Abstract
Background:
Lead is an established neurotoxin and early life exposure to lead is associated with detrimental impacts on IQ and several neurobehavioral domains. Less is known, however, about effects of prenatal lead exposure below the CDC reference level on executive function and on social, emotional and self-regulatory behaviors in childhood.
Objectives:
To examine the association between prenatal lead exposure and mid-childhood executive function and social, emotional and self-regulatory behaviors.
Methods:
We included 1006 mother-child pairs from the Project Viva pre-birth cohort. We measured prenatal maternal lead in second-trimester erythrocytes. In mid-childhood (median 7.7 years), parents and teachers rated executive function related behaviors using the Behavior Rating Inventory of Executive Function (BRIEF) and behavioral difficulties using the Strengths and Difficulties Questionnaire (SDQ). We used multivariable linear regression models adjusted for maternal, paternal, and child characteristics and metal co-exposures.
Results:
Mean maternal erythrocyte lead concentration was 1.2 μg/dL (interquartile range [IQR] 0.8-1.5 μg /dL), which is approximately 0.4 μg/dL of lead in whole blood. For an IQR increase in lead, BRIEF Global Executive Composite (GEC) was 0.73 (95% CI: −0.06, 1.52) points higher for parent-rated scores and 0.42 (95% CI: −0.39, 1.23) points higher for teacher-rated scores, with higher scores indicating worse neurobehavioral outcomes. Associations were strongest for parent-rated BRIEF plan/organize (β= 0.85; 95% CI: 0.12, 1.59) and shift (β= 0.88; 95% CI: 0.01, 1.75) subscales, as well as the SDQ emotional problems subscale (β= 0.18; 95% CI: 0.03, 0.33).
Discussion:
In this cohort with lead levels an order of magnitude below the CDC reference level of 5 μg/dL in whole blood, greater prenatal lead concentrations were associated with poorer observed mid-childhood emotional problems and capacity to plan/organize and shift. Our findings suggest that prenatal maternal lead exposure can impair child behavior, even at levels relevant to current population concentrations, highlighting the importance of continuing efforts to eliminate lead exposure in the general population.
Keywords: environmental epidemiology, lead, prenatal exposure, neurobehavior, childhood
1. Introduction
Lead is a neurotoxic metal that is ubiquitous in the environment (ATSDR (Agency for Toxic Substances and Disease Registry), 2007; Sanders et al., 2009). Although the U.S. ban of leaded gasoline and paint reduced population lead levels and resulted in major health benefits (Centers for Disease Control (CDC), 2010; Schwartz et al., 1985; Silbergeld, 1997), the ubiquity of environmental lead from past industrial usage has made total eradication of exposure difficult. In pockets of urban and rural America, adverse neurological effects of lead remain a heavy burden since there is no known safe level of exposure (CDC, 2018a). Additionally, recent research has shown detrimental neurological impacts of childhood lead concentrations below the CDC reference level (5 μg/dL) that are still commonly measured among children today. Research interest has also turned to examine exposure during pregnancy, which is a vulnerable time of rapid neurodevelopment. Lead crosses the placenta and exposure to the developing fetus represents an even greater dose of lead per unit of body weight when compared to childhood exposure (ATSDR, 2007; CDC, 2010; World Health Organization (WHO), 2017). Lead in maternal bone can also be mobilized during pregnancy, acting as an endogenous source of lead (CDC, 2010; Gulson et al., 2003; Manton et al., 2003). An estimated 79% of fetal lead exposure is from mobilized maternal bone lead (Gulson et al., 2003). Furthermore, lead can cross the blood-brain barrier and penetrate fetal brain tissue (CDC, 2010; Goyer, 1990), raising concerns about fetal and child neurological health following prenatal lead exposure, even at current population levels.
Epidemiologic research has consistently demonstrated associations between lead exposure, occurring from early childhood through school age, and worse performance on tests of child central nervous system function (Bellinger et al., 1991, 1987; Schwartz, 1994; Surkan et al., 2007). Similarly, childhood lead exposure has been widely reported to have detrimental effects in multiple behavioral domains, including attention, cognitive efficiency, social behavior, visual-motor reasoning, and on tests related to executive function (Berent and Albers, 2010; Chiodo et al., 2004; Mazzocco and Ross, 2007). Executive function (EF) refers to a set of cognitive processes that facilitate regulation of behavior and achievement of goals (Espy et al., 2011; Skogan et al., 2016). These processes support goal motivated behaviors and are essential for successful response to shifting life demands (Rinsky and Hinshaw, 2011).
Previous childhood studies of the association between lead and EF have largely assessed EF through performance-based tests under standardized conditions and optimal performance situations (Toplak et al., 2013). For example, prior studies of childhood lead exposure have utilized various standardized performance-based tests to assess EF skills including working memory (Chiodo et al. 2004; Min et al. 2007; Ris et al. 2004; Surkan et al. 2007), inhibition (Chiodo et al., 2004; Kordas et al., 2006; Stewart et al., 2006), cognitive flexibility (Canfield et al., 2003; Chiodo et al., 2004; Surkan et al., 2007), and planning (Canfield et al. 2003; Chiodo et al. 2004; Surkan et al. 2007). Findings, however, have been inconclusive, and none of these aforementioned performance-based studies have evaluated EF capabilities in the child’s everyday environment. EF-related behavioral assessments such as EF rating scales may add a dimension to our understanding of lead’s impact on EF problem-solving processes and behavioral manifestations of EF in the real world (Gioia et al., 2002; Goldberg and Podell, 2000), but childhood lead research utilizing these scales to assess EF-related behavior is limited (Barg et al., 2018; Roy et al., 2009). Rating scales measure the extent to which individuals pursue goals under typical performance situations and unstructured conditions in daily life (Toplak et al., 2013).
Moreover, previous literature exploring the association between lead and EF in childhood has mainly examined effects of lead at levels that exceed US average concentrations and are therefore not generalizable to the current population (CDC, 2017). Prior work examining prenatal lead exposure has also generally not reported effects on EF or EF-related behaviors, but has primarily reported adverse associations of prenatal lead with childhood IQ (Taylor et al., 2017; Wasserman et al., 2000) as well as tasks of infant and toddler development and child cognition (Kim et al. 2018; Liu et al. 2014; Parajuli et al. 2015).
Our study evaluates the association of prenatal lead at levels relevant to current U.S. population averages and that are below the CDC reference level, with observations of EF-related tasks in the child’s everyday environment and social, emotional and self-regulatory behaviors. We investigated this relationship in Project Viva, a prospective pre-birth cohort. We hypothesized that higher prenatal lead levels would be associated with worse EF-related behaviors and social, emotional and self-regulatory behaviors in mid-childhood, even at prenatal exposures below the CDC reference level of 5 μg/dL whole blood, while simultaneously accounting for other neurotoxic metals (mercury, manganese). Additionally, we evaluated potential sex-specific associations of lead with EF and with behavioral difficulties given evidence from previous studies (Llop et al., 2013).
2. Methods
2.1. Study Participants
Study participants were mother-child pairs from Project Viva, a prospective, pre-birth cohort study. Details of Project Viva have been described previously (Oken et al., 2015). Briefly, pregnant women were recruited from eight clinical facilities (Atrius Harvard Vanguard Medical Associates) across eastern Massachusetts during their first prenatal visit from 1999–2002. Eligibility criteria included ability to understand English, less than 22 weeks gestation at the first prenatal visit, singleton births, and having no plans to move from the geographic area prior to delivery. The Harvard Pilgrim Health Care Institutional Review Board approved the recruitment procedure and mothers provided written informed consent at recruitment and follow-up. A total of 2128 live births were enrolled in Project Viva. Second trimester blood was collected from 1617 enrolled mothers. Of those, we included 1006 children with a mid-childhood assessment (at the 7-year visit) in the final sample for this analysis.
2.2. Measurement of Lead
We collected venous erythrocyte blood from pregnant women at the second trimester visit (median 27.9 weeks gestation). Lead was measured in erythrocytes as a secondary analysis, because the original study aims were related to gestational nutrition, birth outcomes and neurodevelopment, and we did not retain whole blood but did retain erythrocytes. More than 99% of lead in whole blood is found in the erythrocytes and this portion can be used to estimate lead exposure (Smith et al., 1998). Samples were collected in ethylenediaminetetraacetic acid vacutainer tubes and stored on ice for 24 hours before being sent to the Trace Metals Laboratory at Harvard T.H. Chan School of Public Health in Boston, MA. Samples were then centrifuged to separate plasma and erythrocytes, and erythrocytes were stored in −80° C freezers. Erythrocyte samples were prepared by digesting in nitric acid (HNO3) and hydrogen peroxide (H2O2) and then diluting with deionized water (Perkins et al., 2014). The concentration of lead in erythrocytes was measured as described previously using a dynamic reaction cell-inductively coupled plasma mass spectrometer (ICP-MS) (Perkins et al., 2014) (Elan DRC II, Perkin Elmer, Norwalk, CT). The limit of detection for lead in this study was 0.2 ng/ml in erythrocytes and one lead sample was below this LOD (0.1%). Quality control measures were analyzed with the calibration verification standards, procedural blanks, 1-ppb lead standard, and QC standard (Wu et al., 2017). Lead measurements were computed as the mean of five replicates (Wu et al., 2017). Recovery of the QC standard and spiked samples by this procedure was 90–110% (Perkins et al., 2014).
