Abstract
The present study aimed to evaluate the in vitro efficacy of four medicinal plant extracts: petroleum ether and ethyl alcohol extracts of the ripen fruits of Melia azedarach and whole aerial parts of Artemisia herba-alba against the two inactive stages of the camel tick Hyalomma dromedarii, embryonated eggs and engorged nymphs in comparison to reference acaricide Butox®5.0 (Deltamethrin). Egg and nymphal immersion tests at four concentrations with three replicates were used. The deformity in larvae hatched from treated eggs and adults moulted from treated nymphs were observed and photographed by light microscope (LM) and scanning electron microscope (SEM). The results showed that M. azedarach and A. herba-alba extracts revealed higher significant toxic effects on embryonated eggs and engorged nymphs comparing with the reference acaricide (Butox®5.0) and control. In egg emmersion test, the LC50 of petroleum ether extracts of M. azedarach and A. herba-alba was 3.14 and 3.91%, respectively and LC50 of the respective ethyl alcohol extracts was 1.77 and 2.45%. In nymphal immersion test, LC50 of petroleum ether extracts of M. azedarach and A. herba-alba was 0.26 and 1%, respectively, and LC50 of the respective ethyl alcohol extracts was 4.17 and 8.7%. Abnormalities were observed by LM and SEM in the larvae hatched from the treated eggs as incomplete development of legs and mouth parts as well as shrinkage mainly in legs and mouthparts of adults emerged from treated nymphs. In conclusion, all extracts and petroleum ether extracts of the two plants have great potential to be developed as a novel acaricidal for controlling eggs and nymphs of H. dromedarii, respectively.
Keywords: Hyalomma dromedarii, Acaricidal activity, Tick, Control, Melia azedarach, Artemisia herba-alba
Introduction
Ectoparasites cause serious problem to animal health and economy all over the world. They can cause annoyance, skin infection, irritation, anaemia, fever and also act as a vector for various destructive diseases to animals (Fahmy 1980, 1982; Liebisch et al. 1984; Abbas et al. 2014; Yadav et al. 2017). Ticks are very important ectoparasites of domestic and wild animals (Roberts and Janovy 2005). Ticks can cause harm to animals through blood loss, depression of immune function, general stress and irritation and depreciation of the skin and hide value up to 20–30% (Ghosh et al. 2007). Ticks also act as vectors of a variety of infectious diseases harmful to humans and animals, including babesiosis, anaplasmosis, borreliosis, ehrlichiosis and rickettsoises (Abdel-Shafy et al. 2012; Guglielmone et al. 2014; Abdullah et al. 2016). The camel tick, Hyalomma (H.) dromedarii (Acari: Ixodidae), is the predominant tick species infesting camels, 95.6% in Sinai (Van Straten and Jongejan 1993) as well as 89% in Sudan (Elghali and Hassan 2009) and 57.13% in Benha and Belbis, Egypt (Ramadan 1997). H. dromedarii behaved as a three or two host species, although the most common is the two host life cycle.
Ectoparasites infecting various species of animals are controlled by using chemical acaricides which is mostly the method used throughout the world in spite of several problems like resistance development and negative effects on the non target organisms including humans (Maxwell et al. 2002; El-Seedi et al. 2017; Showler 2017). Control of ticks should be a priority, especially in developing countries due to the significant economic impact of ticks and tick borne diseases on the national economics (Bansal 2005). Due to resistance problems, alternative options are being incorporated in strategic and integrated control programs of the parasites (Masood et al. 2013; Abbas et al. 2017; Idris et al. 2017; Khan et al. 2017; Aboelhadid et al. 2018). Products derived from plants have been extensively used as an alternative way to control parasites, aiming to decrease the resistance development and obtain low-cost biodegradable parasiticides (Chagas et al. 2012). There were many previous reports on the acaricidal activity of plant extracts against H. dromedarii, Ascardiac glycosidal extract from Calotropis procera, azadirachtin and neem oil from Azadirachta indica against larvae and adults of H. dromedarii (Al-Rajhy et al. 2003), Artemisia herba-alba, Artemisia monosperma, Euphorbia aegyptiaca, Francoeuria crispa, Mesembryanthemum forskalii and Reaumuria hirtella extracted with successive solvents; hexan, diethyl ether, ethyl acetate and ethanol against the larvae of H. dromedarii (Abdel-Shafy et al. 2007) and essential oil from Citrus sinensis against H. dromedarii eggs (Habeeb et al. 2007).
Melia azedarach Linn. is wide spread and naturalized in most of the tropics and subtropical countries. It was introduced and naturalized in United States of America, Philippines, Argentine, Brazil, many African countries and many Arab countries (Rubae 2009). The effect of extracts from different parts of M. azedarach on many pests has been already evaluated. Also the efficacy of hexane, chloroform and ethanolic extracts of M. azedarach Linn. ripen fruit was reported against the tick Rhipicephalus (Boophilus) microplus (Borges et al. 2003) and Dermanyssus gallinae (Sariosseiri et al. 2018). Artemisia herba-alba is one of the plants that grown in Sinai, Egypt. It is a greyish-white perennial dwarf shrub, with small flowers. It is commonly grown in the central and southern wadis (Mohamed et al. 2010). Essential oils from A. herba-alba against Ixodes ricinus ticks (El-Seedi et al. 2017) and hexan, diethyl ether, ethyl acetate and ethanol extracts of A. herba-alba against larvae of H. dromedarii (Abdel-Shafy et al. 2007) have been evaluated.
The aim of the present study was to evaluate in vitro acaricidal activity of petroleum ether and ethyl alcohol extracts of M. azedarach Linn. and A. herba-alba in controlling the inactive stages; emeryonated eggs and engorged nymphs of H. dromedarii.
Materials and methods
Collection of plant materials
The ripen fruits of M. azedarach (Family: Meliaceae) and whole aerial parts of A. herba-alba (Family: Asteraceae) were obtained from Genetics and Cytology Department, Biotechnology Division, National Research Centre.
Preparation of petroleum ether and ethyl alcohol extracts
Melia azedarach ripen fruits and A. herba-alba aerial parts were grinded using stainless steel knife mill. Petroleum ether and ethyl alcohol extracts were prepared by adding 150 g powder for each plant to one litter of petroleum ether (grade 40–60 °C) or 70% ethanol and the mixtures were kept for 72 h in Soxhlet extractor. Then, the obtained extracts were filtrated using Whatman filter paper No. 1. Extracts were then concentrated using vacuum rotary evaporator at 40 ºC, and a rotation speed of 20 rpm. The plant extracts were transferred to dark glass vials and kept under 4 ºC until use.
Ticks
Engorged females of H. dromedarii (Acari: Ixodidae) were collected from naturally infested camels at Birqash camel market (30°09′58.4″N 31°02′13.2″E), Giza province, Egypt. The ticks were identified according to (Walker 2003; Estrada-Pena et al. 2004). Females were kept in an incubator in plastic cups (one female/cup) at 25 ± 1 °C and 75–80% RH to provide optimum conditions for oviposition. The eggs laid by each female were collected daily then kept in the incubator. Lots of eggs were used for the immersion test and the remaining eggs were put in labeled plastic tubes, closed by nylon gauze and kept at the same condition to hatch. Hatched larvae were allowed to fed on healthy pathogen free rabbits at room temperature using a capsule technique (Madbouly 1965; El-Kammah 1969) to obtain nymphs which used for nymph immersion test (Osman et al.2014).
