Abstract
Since the past 2 decades, an increasing number of resistance to the Benzimidazoles (BZs) have been reported in nematode parasites of livestock. More recently, detection of single-nucleotide polymorphisms (SNPs) at codons of 167, 198 or 200 of the β-tubulin gene has been attributed to the occurrence of resistance. In the present study, we investigated the presence of those SNPs in the β-tubulin isotype-1 gene in different isolates of Parascaris in horse. Also, the mitochondrial (mt) and ribosomal genes were sequenced for species confirmation of the isolates. The analysis of sequences inferred from COII gene confirmed that those isolates were P. equorum. The distance between mt genes obtained here and several ascarid species in equids and other hosts suggests the need for the combination of more genetic data with morphologic and other diagnostic measures. The analysis on β-tubulin isotype-1 gene revealed no resistance-related SNPs or substitutions at the expected codon positions and selection pressure with BZs has not occurred for Parascaris worms. Although the molecular data showed the susceptibility of Parascaris isolates against BZs, other mechanisms of resistance should be also investigated to confirm the validity of molecular results.
Keywords: Parascaris equorum, Cytochrome oxidase, 18s rRNA gene, β-Tubulin gene, Phylogeny
Introduction
Parascaris equorum is a nematode parasite belonging to the family Ascarididae. The infection is widely distributed causing serious pathogenicity in foals (Reinemeyer and Marchiondo 2010).
Until recently, the equine ascarid parasites have been traditionally referred to one species and considered as P. equorum. However, the literature review shows another ascarid species infecting horses, namely Parascaris univalens. The mitochondrial (mt) genomic marker has been applied for diagnosis of parasitic Ascarids (Gasser 2006; Jabbar et al. 2014). Recently, the mt genome was also amplified from total genomic DNA of P. univalens and phylogenetically analyzed (Jabbar et al. 2014). In addition, ribosomal primers targeting the internal transcribed spacers 2 (ITS-2) of the ribosomal rDNA gene was suggested for estimation of genetic diversity in samples of Parascaris isolates (Tydén et al. 2013).
The ascarid roundworms has been controlled by strategic use of different anthelmintics, including Benzimidazoles (BZs), macrocyclic lactones, and tetrahydropyrimidines (Reinemeyer 2012). Despite the drug efficacy, field studies have shown that heavy deworming programs over many years have lead to selection of resistant populations in different nematode species. This phenomenon has become a major threat to sustainable livestock production (Kaplan 2004).
In the past 2 decades, several cases of BZ resistance have been reported in nematode infected ruminants or equids worldwide (Coles et al. 2006). The underlying mechanisms remained unknown; however it was proposed that changes in the targeted genomes encoding the binding site of the BZs are mainly responsible for reduced efficacy of the following treatments (Wolstenholme et al. 2004). Among the commonly used anthelmintics, molecular analyses have been most focused on the resistance to the BZsin trichostrongylid nematodes, particularly in Haemonchus contortus (von Samson-Himmelstjerna et al. 2007, 2009). This is mainly attributed to the occurrence of single nucleotide polymorphisms (SNPs) in parasite β-tubulin gene at codon 200 (Kwa et al. 1994) and also at codons 167 (Silvestre and Cabaret 2002) and 198 (Ghisi et al. 2007). Among the equid nematodes, a TAC mutation in codon 167 has been indicated in cyathostomins, even in horses that have never received any anthelmintic treatment (Blackhall et al. 2011). In contrast, no SNPs were detected in β-tubulin genes of P. equorum (Tydén et al. 2013, 2014). Thus, the association between β-tubulin encoding gene and the occurrence of BZ resistance in equine nematodes is still unclear.
The present study aimed to characterize some of mitochondrial and ribosomal genes among Parascaris populations in horses in Iran. Then, the β-tubulin isotype-1 gene was investigated in larvae of different isolates to determine any hypothetical BZ resistance considering the SNPs in codons 167, 198 or 200.
