Abstract
The current work was carried out to evaluate the potency of larval and adult somatic Haemonchus contortus (H. contortus) antigens in detection of haemonchosis among sheep and goats using ELISA. Two hundred and forty-three fecal and blood samples were randomly collected from small ruminants (107 sheep and 136 goats) in Beni-Suef Governorate, Egypt, during the period from June to August 2018. The fecal analysis exhibited that 26.33% of the small ruminants were infected with gastrointestinal nematodes. The overall prevalence of H. contortus was reached 22.22% whereas it was 27.10% and 18.38% among sheep and goats, respectively. The current study elucidated that the larval antigen has claimed more superior diagnostic results compared to the adult somatic H. contortus antigen. The apparent overall sero-prevalence among small ruminants was reached 51.85%. Separately, it was 64.48% in sheep and 41.91% in goats. The larval antigen had proved 96.55% sensitivity and 47.43% specificity, for sheep serum samples. Meanwhile, sensitivity and specificity for goats’ sera were 100% and 71.17%, respectively. Diagnostic efficacy of ELISA was recorded 60.74% in sheep and 76.47% in goats. This study deduced that the larval antigen has proved the priority and the potency for diagnosis of H. contortus infection. Moreover, haemonchosis is a prevalent disease among the examined sheep and goats.
Keywords: Haemonchus contortus, Larval antigen, Adult somatic antigen, ELISA, Sheep and goats
Introduction
Helminthosis remains responsible for impeding productivity in small ruminants worldwide (El-Ashram et al. 2017). GINs are deemed to be one of the serious livestock industry obstacles (Jackson et al. 2009; Díaz et al. 2015). Haemonchosis is the most extremely pathogenic disease affecting small ruminants caused by the blood sucking abomasal nematode; H. contortus (Gowda 2016; Selemon 2018; Zhang et al. 2019). Sheep and goats breeding represents a vital effective global sector including Egypt. Several studies confirmed that H. contortus is one of most prevalent species in Egypt among sheep and goats (El-Dakhly et al. 2012; Elshahawy et al. 2014; Hassan et al. 2019); the primary susceptive hosts (Sutherland and Scott 2010; Selemon 2018). Actually, the conventional diagnosis of H. contortus infection is relied on the clinical signs and confirmed by the coprological examination. The clinical signs could be observed in heavy infection after animal undergoes serious damages, beside that H. contortus eggs could be only detected in feces after the end of the prepatent period (Soulsby 1983). Thus, due to the drawbacks of such approaches, reliable, accurate and early diagnostic methods are urgent. ELISA might be an alternative credible tool (Schallig et al. 1995; Lone et al. 2012). ELISA has been utilized for revealing of infection and in sero-epidemiological studies among considerable number of livestock (Prasad et al. 2008; Demeler et al. 2012). Different H. contortus antigens had showed variable degree of sensitivity and specificity in detecting anti-H. contortus antibodies (Lone et al. 2012; Arab et al. 2013; Kandil et al. 2016). Periodical assessment of the parasitic loads of livestock in different localities is deemed to be beneficial, especially for endemic and prevalent disease that has anthelmintic resistance as heamonchosis. Thus the current study aimed to assess the potency of somatic adult and larval H. contortus antigens through ELISA in sero -diagnosis of haemonchosis among naturally infected sheep and goats, in comparison with the results of coprological examination of the corresponding fecal samples, in addition to determination of haemonchosis prevalence among examined sheep and goats in Beni-Suef Governorate, Egypt.
Materials and methods
Ethics approval
This study was conducted according to the guidelines of the Institutional Animal Care and Use Committee at the National Research Centre, Giza, Egypt, Approval Protocol No. (19/044). In this study, all procedures performed involving animals were in accordance with the ethical standards of the institution or practice at which the study was conducted.
Animals and samples
This study was conducted on two hundred and forty-three selected small ruminants, 107 sheep and 136 goats reared by small holders in Beni-Suef Governorate, Egypt during the period from June to August 2018. They were suffering from pallor mucous membrane, rough dull-coat, sluggishness, sub mandibular edema and poor body condition. Fecal and its corresponding blood samples were assembled for all the animals under experiment. Each fecal sample was collected in a separate labeled plastic bag. The blood samples of the examined animals were compiled by jugular vein puncture.
