Abstract
The preferred post-remission therapy for older patients with acute myeloid leukemia (AML) in first complete remission (CR1) remains uncertain. In this retrospective, multicenter study, we compared outcomes for older AML patients (age 60–77 years) receiving allogeneic hematopoietic cell transplantation (alloHCT) (n=431) with those treated on prospective National Clinical Trials Network induction and non-transplantation chemotherapy consolidation (CT) trials (n=211). AlloHCT patients were younger (median age: 64.2 vs. 67.9 years, p<0.001), but more frequently had high-risk AML (high WBC, secondary AML and unfavorable cytogenetics). Overall survival (OS) was worse in alloHCT during the first 9 months after CR1 (HR=1.52, p=0.02), but was significantly better thereafter (HR= 0.53, p<0.0001) relative to CT. Treatment-related mortality (TRM) following HCT was worse in the first 9 months (HR=2.8, CI: 1.5 −5.2, p=0.0009), while post-HCT relapse was significantly less frequent beyond 9 months (HR = 0.42, CI: 0.29 to 0.61, p<0.0001). Despite higher early TRM, alloHCT recipients had superior long-term OS [29% (24–34%) vs. CT 13.8% (9 −21%) at 5-year]. Although this is a retrospective analysis with potential biases, it indicates that alloHCT led to heightened early risks from TRM, yet reduced relapse and superior long-term survival relative to CT in older AML patients in CR1.
Keywords: Acute myeloid leukemia, older, elderly, relapse, non-relapse mortality, complete remission, consolidation, allogeneic hematopoietic cell transplantation, survival
Introduction :
Acute myeloid leukemia (AML) most often affects older patients (≥60 years) with a median age of 68 years,1–3 who generally have worse outcomes.4 Overall survival (OS) remains dismal due to frequent disease relapse.5, 6 Although intensive remission induction regimens can induce complete remission (CR) in 45 to 65% of patients,7, 8 relapse frequently occurs within the first 12–18 months.9 Adverse disease biology of AML in older patients drives high relapse as a consequence of unfavorable cytogenetics/molecular profile, intolerance to intensive chemotherapy and overexpression of multidrug resistance genes.4, 5, 10–13
Allogeneic hematopoietic cell transplantation (AlloHCT) is a highly potent antileukemic consolidation strategy; however, treatment-related mortality (TRM) presents a major limitation, particularly in older patients.14 Advances in alloHCT using nonmyeloablative/reduced-intensity conditioning (NMA/RIC) regimens15, 16 have permitted more widespread HCT application, even in the eighth decade of life.17, 18 Several studies have shown that when compared with conventional myeloablative conditioning (MAC) regimens, RIC HCT yields comparable or lower peri-HCT toxicities but higher relapse rates (CTN 0910) - despite its use in older patients or those with high pre-HCT comorbidity scores.19–22
The benefit of alloHCT in younger AML patients has been evaluated in biologic randomization studies of those with and without an available HLA matched donor.14, 23, 24 Studies among older AML patients reporting 30–50% 2-year survival after RIC appear promising relative to non-HCT chemotherapy (CT) approaches25, 26 but are hampered by the lack of control groups. Since most older AML patients never pursue alloHCT3, 27, adjusting for selection bias among older alloHCT recipients poses a major limitation in generalizing transplantation results. Studies addressing the benefits of alloHCT in an older AML population compared to CT consolidations are limited and no well-designed prospective studies have been reported28–30. To better delineate the risks and benefits of alloHCT, here, we compare AML outcomes in older patients receiving alloHCT with those receiving CT consolidation in prospective cooperative group trials.
Materials and Methods:
Patients
Patients 60 to 75 years of age with AML in CR1 receiving a first alloHCT between 2008 and 2013 included in the Center for International Blood and Marrow Transplantation (CIBMTR) database comprised the alloHCT cohort. As reporting of allogeneic stem cell transplant outcomes is a federal requirement in the US, this data set includes almost all such transplants performed in the US during this time. Older AML patients enrolled on US cooperative group trials (now National Clinical Trials Network, NCTN) achieving CR1 receiving postinduction therapy in Alliance for Clinical Trials in Oncology (Alliance)/Cancer and Leukemia Group B trials [10502, 10801, 1100131, 32 and 11002 (https://clinicaltrials.gov/ct2/show/NCT01420926)] between and 2008 and 2013, ECOG-ACRIN Cancer Research Group trial E399933 between 2002 and 2005 and Southwest Oncology Group (SWOG) protocols (S0432 and S0703)34, 35 between 2005 and 2012 constituted the chemotherapy (CT) consolidation group (Supplemental Table 1). All subjects signed written informed consent for treatment trials in the cooperative groups and for data capture in the CIBMTR. Each NCTN and the CIBMTR approved this retrospective study. Any patient in the CT group who later underwent alloHCT was excluded in an attempt to preserve homogeneity within each cohort.
De novo or treatment-associated AML or AML evolving from a previous myelodysplastic (MDS) or myeloproliferative (MPN) disorder were eligible. All types of donors [sibling, unrelated (URD), and umbilical cord blood (UCB)] except haploidentical donors, and any conditioning intensity regimens were eligible.36 Cytogenetic reports from the Alliance studies were reviewed and categorized by the 2016 European Leukemia Net although molecular data were not included since this information was not available for the majority of patients enrolled in these studies.37 Cytogenetic risk classification generally followed the classification by Slovak for ECOG-ACRIN and SWOG.38 The CIBMTR cytogenetic characterization mirrored the Alliance schema (Supplemental Table 2).
Karnofsky (or Zubrod for only in the SWOG study) performance score (KPS) for CT cohort was collected prior to induction therapy while alloHCT cohort KPS was reported before alloHCT.
Statistical Considerations:
Categorical variables were summarized by frequency (percent) and compared using a Chi-square or Fisher exact test as appropriate. Continuous variables were summarized by median (range) and compared using a two-sample t-test or a Wilcoxon rank-sum test.