Materials and methods
Parasite material and harvesting of larvae
Eight isolates of Parascaris worms (n = 8 for each) were collected from feces of naturally infected foals or horse died due to an overwhelming disease and gastrointestinal roundworm impaction with unknown history of anthelmintic use from different localities in South of Iran. A part of specimens from each isolate were washed carefully with normal saline solution and fixed in 70% (v/v) ethanol for molecular diagnosis. A number of adult females were separately dissected out to obtain the egg material. The eggs were placed in plates with layers of 2.5% (w/v) aqueous potassium dichromate, for development and hatching of larvae (Rakhshandehroo et al. 2017). Larvae were stored at − 80 °C until use.
PCR amplification and DNA sequencing
Genomic DNA was extracted from the collected (male, female or larvae) samples of each isolate and purified individually using the DNA extraction Kit (MBST, Iran) according to the manufacturer’s recommended protocol and used as a template DNA for the PCR. A set of primers, F1 (5′-TTTTCTAGTTATATAGATTGGTTTCAT-3′) and R1 (5′-CACCAACTCTTAAAATTATC-3′) were used for the assessment of mitochondrial cytochrome oxidase subunit 2 (COII) gene sequence (Nadler and Hudspeth 2000). PCR amplification was carried out with an initial DNA denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 45 s, 48 °C for 45 s, and 72 °C for 1 min, with a post amplification extension for 5 min at 72 °C.
To amplify partial fragment of 18s rRNA gene, a one step PCR was done using primers, Forward (5′-CCCGATTGATTCTGTCGGC-3′) and Reverse (5′-TGATCCTTCTGCAGGTTCACCTAC-3′) (Nadler and Hudspeth 2000). The PCR cycling conditions were 94 °C for 4 min, followed by 35 cycles of 94 °C for 45 s, 60 °C for 30 s and 72 °C for 50 s, a final extension of 72 °C for 5 min and 10 °C for 15 min. The conditions were adjusted empirically to improve the quality of reactions. Sterile water was used as the negative controls and a DNA sample referring to a previously confirmed P. equorum worm was used as positive one.
The expected amplicon size were assessed by electrophoresis in 1.2% (w/v) Tris-acetate/EDTA agarose gel and visualized under ultraviolet illumination.
RNA extraction, cDNA synthesis and β-tubulin isolation
Total RNA was extracted from larvae of each isolate. The larvae (up to 30 mg of weight) were disrupted by grinding, lysed and the total RNA extraction performed using GeneJET RNA Purification Kit (Thermo Scientific, Lithuania). The RNA was reverse transcribed with the oligo-dt primer using SinaClon cDNA synthesis kit (Cinnagen Co., Iran) according to the manufacturer’s instructions. A set of primers were designed for the amplification of the β-tubulin isotype 1 gene on the basis of the sequence available at Genbank database (GenBank Accession No. KC713797) (F: GCGGCCATGAGAGAAATTGTGCATG and R: TGTTCGGTATACTCGGCCTCGCC). The PCR reaction mix included 12.5 μl of PCR premix (Ampliqon, Denmark, Cat. No. A180301), 1 μl of each primer, 6.5 μl H2O and 4 μl of cDNA as template. The cycling parameters for the amplification consisted of an initial denaturation at 94 °C for 2 min, 94 °C for 40 s, followed by 40 cycles of 58 °C for 1 min, the extension at 72 °C for 1 min, the final extension at 72 °C for 5 min and 15 °C for 15 min.
Molecular analysis
Products from each assay were sequenced in both directions (ABI 3730 DNA analyzer; Bioneer, Korea). Forward and reverse sequences data were used to construct a consensus DNA sequence using CLC Main Work bench 5.5 software (Tamura et al. 2007). The comparison of the sequences was made with other available sequences in NCBI using BLAST search. The sequence data was edited and aligned against homologous sequences existing in Genbank using Clustal W program by MEGA 6 software (Tamura et al. 2013). The phylogenetic tree was constructed by maximum likelihood (ML) method and analyses was carried out using the Kimura 2-parameter distance estimate (Kimura 1980). Support values for internal nodes were estimated using a bootstrap resampling procedure with 2000 replicates. The pairwise similarities and distances were calculated by CLC Main Work bench 5.5 software (Tamura et al. 2007).
