Abstract
Background & Aims:
Endoscopic eradication therapy (EET) for Barrett’s esophagus (BE) has unclear effects on the gastric cardia. We investigated the prevalence of intestinal metaplasia (IM) and dysplasia in the cardia after complete eradication of IM (CEIM) and the incidence of newly diagnosed cardia IM or dysplasia after EET.
Methods:
We performed a prospective study, from 2013 through 2016, of patients with previously successful EET undergoing surveillance after CEIM (cross-sectional group) and treatment-naive patients with BE undergoing EET (longitudinal group). Standard biopsies were collected from multiple levels in the cardia and analyzed histologically. We calculated the prevalence (cross-sectional group) and the incidence (longitudinal group) of cardia IM or dysplasia after EET.
Results:
Of the 116 patients in the cross-sectional group, 17 (15%) had cardia IM or dysplasia after CEIM: 12 patients had IM, 2 patients were indefinite for dysplasia, and 3 patients had low-grade dysplasia. Cardia IM or dysplasia were most commonly found at the tops of gastric folds. Among 42 subjects in the longitudinal group, the pre-treatment prevalence of cardia IM or dysplasia was 28.5% (3 with non-dysplastic IM, 9 with dysplastic IM, 1 indefinite for dysplasia, 2 with low-grade dysplasia, 3 with high-grade dysplasia, and 3 with intramucosal cancer). All achieved CEIM. The incidence of cardia IM or dysplasia was 11.9% after 18 months of follow up. IM or dysplasia was more higher in the cardia after CEIM than in the tubular esophagus (P<.01).
Conclusions:
In a prospective study, we found that cardia dysplasia becomes less, not more, common, after successful EET; recurrence of IM or dysplasia was more frequent in the cardia than the esophagus. Patients with BE undergoing EET should have careful examination of the cardia, with a single set of surveillance biopsies at the top of the gastric folds.
Keywords: Barrett’s esophagus, radiofrequency ablation, gastric cardia, spray cryoablation
INTRODUCTION
Endoscopic eradication therapy (EET) is an effective management strategy for the treatment of dysplastic Barrett’s esophagus (BE) and early-stage esophageal adenocarcinoma (EAC), achieving complete eradication of intestinal metaplasia (CEIM) and dysplasia in most patients.1–3EET consists of mucosal resection of any nodular areas in the BE segment followed by ablation of flat BE mucosa.4, 5 Ablation modalities consist of thermal or freezing techniques to destroy metaplastic and dysplastic cells and promote regeneration of squamous epithelium. Of all ablation modalities, radiofrequency ablation (RFA) is most commonly used based on its safety and efficacy profile.1, 6 Following CEIM, surveillance exams are more likely to discover dysplasia in the cardia than in the tubular esophagus.7 It is unknown whether patients are at an increased risk of developing dysplasia or carcinoma of the gastric cardia following EET due to increased proliferation and mutations at the new squamocolumnar junction, and whether successful ablation itself is somehow associated with the development of neoplasia in the cardia.8
Prior studies have shown an association with BE and adenocarcinomas of the gastric cardia in those who did not undergo ablative therapy. Two cross-sectional studies9, 10 of surgical esophagectomy samples demonstrated that 42% of patients with cardiac adenocarcinomas had BE, with one study showing a stronger association with short segment BE.10 One prior study11 investigated endoscopic and histologic changes in the cardia before and after ablation in BE patients. In this study, prevalence of intestinal metaplasia (IM) and dysplasia increased from 8.5% and 0% before ablation to 28% and 5.3% after successful ablation, respectively. However, none of these patients were treated with RFA, which is the most commonly used ablation modality currently. In addition, four quadrant biopsies were taken from a single level in the cardia, at the top of the gastric folds, allowing for sampling error, which could underestimate the risk of dysplasia.