Parasites
Adult worms
The adult worms were harvested from the abomasa of slaughtered infected sheep in El-Monieb abattoir at Giza Governorate. The worms were thoroughly washed several times in phosphate buffer saline (pH 7.2). The characteristic barber’s pole worms were identified as illustrated by Soulsby (1986).
Larvae
H. contortus larvae were obtained through homogenizing the collected female worms, and adding emerging eggs to saw dust and sterilized feces, previously subjected to oven. The culture was kept aerated and wetted by daily addition of few drops of water and mixing for 10 days, at room temperature as described by Soulsby (1983), Tariq et al. (2008). The larvae were harvested using Bearman Wetzal funnel technique as outlined by Baerman and Wetzal (1953), identified according to Whitlock (1960) and counted in five aliquots under microscope 10X and 40X to be utilized in antigen preparation and experimental infection.
Experimental infection
Two of sheep and goats, 5 months age 15–20 kg, were utilized. They have been subjected to clinical and fecal examinations to insure that they were free from parasitic infections. The animals were kept in healthy separate pens and provided with clean rations and tape water. Oral infection was done for each animal, utilizing about 5000 H. contortus third stage larvae. Detection of infection was achieved three weeks post infection. These mono-specific H. contortus infected animals were served as larval donors. Blood samples were collected from the experimentally H. contortus infected animals to be used as control positive sera.
Fecal examination
Fecal examination was performed through the concentration floatation technique for the naturally and experimentally infected animals as illustrated by Soulsby (1986) for detection of GINs and H. contortus eggs respectively.
Fecal culture
Preparation of fecal culture was done for the fecal samples of the naturally and experimentally infected sheep and goats according to Soulsby (1983). H. contortus larvae were assembled as reported by Baerman and Wetzal (1953) and identified as outlined by Whitlock (1960).
Antigens preparation
Adult somatic antigen
Adult somatic antigen was prepared according to Prasad et al. (2007).
Larval antigen
Larval antigen was prepared as described by Alunda et al. (2003).
The protein content of both antigens was determined according to Lowry et al. (1951).
Sera preparation
Serum samples were separated from the collected blood samples of sheep and goats under the experiment. The control positive sera were prepared from blood samples of the experimentally H. contortus infected animals. Negative sera were obtained from one day old newly born lamb and goat kid blood samples’ were used as negative sera. Serum samples were kept at – 20 °C till utilized.
Enzyme linked immunosorbent assay (ELISA)
Firstly, it was adopted to evaluate the diagnostic potency of both somatic and larval antigens using two fold serial dilutions of the experimentally infected animals. Then H. contortus antibodies in randomly collected sheep and goats serum samples were detected using the most potent evaluated antigen. The optimum antigen and serum concentrations were assigned by checkerboard titration and the test procedures was carried out as described by Schallig et al. (1995). The cut-off value was estimated according to the methods of Lone et al. (2012).
The sensitivity, specificity, accuracy, apparent prevalence, positive predictive value, negative predictive value and diagnostic efficacy were estimated according to Thrusfield (1997).
Statistical analysis
Expression of OD data was achieved as arithmetic mean with standard deviation. T Test was used to detect the significant difference of the OD values between larva and adult antigens. The statistical significant difference of the apparent prevalence was detected by Chi square (χ2) test. All statistical analyses were performed by using statistical computer package for social science (SPSS) version 14.