Results
Molecular diagnosis of samples
In the present study, the sequences of COII and 18s rRNA genes were detected in Parascaris samples and successfully amplified.
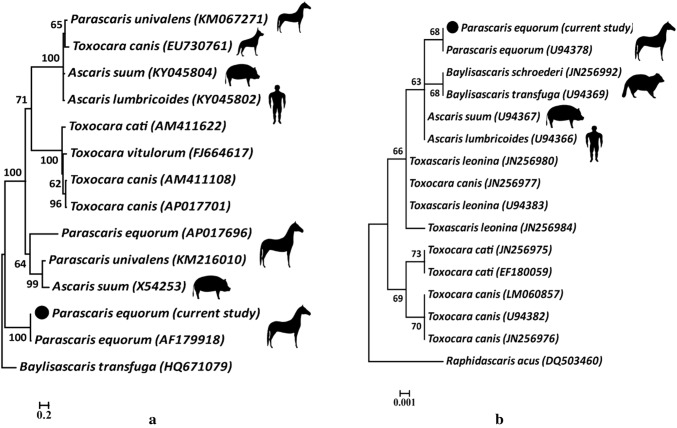
The obtained DNA sequences of 537 bp for partial COII gene in both directions were trimmed and the sequence was deposited in the Genbank under the Accession number: MG676884. A primary BLAST analysis confirmed that the specimens were isolates of P. equorum. Based on this region, the isolates had about 98.0% identity to each other. Comparison of the Iranian Parascaris COII gene with other sequences deposited in the GenBank database revealed 99.0% similarity with P. equorum previously reported from America (AF179918) (Nadler and Hudspeth 2000) (Table 1). However, interestingly, present isolates showed low homology (~ 33%) with other report of mt gene from P. equorum in the GenBank (AP017696). Also, a high degree of genetic difference with another species of Parascaris (P. univalens) and other ascarid worms in cattle (Toxocara vitulorum), carnivores (T. canis, T. cati), pigs (A. suum) and human (Ascaris lumbricoides) was observed. The phylogenetic tree confirmed that our specimens and the American record of P. equorum had close phylogenetic association. In addition, P. univalens had not a fixed position and categorized with P. equorum and A. suum in one clade and T. canis, A. suum and A. lumbricoides in a separate one (Fig. 1A).
Table 1.
Pairwise comparison of aligned COX2 gene sequences showing the genetic percent identity of P. equorum in the present study and other ascarids from equids and other hosts
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parascaris equorum (current study) | – | ||||||||||
| Parascaris equorum (AF179918) | 99.21 | – | |||||||||
| Parascaris equorum (AP017696) | 33.27 | 33.27 | – | ||||||||
| Parascaris univalens (KM067271) | 35.30 | 35.30 | 39.28 | – | |||||||
| Parascaris univalens (KM216010) | 38.01 | 37.64 | 31.58 | 39.20 | – | ||||||
| Toxocara vitulorum (FJ664617) | 36.75 | 36.57 | 45.42 | 43.66 | 39.32 | – | |||||
| Toxocara canis (AP017701) | 37.69 | 37.50 | 46.59 | 45.34 | 38.75 | 86.90 | – | ||||
| Toxocara canis (AM411108) | 38.25 | 38.06 | 47.17 | 45.15 | 38.75 | 87.30 | 97.82 | – | |||
| Toxocara cati (AM411622) | 37.87 | 37.50 | 45.42 | 44.40 | 38.94 | 87.10 | 87.70 | 87.10 | – | ||
| Ascaris suum (KY045804) | 34.01 | 34.01 | 39.09 | 90.59 | 40.34 | 44.59 | 44.22 | 43.84 | 44.40 | – | |
| Ascaris lumbricoides (KY045802) | 34.57 | 34.57 | 38.14 | 91.62 | 38.81 | 44.22 | 44.59 | 44.59 | 44.22 | 95.50 | – |
Fig. 1.
