Abstract
Opioid use during pregnancy is rising, with an estimated 14–22% of women obtaining an opioid prescription during pregnancy. Methadone maintenance therapy (MMT) has been the gold standard for treatment of opioid use disorders during pregnancy; however, its use is limited in clinical practice due to availability, stigma, and reluctance on the part of clinicians. The present study compared against medical advice (AMA) treatment dropout from seven days of residential care between pregnant women diagnosed with opioid dependence who elected either MMT (n=119) or non-pharmacological treatment (NPT) (n=91) within the same treatment program in Baltimore, Maryland from 1996 to 1998. Multiple logistic regression analysis was conducted to compare the rate of AMA drop out between the two modalities. Patients who elected NPT were 2.77 times as likely to leave residential treatment as patients who elected MMT (adjusted odds ratio [OR = 2.77, 95% confidence interval [CI]: 1.23–6.17). AMA was associated with interviewer-assessed drug severity and patient’s rating of the importance of psychiatric treatment. The present findings further support the clinical utility of MMT and suggest that policies that facilitate the implementation of MMT in clinical practice would be beneficial to the engagement and retention of pregnant women with opioid use disorders.
Keywords: pregnancy, drug use, mental health, alcohol
INTRODUCTION
The United States is currently experiencing an opioid epidemic, with deaths from prescription opioids having more than quadrupled since 1999 (CDC 2016). Rates of heroin use have also increased over this time, with a 6.2-fold increase in deaths due to heroin overdoses from 2002 to 2015. As opioid use continues to rise, one population of particular concern is pregnant women. An estimated 14–22% of women in the United States receive an opioid prescription during pregnancy (Lind et al. 2017). Opioid use during pregnancy is associated with negative health consequences, including preterm birth, low birth weight, reduced head circumference, neonatal abstinence syndrome (NAS), and sudden infant death syndrome (Lind et al. 2017). Further, opioid use in women of reproductive age, both prescription and illegal, is associated with lower socioeconomic status, family instability, inadequate prenatal care, and other substance use (Whiteman et al. 2014).
Since the 1970s, methadone, delivered in combination with counseling and behavior therapy, has been recommended as the standard of care for opioid use during pregnancy (ACOG 2017). Methadone is associated with improved obstetrical care, reduced pregnancy complications, longer periods of abstinence, and improved fetal outcomes (Jones et al. 2012; Park, Meltzer-Brody, and Suzuki 2012). While the American College of Obstetricians and Gynecologists (ACOG) still currently recommends methadone, it also recognizes buprenorphine as another medication-assisted treatment (MAT) option for pregnant women with opioid use disorders (ACOG 2017). The potential benefits of buprenorphine include less severe withdrawal in neonates and shorter stays in the neonatal intensive care unit (Jones et. al 2012; Unger et al. 2011; Jones et al. 2010; Johnson et al. 2001). Taken together, opioid agonist pharmacotherapy is the recommended treatment over other approaches, such as medically supervised withdrawal, which is associated with high relapse rates ranging from 59% to more than 90% (Saia et al. 2016) and poorer treatment retention (Jones, Terplan, and Meyer 2017).
Despite the effectiveness of MAT, its availability is limited. Methadone can only be administered in daily doses by a registered facility, limiting patient access, particularly in rural communities (Laslo et al. 2017). Further, Medicaid does not cover methadone in many states, creating additional financial barriers to care. Availability of buprenorphine is also limited in the United States due to it being prescribed in mostly office-based practices by physicians who have completed eight hours of certification training and obtained approval from the Substance Abuse and Mental Health Services Administration (SAMHSA) (Bishop et al. 2017). Further, in their first year of practicing with their buprenorphine certification, providers are only approved to prescribe to as many as 30 patients, rising up to 275 patients in the following years. As of January 2018, fewer than 34,000 physicians were certified to prescribe buprenorphine to up to 30 patients/year, and fewer than 4,000 providers could prescribe to 275 patients/year (SAMHSA 2018). As a result, a recent survey found that 96% of states reported higher rates of opioid misuse than their buprenorphine treatment capacity (Jones et al. 2015; Huhn and Dunn 2017). Further, research suggests that only a minority of physicians are prescribing to their capacity, with one study reporting that 48.1% of waivered physicians prescribing buprenorphine to five patients or fewer (Sigmon 2015). The primary reasons cited by physicians were lack of belief in agonist treatment, lack of time, and the belief that reimbursement rates were inadequate (Huhn and Dunn 2017).
