Abstract
Background & Aims:
There are few data from prospective studies on the effects of aspirin on fibrosis in patients with nonalcoholic fatty liver disease (NAFLD).
Methods:
We performed a prospective cohort study of 361 adults with biopsy-confirmed NAFLD, from 2006 through 2015, examined every 3–12 months for incident advanced fibrosis defined using serial measurements of validated indices (the Fibrosis-4, NAFLD fibrosis score, and aspartate aminotransferase to platelet ratio indices). Histologic analyses of liver biopsies collected at baseline were performed by a blinded pathologist. Information collected at baseline and at each examination included frequency and duration of aspirin and nonsteroidal anti-inflammatory drug (NSAID) use. Using multivariable-adjusted logistic regression, we estimated the association of aspirin use with prevalent steatohepatitis (NASH) and fibrosis. Using multivariable-adjusted Cox proportional hazards modeling, we estimated the association between aspirin use and risk for fibrosis progression.
Results:
At enrollment, 151 subjects used aspirin daily. Compared with non-regular use, daily aspirin use was associated with significantly lower odds of NASH (adjusted odds ratio, 0.68; 95% CI, 0.37–0.89) and fibrosis (adjusted odds ratio, 0.54; 95% CI, 0.31–0.82). Among individuals with baseline F0–F2 fibrosis (n=317), 86 developed advanced fibrosis over 3692 person-years. Daily aspirin users had significantly lower risk for developing incident advanced fibrosis vs non-regular users (adjusted hazard ratio [aHR], 0.63; 95% CI. 0.43–0.85). This relationship appeared to be duration dependent (adjusted P trend=.026), with the greatest benefit found with at least 4 years or more of aspirin use (aHR, 0.50; 95% CI, 0.35–0.73). Conversely, use of nonaspirin NSAIDs was not associated with risk for advanced fibrosis (aHR, 0.93; 95% CI, 0.81–1.05).
Conclusions:
In a prospective study of patients with biopsy-proven NAFLD, daily aspirin use was associated with less severe histologic features of NAFLD and NASH, and lower risk for progression to advanced fibrosis with time.
Keywords: prevention, chronic liver disease, anti-inflammatory, anti-fibrotic
Introduction
Approximately 50 million Americans have nonalcoholic fatty liver disease (NAFLD), characterized by fatty infiltration of the liver (steatosis) in the absence of excess alcohol consumption1. Among them, 10-25% develop progressive hepatic fibrosis1, which carries an increased risk for cirrhosis, hepatocellular carcinoma (HCC) and death2. Despite evidence suggesting that fibrosis stage is the most important pathologic determinant of NAFLD clinical outcomes2, approved therapies to prevent fibrosis are lacking.
Recent experimental3, 4 data suggest that aspirin is a promising anti-fibrotic strategy for NAFLD. In murine models, aspirin limits hepatic stellate cell activation by inhibiting the pro-inflammatory cyclooxygenase-2 (COX-2) enzyme3, and blocking platelet-derived growth factor (PDGF) signaling4. In humans, two recent cross-sectional studies have focused on aspirin use and NAFLD5, 6. In the first, aspirin use was inversely associated with steatosis, defined by abdominal ultrasound5; in the second, aspirin users had lower surrogate serum indices of liver fibrosis, compared to non-users6. However, despite promising results, these prior studies were limited by cross-sectional design and lack of hepatic histology to stage NAFLD severity.
Given the growing incidence and burden of NAFLD, understanding the potential anti-fibrotic benefits of aspirin remains an important unmet need. Therefore, we investigated the association between aspirin use and NAFLD histology, as well as the impact of aspirin use on risk for NAFLD progression to advanced fibrosis, using a well-phenotyped, prospective cohort with biopsy-confirmed NAFLD and long-term follow-up.
Methods:
Study population:
The Massachusetts General Hospital (MGH) NAFLD Repository is a prospective cohort study of adults over the age of 18 years with biopsy-confirmed NAFLD. All subjects were referred for clinically-indicated liver biopsy from community clinics. Over 90% of biopsies were obtained for persistent unexplained elevation of aminotransferases and/or abnormal hepatic imaging suggesting steatosis and/or fibrosis. This study was approved by the MGH Institutional Review Board and patients provided signed, informed consent.
At the enrollment biopsy, all patients underwent an extensive evaluation by a hepatologist and trained investigators, including a complete physical examination, ascertainment of personal and family medical history, medication use, alcohol intake (drinks/week) and detailed laboratory testing, permitting the calculation of three validated non-invasive indices of liver fibrosis: the fibrosis-4 (FIB-4) Index7, the NAFLD Fibrosis Score (NFS)8 and the aspartate aminotransferase-to-platelet ratio index (APRI)9 (Supplementary Methods). NAFLD diagnoses were confirmed through blinded medical record review.
