Abstract
Binge drinking is characterized by bouts of high-intensity alcohol intake and is associated with an array of health-related harms. Even though the transition from occasional impulsive to addictive alcohol use is not well understood, neurobiological models of addiction suggest that repeated cycles of intoxication and withdrawal contribute to the development of addiction in part through dysregulation of neurofunctional networks. Research on the neural sequelae associated with binge drinking is scant but resting state functional connectivity (RSFC) studies of alcohol use disorders (AUD) indicate that the development and maintenance of long-term excessive drinking may be mediated by network-level disruptions. The present study examined RSFC in young adult binge (BD) and light (LD) drinkers with seeds representing the networks subserving reward (the nucleus accumbens and caudate nucleus), salience (anterior cingulate cortex, ACC), and executive control (inferior frontal cortex, IFC). BDs exhibited enhanced connectivity between the striatal reward areas and the orbitofrontal cortex and the ACC, which is consistent with AUD studies and may be indicative of alcohol-motivated appetitive behaviors. Conversely, BDs demonstrated lower connectivity between the IFC and hippocampus which was associated with higher craving. This may indicate impaired ability to suppress unwanted thoughts and a failure to employ memory of the harmful consequences of heavy drinking in prospective plans and intentions. The observed greater connectivity of the reward/salience network and the lower prefrontal-hippocampal connectivity were associated with hazardous drinking levels indicating that dysregulation of neurofunctional networks may underlie binge drinking patterns.
Keywords: Binge drinking, resting state functional connectivity, reward, salience, control networks, nucleus accumbens, hippocampus, medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex
Introduction
Effective cognitive processing requires efficient communication among different regions in the brain. Neuroimaging techniques such as resting state functional connectivity (RSFC) provide insight into how these regions interact at the level of dynamically coherent neurofunctional systems (Bullmore and Sporns 2009). RSFC analysis is based on correlations of the time series of blood-oxygen-level dependent (BOLD) activity recorded from different brain areas while the subject is at rest. Similarities in brain activity patterns are assumed to underlie functional connectivity between regions comprised in different networks (Prohovnik et al. 1980; Biswal et al. 1995; Greicius et al. 2003). Spontaneous fluctuations in the RSFC BOLD signal are not confounded with task-induced activity related to attention or cognitive demands, rendering the RSFC suitable for investigating the neural assemblies that form intrinsic functional networks (Smith et al. 2009). Large-scale studies indicate that spontaneous activity is reflected in largely replicable networks (Deco et al. 2011; Smith et al. 2009). Furthermore, spatial connectivity maps and network coherence have been associated with variability in behavior (Fox and Raichle 2007), making it possible to examine the disruptions of neural systems associated with different disorders including addiction (Sutherland et al. 2012).
It has been well established that repeated cycles of intoxication and withdrawal can dysregulate the neurofunctional networks necessary for effective cognitive processing (Volkow et al. 2004; Oscar-Berman 2012; Loeber et al. 2009a). Neurobiologically driven models of addiction suggest the shift from impulsive to compulsive drug consumption is mediated by enhanced saliency of drug-related rewards and a simultaneous lowering of a top-down executive control network (Baler and Volkow 2006; Goldstein and Volkow 2011; Volkow et al. 2004; Garavan and Weierstall 2012). This extensive evidence implicates dysregulation of frontal, striatal, and limbic circuitry that underlies progression from recreational to compulsive drug use (Everitt and Robbins 2016; Feil et al. 2010; Haber and Knutson 2010; Koob and Volkow 2016; Van den Oever et al. 2010).
Alcohol use disorder (AUD) is characterized by excessive and compulsive alcohol use, craving and loss of control over alcohol intake, and dysphoric affect when sober, contributing to relapse (Koob 2000; Loeber et al. 2009a; Loeber et al. 2009b). Consistent with neurobiological accounts of addiction, imaging studies indicate that these attributes may be mediated by the disruption of several functional networks including the reward, salience, and the executive control networks (Beck et al. 2012; Muller-Oehring et al. 2015; Kim et al. 2017; Zhu et al. 2017; Sullivan et al. 2013; Camchong et al. 2013a; Oscar-Berman 2012). The reward network plays a key role in the development of addiction by mediating reinforcement of the hedonic effects experienced in the binge/intoxication stage (Baler and Volkow 2006; Goldstein and Volkow 2011). Imaging studies indicate the striatum, as part of the reward circuitry, is affected by chronic alcohol consumption (Schulte et al. 2010; Makris et al. 2008; Muller-Oehring et al. 2015). Alcohol elevates dopamine levels in the nucleus accumbens (NAcc) in the ventral striatum which plays an essential role in the rewarding effects of alcohol and the development of addiction (Engel and Jerlhag 2014; Soderpalm et al. 2009). Indeed, mesolimbic regions have demonstrated enhanced activation in response to alcohol-related cues (Grusser et al. 2004; Myrick et al. 2004; Wrase et al. 2007), while RSFC studies indicate dysregulated communication between regions within the reward network (Muller-Oehring et al. 2015; J. Wang et al. 2016; Fein et al. 2017). Tightly coupled with rewarding behavior is the motivation for completing and reengaging in such behaviors which is mediated by the salience attributed to appetitive cues. The process of detection and evaluation of salient stimuli is dependent on a number of brain regions including the insula, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), amygdala, and thalamus (Menon and Uddin 2010), which have been shown to be dysfunctional in AUD (Schoenbaum et al. 2006; Ivanov et al. 2012; Tremblay and Schultz 1999; Harsay et al. 2012; Taylor et al. 2009). Individuals with AUD have displayed greater OFC and ACC activation in response to alcohol-related cues which was associated with higher alcohol craving and relapse (Grusser et al. 2004; Myrick et al. 2004; Tapert et al. 2004; Oberlin et al. 2016).
Decreased executive control has been heavily implicated in the development and maintenance of AUD (Brion et al. 2017; Le Berre et al. 2017; Muller-Oehring and Schulte 2014; Naim-Feil et al. 2014; Oscar-Berman et al. 2014; Oscar-Berman and Marinkovic 2007) as impaired executive control limits the ability to inhibit alcohol consumption (Crews and Boettiger 2009; Field et al. 2010; Fillmore 2003; Garavan and Weierstall 2012). Neuroimaging studies indicate that regions subserving executive control with primary contributions from lateral and medial prefrontal cortices including the cingulate cortex, are compromised in AUD (Sullivan and Pfefferbaum 2005; Wilcox et al. 2014). Recent studies have focused on changes at the level of networks subserving executive functions and have demonstrated lower connectivity between the lateral prefrontal and medial prefrontal cortices (Muller-Oehring et al. 2015; Kim et al. 2017) but greater connectivity of these regions with the reward network including ventral tegmental area, caudate nucleus, and NAcc (Camchong et al. 2013a; Fein et al. 2017; Kohno et al. 2017; Zhu et al. 2017). Furthermore, studies of acute alcohol challenge point to the medial prefrontal cortex, especially the ACC, as being particularly sensitive to alcohol during cognitive control tasks with implications for self-control (Kovacevic et al. 2012; Marinkovic et al. 2012; Marinkovic et al. 2013; Rosen et al. 2016).
Patterns of alcohol consumption and alcohol-related risk behaviors associated with alcohol use disorder (AUD) derive from a complex interplay of sociocultural, psychological, and heritable dimensions whose influence varies over the lifespan (Begleiter 1991; Brown and Tapert 2004; Finn 2002; Fromme et al. 2004; Schuckit 2000; K.J. Sher et al. 2014). Binge drinking is characterized by the intake of large quantities of alcohol, typically five or more drinks for men and four or more drinks for women within a two-hour time frame, bringing the blood alcohol concentration to approximately 0.08 g/dL (NIAAA 2004) and alternations between periods of consumption and withdrawal (Courtney and Polich 2009; Koob 2013a; Naimi et al. 2010). However, a substantial proportion of drinkers significantly exceed these levels of consumption, imbibing alcohol at much higher levels (Linden-Carmichael et al. 2017; Terry-McElrath and Patrick 2016). Prevalence rates peak in the early 20-ies (Naimi et al. 2010; Patrick et al. 2019) and then subsequently decline as young adults mature out of binge drinking and assume adult responsibilities(Lee and Sher 2018; Patrick et al. 2019; K.J. Sher et al. 2014). However, a subset of heavy drinkers continue drinking at similar or enhanced levels(Witkiewitz et al. 2014). The precipitating factors surrounding the transition from impulsive to compulsive drinking are not well understood but longitudinal studies have reported that binge drinking during the college years is a significant predictor of AUD after a 10-yr period (Jennison 2004; O’Neill et al. 2001). With binge drinking being especially prevalent among young and emerging adults (Johnston et al. 2018), the potential neurotoxic effects (Jacobus and Tapert 2013; Vetreno and Crews 2015), greater risk of developing AUD, and other negative health effects (Colby et al. 2004; Hingson et al. 2017; Wells et al. 2004) are a serious public health issue. Despite these growing concerns, evidence on the functional connectivity in young individuals engaging in binge drinking is exceedingly scant. In a study comprising a large number individuals whose drinking patterns ranged from binge drinking to severe AUD, the connectivity of the executive network was negatively correlated with AUD severity (Weiland et al. 2014). It is not clear, however, whether the connectivity patterns in binge drinkers resemble those reported in individuals with AUD.