2.3. Neurobehavioral Assessment
Parents and teachers completed two neurobehavioral rating scale assessments about child participants in mid-childhood (median 7.7 years): the Behavior Rating Inventory of Executive Function (BRIEF) and the Strengths and Difficulties Questionnaire (SDQ). Mothers completed neurobehavioral assessments at the mid-childhood visit. We asked mothers for permission to contact their child’s teacher and mailed these assessments to the teacher if permission was granted. The BRIEF is a validated questionnaire designed to evaluate behaviors related to EF in children ages 5–18 years based on observer reporting (Gioia et al., 2000). The BRIEF questionnaire for parent- and teacher-rated scales consists of 86 items separated into eight subscales: emotional control, shift, inhibit, initiate, working memory, plan/organize, organization of materials, and monitor. Two indices are created by summing across subscales: (1) Behavioral Regulation Index, which is the sum of emotional control, shift, and inhibit subscale scores; and (2) Metacognition Index, which is the sum of initiate, working memory, plan/organize, organization of materials, and monitor subscale scores. Finally, an overall EF-related behaviors score, the Global Executive Composite (GEC) score, is created by summing the raw scores of all subscales (Sullivan and Riccio, 2007). All scores (subscales, indices, and GEC) are converted to T-scores (mean=50, SD=10) and represent age- and sex-standardized scores. Higher BRIEF scores indicate more executive function related behavioral dysfunction (Sullivan and Riccio, 2007), with T-scores greater than 65 considered clinically significant for executive dysfunction (McCandless and O’ Laughlin, 2007). Test-retest reliability correlations across scales for the normative sample range from 0.83–0.92 for teacher-rated and 0.76–0.85 for parent-rated scales (Gioia et al., 2000). Internal consistency was measured using Cronbach’s alpha and ranged from 0.80 and 0.98 (Gioia et al., 2000).
The SDQ is a screening questionnaire on which parents and teachers rate behavioral difficulties in children aged 4–16 years (Goodman and Goodman, 2009). The SDQ incorporates five scales: peer relationship problems, hyperactivity, emotional problems, conduct problems, and prosocial behavior, which are scored from 0–10 points. Higher scores indicate worse performance on all scales except the prosocial behavior scale, which is scored in the opposite direction. The total difficulties score is a composite SDQ score that is calculated by summing the scales of peer, hyperactivity, emotional, and conduct problems to obtain a maximum of 40 points (Goodman and Goodman, 2009). SDQ scores greater than the 90th percentile are considered within the clinically abnormal range (Goodman, 1997; Kremer et al., 2015; Mellor, 2005), corresponding to a score of 16 points or above for the US population (Bourdon et al., 2005; Goodman, 1997). The SDQ website (www.sdqinfo.com) provides the SDQ normative data and scoring information for the US. Test-retest correlations for the SDQ subscales ranged from 0.65 to 0.76 for parent-rated scales and 0.72 to 0.85 for teacher-rated scales (Stone et al., 2015, 2010). Internal consistency for the normative sample was measured using Cronbach’s alpha, ranging from 0.53 to 0.89 (Stone et al., 2015, 2010).
2.4. Covariates
We collected information on demographics (maternal age, annual household income, education status, marital status), maternal smoking status during pregnancy, and date of last menstrual period (LMP) through interviews and self-administered questionnaires in pregnancy. Data on maternal parity, date of delivery, birthweight, and hemoglobin clinical lab values were abstracted from medical records. We calculated length of gestation in days by subtracting the date of LMP from date of delivery. If gestational age according to the second trimester ultrasound differed from the LMP calculation by greater than 10 days, we used the ultrasound result to determine gestational duration. Gestational age was then converted from days to weeks. Child race/ethnicity was reported by the mother during early childhood. We measured manganese (μg/L) and mercury (ng/g) concentrations in erythrocytes from the same second trimester maternal blood samples used for lead measurements. Manganese was measured using ICP-MS (Elan DRC II, Perkin Elmer, Norwalk, CT) following the same method as described above for lead. The limit of detection for manganese was 2.0 μg/L, with one sample (0.1%) below this LOD. Mercury concentrations were analyzed using the Direct Analyzer 80 (Milestone Inc., Monroe, CT) as previously published (Oken et al., 2016). The detection limit for mercury was 0.5 ng/g of sample, and percent recovery for QC standards was 90–110% (Oken et al., 2016), with thirty-one samples (3.1%) below the LOD. We used the reported instrument values for all samples below the LOD.
At the mid-childhood visit, the Kaufman Brief Intelligence Test (KBIT-2) was administered to mothers as a measure of maternal IQ (Kaufman and Kaufman, 2004). We also administered the Home Observation Measurement of the Environment short form (HOME-SF). The HOME-SF assesses the emotional support and stimulation in a child’s home environment, with higher scores indicating more positive outcomes (Frankenburg and Coons, 1986).
2.5. Statistical Analysis
Summary statistics and distributional plots were examined for all variables. We evaluated distributions of metals and neurobehavioral scores for normality by testing model residuals using histogram inspection and Shapiro-Wilk testing. Model residuals were approximately normally distributed. In addition, we computed Spearman rank correlation coefficients between parent and teacher ratings for each neurobehavioral scale.
We evaluated associations between prenatal lead levels and neurobehavioral outcomes using multivariable regression. We assessed potential nonlinear associations between lead and neurobehavioral outcomes using generalized additive models with penalized splines restricted to four knots. Based on visual inspection of splines, most associations appeared approximately linear; therefore, lead concentrations were modeled as continuous linear terms, and scaled by the interquartile range (IQR) to compare the 75th with the 25th percentile.
In constructing multivariable models, baseline models (Model 1) controlled for age and sex (BRIEF scores are age- and sex-standardized; models of SDQ were adjusted for age and sex as covariates). Additionally, the main model (Model 2) was adjusted for the following covariates, based on prior literature and directed acyclic graphs (Hernán et al., 2002): maternal IQ, maternal smoking during pregnancy (self-reported as former or during pregnancy vs. never), nulliparity (yes vs. no), maternal and paternal education (college graduate yes vs. no), prenatal mercury (ng/g), prenatal manganese (μg/L), child race/ethnicity (black, Hispanic, Asian, other vs. white), HOME-SF score and household income modeled as an ordinal variable (≤$40,000; >$40,000–70,000; >$70,000). We examined sex-specific effects of lead by 1) stratifying models by child sex, and 2) including cross-product terms between lead and child sex in the models.
Data were missing on several important covariates (Table S1). We therefore imputed missing values using multiple imputation by chained equations, assuming data were missing at random (MAR) (Horton and Kleinman, 2007; White et al., 2011). We conducted multiple imputation using the MI procedure in SAS, generating 50 imputed datasets. All participants (n=2128) were included in the imputation, and all variables that might be related to the process causing missingness were included. These imputed datasets were then pooled using the MI analyze procedure in SAS. Primary data analysis was performed on the multiply imputed (MI) data, with mother-child pairs that had second trimester blood drawn and mid-childhood data (n=1006). In two separate sensitivity analyses, we (1) compared the MI results to the complete case analysis (n=625) and (2) adjusted for hemoglobin as a proxy for iron status. Data analysis was performed using SAS Version 9.4 and R Version 3.1.3 (R Foundation for Statistical Computing, Vienna).
3. Results
Of the 1006 mothers, the mean age at enrollment was 32.5 years, 72.1% were college graduates, and 71.4% were never smokers (Table 1). Most women were married or cohabitating (93.6%) and about half were nulliparous before the current pregnancy (48.6%). Mean (SD) gestational age of the infants at birth was 39.6 (1.6) weeks, 69.3% were white and 49.3% were female. Relative to mothers with second trimester lead concentrations below the median (1.1 μg/dL in erythrocytes), mothers with lead concentrations at or above the median were more likely to be former smokers (21.8% vs. 17.3%) or to have smoked during pregnancy (9.8% vs. 8.4%). Children in the higher lead group were also more likely to be black than children in the lower lead group (15.7% vs. 11.4%). Distributions of other characteristics were similar between lead groups (Table 1). Distributions of characteristics were also similar between boys and girls (Table S2). Characteristics of the imputed dataset were comparable to the characteristics of the complete case dataset with data on prenatal lead exposure and a neurobehavioral assessment in mid-childhood (Table S3).
Table 1.
