of M. oliefera on Nf-kB transcription factor may shed light on immune modulatory mechanism of M. oliefera.
In conclusion, this study provides evidence that M. oleifera may have potential therapeutic effects against F. gigantica. However, further studies are warranted to investigate the implicated molecular pathways and cell–cell interaction during the treatment with M. oleifera in naturally infected animals.
Acknowledgements
This study was supported by National Research Centre, Egypt (Grant No. 11020201) entitled: “Evaluation of the antimicrobial effect of propolis and Moringa on Fasciola gigantica and Clostridium oedematiens (Clostridium novyi) type B”.
Author contributions
EHA and AGH designed the study. HGK and EAF clinically diagnosed the infection. EEE, HGK participated in preparation of M. oleifera leaf aqueous extract, and animal’s treatment. EEE, HGK and EAF weighed animals and collected fecal, and blood samples. SEH identified and counted F. gigantica eggs in feces with determination of its morphology. EEE and SEH carried out ELISA for detection of F. gigantica specific antibodies. EEE conducted in cytokines quantification in sheep sera. EAF isolated, identified, characterized and counted C. novyi. AGH, EHA and EEE were conducting in data analyses and interpretation and in manuscript preparation. EEE was write manuscript. All authors have reviewed and approved the final manuscript.
Compliance with ethical standard
Conflict of interest
The authors declare that they have no conflicts or competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Elhakim YM, El Bohi KM, Hassan SK, El Sayed S, Abd-Elmotal SM. Palliative effects of Moringa olifera ethanolic extract on hemato-immunologic impacts of melamine in rats. Food Chem Toxicol. 2018;114:1–10. doi: 10.1016/j.fct.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman EH, Mohamed AH, Abdel-Rahman AAH, El Shanawany EE. The role of Ser-(Arg-Ser-Arg-Ser-GlucNAc)19-GlucNAc Fasciola gigantica glycoprotein in the diagnosis of prepatent fasciolosis in rabbits. JOPD. 2016;40:11–21. doi: 10.1007/s12639-014-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanzor D, Clemente R, Fornillos M, Gomez F, Ladiao B, Calzada BD, Tamodtamod R. In vivo trials of Moringa oleifera Lam. extracts as antischistosomal treatment on Schistosoma japonicum infected mice. IJIMS. 2014;2:49–56. [Google Scholar]
- Almazán C, Avila G, Quiroz H, Ibarra F, Ochoa P. Effect of parasite burden on the detection of Fasciola hepatica antigens in sera and feces of experimentally infected sheep. Vet Parasitol. 2001;97:101–112. doi: 10.1016/S0304-4017(01)00376-4. [DOI] [PubMed] [Google Scholar]
- Attallah AM, Bughdadi FA, El-Shazly AM, Ismail H. Immunodetection of Fasciola gigantica circulating antigen in sera of infected individuals for laboratory diagnosis of human fascioliasis. Clin Vaccine Immunol. 2013;20:1569–1577. doi: 10.1128/cvi.00305-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani M, Rafieian-Kopaei M, Hassanzadazar H, Saki K, Karamati SA, Delfan B. A review on most important herbal and synthetic antihelmintic drugs. Asian Pac J Trop Med. 2014;7:S29–S33. doi: 10.1016/s1995-7645(14)60200-5. [DOI] [PubMed] [Google Scholar]
- Bannam TL, Yan XX, Harrison PF, Seemann T, Keyburn AL, Stubenrauch C, Weeramantri LH, Cheung JK, McClane BA, Boyce JD, Moore RJ, Rood JI. Necrotic enteritis-derived Clostridium perfringens strain with three closely related independently conjugative toxin and antibiotic resistance plasmids. MBio. 2011;2:e00190–e00211. doi: 10.1128/mBio.00190-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, González L, Dagleish M, Pérez V. Diagnostic pathology in microbial diseases of sheep or goats. Vet Microbiol. 2015;181:15–26. doi: 10.1016/j.vetmic.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Black JG. Microbiology: principles and applications. 3. Upper Saddle River: Prentice Hall; 1996. [Google Scholar]