(a, b) Phylogenetic position of P. equorum isolates from Iranian equids and several other ascarid species in different hosts inferred from partial COII (a) and 18s rRNA (b) genes using the maximum-likelihood method. Bootstrap values (2000 psuedoreplicates) are indicated at the nodes. The horizontal distance is proportional to hypothesized evolutionary change as indicated (scale bar)
Based on analyses on Parascaris 18s rRNA gene, an amplicon size of 507 bp was yielded. Our samples had high levels of similarity (about 97.0 to 98.0%) with previous records of P. equorum and other ascarids in different hosts (Table 2). They categorized with previous record for P. equorum and separated from other ascarid nematodes, however with relatively weak bootstrap support (Fig. 1b).
Table 2.
Pairwise comparison derived from aligned 18s ribosomal RNA gene sequences showing the genetic percent identity of P. equorum in the present study and other ascarids
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parascaris equorum (current study) | – | ||||||||||
| Parascaris equorum (current study) | 98.40 | – | |||||||||
| Parascaris equorum (U94378) | 98.40 | 99.20 | – | ||||||||
| Toxocara canis (JN256977) | 98.00 | 98.80 | 99.00 | – | |||||||
| Toxocara canis (U94382) | 97.41 | 98.20 | 99.00 | 98.80 | – | ||||||
| Toxocara leonina (JN256980) | 98.00 | 98.80 | 99.00 | 100.00 | 98.80 | – | |||||
| Ascaris suum (U94367) | 98.20 | 99.00 | 99.80 | 99.20 | 99.20 | 99.20 | – | ||||
| Ascaris lumbricoides (U94366) | 98.20 | 99.00 | 99.80 | 99.20 | 99.20 | 99.20 | 100.00 | – | |||
| Baylis ascaris scroederi (JN256992) | 98.00 | 98.80 | 99.00 | 99.60 | 98.40 | 99.60 | 99.20 | 99.20 | – | ||
| Baylis ascaris procyonis (U94368) | 98.20 | 99.00 | 99.80 | 99.20 | 99.20 | 99.20 | 100.00 | 100.00 | 99.20 | – | |
| Toxocara cati (JN256975) | 97.40 | 98.20 | 98.40 | 99.40 | 99.00 | 99.40 | 98.600 | 98.60 | 99.00 | 98.60 | – |
β-Tubulin gene analysis
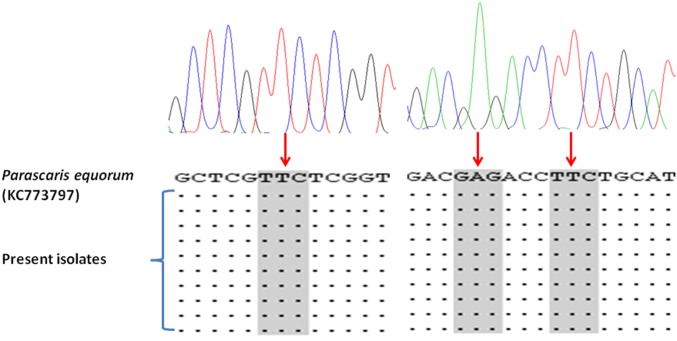
The sequences resulted from the amplification of P. equorum β-tubulin cDNA were analysed and trimmed. Fragments of 812 bp were obtained. The isolates had more than 96.9% similarity with each other. A primary BLAST analysis demonstrated higher identities with the β-tubulin gene of P. equorum, A. lumbricoides and also species of an insect, Drosophila. Data showed high similarities (97.6 to 99.3 percent) with the only available cDNA sequence for P. equorum β-tubulin isotype 1 (Accession number: KC713797); In contrast, our isolates were unmatched with the record for the parasite β-tubulin isotype 2 (Accession number: KC713798) showing about 75.0% similarity. In the present study, 38 polymorphic sites were detected, from which 15 positions were parsimony informative. No occurrence of resistance-related SNPs or substitutions was revealed at codon positions of 167, 198 or 200 (Fig. 2). According to the phylogenetic data (Fig. 3), the present specimens were clustered with P. equorum and the human ascarid worm, A. lumbricoides. At higher taxon levels, Parascaris were grouped with Ascaridia galli and a filarioid nematode, Brugia malayi. The other equid nematode, Cylicocyclus separated from ascarids and showed paraphyletic relation with Trichostrongylids.