Therefore, there is a need to determine whether BE patients undergoing EET using current ablation modalities are at increased risk of gastric cardiac dysplasia or carcinoma, so that BE treatment and surveillance protocols can be modified accordingly. The primary objectives of this study are: 1) To determine the prevalence of intestinal metaplasia and dysplasia in the gastric cardia of BE patients who have achieved CEIM after EET and 2) To assess the incidence of newly diagnosed cardia IM/dysplasia after endoscopic therapy.
METHODS
Study Design, Setting, and Population
This was a single-center study conducted from 2013-2016 at a tertiary care referral center, the University of North Carolina at Chapel Hill. The study consisted of two arms: 1) a cross-sectional arm of patients who had previously undergone successful EET for BE with either LGD, HGD, or early EAC, and were presenting for surveillance endoscopy after achieving CEIM; and 2) a prospective longitudinal arm consisting of treatment naive BE patients who were undergoing EET. The longitudinal arm was designed to assess the incidence of cardia IM/dysplasia after endoscopic therapy and the larger cross-sectional arm was designed to assess prevalence of cardia IM/dysplasia after CEIM. Subjects were excluded if they had a bleeding disorder, were on active anticoagulation, had a history of complete or partial esophagectomy, or had a history of invasive EAC.
Study Procedures: Cross-sectional Group
The cross-sectional group consisted of those who underwent previous EET for dysplastic BE, or early EAC and had at least one prior exam documenting CEIM. CEIM was defined as complete eradication of columnar epithelium in the esophagus on endoscopy with biopsies from the tubular esophagus and at the gastroesophageal junction showing no evidence of IM or dysplasia. EET included endoscopic mucosal resection (EMR) of any nodular areas followed by ablation with radiofrequency ablation (RFA), cryoablation, PDT or APC. All subjects underwent ablation 1-2 cm into the cardia per routine clinical care, after which patients underwent surveillance at intervals ranging from 3 months to a 1 year based on guideline recommendations.12
Participants were screened for eligibility and underwent informed consent prior to their scheduled surveillance upper endoscopy after CEIM. All demographic and clinical data including BE ablation history were collected using standardized case report forms. All endoscopies were performed by a single expert endoscopist (NJS) using high-definition white light and narrowband imaging with careful inspection of the cardia and esophagus. In addition to clinical biopsies,13 standardized biopsies were obtained per research protocol for this study from 4 quadrants in 4 locations for a total of 16 biopsies. The locations included: 1) the distal esophagus, 1 cm proximal to top of gastric folds (TGF-1cm), 2) at top of gastric folds (TGF), 3) 1 cm into the gastric cardia (TGF+1cm), and 4) 2cm into the cardia (TGF+2cm), (Supplementary Figure).
Study Procedures: Longitudinal Group
The treatment-naive group undergoing EET were BE patients with either LGD, HGD, or IMC who were enrolled before their first EET procedure and baseline data was collected as described above with all procedures performed by a single endoscopist. Prior to the first EET session, standardized research protocol biopsies were obtained, as above, from 4 quadrants in the distal esophagus 1 cm proximal to top of gastric folds, (TGF-1cm), at top of gastric folds (TGF), and in the cardia (TGF+1cm and TGF+2cm), for a total of 16 biopsies, avoiding any endoscopically visible abnormalities. Endoscopically visible abnormalities were treated with EMR. After EMR, endoscopic ablation was performed at 2-3 month intervals using either RFA or cryoablation, with APC therapy for any residual minute islands (<5 mm diameter). Patients received endoscopic treatment every 2-3 months until CEIM was achieved. After CEIM, subjects had all surveillance exams performed at UNC with standardized research protocol biopsies of the distal esophagus and cardia obtained at 6 months and 18 months after CEIM to assess for the development of cardia IM or dysplasia.