- Brockwell YM, Elliott TP, Anderson GR, Stanton R, Spithill TW, Sangster NC. Confirmation of Fasciola hepatica resistant to triclabendazole in naturally infected Australian beef and dairy cattle. Int J Parasitol Drugs Drug Resist. 2014;4:48–54. doi: 10.1016/j.ijpddr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabardo DE, Jr, Portugaliza HP. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. IJVSM. 2017;5:30–34. doi: 10.1016/j.ijvsm.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10:735. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- Cox F. Concomitant infections, parasites and immune responses. Parasitol. 2001;122:S23–S38. doi: 10.1017/S003118200001698X. [DOI] [PubMed] [Google Scholar]
- Cullen JM, Stalker MJ. Liver and biliary system. Jubb Kennedy Palmer’s Pathol Domest Anim. 2015;2:258–352. [Google Scholar]
- D’addabbo T, Carbonara T, Leonetti P, Radicci V, Tava A, Avato P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem Rev. 2011;10:503–519. doi: 10.1007/s11101-010-9180-2. [DOI] [Google Scholar]
- El-Kholy K, Barakat SA, Morsy W, Abdel-Maboud K, Seif-Elnaser M, Ghazal MN. Effect of aqueous extract of Moringa oleifera leaves on some production performance and microbial ecology of the gastrointestinal tract in growing rabbits. Pak J Nutr. 2018;17:1–7. doi: 10.3923/pjn.2018.1.7. [DOI] [Google Scholar]
- Fard MT, Arulselvan P, Karthivashan G, Adam SK, Fakurazi S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn Mag. 2015;11:S556. doi: 10.4103/0973-1296.172961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima T, Sajid MS, Jawad-ul-Hassan M, Siddique RM, Iqbal Z. Phytomedicinal value of Moringa oleifera with special reference to antiparasitics Pak. J Agric Sci. 2014;51:251–262. [Google Scholar]
- Gholamiandehkordi A, Eeckhaut V, Lanckriet A, Timbermont L, Bjerrum L, Ducatelle R, Haesebrouck F, Van Immerseel F. Antimicrobial resistance in Clostridium perfringens isolates from broilers in Belgium. Vet Res Commun. 2009;33:1031. doi: 10.1007/s11259-009-9306-4. [DOI] [PubMed] [Google Scholar]
- Hao H, Cheng G, Iqbal Z, Ai X, Hussain HI, Huang L, Dai M, Wang Y, Liu Z, Yuan Z. Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happich F, Boray J. Quantitative diagnosis of chronic fasciolosis: 1. Comparative studies on quantitative faecal examinations for chronic Fasciola hepatica infection in sheep. Aust Vet J. 1969;45:326–328. doi: 10.1111/j.1751-0813.1969.tb05009.x. [DOI] [PubMed] [Google Scholar]
- Hegazi AG, Megeed KNA, Hassan SE, Abdelaziz M, Toaleb NI, El Shanawany EE, Aboelsoued D. Comparative ovicidal activity of Moringa oleifera leaf extracts on Fasciola gigantica eggs. Vet World. 2018;11:215. doi: 10.14202/vetworld.2018.215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006;22:253–261. doi: 10.1016/j.pt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Jayanthi M, Garg SK, Yadav P, Bhatia A, Goel A. Some newer marker phytoconstituents in methanolic extract of Moringa oleifera leaves and evaluation of its immunomodulatory and splenocytes proliferation potential in rats. Indian J Pharmacol. 2015;47:518. doi: 10.4103/0253-7613.165199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JM, Elliott TP, Beddoe T, Anderson G, Skuce P, Spithill TW. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol. 2016;32:458–469. doi: 10.1016/j.pt.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Kooltheat N, Sranujit R, Chumark P, Potup P, Laytragoon-Lewin N, Usuwanthim K. An ethyl acetate fraction of Moringa oleifera Lam inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients. 2014;6:697–710. doi: 10.3390/nu6020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. Adv Exp MedBiol. 2014;766:77–114. doi: 10.1007/978-1-4939-0915-5_4. [DOI] [PubMed] [Google Scholar]
- Molan A, Alexander R, Brookes I, McNabb W (2000) Effects of an extract from sulla (Hedysarum coronarium) containing condensed tannins on the migration of three sheep gastrointestinal nematodes in vitro. In: Proceedings of the New Zealand society of animal production, pp 21–25
- Moyo B, Masika PJ, Muchenje V. Effects of supplementing cross-bred Xhosa lop eared goats with Moringa oleifera Lam. on helminth load and corresponding body condition score, packed cell volume. Afr J Agric. 2013;8:5327–5335. doi: 10.1007/s11250-011-9970-6. [DOI] [Google Scholar]