Fig. 2.
The nucleotide composition of β-tubulin isotype 1 cDNA extracted from larvae of Parascaris equorum at codons of 167, 198 and 200 recorded in a previous study and the present work. Accordingly, no SNPs were detected at the expected positions
Fig. 3.
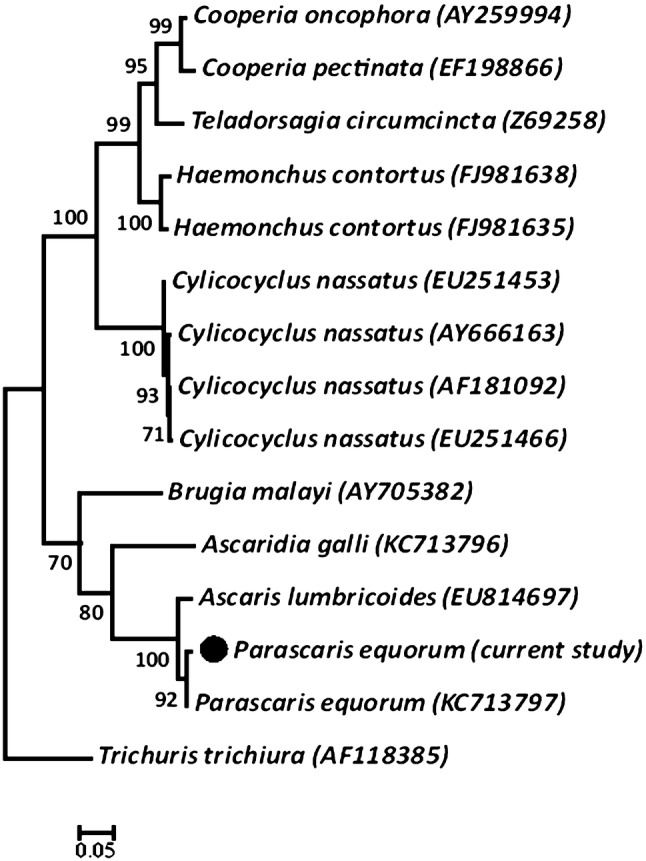
Maximum likelihood phylogenetic analysis of β-tubulin isotype 1 gene in Parascaris equorum and other nematodes. Note the close relationship between ascarids and a filarioid nematode, Brugia malayi
Discussion
The comparison between the sequences inferred from COII and 18s rRNA gene regions with previous published data proved that our specimens were P. equorum. The analysis also showed the close interrelationships of P. equorum with selected ascaridoid nematodes.
The mt genomic sequence has been suggested to provide a foundation for a diagnostic molecular marker. According to the COII sequence, the Iranian isolates showed closed similarity only with a P. equorum sample data previously reported from America (AF179918) (Nadler and Hudspeth 2000). Thus, it seems that geographic borders could not have influence the gene flow for this parasite yet. The high distance between the present samples and other reports of mt gene from P. equorum (AP017696), P. univalens and other ascarid worms in other hosts may not be easily explained. In addition, P. univalens records were categorized with P. equorum and A. suum in one clade and T. canis, A. suum and A. lumbricoides in a separate one. The above data suggest that the COII gene should be more investigated in different ascarid types. Nielsen et al. (2014) derived the whole mt genome sequence from a specimen of P. univalens. The sequence reads for P. univalens had above 99.0% similarity with that of P. equorum. Despite the close relationship, they suggest that the high level of identity could likely implicate that previous sequencing data for P. equorum was mislabeled and some molecular data of Parascaris specimens may belong to P. univalens. In contrast to the COII region, the 18s rRNA gene revealed high sequence conservation. Despite finding some phylogenetic relations between P. equorum and other ascarids of different origins, they did not support with a strong bootstrap measure. According to our data and some other studies (Nadler and Hudspeth 2000), it can be argued that 18s rRNA gene could not be focused for future studies investigating the phylogeny of Ascarids.