We included subjects with at least one year of follow-up. We excluded alternative etiologies of liver disease through appropriate clinical, laboratory, radiographic, and/or histological criteria. We also excluded anyone in whom alcohol consumption could not be ascertained or with a history of heavy alcohol use (i.e. >14 alcoholic beverages/week in men, or >7 in women), bariatric surgery, hepatocellular carcinoma (HCC) or decompensation. Applying these criteria, we identified 361 consecutive patients with confirmed NAFLD enrolled in our prospective cohort between January 1, 2006 and December 31, 2015.
Aspirin exposure:
Self-reported aspirin use, frequency and duration of use were ascertained at enrollment and at each clinical follow-up visit by trained investigators, and confirmed by medical record review. At each visit, individuals reporting daily aspirin use were classified as aspirin users, while less-frequent or non-use was classified as non-regular use. For the longitudinal analysis, to most accurately represent long-term patterns of use, we prospectively updated aspirin use over each clinical follow-up interval, and modeled aspirin use as a time-varying exposure. Duration of use was estimated at baseline or beginning on the date of first reported use, and updated at each visit. Cited reasons for aspirin use included primary cardiovascular disease (CVD) prevention (54%), secondary CVD risk reduction (30%), and musculoskeletal pain/headaches (16%). Anyone reporting non-aspirin nonsteroidal anti-inflammatory drug (NSAID) use at least twice weekly, or who received an NSAID prescription (i.e. ibuprofen, naproxen, ketoprofen, diclofenac, indomethacin, etc) for at least twice weekly use, was classified as a non-aspirin NSAID user, consistent with prior work10. Data regarding other anti-platelet therapies, NSAID dosages or frequency of use were not collected.
Liver histology:
Biopsy slides were read and scored by a blinded, central hepatopathologist. If needed, new cuts of stored paraffin-embedded liver tissue and stains were obtained from blocks. A threshold of 5% hepatocytes showing steatosis was required for the histologic diagnosis of NAFLD, and NAFLD was graded and staged using validated criteria11 (Supplementary Methods).
Follow-up & Outcomes:
Patients were re-evaluated every 3-12 months according to clinical standards of care. At each visit, a complete medical history and physical examination was performed, medication data were updated, and all patients underwent sufficient laboratory testing for calculation of serial FIB-4, NFS and the APRI scores. No follow-up clinical or laboratory data were missing. Similar monitoring and lifestyle recommendations were provided for all patients, in accordance with published guidelines12. Individuals with cirrhosis underwent semi-annual ultrasonographic HCC screening. No patients underwent bariatric surgery.
The primary cross-sectional outcome was prevalent fibrosis. For longitudinal analyses, we limited the cohort to patients with early-stage NAFLD on enrollment biopsy (i.e. F0-2; n=317), consistent with prior studies2, and the primary outcome was incident advanced fibrosis, defined using serial FIB-4, NFS and APRI indices, measured at each clinical follow-up visit, using validated threshold cut-points13–15. Specifically, advanced fibrosis was defined as the first recorded FIB-4 >2.67 or NFS >0.67 or APRI >1.0 during follow-up13–15. We also conducted secondary analyses whereby advanced fibrosis was defined histologically (i.e. F3-4), among those with paired biopsies (n=72).
Statistical Analysis:
Data are expressed as means with standard deviations (SD), or numbers (N) with percentages. To examine the cross-sectional association between aspirin use and fibrosis, we constructed age- and multivariable-adjusted logistic regression models, stratified by calendar year of biopsy. The main multivariable-adjusted model (Model 2) accounted for age (years), sex, race/ethnicity, body mass index (BMI; continuous kg/m2), diabetes, any antidiabetic medication use, hypertension, hyperlipidemia, statin use, smoking status, CAD, and non-aspirin NSAID use.
For longitudinal analyses, the cohort was limited to those with F0-2 fibrosis (n=317). Follow-up accrued from enrollment biopsy to the date of death, outcome or December 31, 2015. No individuals were lost to follow-up. We calculated the cumulative incidence of advanced fibrosis among aspirin users versus non-regular users, modeling aspirin as a time-varying exposure16, and accounting for the competing risk of death, using the method of Fine and Gray17. Using Cox proportional hazard regression models conditioned on age, sex, calendar year and number of follow-up visits, we calculated age- and multivariable-adjusted subdistribution hazard ratios (aHR) and 95% confidence intervals (CI) for incident advanced fibrosis. The main model was adjusted for baseline fibrosis stage and all covariates from the cross-sectional analyses, however for this analysis, all variables were modeled as time-varying covariates. An additional model further adjusted for continuous low-density lipoprotein cholesterol (LDL-C) and hemoglobin A1C (%). In stratified models, we assessed whether the effects of aspirin varied according to pre-specified risk factors, using the log likelihood ratio test. Separately, we examined non-aspirin NSAID use and incident advanced fibrosis risk.
Duration of aspirin use was estimated by summing all previous years of daily aspirin use up to each clinical follow-up visit, and updated over time. We categorized duration of daily aspirin use as: never use, <2 years, 2 to <4 years, and ≥4 years. Linear trend was assessed using the median of each category as a continuous variable, among aspirin users.