Characteristics of study participants, overall and by maternal 2nd trimester lead exposure (n=1006)
| Characteristics | All participantsa |
Participants with < median lead exposure a, b |
Participants with >= median lead exposure a,b |
|---|---|---|---|
| Maternal Characteristics | |||
| Age at Enrollment (Years) (1999-2002), Mean ± SD | 32.5 ± 5.0 | 31.8 ± 5.0 | 33.1 ± 4.8 |
| College graduate, % | 72.1 | 72.1 | 72.2 |
| Nulliparous, % | 48.6 | 50.2 | 47.0 |
| Married or cohabitating, % | 93.6 | 93.8 | 93.5 |
| Smoking Status, % | |||
| Never | 71.4 | 74.4 | 68.5 |
| Former | 19.5 | 17.3 | 21.8 |
| Smoked During Pregnancy | 9.1 | 8.4 | 9.8 |
| Maternal combined KBIT score from age 7 visit, Mean ± SD | 107.9 ± 15.0 | 108.5 ± 15.0 | 107.4 ± 15.0 |
| Child Characteristics | |||
| Female, % | 49.3 | 48.6 | 50.0 |
| Gestational age (weeks) | 39.6 ± 1.6 | 39.7 ± 1.6 | 39.6 ± 1.6 |
| Race/Ethnicity, % | |||
| Asian | 3.2 | 1.8 | 4.6 |
| Black | 13.5 | 11.4 | 15.7 |
| Hispanic | 3.3 | 3.6 | 2.9 |
| White | 69.3 | 72.7 | 65.9 |
| Other | 10.7 | 10.6 | 10.9 |
| Paternal Characteristics | |||
| College graduate, % | 66.9 | 65.8 | 68.0 |
| Household Characteristics | |||
| Household income reported on early pregnancy questionnaire (annual), % | |||
| ≤40k | 14.7 | 14.3 | 15.2 |
| >$40-70K | 22.1 | 21.1 | 23.1 |
| >$70K | 63.2 | 64.6 | 61.7 |
| HOME-SF score, Mean ± SD | 18.4 ± 2.1 | 18.5 ± 2.1 | 18.4 ± 2.2 |
| Metals (2nd Trimester) | |||
| Lead (Pb) concentration (μg/dL), Median (IQR) | 1.1 (0.6) | 0.8 (0.3) | 1.5 (0.6) |
| Manganese (Mn) concentration (μg/L), Median (IQR) | 33.1 (11.8) | 32.2 (12.2) | 33.9 (11.7) |
| Mercury (Hg) concentration (ng/g), Median (IQR) | 3.1 (3.4) | 3.0 (3.3) | 3.3 (3.4) |
| Neurobehavioral Assessments | |||
| Parent BRIEF Global Executive Composite (GEC) score, Mean ± SD | 48.6 ± 9.1 | 48.2 ± 9.0 | 49.1 ± 9.1 |
| Teacher BRIEF GEC score, Mean ± SD | 50.6 ± 9.9 | 50.5 ± 9.9 | 50.7 ± 9.9 |
| SDQ Parent Total Difficulties score, Mean ± SD | 6.5 ± 4.6 | 6.2 ± 4.6 | 6.7 ± 4.7 |
| SDQ Teacher Total Difficulties score, Mean ± SD | 6.3 ± 5.8 | 6.2 ± 5.7 | 6.3 ± 5.8 |
| Child Age at Neurobehavioral Assessment (Years), Mean ± SD | 7.8 ± 0.8 | 7.8 ± 0.8 | 7.8 ± 0.8 |
Includes participants with multiply imputed variables.
Median lead level in this sample is 1.1 μg/dL.
The median (IQR) prenatal lead concentration was 1.1 (0.8 to 1.5) μg/dL in erythrocytes, which is roughly equivalent to 0.4 (0.3 to 0.5) μg/dL in whole blood, given that lead concentrations in erythrocytes are approximately three times higher than lead levels in whole blood during pregnancy (Perkins et al., 2014). Lead levels ranged from below the LOD to 9.8 μg/dL, with a standard deviation of 0.7 μg/dL. The mean ± SD (range) parent- and teacher-rated GEC BRIEF scores were 48.6 ± 9.1 (30–88) points and 50.6 ± 9.9 (30–100) points, respectively (Table 1). The mean ± SD (range) parent- and teacher-rated total difficulties SDQ scores were 6.5 ± 4.6 (0–30) points and 6.3 ± 5.8 (0–34) points, respectively (Table 1). Spearman rank correlations between parent and teacher ratings were moderate and positive (e.g., BRIEF: GEC, r =0.33; subscales, r =0.20–0.40; SDQ: total difficulties, r =0.43; subscales, r =0.24–0.53).
3.1. Associations between prenatal lead and mid-childhood BRIEF scores
From multivariable regression models, associations between prenatal lead levels and BRIEF scores were consistently positive, particularly for the parent-rated BRIEF scales (Figures 1, 2). In the baseline model for parent-rated BRIEF scores (Model 1), each IQR increase in erythrocyte lead concentration was associated with a 0.75 (95% CI: −0.02, 1.52) point higher overall GEC score (Table 2). In the fully adjusted model (Model 2), associations remained similar: each IQR increase in erythrocyte lead was associated with a 0.73 (95% CI: −0.06, 1.52) point higher parent-rated GEC score and a 0.85 (95% CI: 0.12, 1.59) point higher plan/organize subscale score (Model 2). Higher lead levels were also associated with worse performance on the parent-rated shift subscale (β= 0.88; 95% CI: 0.01, 1.75), corresponding to a 0.10 standard deviation increase per IQR increase in lead. Effect estimates from models of teacher-rated BRIEF subscales were smaller in magnitude to models of parent ratings, and all confidence intervals included the null value (Table 2).
Figure 1.
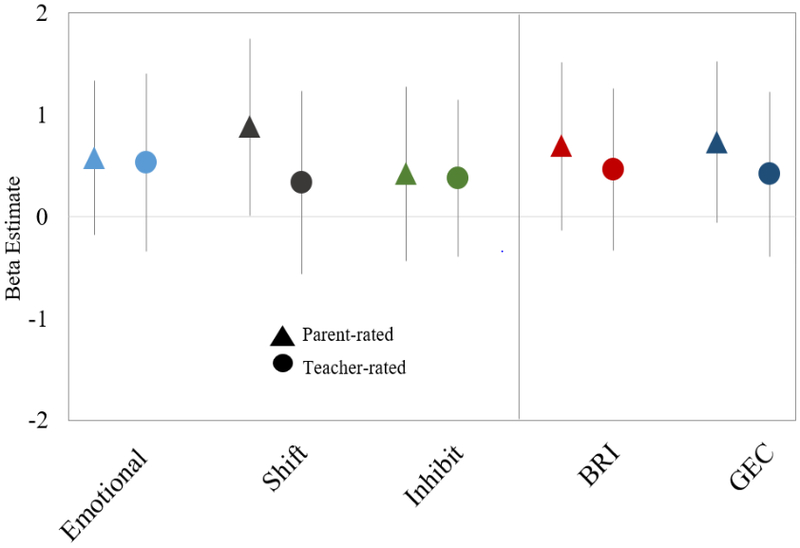
Regression coefficients for associations of maternal 2nd trimester blood lead levels with Behavioral Regulation Index (BRI) BRIEF scores in mid-childhood. Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL). Model 2: Outcomes standardized for child age and sex; models adjusted for maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income; and child race/ethnicity.
Figure 2.
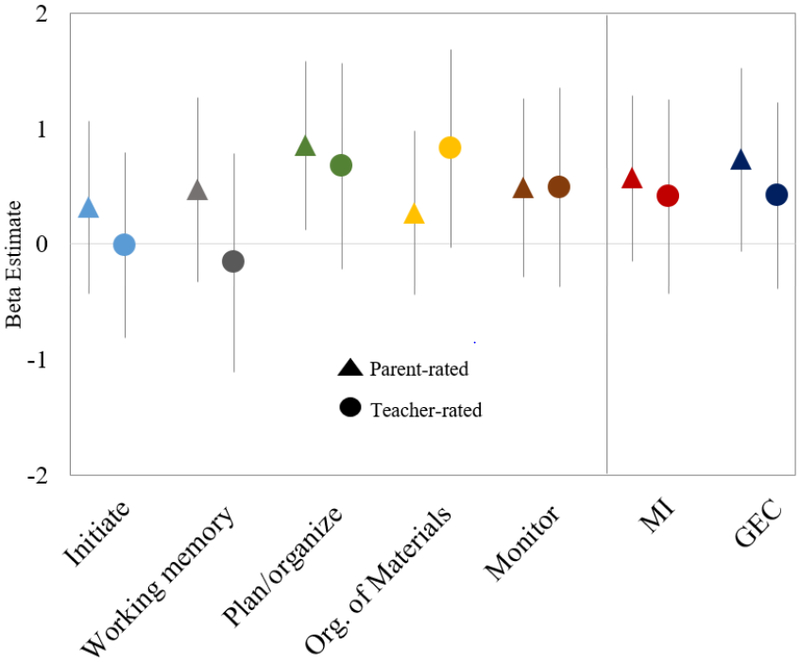
Regression coefficients for associations of maternal 2nd trimester blood lead levels with Metacognition Index (MI) BRIEF scores in mid-childhood. Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL). Model 2: Outcomes standardized for child age and sex; models adjusted for maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income; and child race/ethnicity.