Here we examine the association between binge drinking patterns and functional connectivity during wakeful rest in young, healthy individuals. Given the extensive evidence suggesting that long-term alcohol use induces alterations in the reward, salience and executive control networks, we performed a connectivity analysis by applying “seed” regions of interest in the areas that have been implicated in the reward and top-down regulation in previous reports on AUD (Muller-Oehring et al. 2015; Zhu et al. 2015; Camchong et al. 2013b; Jansen et al. 2015; Kohno et al. 2017; Volkow and Baler 2013). These seeds included the striatum (NAcc and caudate nucleus) for the reward network, the ACC for the salience network, and the IFC for the executive control network. RSFC investigations may reveal dysfunctional networks associated with binge drinking and may provide insight into a potential transitional stage in the development of AUD. In the absence of similar studies in binge drinkers, we based our hypotheses on previous reports in AUD. We expected that, in comparison to LDs, the BDs would show greater connectivity between the caudate nucleus and the ACC (Muller-Oehring et al. 2015) and between the NAcc and the OFC (Zhu et al. 2015), as well as greater connectivity of the IFC with prefrontal regions (Zhu et al. 2015), especially in the context of the compensatory activity in the IFC that we observed in BDs during cognitive challenge (Molnar et al. 2018).
Materials and Methods
Participants
Participants in this study were thirty five young, healthy adults who were 24.5 ± 3.8 yrs old, age range: 18 – 30, 19 females. They were all right-handed and reported no illicit drug or tobacco use for at least one month prior to the study. They had no history of seizures, brain injury, neurological or neuropsychiatric disorders, no vision or hearing problems or learning difficulties, and were medication-free at the time of the study. During screening, they provided extensive information about their current and recent drinking including alcohol consumption rate, frequency, and pattern of intake and were assigned into binge drinking (BD, N = 18) and light drinking (LD, N =17). A binge episode was defined as consuming at least 6 drinks for males (5 for females) within a two hour time span. This criterion was based on empirical evidence indicating that young adults are more likely to reach BAC of 0.08% or above at this level of intake (Lange and Voas 2001). The BD group reported at least five binge episodes in the past six months whereas LDs reported no more than one binge episode in the past six months (see Table 1 1 for details on group characteristics). The two groups were matched on age, gender, education, ethnicity/race, and family history of alcoholism. While the BD group reported higher levels of drinking and scored higher on all alcohol-related variables, no group differences were found on dispositional variables and personality dimensions except for greater impulsivity-related traits reported by BD. They had higher scores on disinhibition and boredom as sensation seeking (Stephenson et al. 2002), and tended to have higher motivation measures (Coutlee et al. 2014).
Table 1.
Participant characteristics for the BD and LD groups.
| BD (n=18) | LD (n=17) | Stat. Value | p< | |
|---|---|---|---|---|
| % Female | 64.3% | 50.0% | .62 | .43a |
| Age | 23.3 ± 3.1 | 25.6 ± 4.2 | 103 | .10 |
| Family History Positive for Alcoholism | 50.0% | 56.3% | .12 | .73a |
| Education years | 15.3 ± 1.8 | 15.8 ± 2.2 | 117 | .48 |
| Undergraduate GPA | 3.3 ± 0.4 | 3.6 ± 0.3 | 72.5 | .03 |
| drinking days/wk | 2.7 ± 1.1 | 1.5 ±1.0 | 63.5 | .003 |
| drinks/occasion | 5.3 ± 2.5 | 2.3 ± 1.2 | 41 | < .001 |
| binge episodes in past 6 mos | 14.9 ± 14.0 | 0.4 ± 0.7 | .000 | < .001 |
| alcohol-induced blackouts in 6 mos | 3.2 ± 2.7 | 0.2 ± 0.5 | 35 | < .001 |
| Max no. of drinks in 24 hrs/past 6 mos | 12.6 ± 8.9 | 3.6 ± 2.0 | 12.5 | < .001 |
| Age Onset of alcohol use | 16.2 ± 1.8 | 18.4 ± 2.0 | 58.5 | .005 |
| AUD symptom severity (SMAST) | 2.4 ± 2.3 | 0.8 ± 1.0 | 76 | .009 |
| AUD Identification Test (AUDIT) | 13.7 ± 5.9 | 4.1 ± 1.5 | 5.5 | < .001 |
| Drinking Motivation (DMQ-R SF) | ||||
| Enhancement | 2.3 ± 0.3 | 1.7 ± 0.4 | 36.5 | < .001 |
| Social | 2.7 ± 0.4 | 2.0 ± 0.4 | 39 | <.001 |
| Conformity | 1.5 ± 0.6 | 1.4 ± 0.5 | 129 | .57 |
| Coping | 1.7 ± 0.5 | 1.2 ± 0.3 | 51.5 | <.001 |
| Drinking consequences (B-YAACQ) | 9.6 ± 6.5 | 2.7 ± 3.1 | 49.5 | .001 |
| Alcohol Craving (PACS) | 8.1 ± 4.9 | 2.8 ± 2.7 | 51 | .001 |
| Anxiety (GAD-7) | 2.7 ± 2.6 | 3.0 ± 2.7 | 147 | .84 |
| Depression (PHQ-9) | 3.4 ± 3.2 | 3.1 ± 3.0 | 144.5 | .77 |
| ADHD Symptoms (ARSS) | 1.7 ± 1.6 | 1.0 ± 1.2 | 120.5 | .26 |
| Impulsivity (ABIS) | ||||
| Attention | 2.1 ± 0.5 | 1.9 ± 0.4 | 116.5 | .22 |
| Motor | 2.2 ± 0.8 | 1.8 ± 0.4 | 1081 | .13 |
| Non-Planning | 2.1 ± 0.6 | 1.8 ± 0.5 | 125 | .35 |
| Sensation Seeking (BSSS) | ||||
| Experience | 4.3 ± 0.8 | 4.0 ± 0.9 | 114 | .27 |
| Boredom | 4.1 ± 0.8 | 3.6± 0.7 | 81.5 | .02 |
| Thrill | 3.6 ± 1.1 | 3.6 ± 1.2 | 138 | .82 |
| Disinhibition | 3.8 ± 0.7 | 3 ± 1.1 | 82 | .03 |
| Eysenck Personality Quest. (EPQ) | ||||
| Neuroticism | 3.7 ± 2.8 | 3.5 ± 3.8 | 126.5 | .53 |
| Psychoticism | 2.5 ± 2.1 | 2.4 ±1.4 | 141 | .91 |
| Extraversion | 9.4 ± 2.6 | 7.9 ± 3.55 | 109.5 | .22 |
| WASI-II intelligence scale (percentile) | 68.3 ± 21.4 | 76.6 ± 19.4 | 110 | .24 |
Included are group characteristics (Mean ± SD or %) for BD and LD groups.
Tested with Chi-Square; all other comparisons performed with the Mann-Whitney U test.
Participants completed an extensive battery that probed their alcohol use including alcohol abuse problem behaviors (Alcohol Use Disorder Identification Test, AUDIT, (Saunders et al. 1993)), alcohol misuse (Short Michigan Alcoholism Screening Test, SMAST, (Selzer et al. 1975)); alcohol cravings (The Penn Alcohol Craving Scale, PACS, (Flannery et al. 1999)), motivational influences on their drinking patterns (Drinking Motive Questionnaire Revised Short Form, DMQ-R SF, (Kuntsche and Kuntsche 2009)), the severity of alcohol consequences (Brief Young Adult Alcohol Consequences Questionnaire, B-YAACQ, (Kahler et al. 2005)). Dispositional traits were assessed with respect to depressive symptomology (Patient Health Questionnaire, PHQ-9, (Kroenke et al. 2002)), anxiety (Generalized Anxiety Disorder, GAD7, (Spitzer et al. 2006)), impulsivity (Abbreviated Impulsiveness Scale, ABIS (Coutlee et al. 2014)), attention deficit and hyperactivity symptomology (Adult ADHD Self-Report Scale, ASRS, (Kessler et al. 2005)), and risk-taking behavior (Brief Measure of Sensation Seeking Scale, BSSS, (Stephenson et al. 2002)). Personality traits including neuroticism, psychoticism, and extraversion were assessed with Eysenck Personality Questionnaire (EPQ, Eysenck reference). Intelligence was assessed with Wechsler Abbreviated Scale of Intelligence (Wechsler 2011)). A modified version of the Family History Assessment Module (FHAM, Rice) was used to evaluate family history for alcoholism (FH+). Participants, who reported at least one first-degree and one first- or second-degree relative, or at least three second-degree relatives diagnosed with AUD, were classified as FH+. On the day of the scan all subjects were screened for illicit substances via urinary analysis and women were additionally tested for pregnancy and all tests were negative. Informed consent was obtained from all individuals participants included in the study.