We conducted five sensitivity analyses. First, we repeated the longitudinal analysis among those with F0-1 fibrosis (n=186). Second, we limited the population to those with paired liver biopsies (n=72). Third, we applied a more stringent definition of incident advanced fibrosis (i.e. two consecutive FIB-4 >2.67 and/or NFS >0.67 and/or APRI > 1.0). Fourth, because subclinical indicators of disease progression may have influenced aspirin use (reverse causation), we assessed the latency of aspirin use and incident advanced fibrosis risk, using a latency of 2 years. For example, we related aspirin use data in 2011 to outcomes in 2013, and we related aspirin use data in 2012 to outcomes in 2014, with aspirin use prospectively updated over time18. Fifth, we compared our results after excluding any user of statins (n=134 excluded), metformin (n=92 excluded), any antidiabetic medication (n=121 excluded), vitamin E (n=33 excluded), and non-aspirin NSAIDs (n=77 excluded). Analyses were performed using SAS Version 9.4 (SAS Inc., Cary NC), with a 2-sided P-value < 0.05.
Results:
Table 1 describes the baseline characteristics of the full study cohort (n=361). Compared to non-regular users, daily aspirin users were significantly older (mean 60±9 years vs. 48±14 years, p<0.0001), and more likely to have diabetes (46% vs. 39%, p=0.001), coronary artery disease (CAD; 21% vs. 9%, p<0.0001), and to be former smokers (39% vs. 25%, p=0.011).
Table 1.
Baseline clinical and demographic characteristics of NAFLD cohort according to daily aspirin use1 at enrollment (n=361)
| Characteristic | Non-Regular Aspirin use1 N=210 |
Daily Aspirin use1 N=151 |
P-value¥ |
|---|---|---|---|
| Age at liver biopsy, years (SD) | 48.2 (13.5) | 59.9 (8.6) | <0.0001 |
| Female sex, % | 119 (56.7) | 79 (52.3) | 0.18 |
| Race, % | 0.22 | ||
| • White | 166 (79.0) | 119 (78.8) | - |
| • Black | 4 (1.9) | 3 (2.0) | - |
| • Asian | 13 (6.2) | 9 (6.0) | - |
| • Other/Not specified | 27 (12.9) | 20 (13.2) | - |
| Hispanic Ethnicity, % | 36 (17.1) | 27 (17.8) | 0.29 |
| Body mass index, kg/m2 (SD) | 33.5 (6.3) | 34.2 (6.3) | 0.08 |
| Diabetes, % | 81 (38.6) | 69 (45.7) | 0.001 |
| Hypertension, % | 116 (55.2) | 97 (64.2) | 0.001 |
| Dyslipidemia, % | 97 (46.2) | 80 (53.0) | <0.0001 |
| Coronary artery disease, % | 18 (8.6) | 45 (29.8) | <0.0001 |
| Prior cardiac catheterization, % | 6 (2.9) | 19 (12.6) | <0.0001 |
| Smoking history, % | 0.011 | ||
| • Current | 26 (12.4) | 19 (12.6) | - |
| • Former | 53 (25.2) | 59 (39.1) | - |
| • Never | 131 (62.4) | 73 (48.3) | - |
| HDL Cholesterol, mg/dL (SD) | 44.1 (13.0) | 43.9 (12.1) | 0.46 |
| Non-HDL Cholesterol, mg/dL (SD) | 126.9 (63.0) | 126.8 (46.9) | 0.98 |
| Triglyerides, mg/dL (SD) | 162.0 (84.9) | 169.6 (95.0) | 0.52 |
| Medications at enrollment | |||
| Anti-hypertensive therapy, % | 92 (43.8) | 82 (54.3) | <0.0001 |
| Statin therapy, % | 75 (35.7) | 59 (39.1) | 0.021 |
| Metformin, % | 49 (23.3) | 43 (28.4) | 0.10 |
| Any anti-diabetic therapy4, % | 64 (30.5) | 57 (37.7) | 0.002 |
| Non-aspirin NSAIDs2, % | 52 (24.8) | 25 (16.6) | 0.37 |
| Vitamin E, % | 24 (11.4) | 9 (6.0) | 0.03 |
| Indices of Liver Fibrosis* at enrollment | |||
| NFS | −1.22 (0.26) | −1.15 (0.38) | 0.33 |
| FIB-4 | 0.84 (0.27) | 0.83 (0.35) | 0.49 |
| APRI | 0.26 (0.19) | 0.25 (0.19) | 0.52 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; HDL, high-density lipoprotein; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation; NFS, NAFLD Fibrosis Score; FIB-4, Fibrosis-4 Index; APRI, aspartate aminotransferase-to-platelet ratio index
Aspirin use was defined as daily use of aspirin. Less frequent use or never-use was defined as non-regular use.
Non-aspirin NSAID use included any recorded or reported use of ibuprofen, naproxen, ketoprofen, diclofenac, indomethacin, or any other nonaspirin NSAID-containing medications.
Any antidiabetic therapy at enrollment included: metformin monotherapy (n=85), metformin + sulfonylurea (n=7), pioglitazone (n=1) and insulin (n=28).
P-values estimated by chi-square or Fisher’s exact test for categorical variables and Mann-Whitney U test or t-test for continuous variables.