Table 2.
Regression coefficients for associations of maternal 2nd trimester blood lead levels with BRIEF scores in mid-childhood (n=1006)
| Model 1a β (95% CI) |
Model 2b β (95% CI) |
|
|---|---|---|
| Parent-rated BRIEF Scales | ||
| Behavioral Regulation Index | 0.63 (−0.16, 1.42) | 0.69 (−0.13, 1.51) |
| Emotional control | 0.50 (−0.24, 1.24) | 0.58 (−0.18, 1.34) |
| Shift | 0.79 (−0.03, 1.61) | 0.88 (0.01, 1.75) |
| Inhibit | 0.42 (−0.39, 1.24) | 0.42 (−0.44, 1.28) |
| Metacognition Index | 0.64 (−0.07, 1.34) | 0.57 (−0.15, 1.29) |
| Initiate | 0.37 (−0.35, 1.09) | 0.31 (−0.44, 1.06) |
| Working memory | 0.64 (−0.13, 1.41) | 0.47 (−0.33, 1.27) |
| Plan/organize | 0.91 (0.20, 1.62) | 0.85 (0.12, 1.59) |
| Organization of materials | 0.21 (−0.50, 0.91) | 0.27 (−0.44, 0.98) |
| Monitor | 0.53 (−0.22, 1.28) | 0.49 (−0.29, 1.26) |
| General Executive Composite | 0.75 (−0.02,1.52) | 0.73 (−0.06, 1.52) |
| Teacher-rated BRIEF Scales | ||
| Behavioral Regulation Index | 0.41 (−0.41, 1.21) | 0.46 (−0.34, 1.26) |
| Emotional control | 0.50 (−0.38, 1.39) | 0.53 (−0.34, 1.41) |
| Shift | 0.22 (−0.67, 1.12) | 0.34 (−0.56, 1.23) |
| Inhibit | 0.37 (−0.39, 1.12) | 0.38 (−0.39, 1.14) |
| Metacognition Index | 0.37 (−0.48, 1.22) | 0.41(−0.43, 1.25) |
| Initiate | −0.002 (−0.81, 0.80) | −0.01 (−0.81, 0.79) |
| Working memory | −0.17 (−1.12, 0.79) | −0.16 (−1.11, 0.78) |
| Plan/organize | 0.61 (−0.30, 1.51) | 0.67 (−0.22, 1.57) |
| Organization of materials | 0.77 (−0.06, 1.61) | 0.82 (−0.03, 1.68) |
| Monitor | 0.44 (−0.43, 1.31) | 0.49 (−0.38, 1.35) |
| General Executive Composite | 0.37 (−0.45, 1.20) | 0.42 (−0.39, 1.23) |
Note: Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL).
Model 1: Outcomes standardized for child age and sex.
Model 2: Model 1 additionally adjusted for maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income; and child race/ethnicity.
We also explored possible sex-specific effects in these data. In fully adjusted models stratified by sex, associations between lead and BRIEF scores were stronger among girls than boys on most subscales. For example, each IQR increase in erythrocyte lead concentration was associated with a 1.17 (95% CI: 0.06, 2.28) point higher (worse) parent-rated GEC score among girls, while the association was weaker among boys (β = 0.47; 95% CI: −0.58, 1.51) (Table 3). Similarly, higher lead levels were associated with worse performance for girls on the parent-rated plan/organize scale (β= 1.05, 95% CI: 0.11, 1.98) and Behavioral Regulation Index (β= 1.03, 95% CI: 0.01, 2.04); among boys, these associations were weaker. However, in models including cross-product terms between lead and sex, there was little evidence that associations with BRIEF scores varied by sex (Table S4). Associations with teacher-rated BRIEF subscales were generally in the same direction as parent-rated subscales, but most were smaller in magnitude, particularly when comparing across scores for girls.
Table 3.
Regression coefficients for associations of maternal 2nd trimester blood lead levels with BRIEF scores in mid-childhood, by sex
| Girlsa β (95% CI) |
Boysa β (95% CI) |
|
|---|---|---|
| Parent-rated BRIEF Scales | ||
| Behavioral Regulation Index | 1.03 (0.01, 2.04) | 0.51 (−0.63, 1.66) |
| Emotional Control | 1.04 (−0.002, 2.08) | 0.36 (−0.70, 1.42) |
| Shift | 0.77 (−0.23, 1.77) | 0.87 (−0.35, 2.10) |
| Inhibit | 0.78 (−0.38, 1.93) | 0.21 (−0.92, 1.34) |
| Metacognition Index | 0.89 (−0.06, 1.85) | 0.36 (−0.63, 1.35) |
| Initiate | 0.90 (−0.14, 1.94) | −0.002 (−1.02, 1.02) |
| Working memory | 0.83 (−0.27, 1.92) | 0.25 (−0.82, 1.32) |
| Plan/organize | 1.05 (0.11, 1.98) | 0.70 (−0.34, 1.74) |
| Organization of materials | 0.49 (−0.46, 1.45) | 0.12 (−0.81, 1.05) |
| Monitor | 0.62 (−0.36, 1.60) | 0.39 (−0.68, 1.45) |
| General Executive Composite | 1.17 (0.06, 2.28) | 0.47 (−0.58, 1.51) |
| Teacher-rated BRIEF Scales | ||
| Behavioral Regulation Index | 0.56 (−0.68, 1.81) | 0.45 (−0.54, 1.44) |
| Emotional control | 0.83 (−0.53, 2.19) | 0.40 (−0.65, 1.46) |
| Shift | 0.45 (−0.91, 1.82) | 0.30 (−0.84, 1.44) |
| Inhibit | 0.24 (−0.93, 1.41) | 0.47 (−0.50, 1.45) |
| Metacognition Index | 0.54 (−0.78, 1.87) | 0.41 (−0.69, 1.50) |
| Initiate | 0.09 (−1.19, 1.37) | −0.02 (−1.06, 1.03) |
| Working memory | −0.02 (−1.45, 1.41) | −0.16 (−1.40, 1.08) |
| Plan/organize | 0.81 (−0.59, 2.21) | 0.65 (−0.49, 1.80) |
| Organization of materials | 0.96 (−0.41, 2.32) | 0.80 (−0.29, 1.89) |
| Monitor | 0.62 (−0.79, 2.03) | 0.49 (−0.57, 1.54) |
| General Executive Composite | 0.53 (−0.75, 1.82) | 0.41 (−0.60, 1.43) |
Note: Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL).
Model 2: Outcomes standardized for child age and sex; models adjusted for maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income; and child race/ethnicity.
3.2. Associations between prenatal lead and mid-childhood SDQ scores
Overall, associations between prenatal lead and SDQ subscales were consistently positive, suggesting adverse effects of lead, for both parent- and teacher-rated SDQ scales (Figure 3). In baseline models for parent-rated SDQ scores adjusted for age and sex only (Model 1), each IQR increase in maternal erythrocyte lead concentration was associated with a 0.18 (95% CI= 0.04, 0.32) point higher emotional problems subscale score and a 0.40 (95% CI= 0.01, 0.79) point higher total difficulties SDQ score (Table 4). In the fully adjusted model (Model 2), associations were similar to the baseline model: an IQR increase in lead was associated with a 0.18 (95% CI= 0.03, 0.33) point higher parent-rated SDQ emotional problems score, and a 0.36 (95% CI: −0.04, 0.77) point higher parent-rated total difficulties score, corresponding to a 0.08 standard deviation increase per IQR increase in lead. Although associations with teacher ratings were similar in direction and trend as parent ratings, confidence intervals were generally wider and all crossed the null (Table 4).
Figure 3.
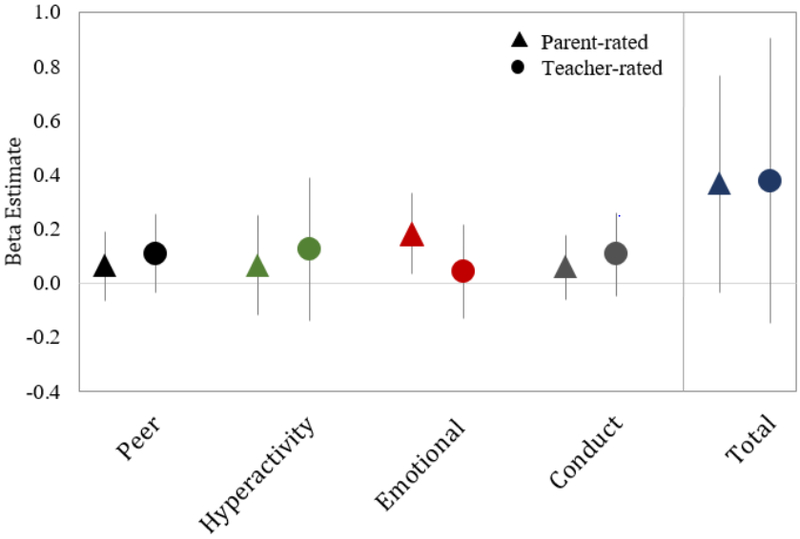
Regression coefficients for associations of maternal 2nd trimester blood lead levels with SDQ scores in mid-childhood. Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL). Total Difficulties is calculated by summing the scales of emotional problems, conduct problems, hyperactivity, and peer relationship problems. Model 2: Adjusted for child age, sex and race/ethnicity; maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income.