Bioassay of plant extracts on ticks
Egg immersion test
For evaluation the acaricidal activities of petroleum ether and ethyl alcohol extract of M. azedarach and A. herba-alba on the eggs approximately, 300 embryonated eggs of H. dromedrii were divided into three replicates contained 100 eggs in each. These eggs were placed in plastic cups and immersed for 1 min in 500 µl of the tested concentration. The concentrations of petroleum ether extract were 19.1, 9.5, 4.7, 2.3% for M. azedarach and 16, 8, 4, 2% for A. herba-alba. The concentrations of ethyl extract were 14.3, 7.1, 3.5, 1.7% for M. azedarach and 9.6, 4.8, 2.4, 1.2% for A. herba-alba. Eggs immersed in ethyl alcohol and petroleum ether for 1 min were considered as solvent control while eggs immersed for 1 min in Butox®5.0 (Deltamethrin) 1 cm/L were considered as reference drug. Subsequently, the solutions were decanted and after evaporation of solvent, the tubes closed with muslin clothes and were incubated at 25 ± 1 °C and relative humidity of 75–80% for 14 days to examine the effect of the extracts by counting the hatched larvae and dead eggs under Carl Zeiss stereo microscope, calculating the mortality percentages in eggs and monitoring the abnormality in the eggs and hatched larvae that photographed by light microscope (LM) and scanning electron microscope (SEM).
Nymphal immersion test
To evaluate the effect of petroleum ether and ethyl alcohol extract of M. azedarach and A. herba-alba extracts on nymphs, 30 engorged nymphs were divided into three replicates (10/replicate). These nymphs were immersed in 5 mL of the tested concentration. The concentrations of petroleum ether extract were 1.1, 0.59, 0.29, 0.14% for M. azedarach and 4, 2, 1.0, 0.5% for A. herba-alba. The concentrations of ethyl alcohol extract were 14.3, 7.1, 3.5, 1.7% for M. azedarach and 19.3, 9.6, 4.8, 2.4% for A. herba-alba. Nymphs immersed in petroleum ether and ethyl alcohol for 1 min were considered as solvent control while nymphs immersed for 1 min in Butox®5.0 (1 cm/L) were considered as reference drug. The tested nymphs were incubated at a temperature of 25 ± 1 °C and 75–80% RH. The number of nymphs failed to moult was counted and their mortality percentages were calculated. The abnormal adults moulted from the treated nymphs were estimated and photographed by LM and SEM.
Preparation of tick specimens for scanning electron microscopy
The tick specimens prepared for scanning electron microscopy (SEM) were; abnormal larvae hatched from eggs treated with the plant extracts, normal larvae hatched from untreated eggs, deformed adults emerged from treated nymphs with the plant extracts and normal adults emerged from untreated nymphs. The tick specimens were well cleaned by overnight immersion in water–glycerol–KCl solution (Homsher and Sonenshine1977; Brody and Wharton,1971). Then, ticks were washed in distilled water several times and immersed one hour in each concentration of 25%, 50%, 70%, 80% ethanol followed by 10 min in each 90% and 100% ethyl alcohol (Keirans et al.1976). Following this, ticks were glued by their dorsal and ventral surfaces to the SEM stub, and were dried by the dryer (Blazer Union, F1-9496 Blazer/Fürstentun Liechtenstein), using liquid carbon dioxide. Ticks mounted on SEM stubs were coated with gold by using a S15OA Sputter Coater. The coated larvae and adults were examined by SEM (QUANTA FEG 250) in National Research Centre.
Statistical analyses
The data was statistically analyzed by one-way ANOVA test following by Tukey test using SPSS program version 20. The lethal concentrations (LC50 and LC99) and their respective 95% confidence intervals (CI) were determined by applying regression equation analysis to the probit transformed data of mortality. The concentration response data were analysed by probit method (Finney 1962) using Ehabsoft.
Results
Effect of plant extracts on embryonated eggs
The results indicated that there was a significant effect for M. azedarach and A. herba-alba extracts on embryonated eggs comparing with the reference acaricide (Butox®5.0) and control (Table 1). The mortality percentage of eggs increased with the increase of the concentration in all plant extracts. The two plants showed strong ovicidal effects comparing with Butox®5.0, especially, in concentrations higher than 9.5% and 8% petroleum ether extracts of M. azedarach and A. herba-alba, respectively. Furthermore, the concentrations 7.1 and 14.3% ethyl extract of M. azedarach and 9.6% ethyl extract of A. herba-alba revealed higher significant mortalities than Butox®5.0. At the highest concentration of petroleum ether and ethyl extract of M. azedarach the mortality rate reached 100%, while at the highest concentration of petroleum ether and ethyl extract of A. herba-alba the mortality rate reached 100% and 93.77% respectively. At concentration of 9.5%, 4.7%, 2.3% and 7.1%, 3.5%, 1.7% the mortality rate reached 92.46%, 66.35%, 36.65% and 90.19%, 73.24%, 50.82% for petroleum ether and ethyl extract of M. azedarach, respectively. The percent mortality caused by the petroleum ether extract of A. herba-alba varied from 16.57 to 100%, when the eggs tested at concentrations ranging from 2 to 16%, while percent mortality caused by ethyl alcohol extract of A. herba-alba varied from 26.35 to 93.77% when the eggs tested at concentration ranging from 1.2 to 9.6%. Lower concentrations of both plant extracts didn’t cause 100% mortality but incomplete hatching and deformed larvae were occurred. The percentage of deformed larvae for petroleum ether extracts of M. azedarach and A. herba-alba was 6.6, 14 and 2.8% at concentrations of 4.7, 2.3 and 2%, respectively. While the percentage of deformed larvae for ethyl alcohol extract of M. azedarach and A. herba-alba was 4, 3.7, 12.5, 7.8 and 7.3% at concentrations of 3.5, 1.7, 4.8, 2.4 and 1.2%, respectively.
Table 1.
Mortality percentages (mean ± SE) of Hyalomma dromedarii eggs treated with petroleum ether and ethylalcohol extracts of Melia azedarach and Artemisia herba-alba
| Melia azedarach | Artemisia herba-alba | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Petroleum ether | Ethyl alcohol | Petroleum ether | Ethyl alcohol | ||||||||
| Conc. (%) | Mortality (%) | Deformed larvae (%) | Conc. (%) | Mortality (%) | Deformed larvae (%) | Conc. (%) | Mortality (%) | Deformed larvae (%) | Conc. (%) | Mortality (%) | Deformed larvae (%) |
| 19.1 | 100 ± 0.00d | 0.00 ± 0.00a | 14.3 | 100 ± 0.00c | 0.00 ± 0.00a | 16 | 100 ± 0.00d | 0.00 ± 0.00a | 9.6 | 93.77 ± 1.04d | 0.00 ± 0.00a |
| 9.5 | 92.46 ± 4.17 cd | 0.00 ± 0.00a | 7.1 | 90.19 ± 9.80bc | 0.00 ± 0.00a | 8 | 81.65 ± 6.16 cd | 0.00 ± 0.00a | 4.8 | 65.53 ± 4.61c | 12.52 ± 3.43b |
| 4.7 | 66.35 ± 10.44c | 6.65 ± 2.10b | 3.5 | 73.24 ± 21.40bc | 4.06 ± 2.03a | 4 | 52.71 ± 21.8bc | 0.00 ± 0.00b | 2.4 | 58.82 ± 4.43c | 7.89 ± 0.55ab |
| 2.3 | 36.65 ± 7.12b | 14.06 ± 1.57c | 1.7 | 50.82 ± 3.800ab | 3.75 ± 1.87a | 2 | 16.57 ± 2.00ab | 2.83 ± 1.42a | 1.2 | 26.35 ± 4.05b | 7.37 ± 1.67ab |
| Butox®5.0 (1 cm/L) | 71.66 ± 4.40c | 0.00 | Butox®5.0 (1 cm/L) | 71.66 ± 4.40bc | 0.00 | Butox®5.0 (1 cm/L) | 71.66 ± 4.40 cd | 0.00 ± 0.00 | Butox®5.0 (1 cm/L) | 71.66 ± 4.40c | 0.00 ± 0.00 |
| Control | 7.09 ± 1.63a | 0.00 ± 0.00a | 8.10 ± 3.03a | 0.00 ± 0.00a | 7.09 ± 1.63a | 0.00 ± 0.00a | 8.10 ± 3.03a | 0.00 ± 0.00a | |||
| F value | 36.93 | 28.24 | 10.86 | 3.00 | 15.08 | 3.99 | 67.75 | 9.98 | |||
| P value | < 0.001 | < 0.001 | < 0.001 | NS | < 0.001 | 0.034 | < 0.001 | 0.002 | |||
a, b… etc., indicate the significant difference between the means of mortality percentages according to Tukey test
Conc. concentration, NS non significant
The calculated LC50 was 3.14, 3.91% and LC99 was 18.64, 18.84% for petroleum ether of M. azedarach and A. herba-alba while LC50 and LC99 for ethyl alcohol extracts of M. azedarach and A. herba alba were 1.77, 2.45% and 17.42, 29.71%, respectively, as shown in Table 2. Furthermore, the LC50 and LC99 confirmed that the two plants had strong ovicidal effects and M. azedarach had slightly higher toxicity on eggs than A. herba-alba.