Calculation of the NFS = −1.675 + (0.037*age[years]) + (0.094*BMI [kg/m2]) + (1.13*Impaired glucose tolerance/diabetes [yes=1, no=0]) + (0.99*AST/ALT ratio) - (0.013*Platelet count [x109/L]) - (0.66*albumin [g/dl]). Calculation of the FIB-4 Index = (Age [years] * AST) / (Platelet count [x109/L] * √(ALT)). Calculation of the APRI = (AST, in IU/L) / (AST Upper Limit of Normal, IU/L) / (Platelet count [x109/L]).
Mean baseline NFS, APRI and FIB-4 scores were similar between groups (all p>0.05). Among daily aspirin users, the median duration of use at enrollment was 2.5 years (range <1 to 6 years).
Aspirin and NAFLD histology:
In the cross-sectional analyses, daily aspirin use was associated with 46% lower adjusted odds of fibrosis, compared to non-regular use (aOR 0.54, 95% CI 0.31-0.82) (Table 2). This association was unchanged after further accounting for nonaspirin NSAID use (aOR 0.57, 95% CI 0.30-0.84). Moreover, compared to non-regular use, daily aspirin use was inversely associated with NASH histology (Table 2), including lower odds of prevalent ballooning (aOR 0.45, 95% CI 0.23-0.88), lobular inflammation (aOR 0.85, 95% CI 0.54-0.98), definite NASH (aOR 0.68, 95% CI 0.37-0.89) and advanced (F3-4) fibrosis (aOR 0.46, 95% CI 0.22-0.89).
Table 2.
Cross-sectional association between daily aspirin use2 and liver histology, in NAFLD cohort (n=361)
| Histological feature1 | Non-Regular Aspirin Use2 (N=210) |
Daily Aspirin use2 (N=151) |
|---|---|---|
| Ballooning (any vs. none>) | ||
| No. of patients with endpoint: | 144 | 68 |
| Age- and sex-adjusted* OR (95% CI) | 1 (ref.) | 0.46 (0.25-0.86) |
| Multivariable Model¥, OR (95% CI) | 1 | 0.45 (0.23-0.88) |
| Lobular Inflammation (any vs. none) | ||
| No. of patients with endpoint: | 148 | 65 |
| Age- and sex-adjusted* OR (95% CI) | 1 (ref.) | 0.79 (0.56-0.94) |
| Multivariable Model¥; OR (95% CI) | 1 | 0.85 (0.54-0.98) |
| Definite Steatohepatitis (NASH) | ||
| No. of patients with endpoint: | 63 | 19 |
| Age- and sex-adjusted* OR (95% CI) | 1 (ref.) | 0.67 (0.39-0.86) |
| Multivariable Model¥; OR (95% CI) | 1 | 0.68 (0.37-0.89) |
| Fibrosis (any vs. none) | ||
| No. of patients with endpoint: | 80 | 48 |
| Age- and sex-adjusted* OR (95% CI) | 1 (ref.) | 0.50 (0.26-0.76) |
| Multivariable Model¥; OR (95% CI) | 1 | 0.54 (0.31-0.82) |
| Advanced Fibrosis stage 3-4 | ||
| No. of patients with endpoint: | 26 | 18 |
| Age- and sex-adjusted* OR (95% CI) | 1 (ref.) | 0.41 (0.19-0.76) |
| Multivariable Model¥; OR (95% CI) | 1 | 0.46 (0.22-0.89) |
Abbreviation: NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval
Model 1: adjusted for age at baseline liver biopsy (years) and sex
Multivariable Model: Model 1 + Hispanic ethnicity, BMI (continuous kg/m2), diabetes, hypertension, hyperlipidemia, smoking history (current vs. prior vs. never), coronary artery disease, statin use and metformin use.
Histology confirmed by a blinded, central pathologist. Definite NASH was defined as the presence of lobular inflammation and hepatocyte ballooning and steatosis (all grade>0).
Aspirin use was defined as daily use of aspirin, assessed at enrollment.
Among current aspirin users at enrollment, a longer duration of pre-enrollment aspirin use was associated with significantly lower odds of prevalent fibrosis (Ptrend=0.016). Specifically, compared to <2 years of use, the aORs for prevalent fibrosis with 2 to <4 years, and ≥4 years of use were: 0.72 (95% CI 0.56-0.88) and 0.48 (95% CI 0.32-0.69), respectively.
The inverse associations between aspirin use and prevalent fibrosis were similar across all pre-specified strata (Pinteractions>0.05; Table S1). Results also were similar after excluding non-aspirin NSAID users (n=77 excluded; Table S2; aOR 0.47, 95% CI 0.24-0.90), statin users (aOR 0.48, 95% CI 0.30-94) and metformin users (aOR 0.52, 95% CI 0.33-0.91).