The time to event for all outcomes started at the time of CR1. Left-truncation was used in all analyses to account for administration of either alloHCT or CT at differing times after CR1 and thus delayed entry into the study. AlloHCT patients enter the risk group at the time of alloHCT and CT patients enter the risk group at the start of first consolidation therapy. Disease-free survival (DFS) was recorded until time of disease relapse or death, whichever occurred first. Overall survival (OS) and DFS were estimated for each cohort using the left-truncated version of the Kaplan-Meier estimator.39 The cumulative incidence of relapse and all-cause treatment related mortality (TRM) estimates used the cumulative incidence function with the risk sets adjusted for left truncation. Relapse was the competing risk for TRM and vice versa; Cox model for cause-specific hazards was used. Outcomes were compared between cohorts using the Cox proportional hazards model with left-truncation. AlloHCT versus consolidation therapy was the primary study comparison with OS as the primary endpoint. The potential confounding effect of age, KPS and cytogenetic risk classification were adjusted for in the multivariate model. Of note, we chose to adjust for these factors as covariates in the multivariate model instead of a stratified analysis so that the interaction between these factors and the main effect (AlloHCT vs. CT) can be evaluated. The proportional hazards assumption comparing alloHCT versus CT was not met for OS and DFS. The maximum partial likelihood approach was then used to determine a cut-point of 9 months post treatment which best segregated post treatment time periods.39 Statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were based on the study database frozen on January 2nd, 2018.
Results:
Baseline Characteristics:
The study evaluated 642 patients comprised of 431 patients in the alloHCT group and 211 patients in the CT group (Supplemental Table 3 for selection). Of note, pruning of the datasets to meet eligibility varied and ultimately relatively few patients met criteria of consolidation therapy on an NCTN trial while in CR1 without subsequent alloHCT. Table 1 summarizes patients′ baseline characteristics. AlloHCT patients were younger, had more secondary AML, more often had high WBC> 100 × 109/L at diagnosis, worse performance scores, less frequent extramedullary disease (EMD) at diagnosis, and less frequent FLT3 mutation in tested patients. Adverse karyotype among those evaluable was similar between alloHCT recipients (38%) versus CT (30%) (p= 0.072). Supplemental Table 2 shows the cytogenetic risk groups among NCTN studies and the alloHCT group. CT patients had more frequent favorable risk cytogenetics 11.3% (17/150) versus only 1.7% (7/416) in the alloHCT cohort (p<0.001). Because of few patients in the favorable cytogenetic risk group, subsequent analyses merged Favorable and intermediate risk groups.
Table 1.
Baseline Characteristics
| Patient Characteristics by Treatment | |||
|---|---|---|---|
| CT (N=211) | alloHCT (N=431) | P value | |
| Age | <0.001 | ||
| 60–65 | 66 (31%) | 234 (54%) | |
| 65–70 | 74 (35%) | 157 (36%) | |
| 70–75 | 70 (33%) | 40 (9%) | |
| Missing | 1 | 0 | |
| Gender | 0.07 | ||
| Male | 114 (54%) | 265 (61%) | |
| Female | 97 (46%) | 166 (39%) | |
| Karnofsky score | <0.001 | ||
| <90 | 135 (64%) | 152 (36%) | |
| >=90 | 76 (36%) | 266 (64%) | |
| Missing | 0 | 13 | |
| White Blood Count at diagnosis | 0.01 | ||
| <= 10 | 145 (69%) | 268 (69%) | |
| 10 – 100 | 64 (30%) | 97 (25%) | |
| > 100 | 2 (1%) | 21 (5%) | |
| Missing | 0 | 45 | |
| Extramedullary disease at diagnosis | <0.001 | ||
| No | 161 (87%) | 415 (97%) | |
| Yes | 24 (13%) | 11 (3%) | |
| Missing | 26 | 5 | |
| Cytogenetics scoring | <0.001 | ||
| Normal, Intermediate or Favorable | 105 (50%) | 257 (60%) | |
| Poor | 45 (21%) | 159 (37%) | |
| Missing | 61 (29%) | 15 (3%) | |
| FLT3 mutation | <0.001 | ||
| No | 10 (43%) | 223 (82%) | |
| Yes | 13 (57%) | 48 (18%) | |
| Missing | 188 | 160 | |
| Type of AML | <0.001 | ||
| De-novo | 170 (81%) | 262 (61%) | |
| Secondary | 41 (19%) | 169 (39%) | |
| Consolidation type | <0.001 | ||
| HiDAC | 183 (87%) | 132 (31%) | |
| Other, non-HiDAC | 28 (13%) | 127 (30%) | |
| None | 0 (0%) | 171 (40%) | |
| Missing | 0 | 1 | |
| Other consolidations | |||
| Azacitine plus gemtumzumab | 15 (54%) | ||
| Tipifarnib | 9 (32%) | ||
| Other | 4 (14%) | ||
| Not applicable | 183 | ||
| Number of consolidation cycles | <0.001 | ||
| 0 | 0 (0%) | 171 (43%) | |
| 1 | 56 (27%) | 96 (24%) | |
| 2+ | 155 (73%) | 132 (33%) | |
| Missing | 0 | 32 | |
| Donor Grouping | |||
| HLA-identical sibling | 107 (25%) | ||
| Well-matched unrelated | 178 (41%) | ||
| Partially matched unrelated | 39 (9%) | ||
| Mismatched unrelated | 3 (1%) | ||
| Unrelated matching unknown | 2 (0%) | ||
| Cord blood | 102 (24%) | ||
| Time from Diagnosis to CR1 | 0.24 | ||
| N | 209 | 428 | |
| Mean (SD) | 2.0 (1.8) | 2.1 (2.2) | |
| Median | 1.6 | 1.5 | |
| Q1, Q3 | 1.3,2.2 | 1.0, 2.4 | |
| Range | (0.6–22.5) | (0.2–21.5) | |
| Time from CR1 to consolidation or HCT (months) | <0.001 | ||
| N | 211 | 431 | |
| Mean (SD) | 0.5 (0.5) | 3.5 (2.5) | |
| Median | 0.5 | 3.2 | |
| Q1, Q3 | 0.2, 0.7 | 1.9, 4.7 | |
| Range | (0.0–3.7) | (0.2–20.6) | |
Abbreviations: AlloHCT: allogeneic hematopoietic cell transplantation, CT: chemotherapy consolidation, CR1: first complete remission, HiDac: high dose cytarabine, SD: standard deviation.
The majority (60%) of alloHCT patients received at least one cycle of pre-alloHCT consolidation therapy. As a result, time from CR1 to alloHCT (median 3.2 months, IQR 1.9 – 4.7) was longer than the time from CR1 to consolidation therapy [median 0.5 months, interquartile range (IQR) 0.2 – 0. 7, p<0.0001].