In addition to limited availability, the use of these treatment options remains controversial in clinical practice. Communities have opposed the implementation of MAT in their neighborhoods. Arbitrary limits on the duration of medication use for opioid use disorders have been imposed by insurers, and some clinicians still view MAT for opioid use as simply substituting one addiction for another (Olsen and Sharfstein 2014). Further, MAT during pregnancy presents additional barriers to care, including stigma, criminalization of substance use during pregnancy, fear of disclosing substance use, and associated lifestyle barriers (e.g., transportation, child care) (Lester, Andreozzi, and Appiah 2004; Allen 2015). As a result, in many settings, detoxification remains the favored choice or the only available option. The National Institutes of Health has solicited research on this topic as well, with a request for studies of medically-supervised opioid withdrawal during pregnancy and observational and cohort studies evaluating the effects of opioid agonist treatment on maternal, fetal and neonatal outcomes (NIH 2017).
The present study offered a unique opportunity to examine pregnant women with opioid use disorders in an intensive residential and outpatient program, comparing those electing methadone as a therapeutic adjunct to those electing non-pharmacological treatment (NPT). Historically, comparisons between these two treatment modalities were difficult because they are typically operated separately, and today direct comparisons or random assignment by modality would be ethically prohibited. Further, the present study provides a historical perspective on MMT and NPT when less was known about these treatment approaches, and a ‘standard of care’ had not been established for women with opioid use disorders.
METHODS
This quasi-experimental study used data from pregnant women entering a comprehensive treatment program in Baltimore, MD (Svikis et al.1997a). A de-identified dataset was analyzed using a series of behavioral intervention treatment studies from the National Institute on Drug Abuse behavioral treatment research center at The Johns Hopkins University School of Medicine. Data were collected from February 1996 to August 1998 (Svikis et al. 2007; Svikis et al. 1997b). Included were primarily African-American, unemployed women 18 years of age or older, who met DSM-III-R criteria for opioid dependence (American Psychiatric Association 1987). Institutional Review Board approval for the study protocol was acquired, and participants provided written informed consent to participate.
Treatment Program
The treatment program offered intensive interdisciplinary care for substance abuse, mental health, obstetrics/gynecology, pediatric, and nursing/medical needs. Treatment consisted of a seven-day residential stay during which patients underwent detoxification or induction for methadone and became oriented and engaged with the treatment program. Treatment services were provided predominantly through group counseling with once weekly individual sessions. The brief residential stay was followed by intensive outpatient treatment. The treatment program was designed to facilitate attendance and reduce treatment barriers with the provision of transportation, nutritional support, and childcare services, along with onsite obstetrical, pediatric, and psychiatric services (Jansson et al. 1996; Svikis et al. 1997a). In the sample of 210 pregnant women with opioid use disorders, 119 (57%) initiated MMT, while 91 (43%) elected not to initiate MMT. Treatment with MMT was based first on physician’s judgment regarding clinical factors, including drug use severity (lifetime and during pregnancy), treatment history and health status, as well as patient preference. All other components of treatment were equivalent for MMT and NPT receiving women.
Assessments
During the seven-day residential phase of treatment, patients completed the Addiction Severity Index (ASI) within 72 hours of admission as part of standard clinical intake procedures (McLellan et al. 1992; McLellan et al. 1985). All ASI interviewers were trained and certified in ASI administration, and to avoid “interviewer drift”, they attended monthly ASI rounds, where consistency of ASI administration procedures was emphasized and inter-rater reliabilities for the Interviewer Severity Ratings (ISRs) in the seven ASI domains were calculated to ensure adequate reporting (typically over 80% agreement).