Aspirin use and incident advanced fibrosis:
The longitudinal analysis included only those with baseline early-stage (F0-2) fibrosis (n=317). Over a median of 3,692 person-years, we recorded 86 cases of incident advanced fibrosis (i.e. first FIB-4>2.67 or NFS>0.67 or APRI>1.0). There were 26 cases of hepatic decompensation, and 18 patients died, including 8 liver-related and 10 non-liver-related deaths. Median overall follow-up was similar (7.4 years) in aspirin user and non-user groups. The median interval between visits was also similar (6 months, IQR 3-10 months) in both groups.
Compared to non-regular use, daily aspirin use was associated with significantly lower cumulative incidence of advanced fibrosis (Gray p-value <0.001; Figure 1). After multivariable adjustment, the risk for incident advanced fibrosis was 37% lower among daily aspirin users, compared to non-regular users (aHR 0.63, 95% CI 0.43-0.85; Table 3). These findings were similar after accounting for nonaspirin NSAIDs (aHR 0.60, 95% CI 0.39-0.86). Further adjustimg for continuous, updated LDL and hemoglobin A1c slightly attenuated these estimates, but the association remained statistically significant (aHR 0.71, 95% CI 0.43-0.95).
Figure 1. Cumulative incidence of advanced fibrosis1 with long-term daily aspirin use2, among patients with baseline early-stage NAFLD* (n=317).
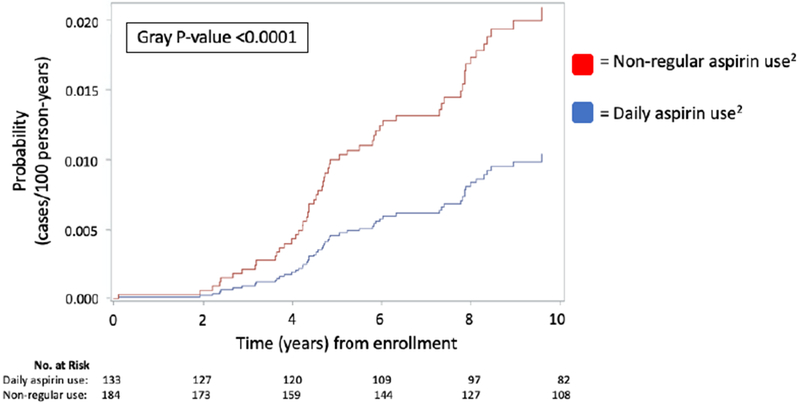
Abbreviations: NAFLD, nonalcoholic fatty liver disease; No., number
1Incident advanced fibrosis was defined by the first recorded Fibrosis-4 (FIB-4) score > 2.67 or NAFLD Fibrosis Score (NFS) > 0.67, or aspartate aminotransferase-to-platelet ratio index (APRI) > 1.0 during study follow-up. Follow-up measurements of each index score was obtained at least annually in all included participants.
2Daily aspirin use was ascertained and verified by trained study staff at enrollment and at each clinical visit. Non-regular aspirin use was defined as less than daily use or non-use of aspirin. This variable was prospectively-updated during study follow-up and modeled as a time-varying exposure..
*Early-stage NAFLD defined as baseline fibrosis stage 0-2 on enrollment liver biopsy, confirmed by a blinded pathologist.
Table 3.
Association between aspirin use1 and risk for developing incident advanced fibrosis/cirrhosis2, among patients with NAFLD fibrosis stage 0-2 at enrollment (n=317)
| Indices of advanced fibrosis2 | Non-Regular Aspirin Use (ref.) N=184 |
Daily Aspirin Use1 N=133 |
|---|---|---|
| Composite2 | ||
| No. with endpoint | 54 | 32 |
| Age- and sex-adjusted* HR (95% CI) | 1 | 0.66 (0.49-0.84) |
| Multivariable Model¥; HR (95% CI) | 1 | 0.63 (0.43-0.85) |
| FIB-4 > 2.67 | ||
| No. with endpoint | 52 | 29 |
| • Age- and sex-adjusted* HR (95% CI) | 1 | 0.66 (0.51-0.86) |
| • Multivariable Model¥; HR (95% CI) | 1 | 0.61 (0.42-0.89) |
| NFS > 0.676 | ||
| No. with endpoint | 50 | 31 |
| • Age- and sex-adjusted* HR (95% CI) | 1 | 0.64 (0.49-0.85) |
| • Multivariable Model¥; HR (95% CI) | 1 | 0.53 (0.38-0.72) |
| APRI > 1.0 | ||
| No. with endpoint | 33 | 16 |
| • Age- and sex-adjusted* HR (95% CI) | 1 | 0.65 (0.51-0.85) |
| • Multivariable Model¥; HR (95% CI) | 1 | 0.61 (0.43-0.87) |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; HR, hazard ratio; CI, confidence interval; NFS, NAFLD Fibrosis Score; APRI, aspartate aminotransferase-to-platelet ratio; FIB-4, fibrosis-4
Model 1: adjusted for age (years), sex, biopsy calendar year and number of follow-up visits.
Multivariable Model: Model 1 + Hispanic ethnicity, BMI (continuous kg/m2), diabetes, hypertension, hyperlipidemia, smoking history (current vs. prior vs. never), coronary artery disease, statin use and metformin use.