Results
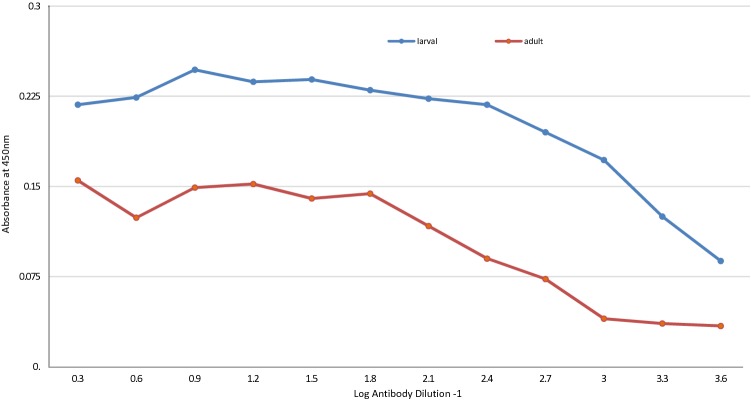
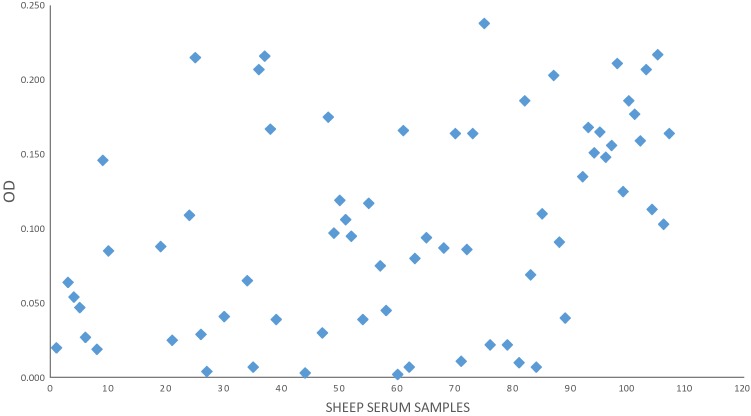
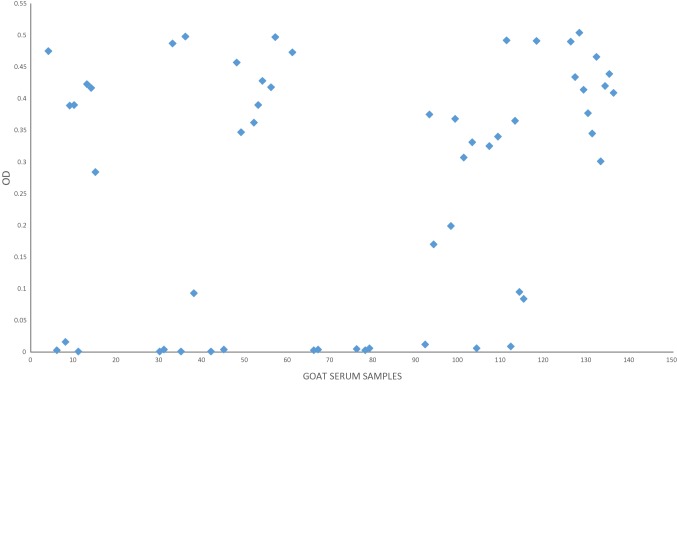
The fecal analysis of the examined small ruminants elucidated that overall prevalence of GINs was 26.33% whereas it was 33.64% in sheep and 20.5% in goats. Based on the fecal culture results, 22.22% of the animals were infected by H. contortus. The prevalence of haemonchosis was reached 27.10% and 18.38%, in sheep and goats, respectively. The statistical analysis of the GINs prevalence in sheep showed insignificant difference versus goats (χ2 = 3.073, p > 0.05). For haemonchosis, also there was insignificant difference of H. contortus prevalence among sheep versus goats (χ2 = 1.8, p > 0.05). The result of the comparative diagnostic potency of adult somatic and larval antigens using monospecific H. contortus sera revealed that the larval antigen was the preferable in detecting H. contortus antibodies as proved by ELISA (Fig. 1). The statistical analysis revealed significant difference of the OD values of larval antigen versus adult antigens (t = 3.321, p < 0.05). So that, the larval antigen was utilized for sero-diagnosis of haemonchosis (Figs. 2, 3). The cut-off OD values for the standardized ELISA taken in consideration for sheep and goats were 0.4 and 0.311 respectively.
Fig. 1.
Comparative diagnostic potential of adult and larval antigens of H. contortus
Fig. 2.
Diagnostic potential of H. contortus larval antigen in the diagnosis of haemonchosis in sheep
Fig. 3.
Diagnostic potential of H. contortus larval antigen in the diagnosis of haemonchosis in goats
Out of the total 243 sheep and goats serum samples, 126 were found to be positive utilizing ELISA. The result disclosed that the sero-prevalence of haemonchosis among the examined small ruminants was 51.85%. The apparent prevalence of haemonchosis was 64.48% and 41.91% for sheep and goats, successively. The sero-prevalence among sheep was higher than goats. A statistical significant difference of the apparent sero-prevalence of sheep versus goats was detected (χ2 = 5.038, p < 0.05). Comparative of H. contortus prevalence brought about by fecal examination and ELISA was illustrated in Table 1.
Table 1.