Imaging data acquisition
MRI scans were acquired with a GE Discovery MR750 3 T scanner (General Electric) equipped with an Invivo HD 8 channel high resolution head coil. A 6 minute resting-state echo-planar imaging (EPI) scan was acquired for each participant with 35axial slices; slice thickness = 4 mm; TR = 1800 ms; TE = 30 ms; flip angle 70°; matrix 64 × 64; FOV 24 cm; 200 volumes. Participants were instructed to rest quietly with their eyes open while focusing on a fixation point on a screen. High resolution IR-Prepped 3 FSPGR T1-weighted anatomical images with 166 contiguous axial 1.2 mm thick slices were also acquired (TR = 7.38 ms; TE = 2.984 ms; flip angle 8°; matrix 256 × 192; FOV 24 cm).
Resting-state functional connectivity analyses
Functional connectivity analysis was carried out using the CONN-fMRI Functional Connectivity toolbox v17 (Whitfield-Gabrieli and Nieto-Castanon 2012). Data were pre-processed using CONN’s default MNI pipeline which includes the typical steps used in a functional activation analysis: slice-timing correction, realignment, co-registration, normalization, and spatial smoothing. Additionally, white matter and CSF masks were created and Principal Component Analysis (PCA) was applied to the BOLD time series to estimate noise within these masks. These components and motion parameters were then regressed from the BOLD time series across all voxels. The residual time series were band-pass filtered within a frequency window of 0.008 to 0.09 Hz.(Whitfield-Gabrieli and Nieto-Castanon 2012).
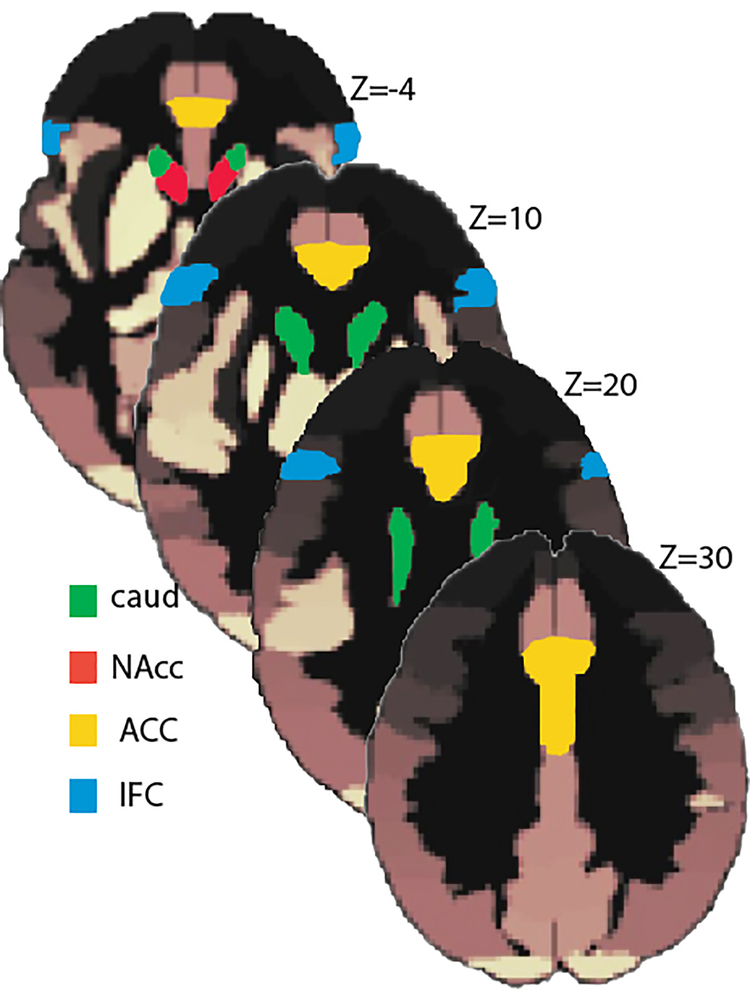
To analyze the RSFC data, seed-to-voxel functional connectivity maps were created for each participant. The same a priori regions-of-interest (ROIs) were chosen as seeds of interest for all subjects based on their theoretical relevance from a set of default pre-defined areas provided by CONN-fMRI Functional Connectivity toolbox. They comprised bilateral regions in the reward network including the ventral striatum (nucleus accumbens, NAcc) and the dorsal striatum (caudate nucleus). The salience network was represented by the anterior cingulate cortex (ACC), and the executive control network was represented by the lateral inferior frontal cortices (IFC) (Fig. 1).
Fig. 1.
Seed regions shown in slices along the z-axis are based on the atlas used by CONN-fMRI Functional Connectivity toolbox
The mean BOLD time series was computed across all voxels within each ROI. The seed-to-voxel analysis computes the correlation between these average time series and the BOLD time series for all other voxels in the brain. Bivariate-correlation analyses were used to determine the linear association of the BOLD time series between each pair of sources and a Fisher Z transformation was applied. Individual seed-to-voxel maps were entered into a group-level analysis.
A peak voxel threshold of p ≤ 0.001 and a cluster extent threshold of p ≤ 0.05 were set for bidirectional (i.e. positive and negative) explorations of connectivity associations. Results were considered significant if they survived correction for multiple comparisons with Family-Wise Error (FWE) p ≤ 0.05. Functional Connectivity values (mean z-scores) for significant clusters were extracted using the REX toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012). There were no significant correlations between head motion and the functional connectivity indices.
A limitation of this method is that the direction of the connectivity findings cannot be determined. The RSFC analysis is based on correlations and is, therefore, not sensitive to the direction, causality, or temporal relationships between the BOLD time series. In order to infer directionality, connectivity methods based on Granger causality or Dynamic Casual Modelling will have to be explored in future studies.
Statistical analysis
SPSS 24 was used for all statistical analyses (IBM Corp., Armonk, NY, USA). Nonparametric (Spearman’s rho) correlations were conducted to characterize the relationship between individuals’ connectivity values (i.e., seed-to-cluster) and variables related to alcohol use and dispositional traits. Correction for multiple correlations was achieved with a false discovery rate approach relying on Benjamini-Hochberg procedure (Hochberg and Benjamini 1990).
Results
Participant characteristics(Table 1)
BD and LD groups were matched on age, education, FH+, and intelligence. As expected, the BD group reported higher levels of drinking and had higher scores on all alcohol-related variables. There were no group differences on the measures of anxiety, depression, attention deficit disorder, and personality including psychoticism, neuroticism, extraversion, and social desirability. However, BD had higher scores on measures of sensation seeking including disinhibition and boredom. BD also had lower undergraduate grade point average (GPA).
Seed-to-voxel analysis
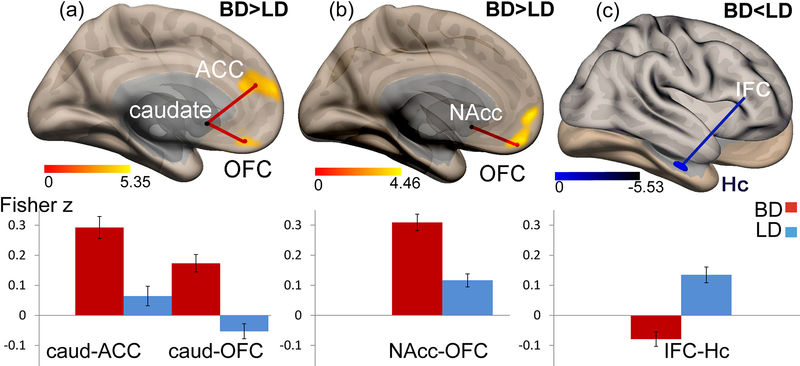
BD and LD groups showed different functional connectivity patterns in three ROIs used as seeds in the seed-to-voxel analysis (Fig 2, Table 2). In line with our expectations, the BDs demonstrated greater functional connectivity relative to LDs for two ROIs. More specifically, BD participants had significantly higher connectivity between the left caudate and the left OFC and bilateral ACC (Fig. 2a), and between the right NAcc and the bilateral OFC (Fig. 2b). In contrast, BD participants had lower connectivity than LD subjects between the right IFC and the left hippocampus (Hc) (Fig 2c). There were no main effects of gender or group × gender interactions in any of the analyses so the factor of gender was omitted from subsequent analyses.