Statistical Analysis
Prevalence of dysplasia and IM were calculated before ablation in the longitudinal group, and incidence of IM and dysplasia was calculated after CEIM. Prevalence of IM and dysplasia in the cardia was calculated in the cross-sectional group. Clinical, histologic, endoscopic, and ablation history features were compared between the groups with and without cardia IM/dysplasia in the cross-sectional cohort and between the groups with and without nodularity in the longitudinal cohort using Chi-square for categorical variables, and t-tests or Wilcoxon Rank-sum for continuous variables as appropriate. A post hoc analysis of both the prevalence of cardia IM/dysplasia in the cross-sectional group and the incidence of cardia IM/dysplasia in the longitudinal group was performed, to assess these variables in a group who uniformly received RFA as their main treatment modality. All analyses were performed using Stata 13.1 (Statacorp, College Station, TX).
RESULTS
The Cross-sectional Group
Among 116 subjects who previously achieved CEIM presenting for surveillance, mean age was 67 years with a majority being white (97%) men (78%) (Table 1). Median BE segment length was 4 cm (range 1-14) and most common baseline pathology was HGD (57%). RFA was used in 93% (n=108) of patients, and 39% (n=45) underwent EMR of nodular lesions in the tubular esophagus or cardia prior to ablation. Median time from first treatment to CEIM was 245 days and 49% required 3-4 treatments to achieve CEIM. Endoscopic findings after EET showed new onset nodularity of the cardia in 3% of the sample.
Table 1:
Baseline characteristics and post-ablation endoscopic findings in the cross-sectional group after complete eradication of intestinal metaplasia (CEIM) between groups with and without cardia IM/dysplasia
| Baseline Demographic and Ablation History | Total Sample (n=116) | No Cardia IM/Dysplasia (n=99) | Cardia IM/Dysplasia (n=17) | p value |
|---|---|---|---|---|
| Age, median years (range) | 67 (40-80) | 67 (40-80) | 69 (56-80) | 0.37 |
| White, n (%) | 112 (97) | 95 (96) | 17 (100) | 0.40 |
| Male, n (%) | 91 (78) | 76 (77) | 15 (88) | 0.30 |
| BE length, cm, median (range) | 4 (1-14) | 4 (1-13) | 5 (1-14) | 0.66 |
| Baseline histology, n (%) | 0.35 | |||
| Indefinite dysplasia | 2 (2) | 2 (2) | 0 (0) | |
| Low-grade dysplasia | 32 (27) | 24 (24) | 8 (47) | |
| High-grade dysplasia | 66 (57) | 59(60) | 7 (41) | |
| Intramucosal Adenocarcinoma | 13 (11) | 11 (11) | 2 (12) | |
| Invasive EAC | 3 (3) | 3 (3) | 0 (0) | |
| Ablation modality, n (%)* | ||||
| RFA | 108 (93) | 89 (97) | 14 (88) | 0.10 |
| EMR | 45 (39) | 39 (52) | 6 (43) | 0.53 |
| Cryoablation | 11 (9) | 11 (17) | 0 (0) | 0.11 |
| PDT | 3 (3) | 2 (3) | 1 (8) | 0.45 |
| Number of ablation sessions, n (%) | 0.26 | |||
| 1-2 | 41 (35) | 32 (32) | 9 (53) | |
| 3-4 | 49 (42) | 44 (44) | 5 (29) | |
| 5-10 | 26 (23) | 23 (23) | 3 (18) | |
| Number of ablation sessions, mean ± SD | 3.4 ± 1.8 | 3.5 ± 1.8 | 2.8 ± 1.4 | 0.07 |
| Time to CEIM, days, median (range) | 245 (56-1539) | 248 (56-1539) | 245 (70-634) | 0.77 |
| Endoscopic Findings on Post-Ablation Exam | ||||
| Time CEIM to cardia biopsy, median days (range) | 944 (98-3109) | 965 (98-3109) | 749 (175-3102) | 0.08 |
| Hiatal hernia, mean cm ± SD | 3.3 ± 1.4 | 3.3 ± 1.4 | 3.4 ± 1.7 | 0.60 |
| Cardia nodularity, n (%) | 3 (3) | 1 (1) | 2 (12) | 0.01 |
| Esophagitis, n (%) | 11 (9) | 11 (11) | 0 (0) | 0.15 |
| Stricture, n (%) | 2 (2) | 2 (2) | 0 (0) | 0.79 |
Total number of ablation modalities sum to >100%, since individual patients underwent more than one modality.