Comparison between the fecal examination and ELISA in detection of H. contortus infection in sheep and goats
| Animals | Number of samples | Fecal analysis | ELISA | ||
|---|---|---|---|---|---|
| Positive samples | Infection % | Positive samples | Infection % | ||
| Sheep | 107 | 29 | 27.10 | 69 | 64.48 |
| Goats | 136 | 25 | 18.38 | 57 | 41.91 |
| Total | 243 | 54 | 22.22 | 126 | 51.85 |
ELISA was able to detect H. contortus antibodies in all positive samples that harboring larvae in their feces except one serum sample among sheep. However, among 78 sheep and 111 goats which were negative for H. contortus larvae, there were (41 and 32) serum samples revealed positive reaction using ELISA, successively.
The larval antigen revealed (96.55%, 100) sensitivity, (47.43%, 71.17%) specificity and (60.74%, 76.47%) diagnostic efficacy, in sheep and goats, respectively. Meanwhile, positive and negative predictive values were (40.57%, 97.36%) for sheep and (43.85%, 100%) for goats (Table 2).
Table 2.
Efficacy parameters of H. contortus larval antigen in diagnosis of haemonchosis in sheep and goats using ELISA
| Parameters | Examined animals | |
|---|---|---|
| Sheep | Goats | |
| Sensitivity % | 96.55 | 100 |
| Specificity % | 47.43 | 71.17 |
| Positive predictive value % | 40.57 | 43.85 |
| Negative predictive value % | 97.36 | 100 |
| Diagnostic efficacy % | 60.74 | 76.47 |
Discussion
The GINs are considered to be one of the major global constraints adversely affecting small ruminants’ productivity (Torres-Acosta and Hoste (2008); Calvete et al. 2014; Brik et al. 2019). The present study declared that GINs were prevalent among the examined animals and the overall prevalence was reached 26.33% whereas 33.64% of sheep and 20.58% of goats were being infected. The results might be close to that obtained by ELshahawy et al. (2014) in Egypt who found that the prevalence of GINs among goats was 24.44%. As well as, Razzaq et al. (2014) in Pakistan who recorded that 23.92% of sheep were infected with GINs. On other hand, Muluneh et al. (2014) and Dugassa et al. (2018) in Ethiopia who studied the GINs infecting small ruminants and found that overall prevalence was (43.2% and 71.88%, respectively). These variations might be attributed to host breed, samples number, immunity level, different climatic, geographical and management factors in various areas.
The present study disclosed that haemonchosis was a prevalent disease among small ruminants in the investigated locality. This was in agreement with related studies on the epidemiology of gastrointestinal helminths which were recorded Haemonchus as the most prevalent nematode (Tsotetsi and Mbati 2003; Ntonifor et al. 2013; Zvinorova, et al. 2016; Hassan et al. 2019). The results exposed that the overall prevalence of H. contortus based on the findings of fecal culture, was 22.22% whereas it was 27.10% for sheep and 18.38% for goats. This was almost in accordance with KC and Kedar (2012) in Nepal who noticed that 20.89% of goats had H. contortus eggs in the feces. Moreover, Elshahawy et al. (2014) in Egypt who mentioned that prevalence of H. contortus among goats was 15.5%. In Morocco, Brik et al. (2019) observed that (23.92%) of 1154 sheep were infected with H. contortus. While Gowda (2016) in Pakistan reported high prevalence and he found that 57% of sheep were infected with H. contortus. Furthermore, Fentahun and Luke (2012) in Ethiopia who showed higher overall prevalence that reached 80.21% whereas it was 81.2% in sheep and 73.5% in goats. Lower infection rate was reported compared to the presented study, by Sultan et al. (2010) in Egypt who declared that 7.9% of the examined sheep were suffered from haemonchosis. The relative high prevalence of H. contortus in the current study might be due to the climatic conditions and management system of the investigated locality. Beside that, the drugs resistance ability of H. contortus might be interfered with treatment and control of the disease as documented by Kotze and Prichard (2016).