- Nyindo M, Lukambagire A-H. Fascioliasis: an ongoing zoonotic trematode infection. BioMed Res Int. 2015 doi: 10.1155/2015/786195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeka S, Obia O, Dapper D. Effect of acute administration of aqueous leaf extract of Moringa oleifera on immunoglobulin levels in wistar rats. EJMP. 2018;14:1–7. doi: 10.9734/ejmp/2016/24880. [DOI] [Google Scholar]
- Oldham G. Antibodies to Fasciola hepatica antigens during experimental infections in cattle measured by ELISA. Vet Parasitol. 1983;13:151–158. doi: 10.1016/0304-4017(83)90075-4. [DOI] [PubMed] [Google Scholar]
- Ortiz P, Scarcella S, Cerna C, Rosales C, Cabrera M, Guzmán M, Lamenza P, Solana H. Resistance of Fasciola hepatica against Triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheep. Vet Parasitol. 2013;195:118–121. doi: 10.1016/j.vetpar.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF, Tang MK. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Complement Altern Med. 2013;2013:627375. doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance J, Wiedosari E, Raadsma H, Meeusen E, Piedrafita D. Resistance to liver fluke infection in the natural sheep host is correlated with a type-1 cytokine response. Parasite Immunol. 2011;33:495–505. doi: 10.1111/j.1365-3024.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- Redford T, Cubberley JC, Hengeveld P, Zabek E, Britton AP. Myocardial necrosis associated with Clostridium novyi infection in a bighorn sheep (Ovis canadensis) J Wildl Dis. 2017;53:695–698. doi: 10.7589/2016-09-221. [DOI] [PubMed] [Google Scholar]
- Russel A, Doney J, Gunn R. Subjective assessment of body fat in live sheep. J Agric Sci. 1969;72:451–454. doi: 10.1017/S0021859600024874. [DOI] [Google Scholar]
- Sashidhara KV, Rosaiah JN, Tyagi E, Shukla R, Raghubir R, Rajendran SM. Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agents. Eur J Med Chem. 2009;44:432–436. doi: 10.1016/j.ejmech.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015;29:796–804. doi: 10.1002/ptr.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha P, Asdaq S, Dhamingi SS, Chandrakala GK. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in animals Indian. J Physiol Pharmacol. 2010;54:133–140. [PubMed] [Google Scholar]
- Tan WS, Arulselvan P, Karthivashan G, Fakurazi S. Moringa oleifera flower extract suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 macrophages via NF-κB pathway. Mediat Inflamm. 2015;2015:1–11. doi: 10.1155/2015/720171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzal FA, Songer JG, Prescott JF, Popoff MR. Clostridial diseases of animals. Hoboken: Wiley Online Library; 2016. [Google Scholar]
- Vats S, Gupta T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol Mol Biol Plants. 2017;23:239–248. doi: 10.1007/s12298-016-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaumourin E, Vourc’h G, Gasqui P, Vayssier-Taussat M. The importance of multiparasitism: examining the consequences of co-infections for human and animal health. Parasit Vectors. 2015;8:545. doi: 10.1186/s13071-015-1167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. Geneva: World Health Organization; 2015. [Google Scholar]
- Zhang FK, Guo AJ, Hou JL, Sun MM, Sheng ZA, Zhang XX, Huang WY, Elsheikha HM, Zhu XQ. Serum levels of cytokines in water buffaloes experimentally infected with Fasciola gigantica. Vet Parasitol. 2017;244:97–101. doi: 10.1016/j.vetpar.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Zhang W, Moreau E, Hope J, Howard C, Huang W, Chauvin A. Fasciola hepatica and Fasciola gigantica: comparison of cellular response to experimental infection in sheep. Exp Parasitol. 2005;111:154–159. doi: 10.1016/j.exppara.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Zidaric V, Pardon B, dos Vultos T, Deprez P, Brouwer MSM, Roberts AP, Henriques AO. Different antibiotic resistance and sporulation properties within multiclonal Clostridium difficile PCR ribotypes 078, 126, and 033 in a single calf farm. Appl Environ Microbiol. 2012;78:8515–8522. doi: 10.1128/AEM.02185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]