After CEIM, findings of cardia IM or dysplasia were common. Of the 116 subjects in the cross-sectional group, 15% (n=17) had either IM or dysplasia in the cardia after CEIM. Of the 17, 12 (10%) had IM, 2 (2%) were indefinite for dysplasia, and 3 (3%) had LGD (Table 2). In contrast to the common nature of cardia IM/dysplasia, only a single patient had IM found in the tubular esophagus during the surveillance endoscopy; this patient was also found to have IM at all three levels in the cardia. Of the 12 patients with cardia IM, 83% (n=10) had IM at the level of TGF, 50% (n=6) at TGF+1, and 25% (n=3) at TGF+2 (Figure 1). Only two cases (12%) had IM solely at TGF+1 or TGF+2 without concurrent IM at TGF. All cases of LGD and indefinite for dysplasia were found at the level of TGF. Of the three cases with nodularity noted in the cardia, one demonstrated LGD, one demonstrated IM, and one only cardiac mucosa.
Table 2:
Distribution of intestinal metaplasia (IM) and dysplasia in the cardia and distal esophagus in Barrett’s patients after complete eradication of intestinal metaplasia (CEIM) in the cross-sectional group
| Cardia | Esophagus | |||
|---|---|---|---|---|
| TGF n (%) | TGF + 1 n (%) | TGF + 2 n (%) | TGF − 1 n (%) | |
| Intestinal metaplasia | 10 (8.6) | 6 (5.2) | 3 (2.6) | 1 (0.9) |
| Indefinite for dysplasia | 2 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Low-grade dysplasia | 3 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| High-grade dysplasia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No significant findings | 101 (87.1) | 110 (94.8) | 113 (97.4) | 115 (99.1) |
TGF= top of gastric folds; TGF+1= cardia 1 cm below TGF; TGF+2: cardia 2 cm below TGF; TGF-1= esophagus 1 cm above TGF
Figure 1:
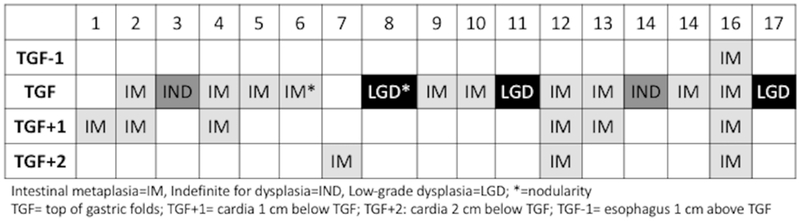
Location of intestinal metaplasia (IM) or dysplasia in the distal esophagus (TGF-1) and cardia (TGF, TGF+1, TGF+2) in the 17 cases with IM/dysplasia in the post-endoscopic eradication therapy (EET) cross-sectional cohort
There were no significant differences between those with and without cardia IM/dysplasia, other than presence of cardiac nodularity (Table 1). While there was a trend towards those with cardia/IM or dysplasia having a shorter duration between time from CEIM to the time of this study initiation, the difference between those with and without cardia findings was not significant. A significantly higher proportion of patients with cardia IM/dysplasia had nodularity in the cardia (1% vs. 12%; p<0.01).
The Longitudinal Group
Among 42 subjects followed longitudinally while undergoing EET, mean age was 66 years with a majority being white (98%) men (76%) (Table 3). On baseline endoscopic exam before EET, nodularity of the cardia was found in 24% (n=10). Mean BE segment length was 4 cm and the most common baseline pathology was HGD (60%), followed by LGD (24%), and IMC (14%). Of 42 subjects, 69% had EMR prior to ablation, 98% had RFA, and 50% required on average 1-2 ablation sessions to achieve CEIM. All subjects achieved CEIM over a median of 193 days.