Table 2.
LC50 and LC99 values with their confidence limits for Hyalomma dromedarii eggs treated with petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba
| Solvent | Plant | LC50 (%) | LC99 (%) | Confidence limits | Slope ± SE | |||
|---|---|---|---|---|---|---|---|---|
| LC50 (%) | LC99 (%) | |||||||
| Lower | Upper | Lower | Upper | |||||
| Petroleum ether | Melia azedarach | 3.14 | 18.64 | 2.69 | 3.57 | 14.27 | 27.46 | 3.00 ± 0.30 |
| Artemisia herba-alba | 3.91 | 18.84 | 3.50 | 4.345 | 14.94 | 25.87 | 3.40 ± 0.29 | |
| Ethyl alcohol | Melia azedarach | 1.77 | 17.42 | 1.371 | 2.12 | 12.34 | 29.78 | 2.34 ± 0.27 |
| Artemisia herba-alba | 2.45 | 29.71 | 2.08 | 2.84 | 19.78 | 54.25 | 2.14 ± 0.22 | |
LC50: lethal concentration for 50% of individuals, LC99: lethal concentration for 99% of individuals
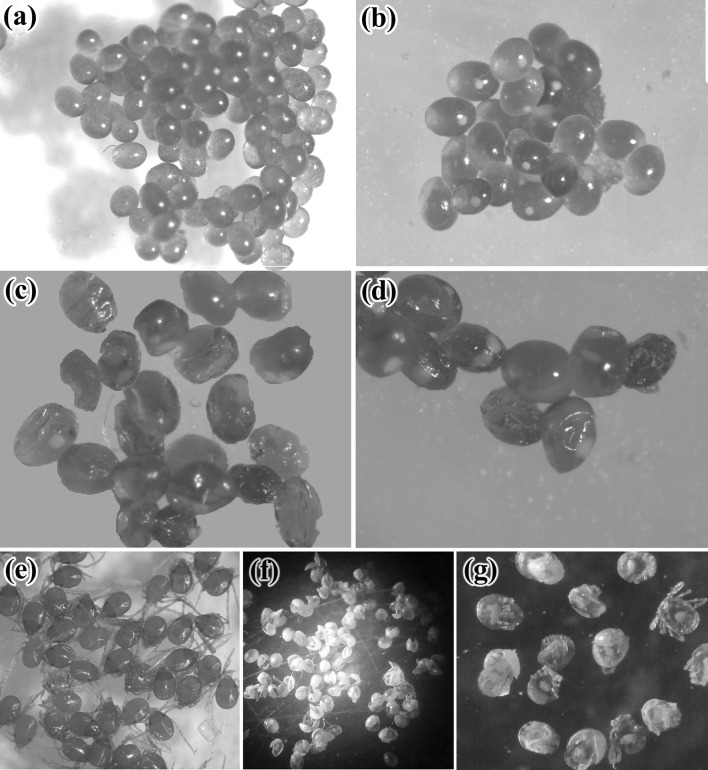
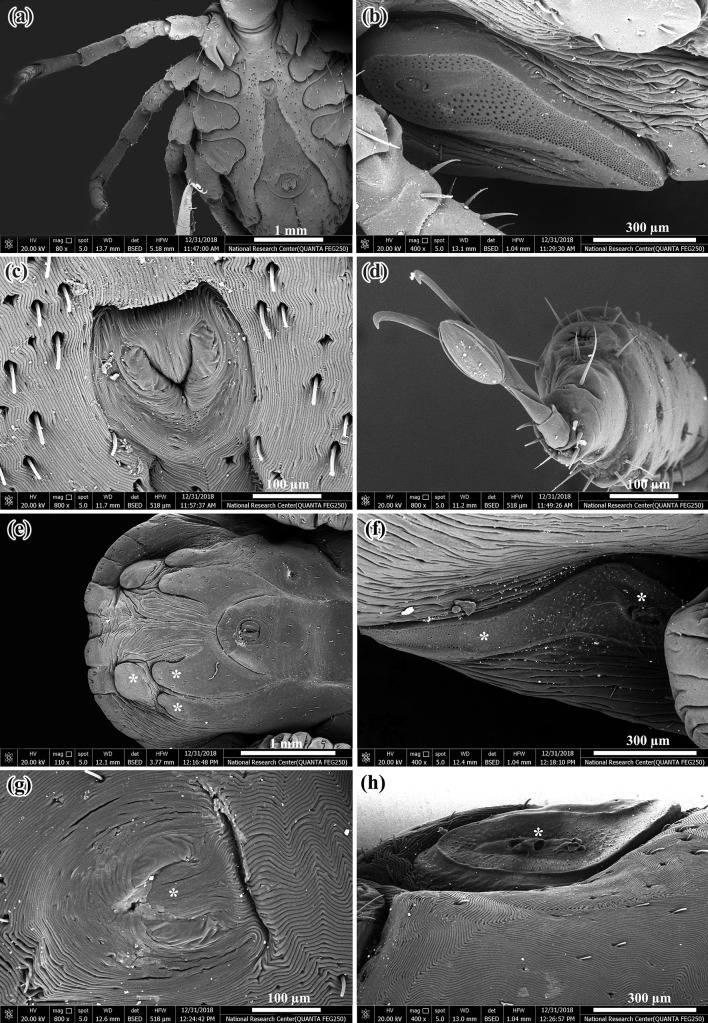
Morphological abnormalities of treated eggs were noticed by light microscope (LM) for the two applied plant extracts (Fig. 1a–g), where eggs appeared dark in color shrinked and dull in appearance (Fig. 1c, d). Also there were several abnormalities appeared in the larvae hatched from the treated eggs as incomplete development of legs and mouth parts. Some of larvae partially hatched from treated eggs and maintained inside the egg shell (Fig. 1f, g).
Fig. 1.