On days 3–7 of residential care, women completed the Structured Clinical Interview for DSM-III-R, Patient Edition (SCID-P) (Spitzer, Williams, and Gibbon 1992; Williams et al. 1992). A master-level research supervisor or associate administered the SCID-P to the participants individually to determine the presence of psychiatric disorders, including affective disorders, anxiety disorders, alcohol and substance-use disorders, somatoform disorders, and eating disorders. Interviewers received training from SCID training videotapes and supervision with master-level and doctorate-level research supervisors. Diagnoses in question were reviewed for consensus.
Study Variables
The primary outcome of interest was premature treatment dropout or departure from residential treatment against medical advice (AMA). AMA treatment dropout was defined as staying in treatment six days or less, and non-AMA was defined as completing seven days or more. This time period was selected as a measure of early treatment engagement and retention as all women completed seven days of residential care before transferring to intensive outpatient services. For the present study, each woman’s retention in treatment was calculated by subtracting the date of admission from the first date of discharge from the program.
The primary exposure of interest was the treatment modality, MMT or NPT. Participants were educated about methadone, the recommended treatment for opioid use during pregnancy, as well as the NPT alternative, and it was ultimately their decision which treatment they elected to receive. In most cases, NPT consisted of a three-day methadone-assisted withdrawal (see Jones et al. 2008 for protocol). Covariates measured included demographic variables from the ASI, such as race (Black vs. Caucasian), age (years), education completed (years), employment pattern for the past three years (unemployed vs. employed), and marital status (single vs. married/living as married). Substance use and psychosocial measures included recent use of alcohol, heroin, cocaine, number of previous drug treatments (lifetime, excluding the current admission), days experiencing drug problems (past 30 days) prior to treatment enrollment, patient ratings for the importance of drug and psychiatric treatment (range 0–4, with 0 = not at all and 4 = extremely), and interviewer severity ratings (range 0–9, with 0 = No real problem, treatment not indicated and 9 = extreme problem, treatment absolutely necessary) for each of the seven domains (McLellan et al. 1992). From the SCID-P, the lifetime prevalence of alcohol, opioid and/or cocaine dependence diagnoses were used to describe the study population.
Data Analysis
All statistical analysis and test procedures were performed using SAS software version 9.1, SAS Institute, Cary, NC. Univariate and multivariate analyses were conducted to compare AMA dropout rate between opioid-dependent pregnant women who received MMT and NPT. Comparisons were made using chi-square analyses for categorical variables and t-tests for continuous measures. Unadjusted and adjusted logistic regression models were employed, and odds ratios (OR) and 95% confidence intervals (CI) were calculated after controlling for potential confounding factors. The likelihood ratio test was used to assess first-order interactions and the change-in-estimate strategy was used for predictive model building, with confounders included defined as those variables that altered the OR by 10% or greater. First order interactions (methadone status*age, methadone status*drug interviewer severity rating, and methadone status*race) were assessed and were not statistically significant. The final model was adjusted for the following measures: methadone status, age, patient rating for the importance of drug treatment, and psychiatric interviewer severity rating.
RESULTS
Overall, the two groups were comparable across demographic and pre-treatment substance use characteristics (Table 1). However, three times more Caucasian women comprised the MMT than the NPT study group (27.1% vs. 7.7%, p<0.001). Additionally, the two groups were comparable on most ASI domains and substance use measures. While MMT women reported more days of recent heroin use (mean=27 vs. 23.8, p=0.01), more days of drug problems (mean=27.3 vs 24.1, p=0.01), and more previous drug treatments (mean=2.2 vs. 1.5, p<0.001), the NPT group reported greater importance concerning receiving psychiatric treatment compared to MMT women (1.3 vs. 0.8, p=0.03).