The analysis on the β-tubulin isotype 1 gene in the present Parascaris isolates did not show significant diversity. Also, the present isolates demonstrated no resistance-related SNPs at previously determined positions at codons 167, 198 and 200. This implies that there is no genetic resistance marker against BZs in Parascaris worms at present time in South of Iran. However due to the frequent use of BZs, it is expected that future studies could be able to detect the resistance development even in cases with no indication of clinical infections.
Isotypes of the β-tubulin family are action sites for the BZs. Although BZs are among the effective anthelmintic drugs in livestock, different reports have revealed the occurrence of resistance in a number of nematode species. In parallel to the faecal egg count reduction test (FECRT), molecular investigations have also assessed the alterations in β-tubulin gene (Coles et al. 2006). The molecular approach for BZ resistance has mainly focused on H. contortus and some of gastrointestinal nematodes of ruminants. In Ascarid roundworms, particularly Parascaris spp., few studies have traced the molecular changes in β-tubulin in field isolates. For P. equorum, only two β-tubulin isotypes have been described (Tydén et al. 2013). In line with our data, all of the examined specimens (including pooled larvae and the adult worms) of P. equorum and/or A. galli have represented susceptibility in all three codons of β-tubulin isotypes (Tydén et al. 2013, 2014). These results indicate that the possibility of BZ-associated SNPs in Parascaris is still low and likely that the BZ selection pressure cannot be regarded as a choice for the equid ascarid now. In addition, the restricted use of BZs for equid parasites has also been assigned to lack of any previous reports of BZ resistance in P. equorum (Tydén et al. 2014).
Among nematodes which infect equids, the presence of TAC mutations was reported to occur at codons 167 and 200 of representatives of cyathostomins recovered from horses with no history of anthelmintic treatment and with substantial BZ resistance (Blackhall et al. 2011). On the other side, the lack of BZ-associated SNPs in A. lumbricoides (Diawara et al. 2009) and some strongylids such as Ancylostoma caninum (Schwenkenbecher et al. 2007) and Trichostrongylus tenuis confirms the need for criticizing the connection between known mutations in β-tubulin gene and BZ resistance in different nematodes. According to these investigations, this is arguable that the genetic data may not be necessarily a reliable factor for the assessment of resistance in ascarids. Also, this indicates the difficulty to infer the resistance mechanism between different parasite groups (Tydén et al. 2013).
The phylogenetic studies have indicated a great diversity of β-tubulin genes in the phylum Nematoda (Tydén et al. 2013). Interestingly, our phylogenetic data showed a close relation between Parascaris isolates and a filarioid nematode, B. malayi. This is somewhat surprising and indicates that the β-tubulin isotypes 1 gene may have shared between Ascarid and Filarioid nematodes before they could separate. Consistently, Tydén et al. (2013) stated that the original gene duplication occurred before the divergence of both superfamilies. They conclude that the diversity of isotype patterns among nematodes is likely due to the fact that interpretation of BZ-resistance from genetic data cannot be generalised from one taxonomic group to another. Unfortunately, we did not find recorded β-tubulin gene sequences for other prevalent ascarid worms such as Toxocara, Ascaris and other species. Thus, we need more information to make a phylogenetic connection between different nematode families and ascarid members on the basis of the BZ resistance.
Conclusion
P. equorum was recognized as the current ascarid species infecting horses in Iran. According to the mt gene (COII) the present helminths had a close relationship with a record of American P. equorum. In the present isolates, no molecular evidence for the resistance was obtained. However, the other possible alterations in the β-tubulin gene should be investigated to confirm lacking or the emergence of resistance in the parasites.
Acknowledgements
This study was granted by Shiraz University, Shiraz, Iran (Grant No: SHM-95).