Outcomes:
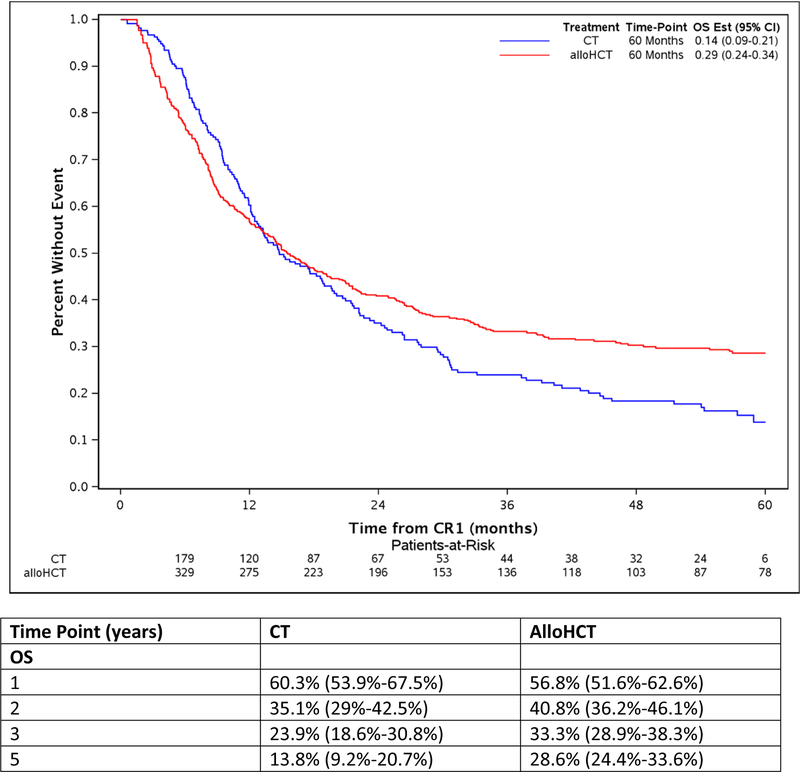
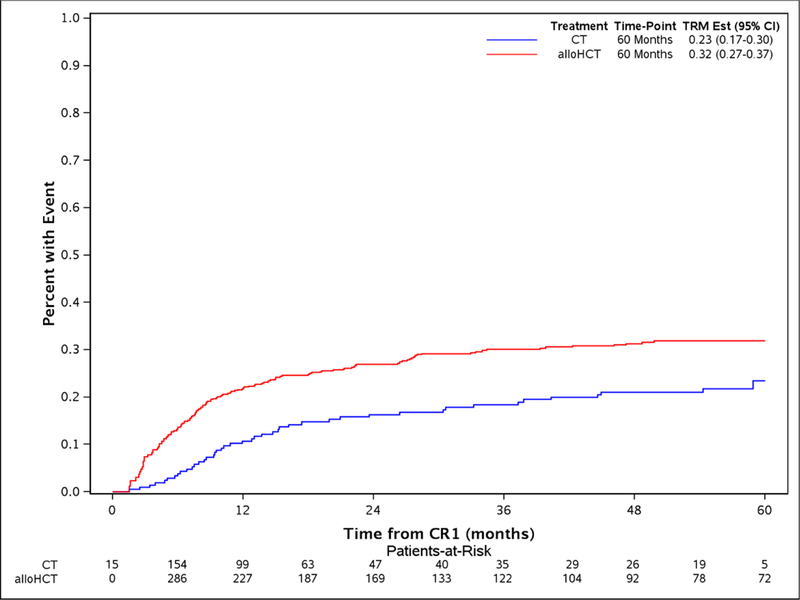
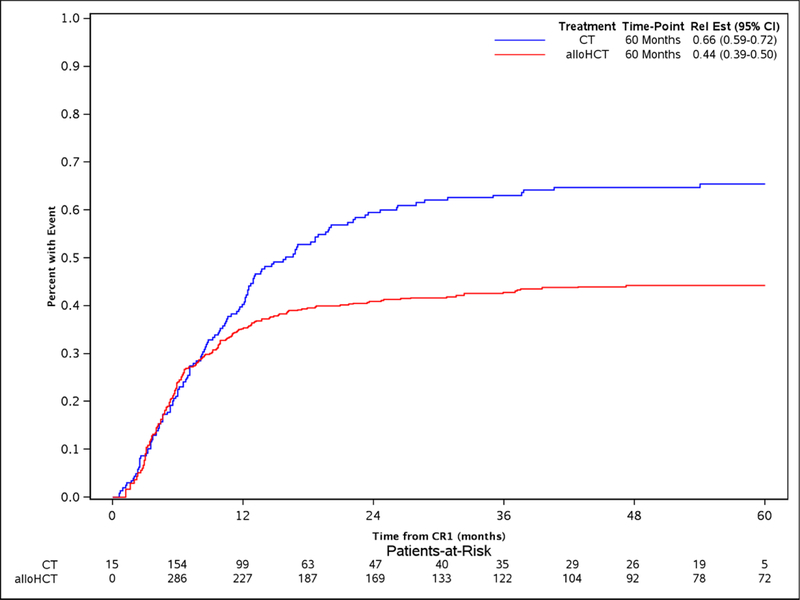
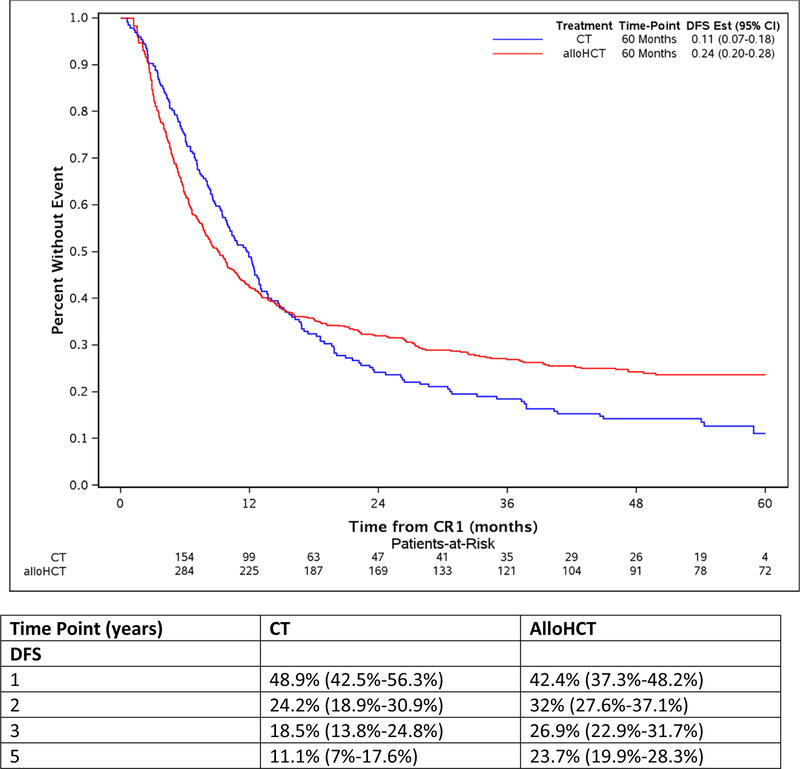
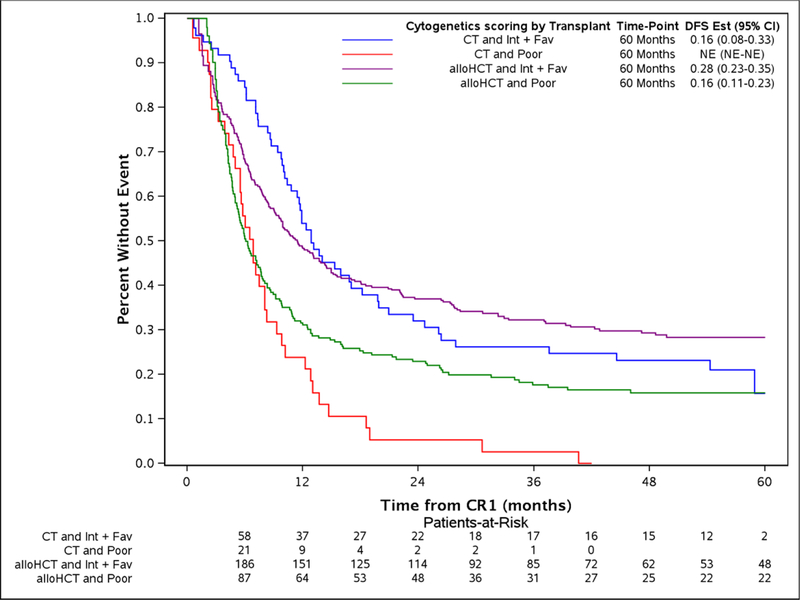
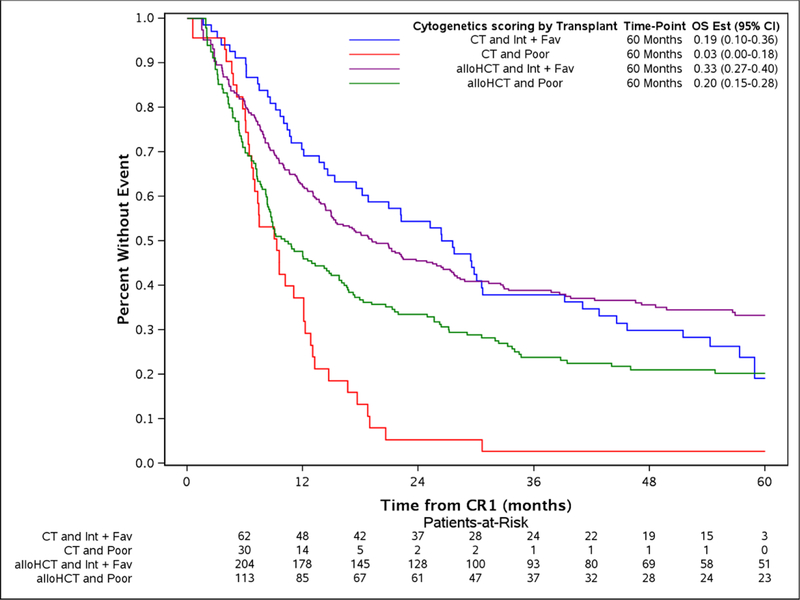
The study took advantage of the long median follow of 56.9 (2–96.3) and 53.1 (8.6 −84.5) months for alloHCT and CT, respectively. The comparison of alloHCT relative to CT outcomes differed over time as OS and DFS did not meet the proportional hazards assumptions (Figure 1A and Figure 1D, respectively) prompting creation of an early and later period at the 9 month timepoint. In the early consolidation period (within the first 9 months), alloHCT resulted in lower OS (HR, 1.52, 95%CI: 1.07 – 2.07, p=0.02) due to higher TRM (HR, 2.81, 95%CI: 1.53 – 5.16, p=0.001) (Table 2, Figures 1A and 1B, respectively). In contrast, beyond 9 months after CR1, alloHCT achieved superior OS (Figure 1A, HR, 0.53, 95%CI 0.40 – 0.70, p<0.0001) and DFS (Figure 1D, HR, 0.53, 95%CI 0.40 – 0.70, p<0.0001) due to a significantly lower incidence of relapse (Figure 1C, HR, 0.42, 95%CI 0.29 – 0.61, p<0.0001). After 9 months, the incidence of TRM (Figure 1B) did not differ between the alloHCT and CT groups. The long-term benefit of alloHCT was more apparent among patients with poor risk cytogenetics (Figures 2A and B). Time point estimates of OS and DFS at years 1, 2, 3 and 5 for each group are shown under Figure 1A and 1D, respectively. Of note, at 5-years OS and DFS significantly favored alloHCT: 5-year OS alloHCT of 28.6% (CI: 24.4–33.6) compared to CT of 13.8% (CI: 9.2–20.7) and 5-year DFS of 23.7% (CI 19.9–28.3) versus CT at 11.1% (CI: 7 – 17.6). In the alloHCT group, OS did not significantly differ by receiving consolidation or consolidation number (Supplemental Figure 1).
Figure 1:
Overall survival for alloHCT and CT with 5-year point estimate (1A), treatment-related mortality for alloHCT and CT with 5-year point estimate (1B), cumulative incidence of relapse for alloHCT and CT with 5-year point estimate (1C), disease-free survival for alloHCT and CT with 5-year point estimate (1D)
Table 2.