Table 1:
Characteristics of Women by MMT and NPT
| Variable | MMT (n=119) |
NPT (n=91) |
P-value |
|---|---|---|---|
| Demographics | |||
| Mean age* | 28.3 | 27.5 | 0.24 |
| Race (% Caucasian) * | 27.1 | 7.7 | <0.001 |
| Mean education (years)* | 10.9 | 11.3 | 0.08 |
| Employment (past 3 years, % unemployed)* | 81.4 | 74.7 | 0.25 |
| Marital Status (% single)* | 94.1 | 96.7 | 0.38 |
| Substance Use | |||
| DSM-III-R diagnosis (lifetime) | |||
| Opiate dependence (%) | 100.0 | 100.0 | |
| Cocaine dependence (%) | 66.4 | 73.6 | 0.26 |
| Alcohol dependence (%) | 20.2 | 12.1 | 0.12 |
| Recent Use (days in past 30) | |||
| Alcohol* | 1.7 | 2.4 | 0.31 |
| Heroin* | 27.0 | 23.8 | 0.01 |
| Cocaine* | 12.1 | 13.0 | 0.60 |
| Severity | |||
| Previous drug treatments † | 2.2 | 1.5 | <0.01 |
| Days with drug problems (past 30)* | 27.3 | 24.1 | 0.01 |
| Patient Rating | |||
| Importance of drug treatment* | 4.0 | 3.9 | 0.12 |
| Importance of psychiatric treatment* | 0.8 | 1.3 | 0.03 |
| ASI Interviewer Severity Ratings | |||
| Medical | 4.3 | 4.3 | 0.72 |
| Employment* | 5.8 | 5.6 | 0.43 |
| Alcohol* | 0.6 | 0.8 | 0.27 |
| Drug* | 5.9 | 5.7 | 0.14 |
| Family/Social* | 3.6 | 3.5 | 0.78 |
| Psychiatric* | 1.5 | 1.9 | 0.14 |
| Legal* | 1.5 | 1.2 | 0.30 |
MMT (n=117), NPT (n=91)
MMT (n=118), NPT (n=91)
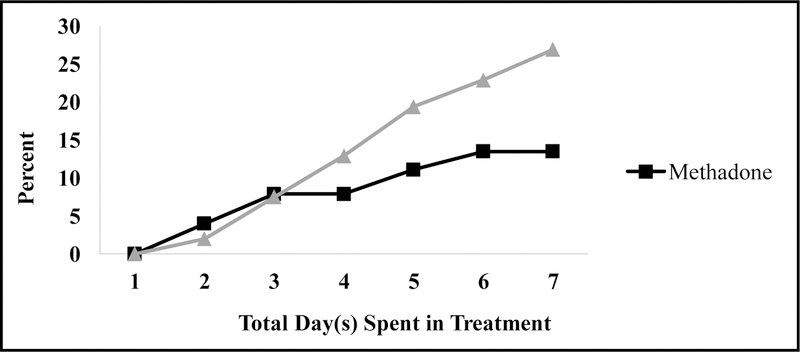
Women who were on NPT were more likely to drop out of the program AMA than those prescribed MMT (Figure 1). The majority of women in NPT left treatment AMA by day six. The unadjusted analyses (Table 2), showed that the odds of women dropping out of the program AMA among the NPT group was 2.57 times higher than women who were on MMT (OR=2.57, 95% CI=1.19–5.58). Leaving treatment AMA was significantly associated with increasing age, cocaine use, patient’s rating of the importance of drug abuse and psychiatric treatment, drug interview severity rating, and family/social interviewer severity rating. However, only interviewer’s severity rating and patient’s rating of the importance of psychiatric treatment were statistically significant in the adjusted analysis (Table 3).
Figure 1.
Proportion of Against Medical Advice Dropout by Treatment Type.
Table 2.