Compliance with ethical standards
Conflict of interest
Authors declared that there is no any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Blackhall WJ, Kuzmina T, Von Samson-Himmelstjerna G. β-Tubulin genotypes in six species of cyathostomins from anthelmintic-naïve Przewalski and benzimidazole-resistant brood horses in Ukraine. Parasitol Res. 2011;109:1199–1203. doi: 10.1007/s00436-011-2426-0. [DOI] [PubMed] [Google Scholar]
- Coles GC, Jackson F, Pomroy WE, Prichard RK, Von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136:167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DA, Scott ME, Halpenny C, Stothard JR, Prichard RK. Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl Trop Dis. 2009;3:e397. doi: 10.1371/journal.pntd.0000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser RB. Molecular tools—advances, opportunities and prospects. Vet Parasitol. 2006;136:69–89. doi: 10.1016/j.vetpar.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ghisi M, Kaminsky R, Mäser P. Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Vet Parasitol. 2007;144:313–320. doi: 10.1016/j.vetpar.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jabbar A, Littlewood DT, Mohandas N, Briscoe AG, Foster PG, Müller F, von Samson-Himmelstjerna G, Jex AR, Gasser RB. The mitochondrial genome of Parascaris univalens-implications for a “forgotten” parasite. Parasites Vector. 2014;7:428. doi: 10.1186/1756-3305-7-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kwa MS, Veenstra JG, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta tubulin isotype 1. Mol Biochem Parasitol. 1994;63:299–303. doi: 10.1016/0166-6851(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Nadler SA, Hudspeth DSS. Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J Parasitol. 2000;86:380–393. doi: 10.1645/0022-3395(2000)086[0380:POTANA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nielsen MK, Wang J, Davis R, Bellaw JL, Lyons ET, Lear TL, Goday C. Parascaris univalens-a victim of large-scale misidentification? Parasitol Res. 2014;113(12):4485–4490. doi: 10.1007/s00436-014-4135-y. [DOI] [PubMed] [Google Scholar]
- Rakhshandehroo E, Asadpour M, Malekpour SH, Jafari A. The anthelmintic effects of five plant extracts on the viability of Parascaris equorum larvae. Equine Vet Edu. 2017;29(4):219–224. doi: 10.1111/eve.12676. [DOI] [Google Scholar]
- Reinemeyer CR. Anthelmintic resistance in non-strongylid parasites of horses. Vet Parasitol. 2012;185:9–15. doi: 10.1016/j.vetpar.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C, Marchiondo A. Efficacy of pyrantel pamoate in horses against a macrocyclic lactone-resistant isolate of Parascaris equorum in horses. Vet Parasitol. 2010;171:111–115. doi: 10.1016/j.vetpar.2010.02.041. [DOI] [PubMed] [Google Scholar]
- Schwenkenbecher JM, Albonico M, Bickle Q, Kaplan RM. Characterization of beta-tubulin genes in hookworms and investigation of resistance-associated mutations using real-time PCR. Mol Biochem Parasitol. 2007;156:167–174. doi: 10.1016/j.molbiopara.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Silvestre A, Cabaret J. Mutation in position 167 of isotype 1 b-tubulin gene of Trichostrongylid nematodes: role in benzimidazole resistance? Mol Biochem Parasitol. 2002;120:297–300. doi: 10.1016/S0166-6851(01)00455-8. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tydén E, Morrison D, Engström A, Nielsen M, Eydal M, Höglund J. Population genetics of Parascaris equorum based on DNA fingerprinting. Infect Genet Evol. 2013;13:236–241. doi: 10.1016/j.meegid.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Tydén E, Dahlberg J, Karlberg O, Höglund J. Deep amplicon sequencing of preselected isolates of Parascaris equorum in β-tubulin codons associated with benzimidazole resistance in other nematodes. Parasites Vector. 2014;7(1):410. doi: 10.1186/1756-3305-7-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G, Blackhall W, McCarthey JS, Skuce PJ. Single nucleotide polymorphism (SNP) markers for benzimidadazole resistance in veterinary nematodes. Parasitology. 2007;134:1077–1086. doi: 10.1017/S0031182007000054. [DOI] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G, Walsh TK, Donnan A, Carriere S, Jackson F, Skuce P, Rohn K, Wolstenholme AJ. Molecular detection of benzimidazole resistance in Haemonchus contortus as a tool for routine field diagnosis. Parasitology. 2009;136:349–358. doi: 10.1017/S003118200800543X. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]