Multivariate analysis for TRM, relapse, DFS and OS
| TRM | Relapse | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (99% CI) | P-value |
| Therapy | 0.0003† | 0.0002† | <0.0001† | <0.0001† | ||||
| CT before 9 mo | 1 | 1 | 1 | 1 | ||||
| AlloHCT before 9 mo | 2.81 (1.53–5.16) | 0.001 | 1.04 (0.73–1.48) | 0.82 | 1.38 (1.03–1.87) | 0.03 | 1.52 (1.07–2.17) | 0.02 |
| CT after 9 mo | 1 | 1 | 1 | 1 | ||||
| AlloHCT after 9 mo | 0.74 (0.45,1.20) | 0.22 | 0.42 (0.29–0.61) | <0.0001 | 0.52 (0.39–0.70) | <0.0001 | 0.53 (0.40–0.70) | <0.0001 |
| Age | 0.5 | 0.77 | 0.57 | 0.28 | ||||
| 60–65 years | 1 | 1 | 1 | 1 | ||||
| 65–70 | 1.22 (0.87–1.71) | 0.25 | 0.99 (0.76–1.29) | 0.96 | 1.08 (0.87–1.32) | 0.49 | 1.10 (0.89–1.37) | 0.37 |
| 70–75 | 1.18 (0.74–1.89) | 0.49 | 1.11 (0.8–1.54) | 0.52 | 1.15 (0.88–1.5) | 0.31 | 1.25 (0.95–1.64) | 0.11 |
| KPS | ||||||||
| KPS >90 | 1 | 1 | 1 | 1 | ||||
| KPS ≤90 | 0.99 (0.72–1.36) | 0.94 | 1.22 (0.97–1.55) | 0.1 | 1.13 (0.94–1.37) | 0.2 | 1.15 (0.94–1.39) | 0.17 |
| Cytogenetic risk group | 0.52 | <0.0001 | <0.0001 | <0.0001 | ||||
| Favorable/intermediate | 1 | 1 | 1 | 1 | ||||
| Poor | 1.23 (0.87–1.74) | 0.25 | 2.12 (1.65–2.73) | <0.0001 | 1.74 (1.42–2.13) | <0.0001 | 1.74 (1.41–2.14) | <0.0001 |
| Missing | 1.03 (0.6–1.77) | 0.91 | 1.32 (0.93–1.89) | 0.12 | 1.20 (0.90–1.62) | 0.22 | 1.13 (0.83–1.54) | 0.43 |
Abbreviations: TRM: treatment-related mortality, DFS: Disease free survival, OS: Overall survival, AlloHCT: Allogeneic hematopoietic cell transplantation, CT-chemotherapy alone for consolidation, KPS: karnofsky performance status
early vs. late effect
Figure 2:
Disease-free survival by alloHCT or CT stratified by intermediate or poor cytogenetic categories and 5-year point estimates (2A), overall survival by alloHCT or CT stratified by intermediate or poor cytogenetic categories and 5-year point estimates (2B).
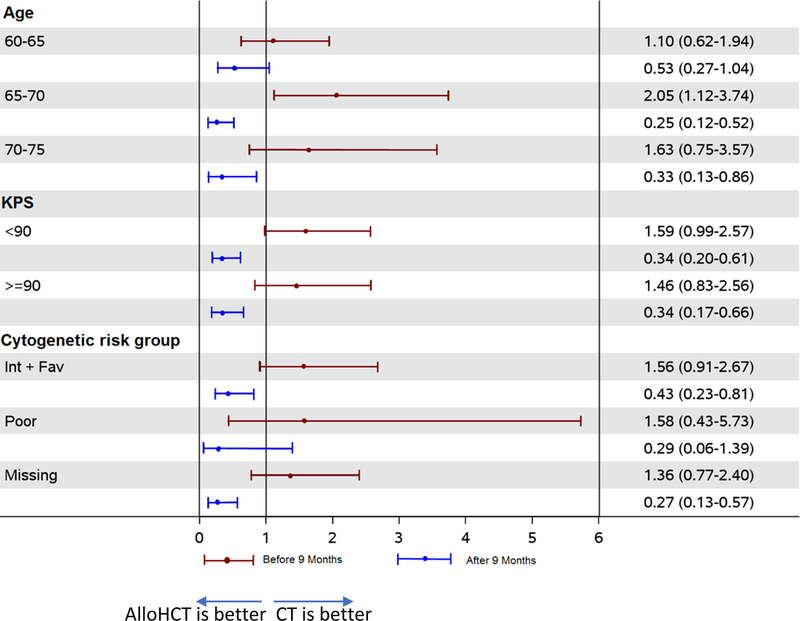
In multivariate analysis, patients harboring poor cytogenetics had higher risks of relapse (HR, 2.12, 95%CI 1.65 – 2.73, p<0.0001), lower DFS (HR, 1.74, 95%CI 1.42 – 2.13, p<0.0001), and OS (HR, 1.74, 95%CI 1.41 – 2.14, p<0.0001) compared with those with favorable or intermediate cytogenetics (Table 2). No significant associations existed for age and KPS with any outcome. The interaction between treatment effect was evaluated for age, KPS and disease risk group and no interactions found (data not shown). In addition, a Forest plot analysis was performed to compare alloHCT vs. CT for subgroup analysis by age, KPS, and cytogenetic risk classification (Figure 3), which confirmed our findings. Adjusted overall survival curves and time-point estimates were also very similar to unadjusted findings (Supplemental Figure 2A and B).
Figure 3.
The Forest Plot represents the effect of each characteristic (age, Karnofsky Performance Status, and cytogenetic risk group) on overall survival per treatment before and after 9 months.
Transplantation Characteristics
Among the 431 patients receiving alloHCT (Table 3), RIC/NMA was used most often (79%), although 21% received MAC. Most received HLA matched related or URD grafts (66%), but 34% received alternative donor grafts: 24% UCB; 10% partially matched URD. Peripheral blood stem cells were used most often (71%) along with tacrolimus-based graft-versus host disease (GVHD) prophylaxis (65%) and in vivo anti-T cell therapies with ATG or alemtuzumab (42%).