Factors Associated with Patients Departing AMA: Unadjusted Analysis
| Odds Ratio Estimates | |||
|---|---|---|---|
| Effect | Point Estimate | 95% Confidence Interval | |
| NPT vs. MMT | 2.57* | 1.19 | 5.58 |
| Age (per year) | 1.16* | 1.02 | 1.31 |
| Race (Black vs. Caucasian) | 0.92 | 0.46 | 1.87 |
| Education (years) | 1.12 | 0.97 | 1.30 |
| Employment (Unemployed vs. Employed) | 1.31 | 0.75 | 2.29 |
| Marital Status (Single vs. Married/Living as Married) | 0.98 | 0.37 | 2.57 |
| DSM-III-R Diagnosis (lifetime) | |||
| Opiate dependence (Yes vs. No) | 0.98 | 0.47 | 2.07 |
| Cocaine dependence (Yes vs. No) | 1.57 | 0.78 | 3.19 |
| Alcohol dependence (Yes vs. No) | 2.89 | 0.99 | 8.47 |
| Recent Use (days in past 30) | |||
| Alcohol | 1.05 | 0.99 | 1.11 |
| Heroin | 0.99 | 0.97 | 1.01 |
| Cocaine | 1.03* | 1.01 | 1.05 |
| Days with Drug Problem (in past 30 days) | 1.00 | 0.98 | 1.02 |
| Importance of Drug Treatment | 2.45* | 1.23 | 4.86 |
| Importance of Psychiatric Treatment | 1.31* | 1.10 | 1.56 |
| ASI Interviewer Severity Ratings | |||
| Medical | 0.98 | 0.73 | 1.32 |
| Employment | 0.90 | 0.77 | 1.06 |
| Alcohol | 1.40* | 1.10 | 1.77 |
| Drugs | 1.20 | 0.95 | 1.50 |
| Family/Social | 1.22* | 1.08 | 1.38 |
| Psychiatric | 1.23* | 1.08 | 1.41 |
| Legal | 1.05 | 0.90 | 1.21 |
p<0.05
Table 3.
Comparison of AMA Departure of Patients in MMT and NPT: Adjusted Analysis
| Odds Ratio Estimates | |||
|---|---|---|---|
| Effect | Point Estimate | 95% Confidence Interval | |
| NPT vs. MMT | 2.77* | 1.25 | 6.17 |
| Drug Interview Severity Rating | 1.26 | 0.91 | 1.75 |
| Importance of Psychiatric Treatment | 1.36* | 1.01 | 1.84 |
Final model after checking each variable for 10% influence/change. Variables with 10% or greater change are listed in table.
Abbreviations: against medical advice (AMA), non-pharmacological treatment (NPT), methadone maintenance therapy (MMT)
After adjusting for drug interviewer’s severity rating and patient’s rating of the importance of psychiatric treatment, NPT exhibited statistically significant higher odds of AMA departure. The odds of AMA departure was nearly three times higher among the NPT group compared to women on MMT (adjusted OR = 2.77, 95% CI= 1.23–6.17). Per unit increase in the patient’s rating of the importance of psychiatric treatment was associated with a 36% increased odds of AMA departure (adjusted OR = 1.36, 95% CI: 1.01–1.84).
DISCUSSION
As predicted, pregnant women with opioid addiction who were treated with methadone, showed higher retention in residential treatment than those who did not initiate methadone maintenance. This is the first study of which we are aware to compare directly early (first week) treatment retention for MMT and NPT for pregnant women in the same residential treatment setting, one that provided both groups with multidisciplinary medical and psychosocial treatment services. These findings extend previous research demonstrating longer treatment durations with MMT (Mattick et al. 2009) to pregnant women. This work further supports MMT as an evidence-based treatment for opioid use disorder during pregnancy (Polak et al., 2015). This remains clinically important even today. In a recent review of national treatment data (1996–2014), Short and colleagues (2018) found only 50% of pregnant women with opioid use disorder received medication-assisted therapy as a component of their addiction treatment.
Pregnant women with opioid use disorders represent a subpopulation of treatment-seekers with a myriad of issues, including a high prevalence of psychiatric co-morbidities, such as depressive and compulsive disorders and traumatic histories, including sexual and violent abuse (Benningfield et al. 2012; Benningfield et al. 2010; Peles and Adelson 2006; Thompson and Kingree 1998). Specialized drug treatment programs are not ‘treatment as usual’ and instead are developed to provide comprehensive services while also addressing the complex needs of pregnant women. For instance, the current study program provided on-site services and an expedited referral for psychiatric services. Our results suggest that women who do not opt for methadone treatment may benefit from psychiatric care early on in treatment, especially if they have a trauma history. However, it is unknown whether this intense program structure may have contributed to the AMA departure of women who viewed themselves as having less severe problems than other women in the program.