Aspirin use was defined as daily use; less frequent or never-use was defined as non-regular use. Aspirin use data was updated at each follow-up visit and modeled as a time-varying exposure.
Incident advanced fibrosis (i.e. stage 3 or 4 fibrosis; composite endpoint) was defined as the first recorded FIB4 > 2.67, or NAFLD Fibrosis Score > 0.676, or APRI > 1.0 during study follow-up.
Longer duration of aspirin use over follow-up was associated with progressively reduced risk for incident advanced fibrosis (Ptrend=0.026; Table 4). Compared to non-regular use, <2 years of aspirin use was not significantly associated with incident advanced fibrosis (aHR 0.90, 95% CI 0.68-1.20). However, the aHRs were 0.64 (95% CI 0.45-0.86) with 2 to <4 years of use, and 0.50 (95% CI 0.35-0.73) for ≥4 years of use. The associations between aspirin use and study outcomes were similar across all pre-defined strata (i.e. age, sex, race/ethnicity, diabetes, smoking history, fibrosis stage, statin or nonaspirin NSAID use; Pinteractions>0.05) (Table S3).
Table 4.
Duration of aspirin use1 and risk for developing incident advanced fibrosis2, among patients with NAFLD fibrosis stage 0-2 at enrollment (n=317)
| Duration of daily aspirin use (years)1 | |||||
|---|---|---|---|---|---|
| Non-regular use (ref.) | 0 to <2 years | 2 to <4 years | ≥4 years | P for trend3 | |
| No. of patients with composite endpoint2 | 54 | 11 | 12 | 9 | |
| Age- and sex-adjusted* HR (95% CI) | 1 | 0.85 (0.65-1.10) | 0.60 (0.42-0.81) | 0.47 (0.34-0.69) | <0.0001 |
| Multivariable Model¥; HR (95% CI) | 1 | 0.90 (0.68-1.20) | 0.64 (0.45-0.86) | 0.50 (0.35-0.73) | 0.026 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; No., number; HR, hazard ratio; CI, confidence interval
Model 1: see footnote for Table 3.
Multivariable Model: see footnote for Table 3.
Aspirin use was defined as daily use of aspirin. Less frequent use or never-use was defined as non-regular use. Aspirin use data was ascertained and updated at each clinical follow-up visit and modeled as a time-varying exposure.
Incident advanced fibrosis was defined as the first recorded follow-up FIB-4 Index >2.67 or NFS >0.67 or APRI > 1.0.
P-trend across increasing categories of duration, among daily aspirin users.
To test the specificity of the observed benefits of aspirin, we examined nonaspirin NSAID use in relation to incident advanced fibrosis (Figure S1). Compared to non-use, nonaspirin NSAID use was not associated with significantly reduced incidence of advanced fibrosis (Gray P-value=0.37). After multivariable adjustment, nonaspirin NSAID use was not associated with significantly reduced risk for incident advanced fibrosis (aHR 0.93, 95% CI 81-1.05).
Sensitivity analyses:
To test the robustness of our results, we conducted numerous sensitivity analyses. First, we limited the cohort to patients with F0-1 fibrosis (n=186), and our results were unchanged (aHR 0.43, 95% CI 0.29-0.64). Second, we limited the cohort to individuals with paired liver biopsies (n=72; median time between biopsies = 4.3 years [IQR 3, 11 years]). Although the small sample size precluded full multivariable adjustment, daily aspirin use nonetheless was associated with a 36% lower odds for incident advanced fibrosis, after accounting for age, sex, baseline fibrosis stage and time between biopsies (aOR 0.64, 95% CI 0.50-0.80; Table S4). Third, we applied a more stringent definition of advanced fibrosis (i.e. two consecutive measurements of FIB-4 >2.67 or NFS >0.67 or APRI > 1.0), and our results were similar (aHR=0.51, 95% CI 0.35-72). Fourth, we assessed the latency of aspirin use and risk for incident advanced fibrosis, using a lag of two years (n=26 excluded), with similar results (aHR 0.54, 95% CI 0.36-0.81). Fifth, our results were similar after excluding (A) statin users (n=134 excluded; aHR 0.46, 95% CI 0.30-0.69); (B) metformin users (n=92 excluded; aHR 0.55, 95% CI 0.34-0.78); (C) any antidiabetic medication user (n=121 excluded; aHR 0.60, 95% CI 0.39-90); and (D) vitamin E users (n=33 excluded; aHR 0.59, 95% CI 0.37-0.82). Finally, we excluded any non-aspirin NSAID user (n=72 excluded), and our findings were unchanged (aHR 0.64, 95% CI 0.49-0.86).
Discussion
In a well-phenotyped, prospective population with biopsy-proven NAFLD, daily aspirin use was inversely associated with NAFLD histological severity and with risk for developing incident advanced fibrosis. Importantly, these findings were consistent in women and men, and among patients with paired liver biopsies. We also found this inverse relationship to be duration-dependent, such that risk for developing advanced fibrosis was significantl reduced after at least 2 years of daily aspirin use. In contrast, similar associations were not found with non-aspirin NSAIDs, suggesting that this benefit may be specific to aspirin.