Light micrograph of normal and treated H. dromedarii eggs with plant extracts. a Freshly normal laid eggs (× 2), b Embryonated normal eggs (× 2.5), c, d Shrunken eggs exposed to plant extracts (× 5), e Normal larvae hatched from untreated eggs (× 2), f Incompletely hatched larvae from treated eggs (× 1.5), g Deformed larvae hatched from treated eggs with incomplete development of their legs and mouth parts (× 2.5)
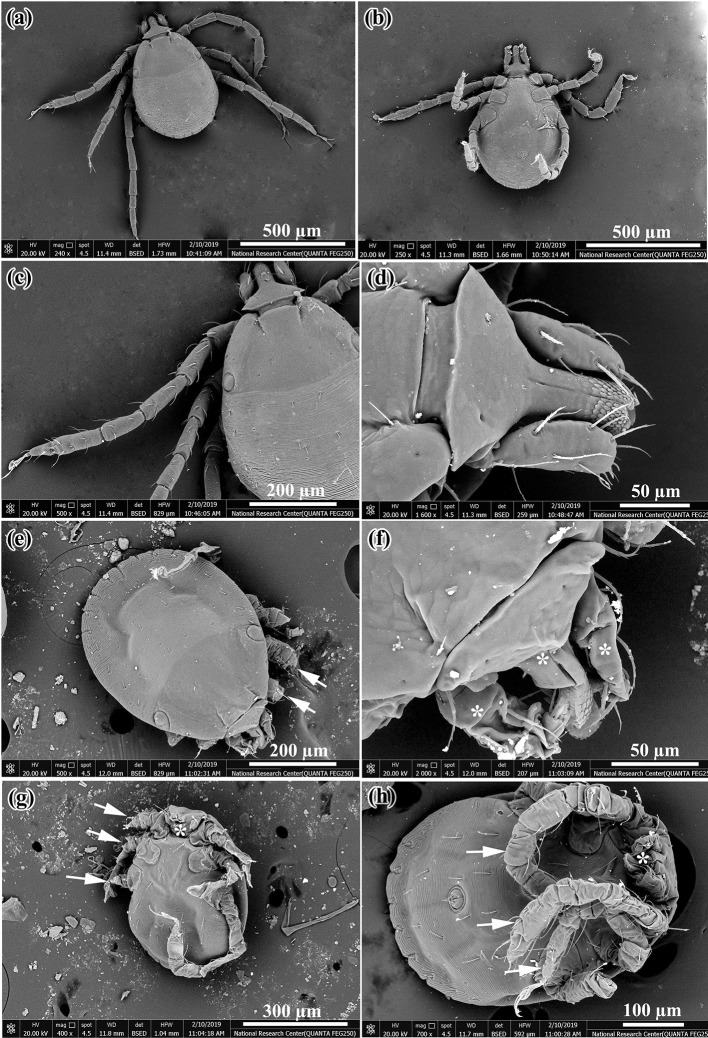
Scanning electron microscopy (SEM) showed normal features of the larva as dorsal surface (Fig. 2a), ventral surface (Fig. 2b), three pairs of legs (Fig. 2c) and mouth parts of larva (Fig. 2d). SEM of abnormal larvae hatched from eggs after treatment with petroleum ether and ethyl extracts of M. azedarach and A. herba-alba showed some alterations on the dorsal and ventral surfaces. At the dorsal surface there was degeneration of legs and mouth parts (Fig. 2e), also distortion of mouth parts with severely wrinkled cuticle (Fig. 2f). At the ventral surface legs were severely degenerated and wrinkled with complete degeneration of mouth parts (Fig. 2g, h).
Fig. 2.
SEM of normal larvae and deformed larvae hatched from H. dromedarii eggs exposed to plant extracts. a Dorsal surface of normal larva, b Ventral surface of normal larva, c Three pair of legs in normal larva, d Mouth parts of normal larva, e Shrinkage with degeneration of legs in deformed larva (white arrows), f Shrunken and distorted mouth parts in deformed larva (white stars), g Complete degeneration of legs (white arrows) and mouth parts (white star) in deformed larva and h Severely wrinkled legs (white arrows) with degenerated mouth parts (white star) in deformed larva
Effect of plant extracts on engorged nymphs
The results demonstrated that there was a significant effect of petroleum ether and ethyl extract of M. azedarach and A. herba-alba on the moulting of engorged nymphs of H. dromedarii comparing with Butox®5.0 and control (Table 3). The effect of M. azedarach extracts was significantly higher than that of A. herba-alba where the petroleum ether and ethyl extracts of M. azedarach at concentrations of 1.1% and 14.3% caused 100% and 86.66% mortality, respectively. While the petroleum ether and ethyl extracts of A.herba-alba at concentration of 4% and 19.3% caused 100% and 70% mortality. However, some deformities were observed in 23.33% and 16.66% of moulted adults which emerged from the nymphs treated with 1% and 0.5% of petroleum ether extracts of A. herba-alba, repcetively. LC50 values for petroleum ether extracts of M. azedarach and A. herba-alba were 0.26 and 1%, respectively, while their LC99 values were 1.3, and 5.29%, respectively. For ethyl extracts of M. azedarach and A. herba-alba, LC50 and LC99 values were (4.17 and 8.7%) and (51.56 and 89.75%), respectively as shown in Table 4. According to LC50 and LC99 values, petroleum ether extract of M. azedarach was the most toxic one to the engorged nymphs followed by petroleum ether extract of A. herba-alab, ethyl extract of M. azedarach and ethyl extract of A. herba-alba.
Table 3.
Mortality percentages (mean ± SE) of Hyalomma dromedarii nymphs treated with petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba
| Melia azedarach | Artemisia herba-alba | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Petroleum ether | Ethyl alcohol | Petroleum ether | Ethyl alcohol | ||||||||
| Conc. (%) | Mortality (%) | Deformed adults (%) | Conc. (%) | Mortality (%) | Deformed adults (%) | Conc. (%) | Mortality (%) | Deformed adults (%) | Conc. (%) | Mortality (%) | Deformed adults (%) |
| 1.1 | 100 ± 0.00d | 00 ± 0.00 | 14.3 | 86.66 ± 3.33d | 00 ± 0.00 | 4 | 100 ± 0.00c | 0.00 ± 0.00a | 19.3 | 70 ± 0.00d | 00 ± 0.00 |
| 0.59 | 86.66 ± 3.33 cd | 00 ± 0.00 | 7.1 | 70 ± 10.00 cd | 00 ± 0.00 | 2 | 76.6 ± 8.81c | 0.00 ± 0.00a | 9.6 | 46.66 ± 3.33c | 00 ± 0.00 |
| 0.29 | 46.66 ± 17.6bc | 00 ± 0.00 | 3.5 | 43.33 ± 6.66bc | 00 ± 0.00 | 1 | 53.33 ± 3.33b | 23.33 ± 3.33b | 4.8 | 33.33 ± 3.33bc | 00 ± 0.00 |
| 0.14 | 23.33 ± 8.81ab | 00 ± 0.00 | 1.7 | 20 ± 5.77ab | 00 ± 0.00 | 0.5 | 16.66 ± 6.66a | 16.66 ± 3.33b | 2.4 | 16.66 ± 3.33ab | 00 ± 0.00 |
| Butox®5.0 (1 cm/L) | 66.66 ± 6.66 cd | 00 ± 0.00 | Butox®5.0 (1 cm/L) | 66.66 ± 6.66 cd | 00 ± 0.00 | Butox®5.0 (1 cm/L) | 66.66 ± 6.66b | 0.00 ± 0.00 | Butox®5.0 (1 cm/L) | 66.66 ± 6.66d | 00 ± 0.00 |
| Control | 0.00 ± 0.00a | 00 ± 0.00 | 3.33 ± 3.33a | 00 ± 0.00 | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.33 ± 3.33a | 00 ± 0.00 | |||
| F value | 19.585 | – | 25.173 | – | 47.80 | 28.25 | 48.42 | – | |||
| P value | < 0.001 | – | < 0.001 | – | < 0.001 | < 0.001 | < 0.001 | – | |||
a, b… etc., indicate the significant difference between the means of mortality percentages according to Tukey test
Conc. concentration
Table 4.