Methadone allows the mother to focus more on treatment and recovery rather than drug craving and symptoms of withdrawal (Mattick et al. 2009; Mattick et al. 2008; Goff and O’Connor 2007; Burns, Mattick, and Cooke 2006; Kaltenbach, Berghella, and Finnegan 1998). In this comprehensive program, because methadone is viewed as a therapeutic adjunct, it also encourages the women to receive other services including prenatal care, parenting classes and drug abuse counseling. Such adjunctive services could be particularly important to address psychiatric and substance use comorbidities that may put women at increased risk of leaving treatment AMA. For example, the present study found women with comorbid alcohol abuse/dependence diagnoses were 2–3 times more likely to leave treatment AMA. Given the negative maternal and fetal/infant outcomes associated with prenatal alcohol use, this subgroup of women deserves particular attention. This is consistent with previous research (Miles et al., 2001) and warrants further study. Although these adjunctive services are made available to all pregnant women with opioid use disorders who meet criteria for initiation of methadone, many refuse due to the stigma of addiction treatment and/or pharmacotherapy during pregnancy.
Buprenorphine provides pregnant women with an alternative pharmacological treatment option that has less controversial side effects (i.e., less severe withdrawal in neonates, shorter stays in neonatal intensive care unit) (Jones et al. 2012; Unger et al. 2011; Jones et al. 2010; Johnson et al. 2001). Unfortunately, buprenorphine was not a treatment option during the data collection for the present study; however, data linking methadone and other medication-assisted therapies, such as buprenorphine, to better treatment retention and participation in health services should be presented when pregnant women are initiating treatment. Further, these data could help guide policy-makers in the development of treatment guidelines for this population, as well as funding programs with integrated pharmacological therapies.
This study had several limitations. First, the data were collected in 1996–1998 before buprenorphine was available. While the present study did not provide information on buprenorphine, methadone still holds an important place in the treatment armamentarium and may be the preferred treatment option for women at risk of treatment discontinuation, those who require higher doses for stabilization, or women who have not had success with buprenorphine (Holbrook 2015). Second, the study was conducted during the time period when cocaine use was prominent, potentially increasing the stigma around methadone maintenance and making it a less attractive treatment option. Third, like most studies involving pregnant women with opioid use disorders, the sample was from a homogeneous treatment population, limiting the generalizability of the results. Fourth, study groups were also not randomly assigned, potentially resulting in group differences and confounding that were not assessed. Lastly, we were limited by available data and could not examine potentially important variables, such as gestational age on admission to the program.
In a 2016 report, the U.S. Surgeon General called for a cultural shift in addiction treatment toward evidence-based practices, as the longstanding public perception of addiction as a moral failing has hindered help-seeking and fostered shame (U.S. Department of Health and Human Services [HHS], 2016). Despite limitations, this study confirmed that MMT has better early treatment engagement and retention than pregnant women receiving NPT during a seven-day residential treatment phase of a specialized treatment program. Dropouts from addiction treatment typically fare poorly due to the chronic nature of substance use disorders. They represent a frustration for providers and constitute a major expense for treatment organizations (McLellan et al. 2000). Policies that support the implementation of methadone treatment in clinical practice and programs that limit treatment barriers for pregnant women may improve both treatment engagement and retention.
Acknowledgements:
We would like to thank Diane Bishop for her contribution to statistical analyses of the data. We would also like to thank Pat Paluzzi, Lauren Jansson, Vickie Walters and Martha Velez for their contributions to the Center for Addiction and Pregnancy (CAP) and to this research.
This research was supported by:
P60 MD002256-01 - NIH/National Center on Minority Health and Health Disparities, Safer Sex Skill Building for Pregnant Drug Abusing Women (PI: Dace Svikis)
DA09258 – NIH/National Institute on Drug Abuse, Behavior Therapy Treatment Research Center (PI: Maxine Stitzer)
References
- Allen S. [March 9, 2018]; The states sending pregnant addicts to jail, not rehab. 2015 Accessed. http://www.thedailybeast.com/the-states-sending-pregnant-addicts-to-jail-not-rehab.
- American College of Obstetricians and Gynecologists (ACOG). [March 1, 2018]; ACOG committee opinion no 711. 2017 Accessed. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Opioid-Use-and-Opioid-Use-Disorder-in-Pregnancy?IsMobileSet=false.