Fig. 2.
Seed-to-voxel resting state connectivity maps (upper panel) and the corresponding bar graphs of connectivity values (lower panel). Compared to LD, BD participants show (A) higher connectivity between the left caudate (seed region) and the clusters in the OFC and ACC; (B) higher connectivity between the right NAcc (seed region) and the clusters in the OFC; (C) lower connectivity between the right IFC (seed region) and the clusters in the hippocampus (Hc)
Table 2.
Seed regions with Functional Connectivity parameters in BD vs LD.
| Cluster regions |
Voxels In region |
BD conn. mean(SD) |
LD conn. mean(SD) |
cluster-size p-FWE corr. |
|
|---|---|---|---|---|---|
|
L-Caudate ↕ |
L-OFC | 137 | .1 (.14) | −.031 (.08) | < .001 |
| L-FP | 63 | ||||
| Cluster coord | MedFC | 20 | |||
| −18, 26, −26 | SubCC | 13 | |||
| L-PCG | 3 | ||||
| ACC | 124 | .29 (.15) | .06 (.1 3) | < .009 | |
| L-FP | 36 | ||||
| Cluster coord | L-SFG | 26 | |||
| −06, 58,18 | L-PCG | 18 | |||
|
R-NAcc ↕ |
OFC | 113 | .31 (.12) | .12 (.09) | < .008 |
| L-PCG | 73 | ||||
| Cluster coord - | R-PCG | 24 | |||
| −12, 52, 0 | L_FP | 16 | |||
| R_FP | 33 | ||||
|
R-IFC ↕ |
L-Hc | 73 | −.08 (.1) | 13 (.1) | < .05 |
| L-TFC | 72 | ||||
| Cluster coord | L-PHc | 18 | |||
| −36, −18, −24 |
Seed regions are listed individually in the first column along with peak-voxel coordinates of the clusters exhibiting significant connectivity with each seed. For each seed region, clusters are then listed together with their number of voxels, the averaged connectivity values for each group and FWE-corrected cluster-level p-values.
NAcc: nucleus accumbens, OFC: orbitofrontal ctx, FP: frontal pole, MedFC: medial frontal ctx, PCG: paracingulate gyrus, SFG: superior frontal gyrus, SubCC: subcallosal ctx, TFC: temporal fusiform ctx, PHc: parahippocampal ctx, Hc: hippocampus.
Correlation analysis
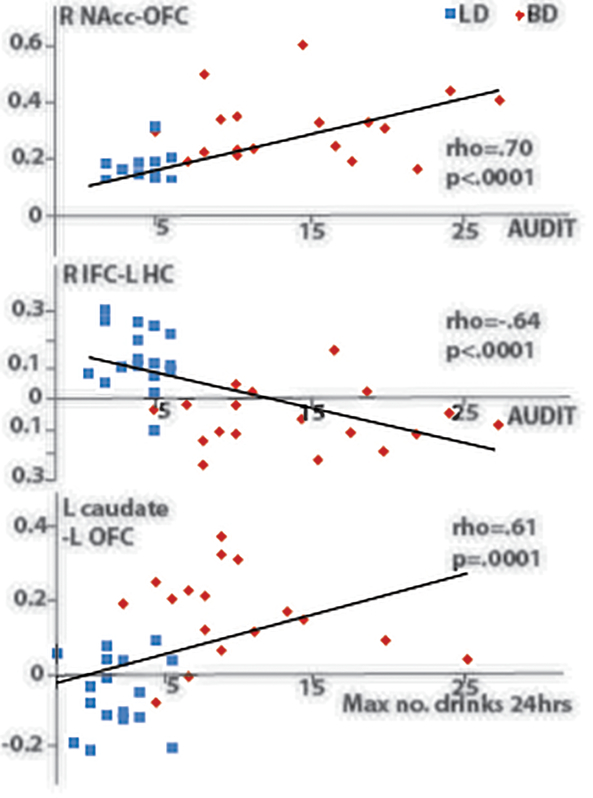
Nonparametric Spearman’s rho correlations were computed to examine the relationship between functional connectivity patterns and alcohol-related and personality variables (Table 3). Multiple correlations were corrected with the FDR-based Benjamini-Hochberg procedure. Across all subjects, greater connectivity values between the striatal seeds and the medial prefrontal cortex, and lower connectivity between the right IFC and the hippocampus were associated with a range of alcohol-related variables with an emphasis on high-intensity drinking including AUDIT scores, the number of binge episodes in the past 6 months, the maximum number of drinks consumed in 24 hrs, alcohol-related harmful consequences, etc. A subset of the representative correlations is shown in Fig 3. In contrast, no significant correlations were observed between any of the connectivity values and personality, disposition, or mood indices.
Table 3.
Spearman’s rho correlations between RSCF and alcohol-related and personality variables
| Alcohol-related variables | L caud-LOFC | L caud-ACC | R NAcc-OFC | R IFC-L HC |
|---|---|---|---|---|
| rho | rho | rho | rho | |
| no. drinks/occasion | .52* | .42 | .58* | −.34 |
| binge episodes in 6 mos | .65* | .55* | .75* | −.68* |
| no. of blackouts in 6 mos | .40 | .37 | .51* | −.56* |
| max no. drinks in 24 hrs/6 mos | .61* | .53* | .65* | −.47 |
| AUD symptoms (SMAST) | .30 | .07 | .31 | −.30 |
| AUD Identif. Test (AUDIT) | .52* | .43 | .70* | −.64* |
| Drinking Consequences | .3 | .24 | .46 | −.63* |
| Alcohol craving (PACS) | .39 | .32 | .51* | −.51* |
| Drinking Motivation (DMQ-R) | .33 | .31 | .41 | −.47 |
| Personality/mood measures | ||||
| Impulsivity (ABIS) | .015 | −.02 | .23 | −.39 |
| Sensation seeking (BSSS) | .13 | .19 | .28 | −.16 |
| Anxiety (GAD-7) | .20 | .02 | −.007 | .001 |
| Depression (PHQ-9) | .19 | .12 | .17 | −.21 |
| Attn. deficit (ASRS) | .25 | .08 | .35 | −.35 |
| Neuroticism (EPQ) | .011 | −.13 | −.014 | −.19 |
| Psychoticism (EPQ) | .014 | −.13 | .12 | .17 |
| Extraversion (EPQ) | .15 | .24 | .23 | −.21 |
SMAST: Short Michigan Alcoholism Screening Test, AUDIT: Alcohol Use Disorder Identification Test, B-YAACQ: Brief Young Adult Alcohol Consequences Questionnaire, PACS: Penn Alcohol Craving Scale, DMQ-R: Drinking Motive Questionnaire Revised, ABIS: Abbreviated Impulsiveness Scale, BSSS: Brief Measure of Sensation Seeking Scale, GAD7: Generalized Anxiety Disorder, PHQ-9: Patient Health Questionnaire, ASRS: Adult ADHD Self-Report Scale. EPQ: Eysenck Personality Questionnaire
Spearman’s rho coefficients are marked with * and written in boldface font if they survived FDR-based Benjamini-Hochberg correction for multiple correlations
Fig. 3.
Scatter plots of the representative correlations between connectivity indices and drinking variables. Red and blue colors signify BD and LD respectively..
Discussion
This study examined functional connectivity during wakeful rest in young individuals as a function of their binge drinking patterns. Of particular interest were the reward, salience, and executive networks which have been implicated in the development and maintenance of AUD. Group comparisons indicate that: (1) BDs showed greater connectivity of the reward network regions (NAcc and the caudate nucleus) with the salience network regions (ACC, OFC) which was positively correlated with AUDIT scores and other variables sensitive to high-intensity drinking; (2) BDs were characterized by lower connectivity of the IFC with the hippocampus which was negatively correlated with AUDIT and other alcohol-related variables. These group differences in RSFC were observed in the absence of differences in intelligence scores, personality traits, and self-reported negative emotional states including depression and anxiety. However, BD participants tended to report higher disinhibition and boredom susceptibility which has been implicated in dysregulated control over alcohol intake (Kuntsche et al. 2006; Leeman et al. 2012; K. J. Sher and Trull 1994; Stautz and Cooper 2013).