Table 4.
Regression coefficients for associations of maternal 2nd trimester erythrocyte lead levels with SDQ scores in mid-childhood (n=1006)
| Model 1a β (95% CI) |
Model 2b β (95% CI) |
|
|---|---|---|
| Parent-rated SDQ Scales | ||
| Total Difficulties | 0.40 (0.01, 0.79) | 0.36 (−0.04, 0.77) |
| Peer | 0.07 (−0.05, 0.20) | 0.06 (−0.06, 0.19) |
| Hyperactivity | 0.10 (−0.08, 0.28) | 0.06 (−0.12, 0.25) |
| Emotional | 0.18 (0.04, 0.32) | 0.18 (0.03, 0.33) |
| Conduct | 0.05 (−0.07, 0.17) | 0.06 (−0.06, 0.18) |
| Prosocial | −0.09 (−0.22, 0.04) | −0.08 (−0.21, 0.05) |
| Teacher-rated SDQ Scales | ||
| Total Difficulties | 0.30 (−0.23, 0.83) | 0.38 (−0.15, 0.91) |
| Peer | 0.09 (−0.06, 0.23) | 0.11 (−0.04, 0.25) |
| Hyperactivity | 0.10 (−0.16, 0.36) | 0.12 (−0.14, 0.39) |
| Emotional | 0.01 (−0.16, 0.18) | 0.04 (−0.13, 0.21) |
| Conduct | 0.10 (−0.05, 0.26) | 0.11 (−0.05, 0.26) |
| Prosocial | −0.11 (−0.29, 0.06) | −0.12 (−0.30, 0.06) |
Note: Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL); Total Difficulties is calculated by summing the scales of emotional problems, conduct problems, hyperactivity, and peer relationship problems; Higher scores indicate worse performance on all scales with the exception of the prosocial behavior scale. The prosocial behavior scale is scored in the opposite direction.
Model 1: Adjusted for child age and sex.
Model 2: Model 1 additionally adjusted for maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income; and child race/ethnicity.
In sex-stratified models, positive (i.e. adverse) associations were stronger among girls than boys on SDQ parent- and teacher-rated subscales. For example, each IQR increase in erythrocyte lead concentration was associated with a 0.72 (95% CI: 0.16, 1.27) point higher parent-rated total difficulties score among girls; in boys, this association was closer to null (β= 0.16; 95% CI: −0.38, 0.70) (Table 5). In addition, higher lead levels were associated with worse performance on parent-rated emotional problems (β= 0.31, 95% CI: 0.11, 0.52) and hyperactivity (β= 0.18, 95% CI: −0.09, 0.45) scales for girls, but this association was attenuated for boys (Table 5). Associations of lead were weaker for teacher-rated subscales, among both boys and girls in stratified models. Furthermore, none of the cross product terms for effect modification by sex suggested that associations between lead and neurobehavioral outcomes varied by sex (Table S5).
Table 5.
Regression coefficients for associations of maternal 2nd trimester erythrocyte lead levels with SDQ scores in mid-childhood, by sex
| Girlsa β (95% CI) |
Boysa β (95% CI) |
|
|---|---|---|
| Parent-rated SDQ Scales | ||
| Total Difficulties | 0.72 (0.16, 1.27) | 0.16 (−0.38, 0.70) |
| Peer | 0.15 (−0.003, 0.30) | 0.01 (−0.16, 0.19) |
| Hyperactivity | 0.18 (−0.09, 0.45) | 0.002 (−0.25, 0.26) |
| Emotional | 0.31 (0.11, 0.52) | 0.10 (−0.10, 0.30) |
| Conduct | 0.08 (−0.08, 0.24) | 0.04 (−0.11, 0.19) |
| Prosocial | −0.12 (−0.32, 0.07) | −0.06 (−0.24, 0.13) |
| Teacher-rated SDQ Scales | ||
| Total Difficulties | 0.51 (−0.19, 1.20) | 0.29 (−0.43, 1.02) |
| Peer | 0.16 (−0.03, 0.36) | 0.06 (−0.15, 0.27) |
| Hyperactivity | 0.17 (−0.16, 0.50) | 0.11 (−0.26, 0.48) |
| Emotional | 0.07 (−0.18, 0.33) | 0.01 (−0.20, 0.22) |
| Conduct | 0.10 (−0.07, 0.28) | 0.11 (−0.10, 0.32) |
| Prosocial | −0.23 (−0.50, 0.05) | −0.06 (−0.31, 0.20) |
Note: Coefficients represent change in score for an IQR increase in maternal erythrocyte lead (0.6 μg/dL); Total Difficulties is calculated by summing the scales of emotional problems, conduct problems, hyperactivity, and peer relationship problems. Higher scores indicate worse performance on all scales with the exception of the prosocial behavior scale. The prosocial behavior scale is scored in the opposite direction.
Model 2: Adjusted for child age and race/ethnicity; maternal 2nd trimester mercury and manganese levels, nulliparity, smoking during pregnancy, IQ, and education; paternal education; HOME composite score and household income.
In sensitivity analyses, results generated by complete case analysis models (n=625) showed consistency in direction when compared with MI models for most subscales (Table S6-S7). Adjusting the model further for hemoglobin levels did not change the results of this analysis.
4. Discussion
In this cohort of school age children, higher average prenatal erythrocyte lead levels predicted poorer childhood ability to plan/organize and shift tasks and more emotional problems, as rated by parents responding to the BRIEF and SDQ assessments. Associations between lead and parent-rated EF-related behaviors were consistent across subscales, indicating poorer observed childhood performance with higher prenatal lead exposures. While effect estimates are modest, these data nonetheless suggest that lead exposure at levels even lower than NHANES average population levels still has the capacity to alter the developing brain. We observed similar trends for teacher-rated scales, though associations were attenuated. The Project Viva cohort is a largely suburban population and the mean erythrocyte lead level observed in this cohort is equivalent to ~0.40 μg/dL in whole blood. These results provide support for the hypothesis that greater exposure to lead prenatally is associated with poorer childhood neurobehavioral function, even in a suburban population not thought to be at high risk of exposure and with blood lead levels that are similar to those common in the U.S. today. These findings underline prior reports (Lanphear, 2017; Lanphear et al., 2005; Shefa and Héroux, 2017) of the lack of a detectable threshold for adverse effects of lead on neurobehavioral outcomes.
Previous research on lead exposure and EF has predominantly focused on exposure during childhood, whereas our study was able to evaluate lead levels during the prenatal period. Notwithstanding this difference in the exposure timeframe, our EF results show consistent directionality with those from a study in India in which childhood lead levels (mean whole blood lead: 11.4 μg/dL) were studied in relation to the BRIEF questionnaire (Roy et al. 2009). This finding lends support to the idea that the prenatal period is an important window of susceptibility to lead with respect to EF, even in a population with relatively higher SES than urban or rural populations. Roy et al. (2009) exclusively measured teacher-rated GEC for the BRIEF and estimated that each 1 μg/dL increase in log blood lead was associated with a 0.42 point higher (worse) age- and sex-standardized GEC score (β = 0.42; 95% CI: 0.18, 0.65) (Roy et al., 2009). Another study from Uruguay evaluating childhood blood lead (mean whole blood lead: 4.2 μg/dL) and BRIEF teacher-rated executive function related behaviors reported poorer ability to inhibit inappropriate behaviors with higher lead levels (prevalence ratio = 1.01; 95% CI: 1.00, 1.03) in a small sample of participants (n=206). This study also found slightly worse overall behavioral regulation in girls when compared to boys, similar to our findings of stronger adverse associations for girls (Barg et al., 2018). We are unaware of any previous studies on lead exposures during prenatal development that evaluated EF-related behavioral assessment rating scales. Our findings were also similar to some studies that utilized EF performance-based tests, such as the Wisconsin Card Sorting Test (WCST) to evaluate cognitive flexibility (set shifting) (Chiodo et al., 2004; Surkan et al., 2007) and the Cambridge Neuropsychological Test Automated Battery (CANTAB) Stockings of Cambridge (SOC) subtest to evaluate planning (Canfield et al., 2004); These studies reported that higher lead levels were associated with worse performance. In contrast to our results, a previous New England study found no association between blood lead and scores on tests of shifting as assessed by the Stroop Color-Word test and the Trail Making Test B, or with planning evaluated using the WISC-III Mazes subtest (Surkan et al. 2007). This analysis, however, was performed on a smaller sample (n<400) and was a cross-sectional analysis. Importantly, none of these previous studies examined associations of EF with prenatal lead. In all of the aforementioned studies, mean lead levels in whole blood ranged from 2.3 to 11.5 μg/dL (Barg et al., 2018; Canfield et al., 2004; Chiodo et al., 2004; Roy et al., 2009; Surkan et al., 2007), which are higher than levels in our study (mean and median equivalent to 0.40 and 0.36 μg/dL, respectively, in whole blood) and higher than the average U.S. lead levels reported in the most recent NHANES 2013–2014 data (geometric mean for women: 0.75 μg/dL; median for women: 0.73 μg/dL) (CDC, 2017).