Table 3:
Transplantation Characteristics
| N | 431 |
|---|---|
| Conditioning intensity | |
| MAC | 92 (21%) |
| RIC/NMA | 337 (79%) |
| Missing | 2 |
| Type of Donor | |
| HLA-identical sibling | 107 (25%) |
| HLA Well-matched unrelated | 178 (41%) |
| Partially matched unrelated/Mismatched unrelated | 42 (10%) |
| UCB | 102 (24%) |
| Missing | 2 |
| Graft Source | |
| Bone marrow | 25 (6%) |
| Peripheral blood | 304 (71%) |
| UCB | 102 (24%) |
| UCB HLA matching | |
| <=4/6 HLA locus match | 57 (58%) |
| 5/6 | 36 (37%) |
| 6/6 | 5 (5%) |
| Missing | 4 |
| GVHD prophylaxis | |
| Ex-vivo TCD | 13 (3%) |
| Tac based | 280 (65%) |
| CsA based | 120 (28%) |
| Other | 18 (4%) |
| In-vivo TCD (ATG or alemtuzumab) | |
| No | 251 (58%) |
| Yes | 180 (42%) |
Abbreviations: MAC: myeloablative conditioning, RIC/NMA: reduced-intensity or nonmyeloablative regimens, UCB: umbilical cord blood, CMV: cytomegalovirus seropositivity, D: donor, R: recipient, GVHD: graft-versus-host disease, TCD: t-cell depletion, tac: Tacrolimus, CsA: cyclosporine A, ATG: antithymocyte globulin.
Risk Factors following AlloHCT
Multivariate risk factor analysis showed that UCB grafts were associated with higher TRM, and lower OS (Table 4). Conditioning intensity, age and KPS had no significant impact on any outcome.
Table 4.
Multivariate analysis for TRM, relapse, DFS and OS within the AlloHCT group
| TRM | Relapse | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (99% CI) | P-value |
| Conditioning Intensity | ||||||||
| MAC | 1 | 1 | 1 | 1 | ||||
| NMA/RIC | 0.99 (0.65–1.51) | 0.95 | 1.39 (0.93–2.08) | 0.11 | 1.19 (0.89–1.59) | 0.24 | 1.14 (0.85–1.54) | 0.39 |
| Donor Type | 0.0004 | 0.07 | 0.0003 | <0.0001 | ||||
| HLA-identical sibling | 1 | 1 | 1 | 1 | ||||
| HLA Partially MURD/MMURD | 1.47 (0.75–2.88) | 0.26 | 0.70 (0.38–1.26) | 0.23 | 0.94 (0.61–1.46) | 0.79 | 1.05 (0.66–1.65) | 0.85 |
| HLA-Well-matched URD | 1.23 (0.74–2.04) | 0.42 | 0.73 (0.5–1.06) | 0.1 | 0.88 (0.66–1.20) | 0.44 | 0.97 (0.71–1.33) | 0.85 |
| UCB | 2.69 (1.59–4.55) | 0.0002 | 1.18 (0.77–1.8) | 0.45 | 1.65 (1.19–2.28) | 0.003 | 1.87 (1.34–2.60) | 0.0002 |
Abbreviations: TRM: treatment-related mortality, DFS: disease-free survival, OS: overall survival, HR: hazard ratio, MAC: myeloablative conditioning, NMA: nonmyeloablative, RIC: reduced-intensity conditioning, MURD: matched unrelated donor, MMURD: mismatched unrelated donor, URD: unrelated donor, UCB: umbilical cord blood.
Discussion:
In this study, alloHCT for older AML patients in CR1 resulted in significantly better long-term DFS and OS relative to chemotherapy consolidation. The rates of failure differed by treatment approach: alloHCT patients suffered from higher rates of early TRM while patients after CT had higher rates of relapse, even beyond the first year (Figure 1C).
Compared to prior studies, the major strengths of this study are inclusion of older patients (17% were 70 years and older), large sample size, available cytogenetic data, and derivation from a recent era reflecting modern treatment and supportive care practices. In addition, the alloHCT cohort represented broad clinical practice by including different donor types and conditioning regimens. The study populations were chosen to minimize bias in comparing the different consolidation strategies. While selection of appropriate alloHCT patients reflected the screening and eligibility at each HCT center, the control comparators also underwent eligibility screening for contemporaneous NCTN group trial participation. We accounted for the delay in alloHCT after CR1 by including only patients receiving chemotherapy on a cooperative group trial study and adjusting for the time to consolidation or HCT.
High quality prospective studies have been primarily conducted in younger patients comparing those with an HLA-matched donor to those without (a.k.a., biologic randomization) showing improved survival in CR1 for intermediate and high-risk disease with an overall hazard ratio of 0.87 to 0.9 in a meta-analysis by Koreth et al.24 Uncontrolled studies among older adults with AML have shown promising survival relative to historical expectations.26, 40, 41 Generalizing the benefits of alloHCT from younger adults to older AML patients is problematic. The higher prevalence of comorbidities and functional impairments influences patient selection for treatments and treatment intensity. However, the more adverse disease biology and higher relapse risks counterbalances efforts to minimize treatment intensity in older patients.21
With inclusion of different donor types, HLA matching and conditioning regimens, the magnitude of benefit observed in our study was similar to prior studies comparing alloHCT to CT consolidation in older adults.28, 29, 42 A retrospective European study compared alloHCT with CT (i.e., additional chemotherapy or autologous HCT) versus no further therapy in 640 older patients (range-62 to 71 years) with AML in CR1. Similar to our results, 5-year overall survival was 35% in the subset of 97 patients receiving HLA matched alloHCT, 26% in the CT, 21% in those receiving no post-remission therapy.42 A retrospective study from Japan including somewhat younger patients (50–70 years) demonstrated higher 3-year OS after alloHCT (n=152) compared to chemotherapy (62% versus 51%, P<0.012).29 Farag et al. compared alloHCT reported to the CIBMTR from an earlier time period (1999 to 2005) to consolidation on CALGB chemotherapy protocols in elderly AML patients in CR1.28 Patients aged 60–70 years who survived at least 4 months in CR1 received either RIC allogeneic HCT (n=96) or chemotherapy (n=94). OS at 3-year was 37% (95% CI, 27%−47%) for alloHCT versus 25% (95% CI, 17%−34%) for chemotherapy (P=0.08). These studies underscore the major value of alloHCT to mitigate relapse evidenced by relapse incidence rates of 22–50% at 3–5 years post alloHCT compared to 66–81% for CT consolidation,25, 28, 29, 42 similar to the relapse reduction that we observed of 66.5% after CT compared to 44.3% for alloHCT. Likewise, these studies also showed increased TRM after alloHCT, especially in the early post HCT months.25, 28, 29, 42
Even though adverse cytogenetics posed the major independent risk for treatment failure among all patients due to higher relapse rates, outcomes were particularly improved in the alloHCT subgroup; 5-year estimated OS and DFS in these high-risk patients were 20.2% and 15.9% after alloHCT compared with 2.6% and 0% after CT, respectively. However, this should be taken cautiously given the small number of patients in the subsets. The Koreth meta-analysis in younger AML patients showed a similar pattern of stronger advantage for alloHCT for adverse karyotype AML with an estimated absolute 11% 5-year OS benefit.24 Patients with favorable or intermediate risk cytogenetics experienced 5-year OS of 33.3% (95% CI 27.5 – 40.3) and DFS of 28.3% (95% CI 22.7 – 35.4) after alloHCT compared to 5 year OS of 19% (95% CI 10.2 – 35.9) and DFS of 15.9 (95% CI 7.5 −33) for CT. An imbalance in favorable cytogenetics favored CT (11%) compared to alloHCT (1.6%).24
Seventeen percent of our study patients were 70 years and older (33% in CT group and 9% in the alloHCT group). Patients aged 70 years and older did not experience worse overall outcomes in either the transplant or CT groups. While a limited sample size, this still represents the largest comparative study including those in their eighth decade and supports efforts to utilize fitness rather than age alone in determining transplant candidacy.