Enhanced RSFC between the subcortical reward regions and the prefrontal salience areas in BD
BDs exhibited greater connectivity between the subcortical reward areas (NAcc and the ventral caudate nucleus) and the OFC and ACC in the medial prefrontal cortex in line with similar reports in AUD studies (Jansen et al. 2015; Kohno et al. 2017; Zhu et al. 2015). Furthermore, Muller-Oehring and colleagues (Muller-Oehring et al. 2015) observed expanded connectivity of the reward network with medial prefrontal regions and a lower within-network connectivity in AUD group. Overall, these results are aligned with a well-established role of the ventral striatum in the mesocorticolimbic network as it subserves alcohol’s rewarding effects (Bjork et al. 2010; Bjork et al. 2008; Haber and Behrens 2014; Jia et al. 2011). Pleasure-inducing, reinforcing effects of alcohol are mediated by dopaminergic pathways from the NAcc which is associated with drug wanting (Robinson and Berridge 1993). Because initiation and development of addiction depend on dysregulation of reward circuitry (Everitt and Robbins 2005; Koob and Volkow 2010), the ventral striatum is considered a key structure in addiction (Koob and Le Moal 2005; Pierce and Kumaresan 2006). Results of the present study are consistent with the prevalent accounts indicating that binge drinking reflects a gradual process as impulsive drinking shifts towards compulsive intake (Enoch 2006; Kimbrough et al. 2017; Koob 2013b; Koob and Le Moal 2008). Indeed, the observed greater connectivity between the ventral striatum and the OFC correlates with daily alcohol intake, binge episodes, and high-level drinking (Table 3). The appetitive dimension has been confirmed in functional imaging studies showing greater activity of the striatum to alcohol-related cues in young heavy drinkers (Ihssen et al. 2011; Vollstadt-Klein et al. 2010). Similar activation has been observed in individuals diagnosed with AUD (Grusser et al. 2004; Myrick et al. 2004). Furthermore, detoxified alcoholics have shown less activation of the ventral striatum to monetary gain but greater activation alcohol-related cues which correlates with craving (Wrase et al. 2007).
Extensive evidence indicates that the ventral striatum is closely coupled with the medial prefrontal cortex including the OFC and ACC which are implicated in the salience valuation and perception of reward (Haber 2011; Rolls 1996, 2004). The mesocortico-ventral striatal circuitry is involved in learning reward associations and in making behavioral choices (O’Doherty et al. 2003; Schultz et al. 2000; Sesack and Grace 2010; Tobler et al. 2006; Rolls 2004). At the neuroanatomical level, the NAcc receives dopaminergic projections from the ventral tegmentum as well as glutamatergic projections from the frontal cortex including the orbitofrontal area (Humphries and Prescott 2010). Both the NAcc and dorsal striatum have reciprocal connections with the medial prefrontal cortex (Haber 2016; Jarbo and Verstynen 2015) which is implicated in reinforcement learning and integrating reward, salience valuation, and learning reward contingencies (Haber and Knutson 2010; Jarbo and Verstynen 2015; Rolls 2004). Imaging studies have reported greater OFC and ACC activation in response to alcohol-related cues in AUD individuals which correlated with higher craving and relapse (Grusser et al. 2004; Myrick et al. 2004; Tapert et al. 2004). Similarly, greater striatal activation was associated with greater craving in heavy drinkers (Vollstadt-Klein et al. 2010). Using a multimodal approach which combined fMRI and PET imaging in heavy drinkers, Oberlin and colleagues have shown that alcohol flavor cues enhanced fMRI activation in the OFC and the right ventral striatum which was associated with craving for alcohol. Furthermore, dopamine release in the NAcc was induced by presenting alcohol flavor cues (Oberlin et al. 2016; Oberlin et al. 2015). Greater connectivity between the ventral striatum and the OFC and ACC observed in the present study in binge drinkers is in agreement with studies in AUD, indicating the importance of the reward/salience network in appetitive behavior as a result of alcohol intake. This connectivity rise is associated with greater alcohol-seeking behaviors such as the number of binge episodes, high-intensity drinking, and AUDIT scores. These data suggest the enhanced saliency of alcohol-related rewards may mediate greater alcohol-seeking behavior as drinking shifts from impulsive to compulsive. Other studies investigating different types of addiction such as pathological gambling (Camara et al. 2008) and substance abuse (Ma et al. 2010; Upadhyay et al. 2010; Y. Wang et al. 2013; Wilcox et al. 2011) have confirmed greater connectivity of the reward/salience network. Even though in the present study it was the ventral aspect of the caudate nucleus that primarily contributed to the greater connectivity with the OFC and ACC, the dorsal striatum is highly functionally connected with the NAcc as they are both involved in contingency and habit learning, and reward processing (Haber 2011; Voorn et al. 2004; Wise 2009). Repeated bouts of heavy drinking reinforce associations with alcohol-seeking behavioral patterns (Tomasi and Volkow 2013), with a shift towards engagement of the dorsal striatum. Habit formation such as engaging in drinking on a regular basis, and goal-directed actions including alcohol seeking and consuming, depend on the dorsal striatum (Balleine et al. 2009; Gremel et al. 2016). Indeed, the fronto-striatal connections are supported by the dopaminergic system (Bromberg-Martin et al. 2010) and are enhanced by alcohol (Everitt and Robbins 2016). The present results confirm that the reward-salience networks are altered in young individuals engaging in bouts of binge drinking in ways that are similar to those in AUD individuals. This observation is consistent with the importance of alcohol-motivated appetitive behaviors in young binge drinkers and could serve as a marker of a transitional stage in a cyclic process leading towards compulsive intake (Koob and Le Moal 2008).
Reduced prefrontal-hippocampal functional connectivity in BD
Previous studies in AUD cohorts have reported stronger resting connectivity within primarily frontal networks presumed to underlie executive control (Camchong et al. 2013a; Jansen et al. 2015; Kohno et al. 2017; Zhu et al. 2015). In comparison to short-term abstinent alcoholics, the resting connectivity of the executive network was especially greater in individuals after long-term abstinence (Camchong et al. 2013a), lending support to a compensatory interpretation of these changes (Chanraud and Sullivan 2014). In that view, strengthening of the executive network serves an adaptive purpose of enhanced recruitment of cognitive control in support of maintaining abstinence (Camchong et al. 2013b; Jansen et al. 2015; Kohno et al. 2017). However, results are inconsistent as other studies reported lower connectivity in the executive network (Kim et al. 2017; Muller-Oehring et al. 2015; Weiland et al. 2014) which is interpreted as lower cognitive control and gretaer likelihood of relapse. In contrast to our expectations, binge drinking patterns in the present study were not associated with altered connectivity of the prefrontal seeds comprising the anterior cingulate cortex, and the left and right inferior frontal cortices. Despite the greater appetitive drive in BD, these results suggest that the frontally-mediated cognitive control is not altered sufficiently to promote lapsing into AUD pattern.
However, compared to light drinkers, BDs exhibited lower connectivity between the right IFC (rIFC) and the hippocampus. It has been well established that the rIFC is an essential node in the cognitive control network with key contributions to response suppression and attentional control (Aron et al. 2014; Hampshire et al. 2010; Levy and Wagner 2011; Munakata et al. 2011; Wessel et al. 2016). A recent study reported lower activity and oscillatory synchrony in rIFC during attentional control in young binge drinkers which correlated with drinking levels (Correas et al. 2018). The hippocampus plays a critical role in memory encoding and retrieval (Eichenbaum and Fortin 2005; Moscovitch et al. 2006; Suzuki 2006) and its connections with the prefrontal cortex underlie a range of memory processes (Barker et al. 2017; Eichenbaum 2017). Prefrontal-hippocampal connectivity and its role in inhibitory control over memory has been examined with functional imaging. In a recent study, a decrease of hippocampal activity observed on “No Think” trials is consistent with memory suppression mediated by well described prefrontal-hippocampal pathways traced in non-human primates (Anderson et al. 2016). Greater activation of the right frontal cortex is associated with downregulation of the hippocampus during inhibition of memory retrieval (Depue et al. 2016; Marinkovic et al. 2009). Numerous studies have reported greater activation in the rIFC during active memory suppression (Benoit et al. 2015; Depue 2012; Depue et al. 2016; Levy and Anderson 2008; Mitchell et al. 2007). The prefrontal-hippocampal interplay is important for cognitive and emotional functions and its dysregulation has been proposed to underlie a range of psychiatric disorders (Godsil et al. 2013) including addiction. In a study of cocaine users, lower right prefrontal activity was associated with the inability to suppress interference by drug-related stimuli (Hester and Garavan 2009). Similarly, in a task investigating the inhibition of memory retrieval, alcoholics were impaired in their ability to suppress unwanted thoughts (Nemeth et al. 2014). In the present study, the prefrontal-hippocampal connectivity was lower among BDs which was associated with higher craving scores, r = −.51, p < .002, suggesting that BDs may be impaired in their ability to suppress intrusive thoughts and memories, potentially related to alcohol craving. These results are consistent with a mechanistic account of GABA-mediated suppression of hippocampal activity. In a study that combined fMRI during a thought suppression task and proton magnetic resonance spectroscopy, lower resting concentration of hippocampal GABA was associated with lower suppression (Schmitz et al. 2017). It has been well established that heavy alcohol use results in neuroadaptive changes reflected in down-regulated GABAA receptors (Most et al. 2014; Roberto and Varodayan 2017). Lower hippocampal GABA underlies impaired prefrontal-hippocampal circuitry needed for thought suppression (Schmitz et al. 2017). Therefore, the inability to suppress unwanted, intrusive thoughts due, in part, to GABA downregulation may contribute to greater cravings in BDs.