While preclinical data suggest that aspirin may protect against the incidence and progression of NAFLD3, 4, clinical evidence focused on aspirin use in NAFLD remains scarce. Published human data are limited to two cross-sectional studies from the Third National Health and Nutrition Examination Survey (NHANES), which evaluated steatosis using ultrasonography5, 6. While broadly consistent with our findings, those prior studies lacked longitudinal follow-up or liver histology. In contrast, the current study benefits from detailed histology and prospectively-updated aspirin data over long-term follow-up, thus providing stronger evidence to support the potential antifibrotic benefits of aspirin in NAFLD.
Several potential mechanisms may underpin the observed benefits of aspirin in NAFLD. In rodent models, activated platelets directly stimulate hepatic stellate cell (HSC) activation, while aspirin prevents fibrosis via platelet-derived growth factor (PDGF) inhibition4. Moreover, progressive NASH is accompanied by enhanced intrahepatic prostaglandin synthase-2 (PTGS-2, or cyclooxygenase [COX-2]) and prostaglandin E2 (PGE2) expression19, which enhance lipid droplet formation, inhibit autophagy20, and further activate HSC21. Accordingly, in preclinical models, antagonism of COX-2 or genetic inhibition of the PGE2 pathway improves NASH and resolves fibrosis22, 23. Collectively these data, together with our current findings, support a potential role for aspirin in the prevention of liver fibrogenesis.
In the present study, we did not find a significant association between nonaspirin NSAID use and risk for fibrosis progression. This might be explained by differences in the actions of aspirin and nonaspirin NSAIDs on COX isoforms. While aspirin irreversibly inhibits COX isoenzymes, NSAIDs do so reversibly24, with transient effects that may reduce anti-fibrotic activity. Nonaspirin NSAIDs also disrupt the intestinal barrier, increasing delivery of proinflammatory cytokines to the liver25. Finally, aspirin uniquely modulates bioactive lipids by stimulating the biosynthesis of proresolving mediators, and inhibiting pro-inflammatory lipids, which in turn may prevent progressive liver damage26.
Given the accelerating incidence and mortality of NAFLD, the potential magnitude of benefit from aspirin could be profound. In 2016, the United States Preventive Services Task Force (USPSTF) released guidelines recommending aspirin use for primary CVD prevention according to age, life expectancy, bleeding risk and estimated 10-year CVD risk27. Although NAFLD is not deemed a CVD risk equivalent by the USPSTF27 or subspecialty societies28, 29, evidence supports a link between NAFLD fibrosis and CVD mortality30. At the same time, in patients with progressive liver disease, any potential benefit from aspirin must be carefully balanced with bleeding risk. Thus, future research is needed to better characterize the potential benefits and hazards of aspirin across the full NAFLD histological spectrum.
To our knowledge this represents the first prospective study of aspirin use and NAFLD to combine liver histology with long-term follow-up. Nevertheless, we acknowledge several limitations. First, we cannot exclude the possibility of residual confounding in this observational study. However, our analysis accounted for variability in clinical follow-up and for known determinants of NAFLD fibrosis. Second, self-reported aspirin use data could result in recall bias and exposure misclassification; however, we minimized this risk by prospectively updating data over study follow-up, and by confirming medication use in the medical record. Third, aspirin use was self-selected, and patients with progressive fibrosis may be advised to avoid aspirin, leading to confounding by indication. However, when the cohort was limited to aspirin users (who presumably shared indications for use), we observed significant, duration-dependent relationships. The results also were similar when we compared F0-1 vs. F2 fibrosis. Fourth, while we cannot exclude the possibility of immortal time bias, we minimized this risk by using time-varying exposures16. Fifth, several of our sensitivity analyses had few cases, and thus require cautious interpretation. Nevertheless, the prevalence of NASH (23%) and F3-4 fibrosis (12%) was comparable to that of prior histology cohorts2, supporting the generalizability of this study. Sixth, this cohort was predominantly white, with relatively few users of certain antidiabetic drug classes; moreover, it lacked data regarding aspirin or nonaspirin NSAID doses, other aspirin use patterns, compliance, adverse events, or use of other anti-platelet agents, highlighting the need for future comparative effectiveness research in diverse cohorts with more detailed medication data. Finally, non-invasive indices are not the gold standard for evaluating NASH or fibrosis; however, they have demonstrated utility for this purpose, and our findings were similar among those with paired biopsies.
In conclusion, within a prospective cohort of patients with biopsy-proven NAFLD, daily aspirin use was inversely associated with prevalent NASH and fibrosis. Among those with early-stage fibrosis, daily aspirin use was associated with significantly lower risk for progressing to advanced fibrosis, in a duration-dependent manner. Our findings add to the growing literature supporting the potential hepatoprotective effects of aspirin in NAFLD5, 6. Research to uncover the mechanisms by which aspirin might prevent fibrogenesis could help develop urgently-needed anti-fibrotic therapies for NAFLD.
Supplementary Material
What You Need to Know:
Background: There is evidence from preclinical studies that aspirin prevents fibrogenesis in fatty liver. However, there are few data from prospective cohort studies of patients with nonalcoholic fatty liver disease (NAFLD).