Our results are also similar to previous literature on lead exposure and behavioral difficulties as assessed by the Strengths and Difficulties Questionnaire (SDQ). A longitudinal study in a Flemish cohort found that the odds of having an abnormal SDQ total difficulties score were 5.08 times higher for children in the highest prenatal lead concentration tertile (95% CI: 1.36, 19.18) compared to children in the lowest tertile (median lead level: 1.4 μg/dL) (Sioen et al., 2013). Additionally, a cross-sectional study of 6–11 year old Canadian children reported positive (i.e. adverse) associations between blood lead levels (geometric mean: 0.90 μg/dL) and total difficulties on the SDQ (Arbuckle et al., 2016). Lead levels in this study were more comparable to current U.S. population levels, but lead was measured during childhood and not prenatally.
In our study, the effect estimates were similar in direction for parent- and teacher-rated assessments, but slightly attenuated in models of teacher-rated scores. Behavioral ratings completed by parents may diverge from teachers’ ratings for several reasons, including the variation of child behaviors in different settings (i.e. contextual differences in school vs. home and comparisons of large groups of children vs. small). Teachers evaluate children relative to other classmates of the same age, although subtle behavioral differences may not be distinguishable given the limited time students spend in school compared to time spent at home. Additionally, parents and teachers may rate students differently based on factors other than the child’s behavior, such as parental stress or classroom stress, allowing for potential rater bias (Dekker 2017; Stone 2010). This bias is a limitation of rating scales, but these rating scales permit us to assess another dimension of the lead-EF landscape in the real world, rather than assessing lead effects with isolated performance-based tests alone. Furthermore, the parents with higher lead exposure may themselves have cognitive impairments that affect their parenting behaviors and the assessment of their child’s EF and behavioral difficulties. Although misclassification is possible for both raters, the consistent trend across parent and teacher ratings in our study reduces concerns about outcome misclassification. Furthermore, HOME score was similar between high and low lead groups (Table 1), indicating comparability of home environment and the potential for similar parental influence, despite differences in lead exposure level.
Although effect estimates were at magnitudes that may be considered subclinical for individual children, these results are important at the population level. For our study, 4.6% and 5.6% of children were above the clinical cut-point for the BRIEF and SDQ, respectively. The increase of 0.73 points on the parent-rated GEC score and 0.36 points on the parent-rated total difficulties score contributes to distributional shifts in the proportion of adverse neurobehavioral outcomes. These shifts increase the percent of children who meet the clinical threshold for behavioral dysfunction population wide, as indicated by poorer EF-related performance and more behavioral difficulties.
Potential implications of poor EF performance are far-reaching. EF is mediated by frontal lobe brain systems and regulates higher order cognitive functions, including working memory, planning and organization, capacity to inhibit inappropriate responses, initiation of activity, monitoring of behavior and cognitive flexibility (set shifting) (Otero and Barker, 2014). Childhood EF deficits have been associated with worse measures of academic achievement (Biederman et al., 2006; Diamantopoulou et al., 2007; Miller and Hinshaw, 2010) and social functioning (Miller and Hinshaw, 2010) that can continue through later childhood and adolescence (Miller and Hinshaw, 2010). EF has also been implicated in attention deficit hyperactivity disorder (ADHD), the most common neurobehavioral disorder in childhood, with an estimated prevalence of 5–10% (Aguiar et al., 2010; Eubig et al., 2010). Deficits in EF are significantly more common for individuals with ADHD and are considered a comorbidity of the disorder (Biederman et al., 2006; Nigg et al., 2005). Furthermore, prior research has described both ADHD and poor EF as predictors of subsequent delinquent behaviors (Burton et al., 2016; Fletcher and Wolfe, 2009; Savolainen et al., 2010; Wright et al., 2008). Shifting our attention to the prenatal period for elimination of early life EF risk factors, such as fetal lead exposure even at low levels, is therefore important for reducing later life impacts.
Though effect estimates for sex-specific cross-product terms were not statistically significant, we estimated stronger adverse associations between lead and neurobehavioral outcomes among girls across most subtests when compared with boys in mid-childhood. The results suggest greater susceptibility to lead among girls for these behavioral outcomes, but these findings should be confirmed by additional studies. Sex-specific differences in the effects of in utero lead exposure have been reported in previous toxicological studies (Bunn et al. 2001a; Ronis et al. 1998; Virgolini et al. 2008) as well as epidemiologic studies of childhood IQ, attention, visuoconstruction, visuomotor performance, and internalizing behaviors (Llop et al., 2013). The direction of these sex-specific associations has been inconsistent, however, and findings have differed across domains. Hormonal factors influencing the bioavailability of lead as well as hormone-based immunological response mechanisms may explain some of these sex-specific differences (Bunn et al., 2001b; Llop et al., 2013).
Another potential explanation for our finding of higher lead sensitivity among girls may relate to ADHD prevalence and the use of medications in the U.S. The U.S. prevalence of ADHD in boys is about twice that of girls (Pastor et al., 2015), and an estimated 69% of 6–11 year olds with an ADHD diagnosis are taking medications (CDC, 2018b). Thus, we might expect that more boys currently take ADHD medications to treat behavioral and/or EF difficulties. Consequently, it is possible that lead appears to be more harmful among girls because a larger proportion of boys are receiving benefits from ADHD medications. However, only 6.1% (4.8% boys, 1.3% girls) of reporting participants (n=933) had ever been told by a health care professional that they had ADHD or Attention Deficit Disorder (ADD). While the lack of data on medication use among children is a limitation of our study, the prevalence of reported ADHD here is low, and the use of ADHD medications is not likely to explain the sex differences we estimated. Our findings related to sex-specific associations between lead with executive function related behaviors and behavioral difficulties are hypothesis-generating and should be investigated further as part of future research.
There are several limitations to our study. We lack information on postnatal lead levels, which precludes us from determining if observed associations are due to prenatal versus postnatal lead exposure, as is the case for all longitudinal studies with a single exposure timepoint. Participants were mostly white and middle class with health insurance. Although this homogeneity limits generalizability of our findings, it also reduces the potential for residual confounding. Exposure to lead was estimated in erythrocytes for this study, a method that is less common than measuring lead in whole blood. While not customary, this erythrocyte biomarker is nonetheless valid for ranking exposure and thus the observed associations are relevant and valid. Additionally, the literature demonstrates that 99% of whole blood lead is contained in erythrocytes and erythrocyte lead is highly correlated with whole blood lead (r=0.998) (ATSDR, 2007; Chen et al., 2014). We converted lead concentrations in erythrocytes to lead concentrations in whole blood to allow for comparability and generalizability, with our median lead levels roughly equivalent to 0.4 μg/dL in whole blood (Perkins et al., 2014). Finally, selection bias from differential loss to follow-up over the seven-year period is possible if, for example, children with higher prenatal lead exposure also had behavioral difficulties that made it challenging to participate in the follow-up visits. This scenario would bias findings toward the null because we would not be capturing participants with high lead exposure that experienced adverse neurobehavioral outcomes, and the true association may actually be stronger. Baseline characteristics of participants included in the analysis were also similar to the characteristics of the entire cohort overall (Table S8).
Our study has many strengths. This study is among the first to evaluate the relationship between EF rating scales and prenatal lead exposure at levels lower than the CDC reference level and that are more reflective of current-day exposure in the U.S. Our rich covariate data allowed us to control for many important potential confounding variables. Furthermore, observed associations were robust to confounder adjustment, as additional adjustment of baseline models by maternal IQ, nulliparity, smoking during pregnancy, maternal education, 2nd trimester mercury and manganese levels, paternal education, HOME composite score, household income, and child race/ethnicity did not substantially change effect estimates. Additionally, our sample (n=1006) was larger than other studies of lead and EF rating scales. Moreover, the prospective design with extended follow-up at 7 years of age permitted us to assess long-term EF-related behavior and behavioral difficulties associated with lead exposure from as early as the second trimester.
Conclusion
Although population lead levels continue to decrease over time, exposure to lead at health relevant levels persists. Our research focused on prenatal lead exposure and executive function, which may be a more sensitive endpoint than IQ, in a suburban, relatively affluent cohort. We found linear associations suggesting that prenatal lead exposure can impair child behavior. Our findings demonstrate lead’s continued impact on neurobehavior, even at levels that are a full order of magnitude below the current CDC reference level of 5 μg/dL and at population relevant concentrations, highlighting the importance of sustained efforts to eliminate lead exposure across the population.