Recent theories also propose that the hippocampus is involved in decision-making through the role of prospective memory, which enables the imagination of future outcomes (Johnson and Redish 2007; Kwan et al. 2012; Schacter et al. 2007). Indeed, memories are essential for planning adaptive, goal-directed behavior which is the essence of cognitive control. Therefore, lower connectivity between the IFC and hippocampus might also suggest impairments in prospective memory or the ability to employ memory in intended plans or actions in the context of adaptive constraints. In the current study, lower connectivity between the IFC and hippocampus was associated with more binge episodes, blackouts, AUDIT scores, habitual alcohol intake levels, and greater alcohol-related negative consequences. These data provide supporting evidence that an impaired ability to suppress intrusive thoughts or cravings together with a failure to employ prospective memory of the harmful consequences of heavy drinking may subserve the transition to more compulsive drinking. This is broadly consistent with a recent study of young binge drinkers that reported lower frontolimbic connectivity which mediated a relationship between impulsivity and rates of alcohol consumption (Crane et al. 2018).
In sum, we found enhanced connectivity between the reward areas in the striatum and the medial prefrontal cortex in young BDs which is consistent with similar observations in AUD cohorts and is indicative of the importance of alcohol-motivated appetitive behaviors. While we did not observe alterations in the connectivity of the prefrontal seeds within the executive network, BDs were characterized by lower prefrontal-hippocampal connectivity which was associated with higher craving. This may indicate impaired ability to suppress unwanted thoughts and a failure to employ memory of the harmful consequences of heavy drinking in prospective plans and intentions. Overall, connectivity indices correlated with a range of alcohol-related variables with sensitivity to high-intensity drinking which is suggestive of the vulnerability of neural networks to hazardous drinking levels. Even though connectivity values did not correlate with any measures of personality or mood, this study cannot address a possibility of preexisting vulnerability. Taken together, these results support neurobiologically based models of addiction suggesting that, in the context of heavy drinking, dysregulation of reward/salience and memory circuitry may mediate the shift from impulsive to compulsive alcohol consumption (Baler and Volkow 2006; Goldstein and Volkow 2011). Thus, the characteristics observed in BDs are suggestive of potential markers for the development of alcohol addiction and may have clinical implications for intervening before alcohol-seeking behavior becomes more severe.
Informed Consent.
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
This work was supported by start-up funds provided by the College of Sciences at San Diego State University, and the National Institute on Alcohol Abuse and Alcoholism (R01-AA016624 and T32 AA013525). We thank Lauren Beaton and Lee Holcomb for their assistance.
Funding:
The study was funded by start-up funds provided by the College of Sciences at San Diego State University, and the National Institute on Alcohol Abuse and Alcoholism (R01-AA016624 and T32 AA013525).
Footnotes
Disclosure of potential conflicts of interest:
The authors declare that they have no conflict of interest.
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
- Anderson MC, Bunce JG, & Barbas H (2016). Prefrontal-hippocampal pathways underlying inhibitory control over memory. Neurobiol Learn Mem, 134 Pt A, 145–161, doi: 10.1016/j.nlm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci, 18(4), 177–185, doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Baler RD, & Volkow ND (2006). Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med, 12(12), 559–566, doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, & Ostlund SB (2009). The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res, 199(1), 43–52, doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Barker GR, Banks PJ, Scott H, Ralph GS, Mitrophanous KA, Wong LF, et al. (2017). Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nat Neurosci, 20(2), 242–250, doi: 10.1038/nn.4472. [DOI] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, et al. (2012). Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry, 69(8), 842–852, doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Begleiter H (1991). Genetic disposition to alcoholism: Overview In Galanter M (Ed.), Recent Developments in Alcoholism: Children of Alcoholics (Vol. 9, pp. 3–4). New York: Plenum. [Google Scholar]
- Benoit RG, Hulbert JC, Huddleston E, & Anderson MC (2015). Adaptive top-down suppression of hippocampal activity and the purging of intrusive memories from consciousness. J Cogn Neurosci, 27(1), 96–111, doi: 10.1162/jocn_a_00696. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, & Hommer DW (2010). Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry, 51(7), 827–837, doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, & Hommer DW (2008). Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction, 103(8), 1308–1319, doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Brion M, D’Hondt F, Pitel AL, Lecomte B, Ferauge M, de Timary P, et al. (2017). Executive functions in alcohol-dependence: A theoretically grounded and integrative exploration. Drug Alcohol Depend, 177, 39–47, doi: 10.1016/j.drugalcdep.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, & Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron, 68(5), 815–834, doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, & Tapert SF (2004). Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann N Y Acad Sci, 1021, 234–244. [DOI] [PubMed] [Google Scholar]
- Bullmore E, & Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 10(3), 186–198, doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, & Munte TF (2008). Functional connectivity of reward processing in the brain. Front Hum Neurosci, 2, 19, doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, & Fein G (2013a). Resting-state synchrony in long-term abstinent alcoholics. Alcohol Clin Exp Res, 37(1), 75–85, doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, & Fein G (2013b). Resting state synchrony in long-term abstinent alcoholics with versus without comorbid drug dependence. Drug Alcohol Depend, 131(1–2), 56–65, doi: 10.1016/j.drugalcdep.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, & Sullivan EV (2014). Compensatory recruitment of neural resources in chronic alcoholism. Handb Clin Neurol, 125, 369–380, doi: 10.1016/B978-0-444-62619-6.00022-7. [DOI] [PubMed] [Google Scholar]
- Colby SM, Lee CS, Lewis-Esquerre J, Esposito-Smythers C, & Monti PM (2004). Adolescent alcohol misuse: methodological issues for enhancing treatment research. Addiction, 99 Suppl 2, 47–62, doi: 10.1111/j.1360-0443.2004.00854.x. [DOI] [PubMed] [Google Scholar]
- Correas A, Lopez-Caneda E, Beaton L, Rodriguez Holguin S, Garcia-Moreno LM, Anton-Toro LF, et al. (2018). Decreased event-related theta power and phase-synchrony in young binge drinkers during target detection: An anatomically-constrained MEG approach. J Psychopharmacol, 269881118805498, doi: 10.1177/0269881118805498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, & Polich J (2009). Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull, 135(1), 142–156, doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee CG, Politzer CS, Hoyle RH, & Huettel SA (2014). An Abbreviated Impulsiveness Scale (ABIS) Constructed through Confirmatory Factor Analysis of the BIS-11. Arch Sci Psychol, 2(1), 1–12, doi: 10.1037/arc0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, & Boettiger CA (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav, 93(3), 237–247, doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, & McIntosh AR (2011). Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci, 12(1), 43–56, doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Depue BE (2012). A neuroanatomical model of prefrontal inhibitory modulation of memory retrieval. Neurosci Biobehav Rev, 36(5), 1382–1399, doi: 10.1016/j.neubiorev.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Orr JM, Smolker HR, Naaz F, & Banich MT (2016). The Organization of Right Prefrontal Networks Reveals Common Mechanisms of Inhibitory Regulation Across Cognitive, Emotional, and Motor Processes. Cereb Cortex, 26(4), 1634–1646, doi: 10.1093/cercor/bhu324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci, 18(9), 547–558, doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, & Fortin NJ (2005). Bridging the gap between brain and behavior: cognitive and neural mechanisms of episodic memory. J Exp Anal Behav, 84(3), 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA, & Jerlhag E (2014). Alcohol: mechanisms along the mesolimbic dopamine system. Prog Brain Res, 211, 201–233, doi: 10.1016/B978-0-444-63425-2.00009-X. [DOI] [PubMed] [Google Scholar]
- Enoch MA (2006). Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann N Y Acad Sci, 1094, 193–201, doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci, 8(11), 1481–1489, doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2016). Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol, 67, 23–50, doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, & Bradshaw JL (2010). Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev, 35(2), 248–275, doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Fein G, Camchong J, Cardenas VA, & Stenger A (2017). Resting state synchrony in long-term abstinent alcoholics: Effects of a current major depressive disorder diagnosis. Alcohol, 59, 17–25, doi: 10.1016/j.alcohol.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, & Verster JC (2010). Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res, 34(8), 1346–1352, doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT (2003). Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev, 2(3), 179–197, doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Finn PR (2002). Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behavioral and Cognitive Neuroscience Reviews, 1(3), 183–205. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, & Pettinati HM (1999). Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res, 23(8), 1289–1295. [PubMed] [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci, 8(9), 700–711, doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fromme K, de Wit H, Hutchison KE, Ray L, Corbin WR, Cook TA, et al. (2004). Biological and behavioral markers of alcohol sensitivity. Alcohol Clin Exp Res, 28(2), 247–256. [DOI] [PubMed] [Google Scholar]