Application of both fecal examination and ELISA was probably the most favorable tool for diagnosis of the current gastrointestinal parasitic infection, this had been achieved by (Johnson et al. 2004; Jas et al. 2010; Lone et al. 2012). In the present study, ELISA had been conducted for the detection of specific serum antibodies in sheep and goats, to H. contortus using larval and somatic adult antigens. The larval antigen had confirmed its potency and priority in detecting the anti-H. contortus antibodies in all animals’ sera under experiment compared to the somatic adult antigen. This agreed with the results obtained by Kandil et al. (2016) who recorded the larval H. contortus antigen efficiency for serological diagnosis compared to excretory secretory product and adult somatic H. contortus. This might be due to the adult worm escape ability from immunity of host through production of several proteases inhibitors and immunomodulatory components to block host effectors mechanisms (Angulo-Cubillán et al. 2007).
Moreover, Antibody level against the antigen might vary due to diversity of host immune system status, circulating immune complexes formation and the immune evasion mechanisms (Lone et al. 2012).
The present study revealed high sero-prevalence of haemonchosis among sheep and goats screened using larval antigen which was reached 64.48% and 41.91%, respectively. These were possibly resemble that recorded by Gowda (2016) who assigned that 58.66% of sheep were infected with H. contortus using adult somatic antigen through ELISA. At the same time, the results might be somewhat lesser than that obtained by Kandil et al. (2016, 2017) who reported 92% and 85.31% apparent prevalence of H. contortus infection among sheep using larval antigen, respectively. These variations in the sero- prevalence could be returned to the type of antigen, the method of preparation of such antigen and the number of sera samples involved in the experiment.
The assay showed high sensitivity (96.55% and 100%) for sheep and goats, successively, that might be returned to the rise number of the true positive sera among the examined animals. One false negative result was revealed among sheep. This was probably due to several factors including low helminth burden, faint immune response of the animal Gasser et al. (2016) and the level of nutrition of the host. Also, the level of antibodies might be impacted by some physiological and environmental factors e.g. recurrent infection or infection with different parasites at the same time (Carmena et al. 2005). In the current study, ELISA specificity was (47.43% and 71.17%) for sheep and goats, respectively. Furthermore, H. contortus antibodies were also detected by ELISA in 41 sheep and 32 goats that were negative for H. contortus larvae in their feces. These false positive results might be due to the presence of the antibodies of a previous infection, immature worm or arrested stage infection and cross reactivity between H. contortus and other helminthes that was limited the evolution of sero-diagnostic method for detection of helminthosis (Philipp and Rumjaneck 1984; Cuquerella et al. 1994; Molina et al. 1999). Moreover, this study was in agreement with the results obtained by Kandil et al. (2017) who carried out ELISA utilizing the crude larval antigen for the revealing of haemonchosis in sheep and found 100% sensitivity. Mir et al. (2008) showed that the sensitivity could be differed according to the antigen types and they found that the ELISA sensitivity relied on excretory-secretory antigen was (87.5%) more than crude somatic antigen (72.22%). These differences might be due to the preparation method and the kind of the antigen utilized in the ELISA. The relatively high diagnostic efficacy obtained from this study (60.74% for sheep and 76.47% for goats) suggested that the crude larval antigen is favorable for detecting H. contortus antibodies in sheep and goats using ELISA.
The study revealed that sheep were more susceptible to H. contortus infection than goats. There was significant difference in sero-prevalence of haemonchosis among sheep versus goats. This was in agreement with Kanyari (1993) who showed that sheep were more exposed to helminthes infection than goats due to their feeding behaviors. Goats might evade from infection by third larval nematode stage through their preference to the woody parts of plants, contrary sheep that select grass, increasing the possibility of larval swallowing Hoste et al. (2005).
It was concluded that haemonchosis is a prevalent disease among sheep and goats in the investigated locality. The larval antigen was more potent than the adult somatic antigen in sero- diagnosis of haemonchosis. ELISA is one of the superior serological tools that applied for studying the helminths prevalence in comparison to fecal analysis.
A periodical assessment for the prevalence of infectious disease is significant to evaluate the management and control measures. The larval antigen is hopeful however, purification of the larval antigen possibly increases the diagnostic potency.
Authors’ contributions
NMTAE, NMFH, SEH designed, supervised and provided the steering for the experiment. NMFH and TKF clinically examined the animals and collected the samples. NMFH, TKF and NMTAE examined the fecal samples. NMFH carried out the experimental infection. SEH, NMFH and DA prepared the antigens and applied ELISA. NMFH, SEH, NMTAE, DA and TKF analyzed and discussed the data. NMFH, SEH, DA and NMTAE edited the manuscript. NMFH revised and reviewed the manuscript for publication. All authors read and approved the final article.