These data also suggest pathways to improve the poor long-term outcomes of older AML patients. This older group still requires special attention in recipient selection and treatment especially among alloHCT to reduce TRM43 perhaps by applying better geriatric performance assessment,44 donor selection16 and education about alloHCT.45
Alternative donors (haploidentical donors, UCB, or combinations of these) have also been increasingly used and can result in approximately 40% 2 year DFS and 50% OS in older patients with AML.17, 46–49 We found that UCB grafts were associated with higher TRM, and thus lower DFS and OS compared to the other donor grafts in the study. However, additional studies will be needed to delineate optimal alloHCT graft and donor type, including newer platforms such as haploidentical T-replete alloHCT.50 With the availability and improved outcomes of alternative donor grafts, prospective donor versus no donor studies have become even more difficult as nearly all patients have a suitable and available donor and reinforce the need for future comparative effectiveness studies of consolidation strategies
Important limitations in this study included the lack of comprehensive data on molecular profiling or minimal residual disease status for either population and having only cytogenetic data for disease characterization. Additionally, both cohorts lacked data on patient health including comorbidities or geriatric assessments. It should also be pointed out that the small number of older patients in the chemotherapy group reflects the relative paucity of cooperative group trials for older AML patients. In this regard, this patient population is likely to be highly selected for eligibility characteristics and likely reflects a healthier population of older AML patients who may not accurately represent the majority of older AML patients. By using left truncation, we excluded early deaths (prior to consolidation for the CT group and prior to HCT in the transplant group); however, this can also had a bias because CT group received consolidation earlier than allogeneic HCT. Regardless of these limitations, we found alloHCT as consolidation for AML patients 60 years and older improved long-term survival and DFS, primarily in the cytogenetically and clinically higher risk populations. We believe alloHCT should be actively considered for older AML patients in first remission with early donor identification and planning for the possibility of alloHCT soon after diagnosis,51 except in those with good or intermediate risk cytogenetics who also have co-morbidities that would increase transplant-related risks. Future studies to reduce TRM among alloHCT recipients and to reduce relapse after HCT or CT consolidation remain high priorities.
Supplementary Material
Acknowledgements:
We express our deepest appreciation to the late Dr. Arti Hurria who contributed to this work from inception to the first draft of the paper. We are forever indebted to her efforts. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 to the Alliance for Clinical Trials in Oncology NCORP Research Base (Jan C. Buckner, M.D., contact PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Other grant award numbers as follow: U10CA180819, CA180820, CA180794, CA180790, CA180791.
Footnotes
Conflict of Interest: The authors have no relevant conflict of interest to disclose
References:
- 1.O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, et al. Acute myeloid leukemia. J Natl Compr Canc Netw 2012. August; 10(8): 984–1021. [DOI] [PubMed] [Google Scholar]
- 2.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol 2014. August 20; 32(24): 2541–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oran B, Weisdorf DG. Survival for older patients with AML: a population based study. Haematologica 2012. July 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood 2006. May 1; 107(9): 3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006. March 1; 106(5): 1090–1098. [DOI] [PubMed] [Google Scholar]
- 6.van der Holt B, Lowenberg B, Burnett AK, Knauf WU, Shepherd J, Piccaluga PP, et al. The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood 2005. October 15; 106(8): 2646–2654. [DOI] [PubMed] [Google Scholar]
- 7.Lowenberg B, Suciu S, Archimbaud E, Ossenkoppele G, Verhoef GE, Vellenga E, et al. Use of recombinant GM-CSF during and after remission induction chemotherapy in patients aged 61 years and older with acute myeloid leukemia: final report of AML-11, a phase III randomized study of the Leukemia Cooperative Group of European Organisation for the Research and Treatment of Cancer and the Dutch Belgian Hemato-Oncology Cooperative Group. Blood 1997. October 15; 90(8): 2952–2961. [PubMed] [Google Scholar]
- 8.Lowenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009. September 24; 361(13): 1235–1248. [DOI] [PubMed] [Google Scholar]
- 9.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010. January 21; 115(3): 453–474. [DOI] [PubMed] [Google Scholar]
- 10.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood 2001. September 1; 98(5): 1312–1320. [DOI] [PubMed] [Google Scholar]
- 11.Frohling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Dohner K, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood 2006. November 15; 108(10): 3280–3288. [DOI] [PubMed] [Google Scholar]
- 12.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J CLin Oncol 2011. February 10; 29(5): 487–494. [DOI] [PubMed] [Google Scholar]
- 13.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood 1997. May 1; 89(9): 3323–3329. [PubMed] [Google Scholar]
- 14.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood 2011. February 24; 117(8): 2307–2318. [DOI] [PubMed] [Google Scholar]
- 15.Pasquini M, Wang Z, Horowitz MM, Gale RP. 2013. report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl 2013: 187–197. [PubMed] [Google Scholar]
- 16.Artz AS. Older patients/older donors: choosing wisely. Hematology Am Soc Hematol Educ Program 2013; 2013: 70–75. [DOI] [PubMed] [Google Scholar]
- 17.Sandhu KS, Brunstein C, DeFor T, Bejanyan N, Arora M, Warlick E, et al. Umbilical Cord Blood Transplantation Outcomes in Acute Myelogenous Leukemia/Myelodysplastic Syndrome Patients Aged >/=70 Years. Biol Blood Marrow Transplant 2016. February; 22(2): 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 2017. August 31; 130(9): 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood 2004. August 15; 104(4): 961–968. [DOI] [PubMed] [Google Scholar]
- 20.Wong R, Giralt SA, Martin T, Couriel DR, Anagnostopoulos A, Hosing C, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood 2003. October 15; 102(8): 3052–3059. [DOI] [PubMed] [Google Scholar]