- Garavan H, & Weierstall K (2012). The neurobiology of reward and cognitive control systems and their role in incentivizing health behavior. Prev Med, 55 Suppl, S17–23, doi: 10.1016/j.ypmed.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, & Jay TM (2013). The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol, 23(10), 1165–1181, doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci, 12(11), 652–669, doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A, 100(1), 253–258, doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, et al. (2016). Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron, 90(6), 1312–1324, doi: 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. (2004). Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl), 175(3), 296–302, doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haber SN (2011). Neuroanatomy of Reward: A View from the Ventral Striatum In Gottfried JA (Ed.), Neurobiology of Sensation and Reward (Frontiers in Neuroscience). Boca Raton (FL). [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues Clin Neurosci, 18(1), 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Behrens TE (2014). The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron, 83(5), 1019–1039, doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26, doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, & Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage, 50(3), 1313–1319, doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, & Ridderinkhof KR (2012). Error awareness and salience processing in the oddball task: shared neural mechanisms. Front Hum Neurosci, 6, 246, doi: 10.3389/fnhum.2012.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, & Garavan H (2009). Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav, 93(3), 270–277, doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, & White AM (2017). Drinking Beyond the Binge Threshold: Predictors, Consequences, and Changes in the U.S. Am J Prev Med, 52(6), 717–727, doi: 10.1016/j.amepre.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, & Benjamini Y (1990). More powerful procedures for multiple significance testing. Stat Med, 9(7), 811–818. [DOI] [PubMed] [Google Scholar]
- Humphries MD, & Prescott TJ (2010). The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol, 90(4), 385–417, doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, & Linden DE (2011). Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex, 21(6), 1408–1415, doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Liu X, Clerkin S, Schulz K, Friston K, Newcorn JH, et al. (2012). Effects of motivation on reward and attentional networks: an fMRI study. Brain Behav, 2(6), 741–753, doi: 10.1002/brb3.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, & Tapert SF (2013). Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol, 9, 703–721, doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, van Wingen G, van den Brink W, & Goudriaan AE (2015). Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur Neuropsychopharmacol, 25(12), 2230–2239, doi: 10.1016/j.euroneuro.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Jarbo K, & Verstynen TD (2015). Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci, 35(9), 3865–3878, doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison KM (2004). The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am J Drug Alcohol Abuse, 30(3), 659–684. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, et al. (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry, 70(6), 553–560, doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, & Redish AD (2007). Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci, 27(45), 12176–12189, doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2018). Monitoring the Future national survey results on drug use: 1975–2017: Volume 1, Secondary school students. Ann Arbor: Institue for Social Research, The University of Michigan. [Google Scholar]
- Kahler CW, Strong DR, & Read JP (2005). Toward efficient and comprehensive measurement of the alcohol problems continuum in college students: the brief young adult alcohol consequences questionnaire. Alcohol Clin Exp Res, 29(7), 1180–1189. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. (2005). The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med, 35(2), 245–256. [DOI] [PubMed] [Google Scholar]
- Kim S, Im S, Lee J, & Lee SG (2017). Disrupted Control Network Connectivity in Abstinent Patients with Alcohol Dependence. Psychiatry Investig, 14(3), 325–332, doi: 10.4306/pi.2017.14.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kim S, Cole M, Brennan M, & George O (2017). Intermittent Access to Ethanol Drinking Facilitates the Transition to Excessive Drinking After Chronic Intermittent Ethanol Vapor Exposure. Alcohol Clin Exp Res, 41(8), 1502–1509, doi: 10.1111/acer.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Dennis LE, McCready H, & Hoffman WF (2017). Executive Control and Striatal Resting-State Network Interact with Risk Factors to Influence Treatment Outcomes in Alcohol-Use Disorder. Front Psychiatry, 8, 182, doi: 10.3389/fpsyt.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2000). Drug addiction. Neurobiol Dis, 7(5), 543–545, doi: 10.1006/nbdi.2000.0351. [DOI] [PubMed] [Google Scholar]
- Koob GF (2013a). Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry, 4, 72, doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013b). Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci, 13, 3–30, doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci, 8(11), 1442–1444, doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2008). Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci, 363(1507), 3113–3123, doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238, doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 3(8), 760–773, doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, & Marinkovic K (2012). Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One, 7(8), e43957, doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2002). The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med, 64(2), 258–266. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2006). Who drinks and why? A review of socio-demographic, personality, and contextual issues behind the drinking motives in young people. Addict Behav, 31(10), 1844–1857, doi: 10.1016/j.addbeh.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, & Kuntsche S (2009). Development and validation of the Drinking Motive Questionnaire Revised Short Form (DMQ-R SF). J Clin Child Adolesc Psychol, 38(6), 899–908, doi: 10.1080/15374410903258967. [DOI] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Boyer P, & Rosenbaum RS (2012). Future decision-making without episodic mental time travel. Hippocampus, 22(6), 1215–1219, doi: 10.1002/hipo.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange JE, & Voas RB (2001). Defining binge drinking quantities through resulting blood alcohol concentrations. Psychol Addict Behav, 15(4), 310–316. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, & Sullivan EV (2017). Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol Clin Exp Res, 41(8), 1432–1443, doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, & Sher KJ (2018). “Maturing Out” of Binge and Problem Drinking. Alcohol Res, 39(1), 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Patock-Peckham JA, & Potenza MN (2012). Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Exp Clin Psychopharmacol, 20(2), 92–106, doi: 10.1037/a0026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, & Anderson MC (2008). Individual differences in the suppression of unwanted memories: the executive deficit hypothesis. Acta Psychol (Amst), 127(3), 623–635, doi: 10.1016/j.actpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Levy BJ, & Wagner AD (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci, 1224, 40–62, doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Vasilenko SA, Lanza ST, & Maggs JL (2017). High-Intensity Drinking Versus Heavy Episodic Drinking: Prevalence Rates and Relative Odds of Alcohol Use Disorder Across Adulthood. Alcohol Clin Exp Res, 41(10), 1754–1759, doi: 10.1111/acer.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, et al. (2009a). Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol, 44(4), 372–381, doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- Loeber S, Vollstadt-Klein S, von der Goltz C, Flor H, Mann K, & Kiefer F (2009b). Attentional bias in alcohol-dependent patients: the role of chronicity and executive functioning. Addict Biol, 14(2), 194–203, doi: 10.1111/j.1369-1600.2009.00146.x. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, et al. (2010). Addiction related alteration in resting-state brain connectivity. Neuroimage, 49(1), 738–744, doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. (2008). Decreased volume of the brain reward system in alcoholism. Biol Psychiatry, 64(3), 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, et al. (2009). Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res, 33(11), 1880–1892, doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, & Artsy E (2012). Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp, 33(2), 319–333, doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E, & Lee AK (2013). Effects of acute alcohol intoxication on saccadic conflict and error processing. Psychopharmacology (Berl), 230(3), 487–497, doi: 10.1007/s00213-013-3173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct, 214(5–6), 655–667, doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, & Neil Macrae C (2007). Separating sustained from transient aspects of cognitive control during thought suppression. Psychol Sci, 18(4), 292–297, doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Molnar SM, Beaton LE, Happer JP, Holcomb LA, Huang S, Arienzo D, et al. (2018). Behavioral and Brain Activity Indices of Cognitive Control Deficits in Binge Drinkers. Brain Sci, 8(1), doi: 10.3390/brainsci8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, & Rosenbaum RS (2006). The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol, 16(2), 179–190, doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Most D, Ferguson L, & Harris RA (2014). Molecular basis of alcoholism. Handb Clin Neurol, 125, 89–111, doi: 10.1016/B978-0-444-62619-6.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, & Schulte T (2015). The Resting Brain of Alcoholics. Cereb Cortex, 25(11), 4155–4168, doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, & Schulte T (2014). Cognition, emotion, and attention. Handb Clin Neurol, 125, 341–354, doi: 10.1016/B978-0-444-62619-6.00020-3. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, & O’Reilly RC (2011). A unified framework for inhibitory control. Trends Cogn Sci, 15(10), 453–459, doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. (2004). Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology, 29(2), 393–402, doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Naim-Feil J, Fitzgerald PB, Bradshaw JL, Lubman DI, & Sheppard D (2014). Neurocognitive deficits, craving, and abstinence among alcohol-dependent individuals following detoxification. Arch Clin Neuropsychol, 29(1), 26–37, doi: 10.1093/arclin/act090. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Nelson DE, & Brewer RD (2010). The intensity of binge alcohol consumption among U.S. adults. Am J Prev Med, 38(2), 201–207, doi: 10.1016/j.amepre.2009.09.039. [DOI] [PubMed] [Google Scholar]