Compliance with ethical standards
Conflict of interest
The authors declare that the article has no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Noha M. F. Hassan, Email: nohamhassan555@yahoo.com
Dina Aboelsoued, Email: dr.dina.aboelsoued@gmail.com.
Tarek K. Farag, Email: tarek.korany@yahoo.com
Soad E. Hassan, Email: soadeh@yahoo.com
Nadia M. T. Abu El Ezz, Email: nadia_talaat60@yahoo.com
References
- Alunda JM, Angulo-Cubillan F, Cupuerella M. Immunization against ovine haemonchosis with three low molecular weight somatic antigens of adult Haemonchus contortus. J Vet Med B. 2003;50:70–74. doi: 10.1046/j.1439-0450.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Angulo-Cubillán FJ, García-Coiradas L, Cuquerella M, de la Fuente C, Alunda JM. Haemonchus contortussheep relationship: a review. Rev Cient. 2007;17:577–587. [Google Scholar]
- Arab RMH, Abu El Ezz NMT, Deghidy NS, Awed WSA, Hassan NMF. Protective value of Haemonchus contortus adult worm purified antigen against haemonchosis in sheep. Glob Vet. 2013;11:614–621. [Google Scholar]
- Baerman G, Wetzal R (1953) Cited after Soulsby EJL (1965). Text book of veterinary clinical parasitology, vol I. Helminths, 1st edn. Black well Scientific Publications, Oxford
- Brik K, Hassouni T, Elkharrim K, Belghytia D. A survey of Haemonchus contortus parasite of sheep from Gharb plain. Morocco: Parasite Epidemiol Control; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete C, Ferrer L, Lacasta D, Calavia R, Ramos J, Ruiz-de-Arkaute M, Uriarte J. Variability of the egg hatch assay to survey Benzimidazole resistance in nematodes of small ruminants under field conditions. Vet Parasitol. 2014;203:102–113. doi: 10.1016/j.vetpar.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Carmena D, Benito A, Martinez J, Guisantes JA. Preliminary study of the presence of antibodies against excretory-secretory antigens from protoscoleces of Echinococcus granulosus in dogs with intestinal echinococcosis. Mem Inst Oswaldo Cruz. 2005;100:311–317. doi: 10.1590/S0074-02762005000300018. [DOI] [PubMed] [Google Scholar]
- Cuquerella M, Gomez-Munoz MT, Carrera L, de la Fuente C. Cross antigenicity among ovine trichostrongyloidea, preliminary report. Vet Parasitol. 1994;53:243–251. doi: 10.1016/0304-4017(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Demeler J, Schein E, Von Samson-Himmelstjerna G. Advances in laboratory diagnosis of parasitic infections of sheep. Vet Parasitol. 2012;189:52–64. doi: 10.1016/j.vetpar.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Díaz A, Arena A, França J, Gomes AL, Machado MA, Sossanovicz M, Yoshitani Ú, Molento MB. Optimization of an immunoenzymatic (ELISA) assay for detecting ovine antibodies against Haemonchus contortus. Cuban J Agric Sci. 2015;49:477–485. [Google Scholar]
- Dugassa J, Hussein A, Kebede A, Mohammed C (2018) Prevalence and associated risk factors of gastrointestinal nematodes of sheep and goats in Ziway Dugda District, Eastern Arsi Zone of Oromia regional state, Ethiopia. Multidisciplinary advances in veterinary science pp. 2301–310
- El-Ashram S, Al Nasr I, Mehmood R, Hu M, He L, Suo X. Haemonchus contortus and ovine host: a retrospective review. Int J Adv Res. 2017;5:972–999. doi: 10.21474/IJAR01/3597. [DOI] [Google Scholar]
- El-Dakhly KM, Abo El-Hadid SM, El Askalany MA, Yanai T. An Abattoir-Based study on Helminths of slaughtered sheep in Beni-Suef, Egypt. Beni-Suef Univ J Appl Sci. 2012;1:49–60. [Google Scholar]
- Elshahawy IS, Metwally AM, Ibrahim DA. An Abattoir-Based Study on Helminthes of Slaughtered goats (Capra hircus L., 1758) in Upper Egypt, Egypt. Helminthologia. 2014;51:67–72. doi: 10.2478/s11687-014-0210-2. [DOI] [Google Scholar]