LC50 and LC99 values with their confidence limits for Hyalomma dromedarii nymphs treated with petroleum ether and ethyl alcohol extracts of Melia azedarach and Artemisia herba-alba
| Solvent | Plant | LC50 (%) | LC99 (%) | Confidence limits | Slope ± SE | |||
|---|---|---|---|---|---|---|---|---|
| LC50 (%) | LC99 (%) | |||||||
| Lower | Upper | Lower | Upper | |||||
| Petroleum ether | Melia azedarach | 0.26 | 1.3 | 0.12 | 0.42 | 1.47 | 15.46 | 3.24 ± 0.28 |
| Artemisia herba-alba | 1.00 | 5.29 | 0.52 | 1.60 | 5.33 | 52.35 | 3.21 ± 0.27 | |
| Ethyl alcohol | Melia azedarach | 4.17 | 51.56 | 3.57 | 4.83 | 33.93 | 95.24 | 2.13 ± 0.21 |
| Artemisia herba-alba | 8.70 | 89.75 | 4.16 | 17.17 | 132.26 | 1407.56 | 2.29 ± 0.17 | |
LC50: lethal concentration for 50% of individuals, LC99: lethal concentration for 99% of individuals
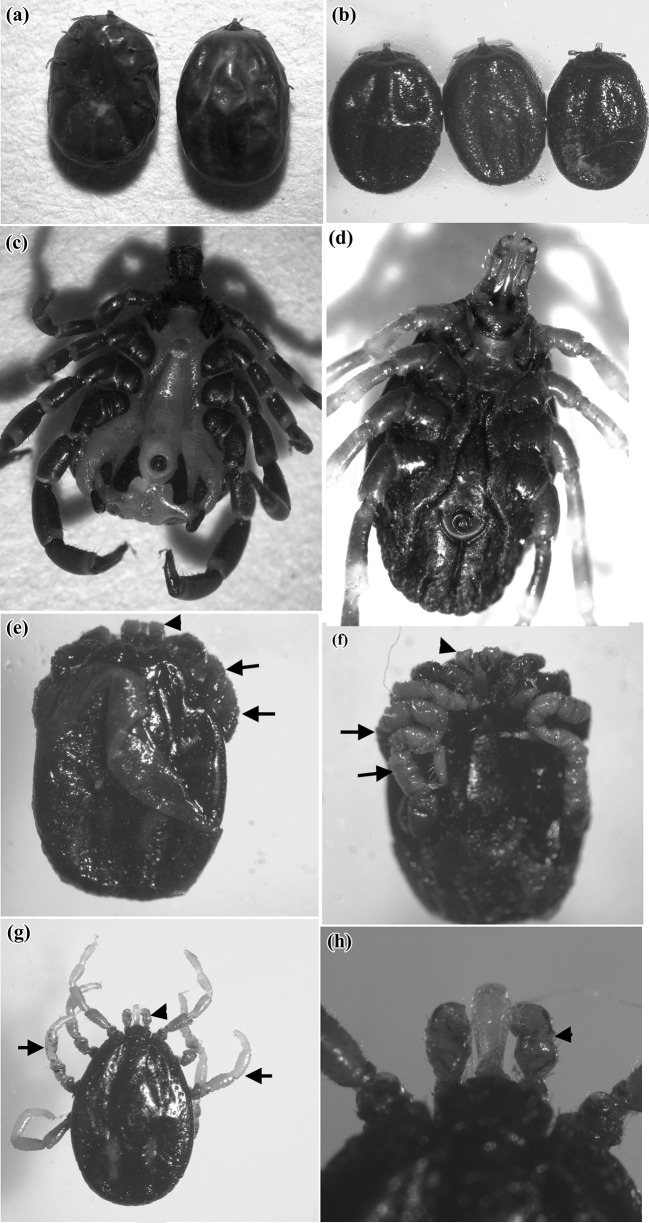
The normal nymphs (control), treated nymphs and adult ticks moulted from both untreated and treated nymphs were photographed by LM (Fig. 3a–h). Normal and dead nymphs were shown in Fig. 3a, b. Dead nymphs appeared with dark colored cuticle (Fig. 3b). Normal features of ventral surface of both male and female were shown in Fig. 3c, d. Emerged adults showed some alterations on appendages such as swollen legs and degenerated mouth parts (Fig. 3e–h).
Fig. 3.
Light micrographs of H. dromedarii nymphs treated by plant extracts and the adults moulted from normal and treated nymphs. a Normal nymphs (× 0.8), b Dead nymphs with dark colored cuticle (× 0.7), c Ventral surface of male moulted from normal nymph (× 1.25), d Ventral surface of female moulted from normal nymph (× 2), e, f Shrunken legs (black arrows) and degenerated mouth parts (black head arrow) of adult moulted from treated nymph (× 1.5), g Swollen leg (black arrows) and mouth parts (black head arrow) of adult moulted from treated nymph (× 1.2) and h Magnification of swollen mouth parts (black head arrow) of the deformed adult (× 5)
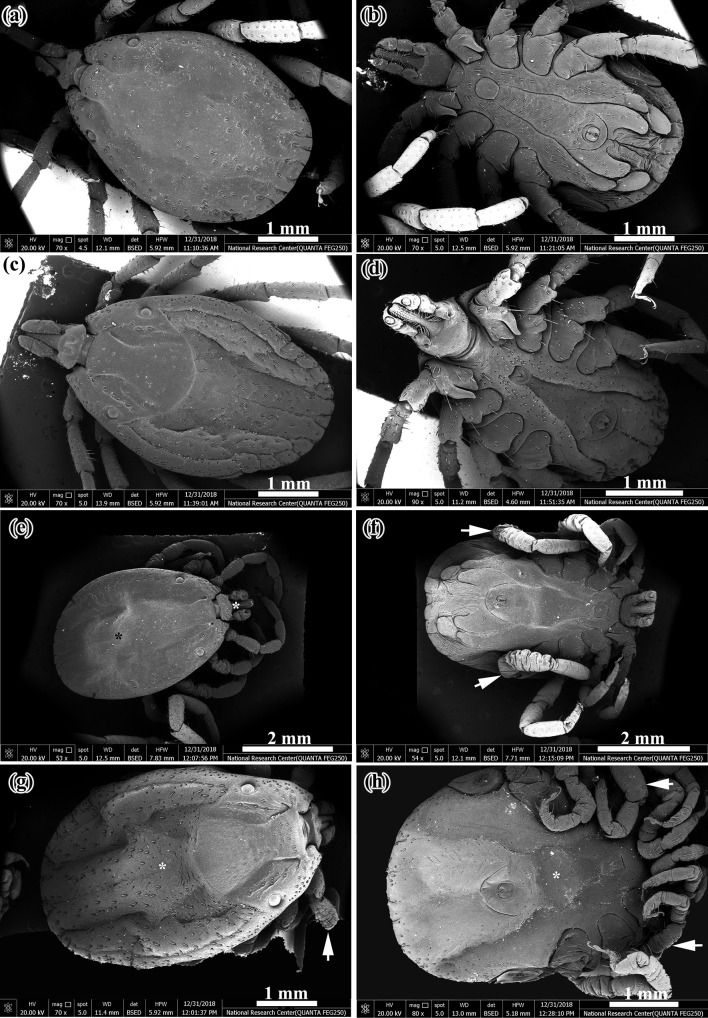
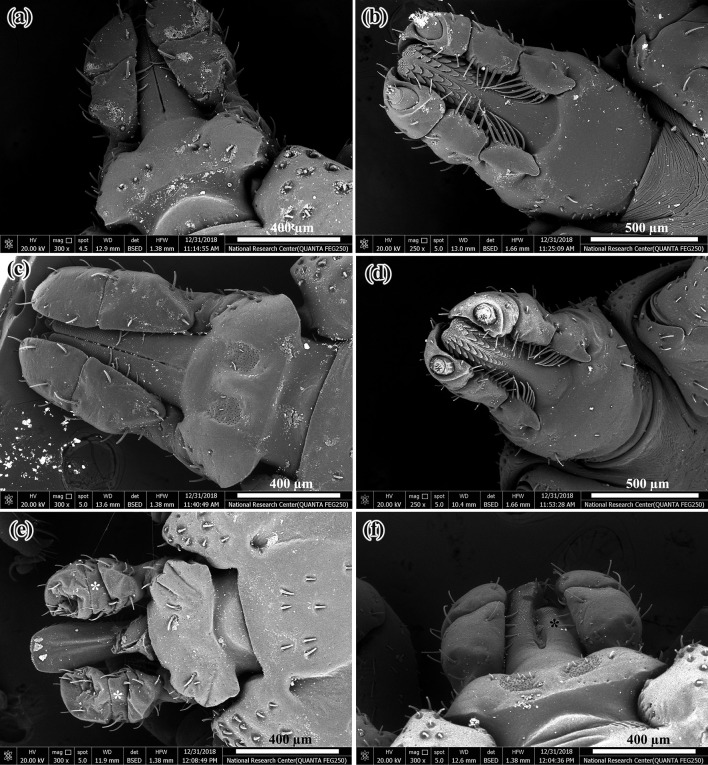
SEM of adults moulted form untreated nymphs (control) showed normal features of dorsal and ventral surfaces of both male and female (Fig. 4a–d). The abnormalities in whole body of the adults moulted from the treated nymphs were observed by SEM as shown in Fig. 4e–h. The dorsal surface of male was corrugated with shrunken mouth parts (Fig. 4e). The ventral surface of male was swollen with severe corrugation of the legs (Fig. 4f). Swelling in whole body and shrinkage in legs were apparent in dorsal and ventral surfaces of female (Fig. 4g, h). Normal characteristics of mouth parts in male and female was shown by SEM (Fig. 5a–d). SEM of mouth parts of the abnormal adults appeared with sever shrinkage of the two palpi which made hypostome to be longer than the palpi, also incomplete development of hypostome and cheliceral sheath was occurred (Fig. 5e, f). Furthermore, SEM of normal adults showed the following structures; ventral surface with normal coxae (Fig. 6a), spiracle plate of male (Fig. 6b), genital aperture of female with posterior lips that form a narrow V shape (Fig. 6c) and tarsus with pulvillus and two claws (Fig. 6d). SEM of abnormal adults emerged from treated nymph appeared swollen ventral plates (Fig. 6e), incomplete development of pores in spiracle plate of male (Fig. 6f), swelling and distortion of the genital aperture of female (Fig. 6g) and degeneration of spiracle plate to such extent that no recognizable structure remained in female (Fig. 6h).