- Nemeth VL, Kurgyis E, Csifcsak G, Maraz A, Almasi DA, Drotos G, et al. (2014). The impact of intermediate-term alcohol abstinence on memory retrieval and suppression. Front Psychol, 5, 1396, doi: 10.3389/fpsyg.2014.01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2004). NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter, 3. [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, & Dolan RJ (2003). Temporal difference models and reward-related learning in the human brain. Neuron, 38(2), 329–337. [DOI] [PubMed] [Google Scholar]
- O’Neill SE, Parra GR, & Sher KJ (2001). Clinical relevance of heavy drinking during the college years: cross-sectional and prospective perspectives. Psychol Addict Behav, 15(4), 350–359. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Harezlak J, Kudela MA, Tran SM, Soeurt CM, et al. (2016). Corticostriatal and Dopaminergic Response to Beer Flavor with Both fMRI and [(11) C]raclopride Positron Emission Tomography. Alcohol Clin Exp Res, 40(9), 1865–1873, doi: 10.1111/acer.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, O’Connor SJ, Yoder KK, et al. (2015). Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology (Berl), 232(5), 861–870, doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M (2012). Function and dysfunction of prefrontal brain circuitry in alcoholic Korsakoff’s syndrome. Neuropsychol Rev, 22(2), 154–169, doi: 10.1007/s11065-012-9198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinkovic K (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev, 17(3), 239–257, doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol, 125, 183–210, doi: 10.1016/B978-0-444-62619-6.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Terry-McElrath YM, Lanza ST, Jager J, Schulenberg JE, & O’Malley PM (2019). Shifting Age of Peak Binge Drinking Prevalence: Historical Changes in Normative Trajectories Among Young Adults Aged 18 to 30. Alcohol Clin Exp Res, doi: 10.1111/acer.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, & Kumaresan V (2006). The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev, 30(2), 215–238, doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Prohovnik I, Hakansson K, & Risberg J (1980). Observations on the functional significance of regional cerebral blood flow in “resting” normal subjects. Neuropsychologia, 18(2), 203–217. [DOI] [PubMed] [Google Scholar]
- Roberto M, & Varodayan FP (2017). Synaptic targets: Chronic alcohol actions. Neuropharmacology, 122, 85–99, doi: 10.1016/j.neuropharm.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Rolls ET (1996). The orbitofrontal cortex. Philos Trans R Soc Lond B Biol Sci, 351(1346), 1433–1443; discussion 1443–1434, doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2004). The functions of the orbitofrontal cortex. Brain Cogn, 55(1), 11–29, doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rosen BQ, Padovan N, & Marinkovic K (2016). Alcohol Hits You When It Is Hard: Intoxication, Task Difficulty, and Theta Brain Oscillations. Alcohol Clin Exp Res, 40(4), 743–752, doi: 10.1111/acer.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, & Buckner RL (2007). Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci, 8(9), 657–661, doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Correia MM, Ferreira CS, Prescot AP, & Anderson MC (2017). Hippocampal GABA enables inhibitory control over unwanted thoughts. Nat Commun, 8(1), 1311, doi: 10.1038/s41467-017-00956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, & Stalnaker TA (2006). Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci, 29(2), 116–124, doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA (2000). Genetics of the risk for alcoholism. Am J Addict, 9(2), 103–112. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, & Sullivan EV (2010). Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues Clin Neurosci, 12(4), 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, & Hollerman JR (2000). Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex, 10(3), 272–284. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, & van Rooijen L (1975). A self-administered Short Michigan Alcoholism Screening Test (SMAST). J Stud Alcohol, 36(1), 117–126. [DOI] [PubMed] [Google Scholar]
- Sesack SR, & Grace AA (2010). Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology, 35(1), 27–47, doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Martinez JA, & Littlefield AK (2014). Alcohol use and alcohol use disorders In Barlow DH (Ed.), The Oxford Handbook of Clinical Psychology (2nd edition) (pp. 405–445). Oxford; New York, NY: Oxford University Press. [Google Scholar]
- Sher KJ, & Trull TJ (1994). Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol, 103(1), 92–102. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A, 106(31), 13040–13045, doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Lof E, & Ericson M (2009). Mechanistic studies of ethanol’s interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry, 42 Suppl 1, S87–94, doi: 10.1055/s-0029-1220690. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Lowe B (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med, 166(10), 1092–1097, doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stautz K, & Cooper A (2013). Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clin Psychol Rev, 33(4), 574–592, doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Stephenson MT, Morgan SE, Lorch EP, Palmgreen P, Donohew L, & Hoyle RH (2002). Predictors of exposure from an antimarijuana media campaign: outcome research assessing sensation seeking targeting. Health Commun, 14(1), 23–43, doi: 10.1207/S15327027HC1401_2. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Muller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, et al. (2013). A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol Psychiatry, 74(7), 547–555, doi: 10.1016/j.biopsych.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl), 180(4), 583–594, doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, & Stein EA (2012). Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage, 62(4), 2281–2295, doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA (2006). Encoding new episodes and making them stick. Neuron, 50(1), 19–21, doi: 10.1016/j.neuron.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, & Brown SA (2004). fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav, 29(1), 33–50. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, & Davis KD (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp, 30(9), 2731–2745, doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, & Patrick ME (2016). Intoxication and binge and high-intensity drinking among US young adults in their mid-20s. Subst Abus, 37(4), 597–605, doi: 10.1080/08897077.2016.1178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, & Schultz W (2006). Human neural learning depends on reward prediction errors in the blocking paradigm. J Neurophysiol, 95(1), 301–310, doi: 10.1152/jn.00762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2013). Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol, 48(1), 1–19, doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, & Schultz W (1999). Relative reward preference in primate orbitofrontal cortex. Nature, 398(6729), 704–708, doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. (2010). Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain, 133(Pt 7), 2098–2114, doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, & De Vries TJ (2010). Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev, 35(2), 276–284, doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, & Crews FT (2015). Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci, 9, 35, doi: 10.3389/fnins.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Baler RD (2013). Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry, 70(7), 661–663, doi: 10.1001/jamapsychiatry.2013.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, & Wang GJ (2004). The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology, 47 Suppl 1, 3–13, doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, et al. (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105(10), 1741–1749, doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, & Pennartz CM (2004). Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci, 27(8), 468–474, doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, et al. (2016). Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. PLoS One, 11(8), e0161956, doi: 10.1371/journal.pone.0161956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu J, Li Q, Li W, Wu N, Zheng Y, et al. (2013). Altered fronto-striatal and fronto-cerebellar circuits in heroin-dependent individuals: a resting-state FMRI study. PLoS One, 8(3), e58098, doi: 10.1371/journal.pone.0058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler (2011). WASI-II: Wechsler Abbreviated Scale of Intelligence. San Antonio, Tex. : Psychological Corporation. [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, et al. (2014). Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res, 38(9), 2445–2453, doi: 10.1111/acer.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, & Fergusson DM (2004). Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction, 99(12), 1529–1541, doi: 10.1111/j.1360-0443.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Jenkinson N, Brittain JS, Voets SH, Aziz TZ, & Aron AR (2016). Surprise disrupts cognition via a fronto-basal ganglia suppressive mechanism. Nat Commun, 7, 11195, doi: 10.1038/ncomms11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2(3), 125–141, doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, & Turner JA (2014). Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev Neurosci, 25(1), 1–24, doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, & Mayer AR (2011). Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend, 115(1–2), 137–144, doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2009). Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci, 32(10), 517–524, doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Dearing RL, & Maisto SA (2014). Alcohol use trajectories among non-treatment-seeking heavy drinkers. J Stud Alcohol Drugs, 75(3), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, et al. (2007). Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage, 36(4), 1253–1262, doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, & Momenan R (2017). Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol, 22(1), 206–217, doi: 10.1111/adb.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Dutta N, Helton SG, Schwandt M, Yan J, Hodgkinson CA, et al. (2015). Resting-state functional connectivity and presynaptic monoamine signaling in Alcohol Dependence. Hum Brain Mapp, 36(12), 4808–4818, doi: 10.1002/hbm.22951. [DOI] [PMC free article] [PubMed] [Google Scholar]