- Fentahun T, Luke G. Small ruminant haemonchosis: prevalence and associated determinants in randomly selected restaurants and hotels of Gondar Town, Ethiopia. Eur J Appl Sci. 2012;4:168–172. [Google Scholar]
- Gasser RB, Schwarz EM, Korhonen PK, Young ND. Understanding H. contortus better through genomics and transcriptomics. Adv Parasitol. 2016;93:519–567. doi: 10.1016/bs.apar.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Gowda AK. Sero-prevalence of Haemonchus contortus infection in sheep by indirect ELISA using somatic antigen. J Parasit Dis. 2016;40:464–468. doi: 10.1007/s12639-014-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan NMF, Tarek KF, Abu El Ezz NMT, Abou-Zeina HAA. Prevalence assessment of gastrointestinal parasitic infections among goats in Giza Governorate, Egypt. Bull Natl Res Centre. 2019;43:127. doi: 10.1186/s42269-019-0151-5. [DOI] [Google Scholar]
- Hoste H, Torres-acosta JFJ, Paolini V, Aguilarcaballero AJ, Etter E, Lefrileux Y, Chartier C, Broqua C. Interactions between nutrition and gastrointestinal infections with parasitic nematodes in goats. Small Rumin Res. 2005;60:141–151. doi: 10.1016/j.smallrumres.2005.06.008. [DOI] [Google Scholar]
- Jackson F, Bartley D, Bartley Y, Kenyon F. Worm control in sheep in the future. Small Rum Res. 2009;86:40–45. doi: 10.1016/j.smallrumres.2009.09.015. [DOI] [Google Scholar]
- Jas R, Ghosh JD, Das K. Polyclonal antibody based coproantigen detection immunoassay for diagnosis of Oesophagostomum columbianum infection in goats. Vet Parasitol. 2010;170:262–267. doi: 10.1016/j.vetpar.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Behnke JM, Coles GC. Coproantigen capture ELISA for the detection of Teladorsagia (Ostertagia) circumcincta in sheep improvement of specificity by heat treatment. Parasitology. 2004;129:115–126. doi: 10.1017/S0031182004005256. [DOI] [PubMed] [Google Scholar]
- Kandil OM, Hendawy SHM, El Namaky AH, Gabrashanska MP, Nanev VN. Evaluation of different Haemonchus contortus antigens for diagnosis of sheep haemonchosis by ELISA and their cross reactivity with other helminthes. J Parasit Dis. 2016 doi: 10.1007/s12639-016-0865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil OM, Gamil IS, Hendawy SHM, Medhat F, El-Habit OH. Efficacy of glutathione-S-transferase purified antigen of the gastro-intestinal nematode Haemonchus contortus in diagnosis of sheep haemonchosis. J Parasit Dis. 2017;41:968–975. doi: 10.1007/s12639-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyari PWN. The relationship between coccidial and helminth infections in sheep and goats in Kenya. Vet Parasitol. 1993;51:137–141. doi: 10.1016/0304-4017(93)90204-Z. [DOI] [PubMed] [Google Scholar]
- KC G, Kedar K (2012) Epidemiology of gastrointestinal helminthes in goats of Chitwan. In: 10th conference, Souvenir, Nepal Veterinary Association
- Kotze AC, Prichard RK. Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv Parasitol. 2016;93:397–428. doi: 10.1016/bs.apar.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Lone BA, Chishti MZ, Ahmad F, Tak H, Hassan J. Immunodiagnosis of H. contortus infection in sheep by indirect enzy ELISA. IJVR. 2012;13:49–53. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AB, Randall RJ. Protin measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mir RA, Chishti MZ, Zargar MA, Tak H, Ganie SA. Excretory- Secretory antigens are better than crude antigens for serodiagnosis of Haemonchus contortus. Asian J Sci Res. 2008;1:171–175. doi: 10.3923/ajsr.2008.171.175. [DOI] [Google Scholar]
- Molina JM, Ruiz A, Rodriguez-Ponce E, Gutierrez AC, Gonzalez J, Hernandez S. Cross-reactive antigens of H. contortus adult worms in Teladorsagia circumcincta infected goats. Vet Res. 1999;30:93–399. [PubMed] [Google Scholar]