Supplementary Material
Acknowledgements:
Funding sources
This work was supported by the National Institutes of Health grant numbers T32ES014562, R00ES022986, R01HD 034568, UG3OD023286, R01ES016314, P30ES023515, R01ES013744.
Footnotes
Declaration of interest: none
References
- Aguiar A, Eubig PA, Schantz SL, 2010. Attention Deficit/Hyperactivity Disorder: A Focused Overview for Children’s Environmental Health Researchers. Environ. Health Perspect. 118, 1646–1653. 10.1289/ehp.1002326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J, 2016. Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6–11 years of age: CHMS 2007–2009. NeuroToxicology 54, 89–98. 10.1016/j.neuro.2016.03.014 [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), 2007. Toxicological Profile for Lead [WWW Document]. URL (accessed 3.12.18). [PubMed]
- Barg G, Daleiro M, Queirolo EI, Ravenscroft J, Mañay N, Peregalli F, Kordas K, 2018. Association of Low Lead Levels with Behavioral Problems and Executive Function Deficits in Schoolers from Montevideo, Uruguay. Int. J. Environ. Res. Public. Health 15, 2735 10.3390/ijerph15122735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M, 1987. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N. Engl. J. Med. 316, 1037–1043. 10.1056/NEJM198704233161701 [DOI] [PubMed] [Google Scholar]
- Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C, 1991. Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics 87, 219–227. [PubMed] [Google Scholar]
- Berent S, Albers JW, 2010. Neurobehavioral Toxicology: Neurological and Neuropsychological Perspectives, Volume III: Central Nervous System. Taylor & Francis. [Google Scholar]
- Biederman J, Petty C, Fried R, Fontanella J, Doyle AE, Seidman LJ, Faraone SV, 2006. Impact of psychometrically defined deficits of executive functioning in adults with attention deficit hyperactivity disorder. Am. J. Psychiatry 163, 1730–1738. 10.1176/ajp.2006.163.10.1730 [DOI] [PubMed] [Google Scholar]
- Bourdon KH, Goodman R, Rae DS, Simpson G, Koretz DS, 2005. The Strengths and Difficulties Questionnaire: U.S. Normative Data and Psychometric Properties. J. Am. Acad. Child Adolesc. Psychiatry 44, 557–564. 10.1097/01.chi.0000159157.57075.c8 [DOI] [PubMed] [Google Scholar]
- Bunn TL, Dietert RR, Ladics GS, Holsapple MP, 2001a. Developmental Immunotoxicology Assessment in the Rat: Age, Gender, and Strain Comparisons After Exposure to Lead. Toxicol. Methods 11, 41–58. 10.1080/105172301300055151 [DOI] [Google Scholar]
- Bunn TL, Parsons, Kao E, Dietert RR, 2001b. Exposure to Lead during Critical Windows of Embryonic Development: Differential Immunotoxic Outcome Based on Stage of Exposure and Gender. Toxicol. Sci. 64, 57–66. 10.1093/toxsci/64.1.57 [DOI] [PubMed] [Google Scholar]
- Burton D, Demuynck S, Yoder JR, 2016. Executive Dysfunction Predicts Delinquency But Not Characteristics of Sexual Aggression Among Adolescent Sexual Offenders. Sex. Abuse 28, 707–721. 10.1177/1079063214556357 [DOI] [PubMed] [Google Scholar]
- Canfield RL, Gendle MH, Cory-Slechta DA, 2004. Impaired Neuropsychological Functioning in Lead-Exposed Children. Dev. Neuropsychol. 26, 513–540. 10.1207/s15326942dn2601_8 [DOI] [PubMed] [Google Scholar]
- Canfield RL, Kreher DA, Cornwell C, Henderson CR, 2003. Low-Level Lead Exposure, Executive Functioning, and Learning in Early Childhood. Child Neuropsychol. Neuropsychol. Dev. Cogn. Sect. C 9, 35–53. 10.1076/chin.9.1.35.14496 [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control), 2018a. Lead [WWW Document]. URL http://www.cdc.gov/nceh/lead/default.htm (accessed 11.27.18).
- CDC (Centers for Disease Control), 2018b. ADHD Data & Statistics [WWW Document]. URL https://www.cdc.gov/ncbddd/adhd/data.html (accessed 4.23.18).
- CDC (Centers for Disease Control), 2017. Fourth National Report on Human Exposure to Environmental Chemicals Update [WWW Document]. URL https://www-cdc-gov.ezproxy.bu.edu/biomonitoring/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf (accessed 5.11.18).
- CDC (Centers for Disease Control), 2010. Guidelines for the identification and management of lead exposure in pregnant women.
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, Ji Y, Hong X, Caruso D, Bartell T, Gong Y, Strickland P, Navas-Acien A, Guallar E, Wang X, 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 24, 537–544. 10.1038/jes.2014.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL, 2004. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol. Teratol. 26, 359–371. 10.1016/j.ntt.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Diamantopoulou S, Rydell A-M, Thorell LB, Bohlin G, 2007. Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Dev. Neuropsychol. 32, 521–542. 10.1080/87565640701360981 [DOI] [PubMed] [Google Scholar]
- Espy KA, Sheffield TD, Wiebe SA, Clark CAC, Moehr M, 2011. Executive Control and Dimensions of Problem Behaviors in Preschool Children. J. Child Psychol. Psychiatry 52, 33–46. 10.1111/j.1469-7610.2010.02265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL, 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 118, 1654–1667. 10.1289/ehp.0901852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J, Wolfe B, 2009. Long-term Consequences of Childhood ADHD on Criminal Activities. J. Ment. Health Policy Econ. 12, 119–138. [PMC free article] [PubMed] [Google Scholar]
- Frankenburg WK, Coons CE, 1986. Home Screening Questionnaire: its validity in assessing home environment. J. Pediatr. 108, 624–626. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L, 2000. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychol. 6, 235–238. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Retzlaff PD, Espy KA, 2002. Confirmatory Factor Analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a Clinical Sample. Child Neuropsychol. 8, 249. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Podell K, 2000. Adaptive Decision Making, Ecological Validity, and the Frontal Lobes. J. Clin. Exp. Neuropsychol. 22, 56–68. 10.1076/1380-3395(200002)22:1;1-8;FT056 [DOI] [PubMed] [Google Scholar]
- Goodman A, Goodman R, 2009. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J. Am. Acad. Child Adolesc. Psychiatry 48, 400–403. 10.1097/CHI.0b013e3181985068 [DOI] [PubMed] [Google Scholar]
- Goodman R, 1997. The Strengths and Difficulties Questionnaire: A Research Note. J. Child Psychol. Psychiatry 38, 581–586. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- Goyer RA, 1990. Transplacental transport of lead. Environ. Health Perspect. 89, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB, 2003. Mobilization of lead from human bone tissue during pregnancy and lactation--a summary of long-term research. Sci. Total Environ. 303, 79–104. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA, 2002. Causal Knowledge as a Prerequisite for Confounding Evaluation: An Application to Birth Defects Epidemiology. Am. J. Epidemiol. 155, 176–184. 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- Horton NJ, Kleinman KP, 2007. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. Am. Stat. 61, 79–90. 10.1198/000313007X172556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL, 2004. Kaufman Brief Intelligence Test-Second Edition (KBIT-2). Bloomington, MN. [Google Scholar]
- Kim Sunmi, Eom S, Kim H-J, Lee JJ, Choi G, Choi S, Kim Sungjoo, Kim SY, Cho G, Kim YD, Suh E, Kim SK, Kim Seunghyo, Kim G-H, Moon H-B, Park J, Kim Sungkyoon, Choi K, Eun S-H, 2018. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age- CHECK cohort study. Sci. Total Environ. 624, 377–384. 10.1016/j.scitotenv.2017.12.058 [DOI] [PubMed] [Google Scholar]
- Kordas K, Canfield RL, López P, Rosado JL, Vargas GG, Cebrián ME, Rico JA, Ronquillo D, Stoltzfus RJ, 2006. Deficits in cognitive function and achievement in Mexican first-graders with low blood lead concentrations. Environ. Res. 100, 371–386. 10.1016/j.envres.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Kremer P, de Silva A, Cleary J, Santoro G, Weston K, Steele E, Nolan T, Waters E, 2015. Normative data for the Strengths and Difficulties Questionnaire for young children in Australia. J. Paediatr. Child Health 51, 970–975. 10.1111/jpc.12897 [DOI] [PubMed] [Google Scholar]
- Lanphear BP, 2017. Low-level toxicity of chemicals: No acceptable levels? PLOS Biol. 15, e2003066 10.1371/journal.pbio.2003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R, 2005. Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis. Environ. Health Perspect. 113, 894–899. 10.1289/ehp.7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen Y, Gao D, Jing J, Hu Q, 2014. Prenatal and postnatal lead exposure and cognitive development of infants followed over the first three years of life: A prospective birth study in the Pearl River Delta region, China. NeuroToxicology 44, 326–334. 10.1016/j.neuro.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa M-J, Rebagliato M, Ballester F, 2013. Gender differences in the neurotoxicity of metals in children. Toxicology, Gender development in Neurotoxicity 311, 3–12. 10.1016/j.tox.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Manton WI, Angle CR, Stanek KL, Kuntzelman D, Reese YR, Kuehnemann TJ, 2003. Release of lead from bone in pregnancy and lactation. Environ. Res. 92, 139–151. [DOI] [PubMed] [Google Scholar]