Table 3:
Patient demographics, baseline endoscopic findings and treatment characteristics in the longitudinal group
| Sample (n=42) | |
|---|---|
| Age, mean ± SD | 65.8 ± 7.5 |
| White, n (%) | 41 (98) |
| Male, n (%) | 32 (76) |
| Hiatal hernia, n (%) | 39 (93) |
| Hiatal hernia, mean cm ± SD | 3.5 ± 1.7 |
| Cardia nodularity, n (%) | 10 (24) |
| Stricture, n (%) | 3 (7) |
| BE length, cm, median (range) | 4 (1-11) |
| Baseline histology, n (%) | |
| Indefinite dysplasia | 1 (2) |
| Low-grade dysplasia | 10 (24) |
| High-grade dysplasia | 25 (60) |
| Intramucosal Adenocarcinoma | 6 (14) |
| Ablation modality, n (%) | |
| RFA | 41 (98) |
| EMR | 29 (69) |
| Cryoablation | 2 (5) |
| PDT | 0 (0) |
| APC | 13 (31) |
| Number of ablation sessions, n (%) | |
| 1-2 | 21 (50) |
| 3-4 | 18 (43) |
| 5-10 | 2 (7) |
| Number of ablation sessions, mean ± SD | 2.9 ± 1.3 |
| Time to CEIM, days, median (range) | 193 (69-572) |
Thirty-five of the 42 subjects provided samples at 6 months after CEIM, and 32 at 18 months after CEIM. All subjects who did not provide standardized protocol cardia biopsies at 6 months after CEIM, had them performed at 18 months after CEIM. Those who did not provide biopsies at 18 months after CEIM underwent endoscopic surveillance from their local referring gastroenterologist. Before ablation, 12 subjects (28%) had IM/dysplasia in the cardia; the prevalence of non-dysplastic IM was 7.1% (n=3) and of dysplastic IM was 21.4% (n=9: indefinite for dysplasia=1, LGD=2, HGD=3, IMC=3) (Figure 2). All cases of dysplasia pre-treatment were found at the level of TGF and one case had dysplasia extending to the level of TGF+1. Six months after first CEIM, none had cardia IM and 8.6% (n=3) had cardia dysplasia of which 2 had LGD and 1 had HGD. All cases of cardia dysplasia at 6 months post-CEIM were located at TGF, except for one case of LGD that extended to TGF+1. These three subjects underwent additional endoscopic therapy and achieved eradication of cardia IM and dysplasia. At 18 months post-CEIM, 6.2% (n=2) had IM and none had dysplasia. In the post-EET longitudinal cohort, the incidence of cardia dysplasia was 7.1% (n=3) 6 months post-CEIM and 0% 18 months post-CEIM, and the incidence of cardia IM was 0% at 6 months post-CEIM and 4.8% (n=2) 18 months-post CEIM.
Figure 2:
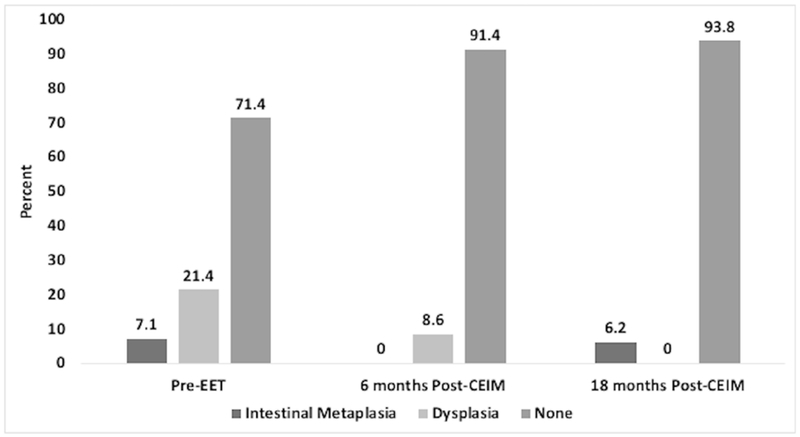
Distribution of non-dysplastic Intestinal metaplasia (IM) and dysplastic IM in the cardia pre-endoscopic eradication therapy (EET) and post-complete eradication of intestinal metaplasia (CEIM)
Prevalence of cardia dysplasia pre-EET was significantly higher in those with nodular lesions in the cardia compared to those who did not have cardia nodularity (Table 4). Seven of 10 patients with nodularity in the cardia had dysplasia, compared to 2 out of 32 cases who were non-nodular (70% vs. 6%; p<0.01). Similarly, all three cases of cardia dysplasia 6 months post-CEIM were found in those with pre-EET cardia nodularity.