- Muluneh J, Bogale B, Chanie M. Major gastrointestinal nematodes of small ruminants in Dembia District, Northwest Ethiopia. Eur J Appl Sci. 2014;6:30–36. [Google Scholar]
- Ntonifor H, Shei S, Ndaleh N, Mbunkur G. Epidemiological studies of gastrointestinal parasitic infections in ruminants in Jakiri, Bui division, North West region of Cameroon. J Vet Med Anim Health. 2013;5:344–352. [Google Scholar]
- Philipp M, Rumjaneck FD. Antigenic and dynamic properties of helminth surface structures. Mol Biochem Parasitol. 1984;10:245–268. doi: 10.1016/0166-6851(84)90025-2. [DOI] [PubMed] [Google Scholar]
- Prasad A, Nasir A, Singh N. Dot-ELISA for the detection of preclinical Haemonchus contortus infections in sheep by using an adult somatic antigen and an immunoaffinity-purified fraction. J Parasit Dis. 2007;31:22–28. [Google Scholar]
- Prasad A, Nasir A, Singh N. Detection of anti-H. contortus antibodies in sheep by dot-ELISA with immunoaffinity purified fraction of ES antigen during prepatency. IJEB. 2008;46:94–99. [PubMed] [Google Scholar]
- Razzaq A, Ashraf K, Maqbool A, Islam M, Hanan A, Awais MM, Khetran MA, Jan S, Shafee M, Essa M, Kakar H. Epidemiology, sero-diagnosis and therapeutic studies on nematodes infection in balochi range-sheep at district Quetta, Balochistan, Pakistan. Iran J Parasitol. 2014;9:169–180. [PMC free article] [PubMed] [Google Scholar]
- Schallig HD, Hornok S, Cornelissen JB. Comparison of two enzyme immunoassays for the detection of H. contortus infections in sheep. Vet Parasitol. 1995;57:329–338. doi: 10.1016/0304-4017(94)00693-7. [DOI] [PubMed] [Google Scholar]
- Selemon M. Review on control of Haemonchus contortus in sheep and goat. J Vet Med Res. 2018;5:1139. [Google Scholar]
- Soulsby EJL (1983) Helminths, arthropods and protozoa of domesticated animals. In: The English language book society, 7th edn. Bailiere Tindall and Cassell Ltd., pp 763–773
- Soulsby EJL. Helminthes, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1986. [Google Scholar]
- Sultan K, Desoukey AY, Elsiefy MA, Elbahy NM. An abattoir study on the prevalence of some gastrointestinal helminths of sheep in Gharbia Governorate, Egypt. Glob Vet. 2010;5:84–87. [Google Scholar]
- Sutherland I, Scott I. Gastrointestinal nematodes of sheep and cattle: biology and control. Chichester: Wiley-Blackwell; 2010. [Google Scholar]
- Tariq KA, Chishti MZ, Fayaz A, Shawl AS. Epidemiology of gastrointestinal nematodes of sheep managed under traditional husbandry system in Kashmir valley. Vet Parasitol. 2008;158:138–143. doi: 10.1016/j.vetpar.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Thrusfield M. Veterinary epidemiology. 2. Oxford: Blackwell Science Ltd.; 1997. pp. 134–135. [Google Scholar]
- Torres-Acosta J, Hoste H. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Rumin Res. 2008;77:159–173. doi: 10.1016/j.smallrumres.2008.03.009. [DOI] [Google Scholar]
- Tsotetsi A, Mbati P. Parasitic helminths of veterinary importance in cattle, sheep and goats on communal farms in the Northeastern Free State, South Africa. J S Afr Vet Assoc. 2003;74:45–48. doi: 10.4102/jsava.v74i2.503. [DOI] [PubMed] [Google Scholar]
- Whitlock JH. Diagnosis of veterinary parasitisms. 1. Philadelphia: Lea and Febiger; 1960. [Google Scholar]
- Zhang R, Liu F, Hunt P, Li C, Zhang L, Ingham A, Li RW. Transcriptome analysis unraveled potential mechanisms of resistance to Haemonchus contortus infection in Merino sheep populations bred for parasite resistance. Vet Res. 2019;50:7. doi: 10.1186/s13567-019-0622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvinorova PI, Halimani TE, Muchadeyi FC, Matika O, Riggio V, Dzama K. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin Res. 2016;143:75–83. doi: 10.1016/j.smallrumres.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]