Fig. 4.
SEM of normal adults emerged from untreated H. dromedarii nymphs (control) and abnormal adults emerged from treated H. dromedarii nymphs. a Dorsal surface of normal adult male, b Ventral surface of normal adult male, c Dorsal surface of normal adult female, d Ventral surface of normal adult female, e Dorsal surface of male appeared corrugated (black star) and the mouth parts appeared shrunken (white star), f At the ventral surface of male, there was swelling and severe corrugation of legs (white arrows). g, h Swelling in whole body (white star) and shrinkage in legs (white arrows) were apparent in dorsal and ventral surfaces of female
Fig. 5.
SEM of male and female mouth parts of H. dromedarii. a Dorsal surface of normal male mouth parts, b Ventral surface of normal male mouth parts, c Dorsal surface of normal female mouth parts, d Ventral surface of normal female mouth parts, e Shrinkage of the two palpi which made hypostome appeared longer than the palpi (white stars) and f Incomplete development of cheliceral sheath (black star)
Fig. 6.
SEM of normal adults emerged from untreated H. dromedarii nymphs (control) and abnormal H. dromedarii adults emerged from treated nymphs. a Ventral surface showed normal coxae, b Spiracle plate of normal male, c Genital aperture of normal female with posterior lips that form a narrow V shape. d Tarsus with normal pulvillus and two claws. e Swollen ventral plates of male (white stars), f Incomplete development of pores in spiracle plate of male (white stars), g Swelling and distortion of the genital aperture of female (whit star) and h Degeneration of spiracle plate with its structure not clear in female (white star)
Discussion
In previous studies, the extracts of M. azedarach and Artemisia spp plants revealed strong acaricidal effects against different Ixodidae tick species. The successive extracts of hexan, CHCl3 and 96% aqueous ethanol from M. azedarach ripen fruits revealed acaricidal effect against larvae and engorged females of the tick Rhipicephalus microplus (Borges et al. 2003). The hexan extract of M. azedarach unripe fruits had effects on engorged females of R. microplus and their egg production and hatching (De Sousa et al. 2014). The ethanolic Artemisia annua extract had acaricidal effect on engorged females of R. microplus (Chagas et al. 2011). The chloroform extract of the plant Artemisia absinthium had acaricidal properties on engorged females, eggs and unfed larvae of the tick R. sanguineus (Godara et al. 2014). The ethanolic extract of the plant A. absinthium had acaricidal properties on adults, eggs and larvae of the tick Hyalomma anatolicum (Godara et al. 2015). A repellent effect of a toluene extract of Artemisia abrotanum and essential oil of A. herba-alba against the nymphs of the tick Ixodes ricinus was occurred (Tunon et al. 2006; El-Seedi et al. 2017). The above mentioned studies indicated that the acaricidal potency of the two plants M. azedarach and A. herba-alba were not evaluated on the camel tick H. dromedarii, excepting a unique study conducted by Abdel-Shafy et al. (2007) showed acaricidal activity of A. herba-alba extracts on the larval stage of H. dromedarii. Furthermore, there is no study evaluated plant extracts on all stages of any tick species. Therefore, we try to evaluate the acaricidal efficacy of the two plant extracts M. azedarach and A. herba-alba on all stages of H. dromedarii. In the current study, the petroleum ether and ethanolic extracts of both M. azedarach and A. herba-alba were evaluated on embryonated eggs and engorged nymphs of H. dromedarii. In forthcoming research, these extracts will be evaluated against larvae and adults of H. dromedarii.
In the present study, the petroleum ether and ethanol extracts of M. azedarach and A. herba-alba revealed ovicidal effect on H. dromedarii eggs. In all extracts, the highest ovicidal activity recorded at concentrations less than 20%. At the lowest concentrations in all extracts, the eggs succeeded to hatch with 2.83 to 14.06% deformities in larvae. By both LM and SEM examination, the malformation in larvae occurred mainly on mouthparts and legs those prevent larvae from feeding and moving to find their hosts. According to the LC50 values, the toxicity of the two plants extracts on H. dromedarii eggs seems to be close to each other. The toxicity of the extracts on H. dromedarii eggs can be ordered as follows; M. azedarach ethanol > A. herba-alba ethanol > M. azedarach petroleum ether > A. herba-alba petroleum ether. This finding means that the polar solvent (ethanol) is better than the less polar solvent (petroleum ether) in the extracting of the tested two plants purposely to control eggs of ticks. In contrary, Borges et al. (2003) stated that the less polar hexan and CHCl3 extracts of M. azedarach were more effective on larvae and engorged females of R. microplus. They found that the ethanolic extract of M. azedarach caused mortality (46–50%) on engorged females and larvae of R. microplus lower than hexan and CHCl3 those caused mortality 98–100%. This may attribute to the differences in the susceptibility of tick stage or the active components of the extracts.
The engorged nymph of H. dromedarii was less susceptible than egg stage to the ethanol extracts of M. azedarach and A. herba-alba. However, the petroleum ether extracts of the two plants were more effective on engorged nymphs. This finding agreed with Laghzaoui et al. (2018) who found that the nymphs of Hyalomma aegyptium was more susceptible than eggs and larvae to the essential oil of A. herba-alba, wherever, the LD50 was 1.105 μL/mL, 0.755 μL/mL and 0.0079 mL/cm2 for eggs, larvae and nymph, respectively. The two lowest concentrations of petroleum ether extract of A. herba-alba only elucidated deformity in adults emerged from treated nymphs. The deformity in adults was not only in appendages like mouthparts and legs but also in the fine structures those photographed by SEM such as spiracular plates, genital opening and anal shields. These abnormalities in the emerged adults can constrain many tick activities such as feeding, moving, mating and finally led to death. LC50 values confirmed that the toxicity of the petroleum ether extracts in the two plants on H. dromedarii nymphs was higher than the ethanol extracts. However, the toxicity of the extracts on H. dromedarii nymphs can be ordered as follows; M. azedarach petroleum ether > A. herba-alba petroleum ether > M. azedarach ethanol > A. herba-alba ethanol. This study is considered the first in evaluation of A. herba-alba and M. azedarach against H. dromedarii nymphs. However, a few studies evaluated Artemisia spp. and M. azedarach as repellents against nymphs of the tick Ixodes ricinus and Amblyomma cajennense. The essential oil of A. herba-alba revealed 84.2% repellency against I. ricinus nymphs (El-Seedi et al. 2017). The repellent effect of toluene A. abrotanum extract against I. ricinus nymphs may be attributed to coumarin and thujyl alcohol those were found in the extract (Tunon et al. 2006). The hexan extract of M. azedarach in concentrations varied from 2.200 to 0.275 mg/cm2 was considered repellent for A. cajennense nymphs (Soares et al. 2010).