- 21.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol 2017. April 10; 35(11): 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustun C, Courville EL, DeFor T, Dolan M, Randall N, Yohe S, et al. Myeloablative, but not Reduced-Intensity, Conditioning Overcomes the Negative Effect of Flow-Cytometric Evidence of Leukemia in Acute Myeloid Leukemia. Biol Blood Marrow Transplant 2016. April; 22(4): 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med 2015. September 17; 373(12): 1136–1152. [DOI] [PubMed] [Google Scholar]
- 24.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009. June 10; 301(22): 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devine SM, Owzar K, Blum W, Mulkey F, Stone RM, Hsu JW, et al. Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol 2015. November 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol 2010. April 10; 28(11): 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood 2007. February 15; 109(4): 1395–1400. [DOI] [PubMed] [Google Scholar]
- 28.Farag SS, Maharry K, Zhang MJ, Perez WS, George SL, Mrozek K, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60–70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant 2011. December; 17(12): 1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosawa S, Yamaguchi T, Uchida N, Miyawaki S, Usuki K, Watanabe M, et al. Comparison of Allogeneic Hematopoietic Cell Transplantation and Chemotherapy in Elderly Patients with Non-M3 Acute Myelogenous Leukemia in First Complete Remission. Biol Blood Marrow Tr 2011. March; 17(3): 401–411. [DOI] [PubMed] [Google Scholar]
- 30.Ustun C, Lazarus HM, Weisdorf D. To transplant or not: a dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transplant 2013. November; 48(12): 1497–1505. [DOI] [PubMed] [Google Scholar]
- 31.Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, DeAngelo DJ, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J Clin Oncol 2013. March 1; 31(7): 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uy GL, Mandrekar SJ, Laumann K, Marcucci G, Zhao WQ, Levis MJ, et al. A phase 2 study incorporating sorafenib into the chemotherapy for older adults with FLT3-mutated acute myeloid leukemia: CALGB 11001. Blood Adv 2017. January 24; 1(5): 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cripe LD, Uno H, Paietta EM, Litzow MR, Ketterling RP, Bennett JM, et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010. November 18; 116(20): 4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nand S, Othus M, Godwin JE, Willman CL, Norwood T, Erba HP, et al. A Phase II Trial of Azacitidine (NSC-102816) and Gemtuzumab Ozogamicin (NSC-720568) As Induction and Post-Remission Therapy in Patients of Age 60 and Older with Previously Untreated Non-M3 Acute Myeloid Leukemia (SWOG S0703): Report On the Poor Risk Patients. Blood 2012. November 16; 120(21). [Google Scholar]
- 35.Erba HP, Othus M, Walter RB, Kirschbaum MH, Tallman MS, Larson RA, et al. Four different regimens of farnesyltransferase inhibitor tipifarnib in older, untreated acute myeloid leukemia patients: North American Intergroup Phase II study SWOG S0432. Leuk Res 2014. March; 38(3): 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009. December; 15(12): 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017. January 26; 129(4): 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000. December 15; 96(13): 4075–4083. [PubMed] [Google Scholar]
- 39.Klein JP, Moeschberger ML. Survival Analysis – Techniques for Censored and Truncated Data, 2nd edn. Springer: New York, 2013. [Google Scholar]
- 40.Deschler B, Binek K, Ihorst G, Marks R, Wasch R, Bertz H, et al. Prognostic factor and quality of life analysis in 160 patients aged > or =60 years with hematologic neoplasias treated with allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2010. July; 16(7): 967–975. [DOI] [PubMed] [Google Scholar]
- 41.Koreth J, Aldridge J, Kim HT, Alyea EP 3rd, Cutler C, Armand P, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant 2010. June; 16(6): 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versluis J, Hazenberg CLE, Passweg JR, van Putten WLJ, Maertens J, Biemond BJ, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol 2015. October; 2(10): E427–E436. [DOI] [PubMed] [Google Scholar]
- 43.Versluis J, Labopin M, Niederwieser D, Socie G, Schlenk RF, Milpied N, et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia 2015. January; 29(1): 51–57. [DOI] [PubMed] [Google Scholar]
- 44.Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014. August; 99(8): 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randall J, Keven K, Atli T, Ustun C. Process of allogeneic hematopoietic cell transplantation decision making for older adults. Bone Marrow Transplant 2015. October 12. [DOI] [PubMed] [Google Scholar]
- 46.Ciurea SO, Shah MV, Saliba RM, Gaballa S, Kongtim P, Rondon G, et al. Haploidentical Transplantation for Older Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant 2017. September 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai SB, Rhodes J, Liu H, Shore T, Bishop M, Cushing MM, et al. Reduced-Intensity Allogeneic Transplant for Acute Myeloid Leukemia and Myelodysplastic Syndrome Using Combined CD34-Selected Haploidentical Graft and a Single Umbilical Cord Unit Compared with Matched Unrelated Donor Stem Cells in Older Adults. Biol Blood Marrow Transplant 2017. December 27. [DOI] [PubMed] [Google Scholar]
- 48.Weisdorf D, Eapen M, Ruggeri A, Zhang MJ, Zhong X, Brunstein C, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant 2014. June; 20(6): 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majhail NS, Brunstein CG, Tomblyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant 2008. March; 14(3): 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasamon YL, Bolanos-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of Nonmyeloablative HLA-Haploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. J Clin Oncol 2015. October 1; 33(28): 3152–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagel JM, Othus M, Garcia-Manero G, Fang M, Radich JP, Rizzieri DA, et al. Feasibility of Allogeneic Hematopoietic Cell Transplantation Among High-Risk AML Patients in First Complete Remission: Results of the Transplant Objective from the SWOG (S1203) Randomized Phase III Study of Induction Therapy Using Standard 7+3 Therapy or Idarubicin with High-Dose Cytarabine (IA) Versus IA Plus Vorinostat. Blood 2016; 128(22): 1166–1166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.