Table 4:
Prevalence of intestinal metaplasia and dysplasia in pre- and post-endoscopic eradication therapy (EET) in patients with and without cardia nodularity in the longitudinal group
| Cardia nodularity (−) | Cardia nodularity (+) | p value | |
|---|---|---|---|
| Pre-EET | |||
| n | 32 | 10 | <0.01 |
| Intestinal metaplasia | 2 (6) | 1 (10) | |
| Dysplasia | 2 (6) | 7 (70) | |
| None | 28 (93) | 2 (20) | |
| 6 months Post-CEIM | |||
| n | 27 | 8 | <0.01 |
| Intestinal metaplasia | 0 (0) | 0 (0) | |
| Dysplasia | 0 (0) | 3 (38) | |
| None | 28(100) | 5 (62) | |
| 18 months Post-CEIM | |||
| n | 25 | 7 | 0.44 |
| Intestinal metaplasia | 2 (8) | 0 (0) | |
| Dysplasia | 0 (0) | 0 (0) | |
| None | 23 (92) | 7 (100) | |
CEIM: complete eradication of intestinal metaplasia
Post Hoc Analysis Excluding Patients Receiving PDT or Cryotherapy
To assess the effect of having patients who received multiple therapeutic modalities in the study, we performed a post hoc analysis excluding patient receiving modalities other than RFA. The cross-sectional prevalence of cardia IM/dysplasia in this group (n=97) was 14%. The longitudinal incidence of cardia IM/dysplasia in this group (n=40) was 7.5%. Neither was statistically significantly different than the overall group (p=NS).
DISCUSSION
We currently do not know what the prevalence of cardia IM or dysplasia is in dysplastic BE patients prior to ablation, or whether incident cardia IM or dysplasia develops with successful ablative therapy. In this single center study, consisting of a cross-sectional post-CEIM group and a longitudinal group followed from prior to therapy to after CEIM, we found that the prevalence of non-dysplastic IM was 7% and the prevalence of dysplastic IM of the cardia was 21% in patients with dysplastic BE before EET. Pre-treatment dysplasia was more commonly found in visibly nodular areas in the cardia, with 70% of nodular areas harboring dysplasia prior to therapy. In addition, all cases of pre-treatment cardia dysplasia were found at the level of TGF. The prevalence of cardia IM and dysplasia decreased after EET, such that 18 months after CEIM was achieved in all patients, none of the patients had dysplasia and only 6.2% had IM of the cardia. The overall incidence of cardia IM/dysplasia was 11.9% in the 18 months follow-up time after CEIM in the longitudinal cohort. In the post-CEIM cross-sectional cohort, 3% had LGD, 2% had indefinite for dysplasia, and 10% had intestinal metaplasia. As in the longitudinal cohort, in all cases of dysplasia and a majority of IM, the lesion was identified at the level of TGF in the cardia, with biopsies deeper into the cardia being normal.