The mode of action of plant extracts almost attribute to their major active constituents. The major active constituent of the Meliaceous trees that including the genera Azadirachta and Melia is azadirachtin that causes negative effects on the development of insects (Mulla and Su 1999). Furthermore, the phytochemical analyses of ethanolic M. azedarach extract revealed the presence of triterpenoids, steroids, alkaloids and tannins. These compounds have ovicidal, feeding inhibition and insecticidal effects on insects (Mulla and Su 1999). Moreover, the active component of the genus Artemisia is artemisinin that revealed toxic effects on many parasites (Pillay et al. 2008; Chagas et al. 2011). This compound reacts with the heme molecules, alters the cell structure, thus affecting the growth and reproduction (Ferreira and Gonzalez 2008).
Conclusion
The ethanolic and petroleum ether extracts of the ripen fruits of M. azedarach and aerial parts of A. herbal alaba revealed ovicidal efficacy against the camel tick H. dromedarii. The petroleum ether extracts of the two plants exhibited higher toxicity than ethanolic extracts on H. dromedarii nymphs. Malformation was observed in larvae hatched from eggs treated with the lowest concentrations of all the tested extracts. Deformed adults were only emerged from the two lowest concentrations of the petroleum ether A. herba-alba extract. It is recommended to use all the tested extracts in controlling H. dromedarii eggs, and petroleum ether extracts of the two plants against H. dromedarii nymphs.
Acknowledgements
This study is a part of a Ph.D. Thesis to be submitted to The Department of Parasitology, Faculty of Veterinary Medicine, Cairo University. The study was carried out by financial support of National Research Centre as a part of Ph.D. Thesis No. 12/2/19.
Authors’ contributions
MMF, SA, MMA, RME, and HSMA designed the experiments. EMH and HSMA participated in the preparation of the plant extracts. MMF, SA, MMA, RME, and HSMA shared in the following protocols: bioassay of plant extracts against both embryonated eggs and engorged nymphs, detection of abnormalities in treated tick specimens by light microscope and scanning electron microscopy. SA and HSMA analysed and tabulated the data. MMF, SA, MMA, and HSMA wrote the draft of the manuscript. All authors revised and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standard
This study was approved by Ethical Committee for Medical Research (MREC) at the National Research Centre (NRC), Egypt in accordance with local laws and regulations (Approval Protocol No. 19085).
Informed consent
Consent was obtained from the owners of camels included in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas A, Abbas RZ, Khan JA, Iqbal Z, Bhatt MMH, Sindhu ZD, Zia MA. Integrated strategies for the control and prevention of dengue vectors with particular reference to Aedes aegypti. Pak Vet J. 2014;34:1–10. [Google Scholar]
- Abbas A, Iqbal Z, Abbas RZ, Khan MK, Khan JA, Sindhu ZD, Mahmood MS, Saleemi MK. In vivo anticoccidial effects of Beta vulgaris (sugar beet) in broiler chickens. Microb Path. 2017;111:139–144. doi: 10.1016/j.micpath.2017.07.052. [DOI] [PubMed] [Google Scholar]
- Abdel-Shafy S, Soliman MM, Habeeb SM. In vitro acaricidal effect of some crude extracts and essential oils of wild plants against certain tick species. Acarologia. 2007;47:33–42. [Google Scholar]
- Abdel-Shafy S, Allam NA, Mediannikov O, Parola P, Raoult D. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector Borne Zoonotic Dis. 2012;12:346–359. doi: 10.1089/vbz.2010.0241. [DOI] [PubMed] [Google Scholar]
- Abdullah HH, El-Molla A, Salib FA, Allam NA, Ghazy AA, Abdel-Shafy S. Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of Rickettsioses in Egypt. Vet World. 2016;9:1087–1101. doi: 10.14202/vetworld.2016.1087-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboelhadid SM, Arafa WM, Mahrous LN, Fahmy MM, Kamel AA. Molecular detection of Rhipicephalus (Boophilus) annulatus resistance against deltamethrin in middle Egypt. Vet Parasitol Reg Stud Rep. 2018;13:198–204. doi: 10.1016/j.vprsr.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Al-Rajhy DH, Alahmed AM, Hussein HI, Kheir SM. Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick, Hyalomma dromedarii (Acari: Ixodidae) Pest Manag Sci. 2003;59:1250–1254. doi: 10.1002/ps.748. [DOI] [PubMed] [Google Scholar]
- Bansal GC. Bovine theileriosis in India: an overview. Proc Nat Acad Sci India. 2005;75:134–143. [Google Scholar]
- Borges LMF, Ferri PH, Silva WJ, Silva WC, Silva JG. In vitro efficacy of extracts of Melia azedarach against the tick Boophilus microplus. Med Vet Entomol. 2003;17:228–231. doi: 10.1046/j.1365-2915.2003.00426.x. [DOI] [PubMed] [Google Scholar]
- Brody AR, Wharton GW. The use of glycerol-kcl in scanning electron microscopy of Acari. Ann Entomol Soc Am. 1971;64:528–530. [Google Scholar]
- Chagas ACDS, Georgetti CS, Carvalho COD, Oliveira MCDS, Rodrigues RA, Foglio MA, Magalhaes PMD. In vitro activity of Artemisia annua L (Asteraceae) extracts against Rhipicephalus (Boophilus) microplus. Rev Bras Parasitol Vet. 2011;20:31–35. doi: 10.1590/s1984-29612011000100007. [DOI] [PubMed] [Google Scholar]
- Chagas ACS, Barros LD, Cotinguiba F, Furlan M, Giglioti R, Oliveira MCS, Bizzo HR. In vitro efficacy of plant extracts and synthesized substances on Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Parasitol Res. 2012;110:295–303. doi: 10.1007/s00436-011-2488-z. [DOI] [PubMed] [Google Scholar]
- De Sousa LA, Da Costa DP, Ferri PH, Showler AT, Borges LM. Soil quality influences efficacy of Melia azedarach (Sapindales: Meliaceae), fruit extracts against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Ann Entomol Soc Am. 2014;107:484–489. [Google Scholar]
- Ehabsoft. http://www.ehabsoft.com/Ldpline
- Elghali A, Hassan SM. Ticks (Acari: Ixodidae) infesting camels (Camelus dromedarius) in Northern Sudan. Onderstepoort J Vet Res. 2009;76:177–185. doi: 10.4102/ojvr.v76i2.43. [DOI] [PubMed] [Google Scholar]
- El-Kammah KM (1969) Definition of the characters of Hyalomma anatolicum (Koch) subspecies anatolicum and excavatum and their hybrids. Ph.D. Thesis. Faculty of Agri, Cairo University, p 103
- El-Seedi HR, Azeem M, Khalil NS, Sakr HH, Khalifa SA, Awang K, Borg-Karlson AK. Essential oils of aromatic Egyptian plants repel nymphs of the tick Ixodes ricinus (Acari: Ixodidae) Exp Appl Acarol. 2017;73:139–157. doi: 10.1007/s10493-017-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A, Bouattour A, Camicas JL, Walker AR. Ticks of domestic animals in the Mediterranean region. Spain: University of Zaragoza; 2004. p. 131. [Google Scholar]
- Fahmy MM (1980) Studies on the cattle ticks and parasitic tick born disease in Giza Governorate. M.V.Sc. Vet. Thesis Fac Vet Med Cairo Univ Egypt