- Mazzocco MMM, Ross JL, 2007. Neurogenetic Developmental Disorders: Variation of Manifestation in Childhood. MIT Press. [Google Scholar]
- McCandless S, O’ Laughlin L, 2007. The Clinical Utility of the Behavior Rating Inventory of Executive Function (BRIEF) in the Diagnosis of ADHD. J. Atten. Disord. 10, 381–389. 10.1177/1087054706292115 [DOI] [PubMed] [Google Scholar]
- Mellor D, 2005. Normative data for the strengths and difficulties questionnaire in Australia. Aust. Psychol. 40, 215–222. 10.1080/00050060500243475 [DOI] [Google Scholar]
- Miller M, Hinshaw SP, 2010. Does childhood executive function predict adolescent functional outcomes in girls with ADHD? J. Abnorm. Child Psychol. 38, 315–326. 10.1007/s10802-009-9369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J-Y, Min K-B, Cho S-I, Kim R, Sakong J, Paek D, 2007. Neurobehavioral function in children with low blood lead concentrations. Neurotoxicology 28, 421–425. 10.1016/j.neuro.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS, 2005. Causal Heterogeneity in Attention-Deficit/Hyperactivity Disorder: Do We Need Neuropsychologically Impaired Subtypes? Biol. Psychiatry 57, 1224–1230. 10.1016/j.biopsych.2004.08.025 [DOI] [PubMed] [Google Scholar]
- Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, Weiss ST, Belfort MB, Burris HH, Camargo CA, Huh SY, Mantzoros C, Parker MG, Gillman MW, 2015. Cohort Profile: Project Viva. Int. J. Epidemiol. 44, 37–48. 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Rifas-Shiman SL, Amarasiriwardena C, Jayawardene I, Bellinger DC, Hibbeln JR, Wright RO, Gillman MW, 2016. Maternal prenatal fish consumption and cognition in mid childhood: Mercury, fatty acids, and selenium. Neurotoxicol. Teratol. 57, 71–78. 10.1016/j.ntt.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero TM, Barker LA, 2014. The Frontal Lobes and Executive Functioning, in: Handbook of Executive Functioning. Springer, New York, NY, pp. 29–44. 10.1007/978-1-4614-8106-5_3 [DOI] [Google Scholar]
- Parajuli RP, Umezaki M, Fujiwara T, Watanabe C, 2015. Association of cord blood levels of lead, arsenic, and zinc and home environment with children neurodevelopment at 36 months living in Chitwan Valley, Nepal. PloS One 10, e0120992 10.1371/journal.pone.0120992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor P, Reuben C, Duran C, Hawkins L, 2015. Association between diagnosed ADHD and selected characteristics among children aged 4–17 years: United States, 2011–2013. NCHS Data Brief 201. [PubMed] [Google Scholar]
- Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E, 2014. Very Low Maternal Lead Level in Pregnancy and Birth Outcomes in an Eastern Massachusetts Population. Ann. Epidemiol. 24, 915–919. 10.1016/j.annepidem.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinsky JR, Hinshaw SP, 2011. Linkages Between Childhood Executive Functioning and Adolescent Social Functioning and Psychopathology in Girls with ADHD. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 17, 368–390. 10.1080/09297049.2010.544649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL, 2004. Early exposure to lead and neuropsychological outcome in adolescence. J. Int. Neuropsychol. Soc. 10, 261–270. 10.1017/S1355617704102154 [DOI] [PubMed] [Google Scholar]
- Ronis M, Gandy J, Badger T, 1998. Endocrine Mechanisms Underlying Reproductive Toxicity in the Developing Rat Chronically Exposed to Dietary Lead. J. Toxicol. Environ. Health A 54, 77–99. 10.1080/009841098158935 [DOI] [PubMed] [Google Scholar]
- Roy A, Bellinger D, Hu H, Schwartz J, Ettinger AS, Wright RO, Bouchard M, Palaniappan K, Balakrishnan K, 2009. Lead Exposure and Behavior among Young Children in Chennai, India. Environ. Health Perspect. 117, 1607–1611. 10.1289/ehp.0900625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, Tchounwou PB, 2009. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 24, 15–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen J, Hurtig TM, Ebeling HE, Moilanen IK, Hughes LA, Taanila AM, 2010. Attention deficit hyperactivity disorder (ADHD) and criminal behaviour: the role of adolescent marginalization. Eur. J. Criminol. 7, 442–459. 10.1177/1477370810376568 [DOI] [Google Scholar]
- Schwartz J, 1994. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ. Res. 65, 42–55. 10.1006/enrs.1994.1020 [DOI] [PubMed] [Google Scholar]
- Schwartz J, Pitcher H, Levin R, Ostro B, Nichols A, 1985. Costs and Benefits of Reducing Lead in Gasoline: Final Regulatory Impact Analysis. Environmental Protection Agency, Office of Policy Analysis. [Google Scholar]
- Shefa ST, Héroux P, 2017. Both physiology and epidemiology support zero tolerable blood lead levels. Toxicol. Lett. 280, 232–237. 10.1016/j.toxlet.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, 1997. Preventing Lead Poisoning in Children. Annu. Rev. Public Health 18, 187–210. 10.1146/annurev.publhealth.18.1.187 [DOI] [PubMed] [Google Scholar]
- Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, Nawrot TS, Schoeters G, 2013. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8years. Environ. Int. 59, 225–231. 10.1016/j.envint.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Skogan AH, Egeland J, Zeiner P, Øvergaard KR, Oerbeck B, Reichborn-Kjennerud T, Aase H, 2016. Factor structure of the Behavior Rating Inventory of Executive Functions (BRIEF-P) at age three years. Child Neuropsychol. 22, 472–492. 10.1080/09297049.2014.992401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Ilustre RP, Osterloh JD, 1998. Methodological considerations for the accurate determination of lead in human plasma and serum. Am. J. Ind. Med. 33, 430–438. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J, 2006. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ. Health Perspect. 114, 1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LL, Janssens JMAM, Vermulst AA, Van Der Maten M, Engels RCME, Otten R, 2015. The Strengths and Difficulties Questionnaire: psychometric properties of the parent and teacher version in children aged 4–7. BMC Psychol. 3 10.1186/s40359-015-0061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LL, Otten R, Engels RCME, Vermulst AA, Janssens JMAM, 2010. Psychometric Properties of the Parent and Teacher Versions of the Strengths and Difficulties Questionnaire for 4- to 12-Year-Olds: A Review. Clin. Child Fam. Psychol. Rev. 13, 254–274. 10.1007/s10567-010-0071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JR, Riccio CA, 2007. Diagnostic Group Differences in Parent and Teacher Ratings on the BRIEF and Conners’ Scales. J. Atten. Disord. 11, 398–406. 10.1177/1087054707299399 [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC, 2007. Neuropsychological function in children with blood lead levels <10 μg/dL. NeuroToxicology 28, 1170–1177. 10.1016/j.neuro.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Kordas K, Golding J, Emond AM, 2017. Data relating to prenatal lead exposure and child IQ at 4 and 8 years old in the Avon Longitudinal Study of Parents and Children. Neurotoxicology 62, 224–230. 10.1016/j.neuro.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, West RF, Stanovich KE, 2013. Practitioner Review: Do performance-based measures and ratings of executive function assess the same construct? J. Child Psychol. Psychiatry 54, 131–143. 10.1111/jcpp.12001 [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA, 2008. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. NeuroToxicology 29, 928–939. 10.1016/j.neuro.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Popovac D, Factor-Litvak P, Kline J, Waternaux C, LoIacono N, Graziano JH, 2000. The Yugoslavia Prospective Lead Study: contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol. Teratol. 22, 811–818. 10.1016/S0892-0362(00)00106-9 [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM, 2011. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 30, 377–399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2017. Lead poisoning and health [WWW Document]. WHO; URL http://www.who.int/mediacentre/factsheets/fs379/en/ (accessed 1.9.18). [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN, 2008. Association of Prenatal and Childhood Blood Lead Concentrations with Criminal Arrests in Early Adulthood. PLoS Med. 5 10.1371/journal.pmed.0050101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Hivert M-F, Cardenas A, Zhong J, Rifas-Shiman SL, Agha G, Colicino E, Just AC, Amarasiriwardena C, Lin X, Litonjua AA, DeMeo DL, Gillman MW, Wright RO, Oken E, Baccarelli AA, 2017. Exposure to Low Levels of Lead in Utero and Umbilical Cord Blood DNA Methylation in Project Viva: An Epigenome-Wide Association Study. Environ. Health Perspect. 125 10.1289/EHP1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