Overall the findings of this study demonstrate that the prevalence of dysplastic IM after ablation and successful CEIM is low in BE patients as shown in the cross-sectional cohort, with 10% prevalence of IM and 5% prevalence of indefinite dysplasia or LGD. These numbers are somewhat lower than that reported in a prior study from our center.7 In the prior study, post-CEIM prevalence of dysplasia was found in 19 out of 218 cases or 9% of the sample of patients from 2006-2015. The discrepancy between the two studies is most likely explained by the practice of ablating 1-2 cm into the gastric cardia as routine care EET at our center, which has evolved in the last 5-6 years. As seen in the longitudinal group, the prevalence of dysplasia was high at 21% before ablation with successful eradication after EET. Because most of the pretreatment cardia dysplasia was found in visibly nodular areas, it is prudent to closely inspect the proximal cardia for mucosal irregularities in addition to the BE segment prior to EET, with mucosal resection of nodular areas followed by ablation of flat BE, as well as ablation 1-2 cm into the cardia. In addition, proximal cardia ablation may explain the difference between our results, where cardia dysplasia decreased after ablation compared to the prior study by Weston et al,11 in which prevalence of cardia dysplasia increased from 0% pre-treatment to 5.3% after treatment. Another possible explanation is that the pre-treatment prevalence of dysplasia was underestimated in the study by Weston et al,11 as biopsies were only obtained from one level at the gastroesophageal junction.
In this study, standardized biopsies were obtained from TGF, 1 cm into the cardia (TGF+1), and 2 cm into the cardia (TGF+2). The pre-treatment location of cardia dysplasia was primarily localized to TGF and TGF+1 with no dysplasia at TGF+2, again supporting the argument that patients benefit from being ablated 1-2 cm into the cardia. Ablating deeper into the cardia during EET is currently not a universal practice due to uncertainty of its clinical implications, but findings of this study support that this should be done, as a clinically significant proportion of patients with dysplastic BE harbor dysplasia in the cardia. In terms of recurrence of IM and dysplasia in the cardia post-CEIM, all cases of dysplasia and a majority of IM was at TGF. Therefore, in addition to routine treatment of the proximal cardia, an implication of this work is that it is sufficient to perform a single set of four quadrant biopsies at TGF in the post-treatment surveillance period to assess for cardia dysplasia, and that biopsies deeper into the cardia, in the absence of nodularity, are unlikely to be of diagnostic utility. Finally, there was only one case of recurrent IM in the tubular esophagus in this study, demonstrating that IM and dysplasia were more common in the cardia than in the tubular esophagus after CEIM, as shown in a prior study.7
There are some limitations to this study. This was a single center study, which can affect the generalizability of the results. Second, multiple ablation modalities were used, although a significant proportion in both groups underwent RFA, which is currently the most commonly used treatment method for dysplastic BE, making the results of the study more applicable to real-world practice. In addition, our post hoc analysis excluding patients receiving PDT or cryotherapy did not significantly change our results. Third, we had some drop out in the longitudinal portion of the study, secondary to patients who lived at a distance choosing to follow-up with their local gastroenterologists for convenience. However, it is likely that this drop-out was non-differential with respect to cardia findings. Finally, because the gastric cardia covers a larger surface area compared to the tubular esophagus, there may be sampling error when biopsying the proximal cardia, despite our extensive biopsy protocol, leading to underestimation of the prevalence of IM and dysplasia.
The study also has multiple strengths, as data were collected prospectively in a standardized manner and complete ablation history was available for all patients. All procedures, including surveillance exams, were performed by a single, experienced endoscopist, so there was standardization of ablation technique and biopsy protocol. This is also the first study to sample the cardia at multiple levels in a systematic manner to determine if biopsying at only one level in the cardia is sufficient during surveillance exams in post-ablation patients. In addition to providing guidance to surveillance protocols, systemic sampling at multiple levels in the cardia allows for a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.