- Fahmy MM (1982) Parasitological and Ecological studies on the ticks Hyalomma anatolicum subspecies (H. a. anatolicum and H. a. excavatum), the main vectors of theileriosis in cattle in Egypt. Cairo Univ PhD. Thesis
- Ferreira JF, Gonzalez JM. Chemical and biological stability of artemisinin in bovine rumen fluid and its kinetic in goats (Capra hircus) Rev Bras Parasitol Vet. 2008;17:103–109. [PubMed] [Google Scholar]
- Finney DJ. Probit analysis a statistical treatment of the response curve. Cambridge: Cambridge University Press; 1962. [Google Scholar]
- Ghosh S, Azhahianambi P, Yadav MP. Upcoming and future strategies of tick control: a review. J Vect Borne Dis. 2007;44:79–89. [PubMed] [Google Scholar]
- Godara R, Parveen S, Katoch R, Yadav A, Verma PK, Katoch M, Kaur D, Ganai A, Raghuvanshi P, Singh NK. Acaricidal activity of extract of Artemisia absinthium against Rhipicephalus sanguineus of dogs. Parasitol Res. 2014;113:747–754. doi: 10.1007/s00436-013-3704-9. [DOI] [PubMed] [Google Scholar]
- Godara R, Parveen S, Katoch R, Yadav A, Katoch M, Khajuria JK, Singh NK. Acaricidal activity of ethanolic extract of Artemisia absinthium against Hyalomma anatolicum ticks. Exp Appl Acarol. 2015;65:141–148. doi: 10.1007/s10493-014-9843-6. [DOI] [PubMed] [Google Scholar]
- Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Pena A, Horak IG. Hard ticks (Acari: Ixodida: Ixodidae) of the world. Heidelberg: Springer; 2014. [Google Scholar]
- Habeeb SM, Abdel-Shafy S, Youssef AA. Light, scanning electron microscopy and SDS-PAGE studies on the effect of the essential oil Citrus sinensis var. balady on the embryonic development of camel tick Hyalomma dromedarii (Koch 1818) (Acari-Ixodidae) Pak J Biol Sci. 2007;10:1151–1160. doi: 10.3923/pjbs.2007.1151.1160. [DOI] [PubMed] [Google Scholar]
- Homsher PJ, Sonenshine DE. Scanning electron microscopy of ticks for systematic studies. 2. Structure of Haller’s organ in Ixodes brunneus and Ixodes frontalis. J Med Entomol. 1977;14:93–97. doi: 10.1093/jmedent/14.1.93. [DOI] [PubMed] [Google Scholar]
- Idris M, Abbas RZ, Masood S, Rehman T, Farooq U, Babar W, Hussain R, Raza A, Riaz U. The potential of antioxidant rich essential oils against avian coccidiosis. World Poult Sci J. 2017;73:89–104. [Google Scholar]
- Keirans JE, Clifford CM, Corwin D. Ixodes sigelos, n. sp. (Acarina: Ixodidae), parasite of rodents in Chile, with a method for preparing ticks for examination by scanning electron microscopy. Acarologia. 1976;18:217–225. [PubMed] [Google Scholar]
- Khan MN, Sajid MS, Rizwan HM, Qudoos A, Abbas RZ, Riaz M, Khan MK. Comparative efficacy of six anthelmintic treatments against natural infection of fasciola species in sheep. Pak Vet J. 2017;37:65–68. [Google Scholar]
- Laghzaoui EM, Kasrati A, Abbad A, Leach D, Spooner-Hart R, El Mouden EH. Acaricidal properties of essential oils from Moroccan plants against immature ticks of Hyalomma aegyptium (Linnaeus, 1758); an external parasite of the spur-thighed tortoise (Testudograeca) Int J Acarol. 2018;44:315–321. [Google Scholar]
- Liebisch A, Abel-Rahman MS, Hoogstraal HC (1984) Studies on the occurrence and veterinary significance of ticks domestic animals in Egypt. A Project report in: Recent German research on problems of parasitology, Animal Health and Animal Breeding in the Tropics and Sub Tropics 74–84
- Madbouly MH (1965) Comparative studies on the biology, behavior and sensory physiology of two Hyalomma ticks in Egypt. Ph.D. Thesis. Dep Entomol Fac Sci Cairo University, pp 301–308
- Masood S, Abbas RZ, Iqbal Z, Mansoor MK, Sindhu ZD, Zia MA, Khan JA. Role of natural antioxidants for the control of coccidiosis in poultry. Pak Vet J. 2013;33:401–407. [Google Scholar]
- Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. Effect of community-wide use of insecticide-treated nets for 3-4 years on malarial morbidity in Tanzania. Trop Med Int Health. 2002;7:1003–1008. doi: 10.1046/j.1365-3156.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- Mohamed AEHH, El-Sayed M, Hegazy ME, Helaly SE, Esmail AM, Mohamed NS. Chemical constituents and biological activities of Artemisia herba-alba. Rec Nat Prod. 2010;4:1–25. [Google Scholar]
- Mulla MS, Su T. Activity of biological effects of neem products against arthropods of medical and veterinary importance. J Am Mosq Cont Assoc. 1999;15:133–152. [PubMed] [Google Scholar]
- Osman IM, Mohammed AS, Abdalla AB. Acaricidal properties of two extracts from Guiera senegalensis JF Gmel. (Combrataceae) against Hyalomma anatolicum (Acari: Ixodidae) Vet Parasitol. 2014;199:201–205. doi: 10.1016/j.vetpar.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Pillay P, Maharaj VJ, Smith PJ. Investigating South African plants as a source of new antimalarial drugs. J Ethnopharmacol. 2008;119:438–454. doi: 10.1016/j.jep.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ramadan MY (1997) Studies on some ectoparasites of camels. MSc thesis, Zagazig University (Benha Branch), Egypt
- Roberts LS, Janovy J. Foundations of parasitology. 7. New York: McGraw Hill; 2005. pp. 644–646. [Google Scholar]
- Rubae AAY. The potential uses of Melia azedarach L. as pesticidal and medicinal plant, review. Am Eurasian J Sustain Agric. 2009;3:185–195. [Google Scholar]
- Sariosseiri A, Moshaverinia A, Khodaparast MHH, Kalidari GA. In vitro acaricidal effect of Melia azedarach ripe fruit extract against Dermanyssus gallinae (Acari: Dermanyssidae) Persian J Acarol. 2018;7:203–208. [Google Scholar]
- Showler AT. Botanically based repellent and insecticidal effects against horn flies and stable flies (Diptera: Muscidae) J Integr Pest Manag. 2017;8:1–11. [Google Scholar]
- Soares SF, Borges LMF, de Sousa Braga R, Ferreira LL, Louly CCB, Tresvenzol LMF, Ferri PH. Repellent activity of plant-derived compounds against Amblyomma cajennense (Acari: Ixodidae) nymphs. Vet Parasitol. 2010;167:67–73. doi: 10.1016/j.vetpar.2009.09.047. [DOI] [PubMed] [Google Scholar]
- Tunon H, Thorsell W, Mikiver A, Malander I. Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum. Fitoterapia. 2006;77:257–261. doi: 10.1016/j.fitote.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Van Straten M, Jongejan F. Ticks (Acari: Ixodidae) infesting the Arabian camel (Camelus dromedarius) in the Sinai, Egypt with a note on the acaricidal efficacy of Ivermectin. Exp Appl Acarol. 1993;17:605–616. doi: 10.1007/BF00053490. [DOI] [PubMed] [Google Scholar]
- Walker AR. Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh: Bioscience Reports; 2003. pp. 3–210. [Google Scholar]
- Yadav PK, Rafiqi SM, Panigrahi PN, Kumar D, Kumar R, Kumar S. Recent trends in control of ectoparasites: a review. J Entomol Zool Stud. 2017;5:808–813. [Google Scholar]