Findings: In a prospective study of 361 patients with biopsy-proven NAFLD, daily aspirin use was associated with less severe histologic features of NAFLD at study enrollment and with significantly lower risk for advanced fibrosis over time, in a duration-dependent manner.
Implications for Patient Care: Our findings provide support for the hepato-protective effects of aspirin in patients with fatty liver disease. Studies are needed to determine the mechanisms by which aspirin prevents fibrogenesis, and whether aspirin might be used in treatment of NAFLD.
Acknowledgments
Grant Support:
NIH K24DK078772 (RTC)
NIH K23DK099422 (KEC)
NIH K24DK098311 (ATC)
NCI R01 CA137178 (ATC)
NIH 5P30DK046200-25 (TGS)
Dr. Chung is a Kevin and Polly Maroni MGH Research Scholar
Dr. Chan is a Stuart and Suzanne Steele MGH Research Scholar
TS is supported by a Clinical and Translational Research Award by the AASLD Foundation.
RM is supported by a Pinnacle Research Award from the AASLD Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and conflicts of interest:
The authors have no disclosures and no conflicts of interest to disclose.
REFERENCES:
- 1.Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2017;46:974–980. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–97 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik YH, Kim JK, Lee JI, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut 2009;58:1517–27. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida S, Ikenaga N, Liu SB, et al. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014;147:1378–92. [DOI] [PubMed] [Google Scholar]
- 5.Devaki P, McCullough A. Association Between Aspirin and Statin Use and the Prevalence of Non-Alcoholic Fatty Liver Disease: A Cross Sectional Study from National Health and Nutrition Examination Survey (Abstract). Gastroenterology 2017;152:S1202–S1203. [Google Scholar]
- 6.Jiang ZG, Feldbrugge L, Tapper EB, et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther 2016;43:734–43. [DOI] [PubMed] [Google Scholar]
- 7.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 8.Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017;66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 9.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated metaanalysis. Hepatology 2011;53:726–36. [DOI] [PubMed] [Google Scholar]
- 10.Simon TG, Ma Y, Ludvigsson JF, et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol 2018;4:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalasani N, Younossi Z, Lavine JE, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 13.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- 15.Mahady SE, Macaskill P, Craig JC, et al. Diagnostic Accuracy of Noninvasive Fibrosis Scores in a Population of Individuals With a Low Prevalence of Fibrosis. Clin Gastroenterol Hepatol 2017;15:1453–1460 e1. [DOI] [PubMed] [Google Scholar]
- 16.Levesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP GR. A proportional hazards model for the subdistribution of a competing risk. . J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 18.Feskanich D, Bain C, Chan AT, et al. Aspirin and lung cancer risk in a cohort study of women: dosage, duration and latency. Br J Cancer 2007;97:1295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Liu D, Bai Q, et al. Celecoxib attenuates liver steatosis and inflammation in non-alcoholic steatohepatitis induced by high-fat diet in rats. Mol Med Rep 2011. ;4:811–6. [DOI] [PubMed] [Google Scholar]
- 20.Ishihara K, Kanai S, Tanaka K, et al. Group IVA phospholipase A(2) deficiency prevents CCl4-induced hepatic cell death through the enhancement of autophagy. Biochem Biophys Res Commun 2016;471:15–20. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Gandhi CR, Gao ZH. Involvement of cytosolic phospholipase A2 alpha signalling pathway in spontaneous and transforming growth factor-beta-induced activation of rat hepatic stellate cells. Liver Int 2011;31:1565–73. [DOI] [PubMed] [Google Scholar]
- 22.Kanai S, Ishihara K, Kawashita E, et al. ASB14780, an Orally Active Inhibitor of Group IVA Phospholipase A2, Is a Pharmacotherapeutic Candidate for Nonalcoholic Fatty Liver Disease. J Pharmacol Exp Ther 2016;356:604–14. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Cifarelli V, Sun S, et al. Major role of adipocyte prostaglandin E2 in lipolysis-induced macrophage recruitment. J Lipid Res 2016;57:663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knights KM, Mangoni AA, Miners JO. Defining the COX inhibitor selectivity of NSAIDs: implications for understanding toxicity. Expert Rev Clin Pharmacol 2010;3:769–76. [DOI] [PubMed] [Google Scholar]
- 25.Tugendreich S, Pearson CI, Sagartz J, et al. NSAID-induced acute phase response is due to increased intestinal permeability and characterized by early and consistent alterations in hepatic gene expression. Toxicol Pathol 2006;34:168–79. [DOI] [PubMed] [Google Scholar]
- 26.Borgeson E, Johnson AM, Lee YS, et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab 2015;22:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bibbins-Domingo K, Force USPST. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836–45. [DOI] [PubMed] [Google Scholar]
- 28.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Diabetes A. 8. Cardiovascular Disease and Risk Management. Diabetes Care 2016;39 Suppl 1:S60–71. [DOI] [PubMed] [Google Scholar]
- 30.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