In conclusion, dysplastic IM of the cardia was common before treatment in patients with dysplastic BE undergoing EET, and was found especially commonly in nodular areas. Therefore, the findings of this study provide additional guidance for management BE patients undergoing EET in that they should have careful examination of the cardia and resection of any nodular areas, in addition to ablative therapy 1-2 cm extending into the cardia. Cardia dysplasia becomes less, not more, common, as patients undergo successful EET. After EET, incidence of IM and dysplasia was more common in the cardia than in the tubular esophagus, making routine surveillance biopsies of the cardia advisable. However, since a majority occurred at TGF, rather than at more distal areas of the cardia, extensive biopsies farther into the cardia are unnecessary during post-EET surveillance exams. The results of this study provide evidence to suggest that in BE patients who have achieved CEIM, it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF, rather than deeper into the cardia, during surveillance exams.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
It is unclear if endoscopic therapy for Barrett’s esophagus results in development of dysplasia of the gastric cardia and whether collecting biopsies from only the top of the gastric folds is sufficient during surveillance after complete eradication of intestinal metaplasia (CEIM).
Findings:
Intestinal metaplasia and dysplasia of the cardia were common in patients with dysplastic Barrett’s esophagus and decreased after successful endoscopic therapy. Cardia dysplasia was more commonly found in visibly nodular areas and more frequently present at the top of gastric folds than at lower levels of the cardia.
Implications for patient care:
Patients with Barrett’s esophagus undergoing endoscopic therapy should have resection of any cardia nodularity and ablation performed 1–2 cm into the cardia. After CEIM, in addition to esophageal biopsies, it is sufficient to collect biopsies from the top of the gastric folds for surveillance.
Acknowledgments
Grant Support: This study was funded in part by T32DK007634 (SE), AGA Research Scholar Award (SE), K24DK100548 (NJS), and CSA Medical Inc.
Abbreviations:
- APC
argon plasma coagulation
- BE
Barrett’s esophagus
- CEIM
complete eradication of intestinal metaplasia
- EAC
esophageal adenocarcinoma
- EET
endoscopic eradication therapy
- EMR
endoscopic mucosal resection
- HGD
high-grade dysplasia
- IM
intestinal metaplasia
- IND
indefinite for dysplasia
- LGD
low-grade dysplasia
- MPEC
multipolar electrocoagulation
- RFA
radiofrequency ablation
- TGF
top of gastric folds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The author authors report no potential related competing interests related to this article.
REFERENCES
- 1.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360(22):2277–88. [DOI] [PubMed] [Google Scholar]
- 2.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62(4):488–98. [DOI] [PubMed] [Google Scholar]
- 3.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11(10):1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology 2013;145(1):87–95. [DOI] [PubMed] [Google Scholar]
- 5.Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65(4):555–62. [DOI] [PubMed] [Google Scholar]
- 6.Qumseya BJ, Wani S, Desai M, et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14(8):1086–95.e6. [DOI] [PubMed] [Google Scholar]
- 7.Guthikonda A, Cotton CC, Madanick RD, et al. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett’s Esophagus With Radiofrequency Ablation. Am J Gastroenterol 2017;112(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampliner RE, Camargo E, Prasad AR. Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19(4):277–9. [DOI] [PubMed] [Google Scholar]
- 9.Cameron AJ, Lomboy CT, Pera M, et al. Adenocarcinoma of the esophagogastric junction and Barrett’s esophagus. Gastroenterology 1995;109(5):1541–6. [DOI] [PubMed] [Google Scholar]
- 10.Clark GW, Smyrk TC, Burdiles P, et al. Is Barrett’s metaplasia the source of adenocarcinomas of the cardia? Arch Surg 1994;129(6):609–14. [DOI] [PubMed] [Google Scholar]
- 11.Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61(4):515–21. [DOI] [PubMed] [Google Scholar]
- 12.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111(1):30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellon ES, Higgins LL, Beitia R, et al. Prospective assessment of serum periostin as a biomarker for diagnosis and monitoring of eosinophilic oesophagitis. Aliment Pharmacol Ther 2016;44(2):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