- American Psychiatric Association. 1987. Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- Benningfield MM, Dietrich MS, Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Gabriele F, et al. 2012. Opioid dependence during pregnancy: relationships of anxiety and depression symptoms to treatment outcomes. Addiction 107(S1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benningfield MM, Arria AM, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Fischer GF, Jones HE, and Martin PR 2010. Co-occurring psychiatric symptoms are associated with increased psychological, social, and medical impairment in opioid dependent pregnant women. The American Journal on Addictions 19(5):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D, Borkowski L, Couillard M, Allina A, Baruch S, and Wood S 2017. Bridging the Divide White Paper: Pregnant Women and Substance Use: Overview of Research & Policy in the United States
- Burns L, Mattick RP, and Cooke M 2006. The use of record linkage to examine illicit drug use in pregnancy. Addiction 101(6):873–882. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). [March 9, 2018]; Wide-ranging online data for epidemiologic research (WONDER) 2016 Accessed. http://wonder.cdc.gov.
- Goff M, and O’Connor M 2007. Perinatal care of women maintained on methadone. Journal of Midwifery & Women’s Health, 52(3):e23–e26. [DOI] [PubMed] [Google Scholar]
- Holbrook AM 2015. Methadone versus buprenorphine for the treatment of opioid abuse in pregnancy: Science and stigma. The American Journal of Drug and Alcohol Abuse 41(5):371–373. [DOI] [PubMed] [Google Scholar]
- Huhn AS, and Dunn KE 2017. Why aren’t physicians prescribing more buprenorphine?. Journal of Substance Abuse Treatment 78:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson LM, Svikis DS, Lee J, Paluzzi P, Rutigliano P and Hackerman F 1996. Pregnancy and addiction: A comprehensive care model. Journal of Substance Abuse Treatment 13(4):321–329. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, Kissin WB, Alpan G, Lantz ME, Cone EJ, et al. 2001. Buprenorphine treatment of pregnant opioid-dependent women: Maternal and neonatal outcomes. Drug & Alcohol Dependence 63(1):97–103. [DOI] [PubMed] [Google Scholar]
- Jones HE, Terplan M, and Meyer M 2017. Medically assisted withdrawal (detoxification): Considering the mother-infant dyad. Journal of Addiction Medicine 11(2):90–92. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, and McCance-Katz E 2015. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health Journal 105(8):e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Heil SH, Baewert A, Arria AM, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, and Fischer G 2012. Buprenorphine treatment of opioid-dependent pregnant women: A comprehensive review. Addiction 107(S1):5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, and Fischer G 2010. Neonatal abstinence syndrome after methadone or buprenorphine exposure. New England Journal of Medicine 363(24):2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, O’Grady KE, Malfi D, and Tuten M 2008. Methadone maintenance vs. methadone taper during pregnancy: Maternal and neonatal outcomes. The American Journal on Addictions 17(5):372–386. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, and Finnegan L 1998. Opioid dependence during pregnancy: Effects and management. Obstetrics and Gynecology Clinics 25(1):139–151. [DOI] [PubMed] [Google Scholar]
- Laslo J, Brunner JM, Burns D, Butler E, Cunningham A, Killpack R, Pyeritz C, Rinard K, Childers J, and Horzempa J 2017. An overview of available drugs for management of opioid abuse during pregnancy. Maternal Health, Neonatology and Perinatology 3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Andreozzi L, and Appiah L 2004. Substance use during pregnancy: Time for policy to catch up with research. Harm Reduction Journal 1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind JN, Interrante JD, Ailes EC, Gilboa SM, Khan S, Frey MT, Dawson AL, Honein MA, Dowling H NF Razzaghi et al. 2017. Maternal use of opioids during pregnancy and congenital malformations: A systematic review. Pediatrics 139(6):e20164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, and Davoli M 2009. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews 3(3). [Google Scholar]
- Mattick RP, Kimber J, Breen C, and Davoli M 2008. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews 2(2). [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, and Kleber HD 2000. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. Jama 284(13):1689–1695. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, and Argeriou M 1992. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment 9(3):199–213. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, and OʼBrien CP 1985. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease 173(7):412–423. [DOI] [PubMed] [Google Scholar]
- Miles DR, Svikis DS, Kulstad JL, and Haug NA 2001. Psychopathology in pregnant drug-dependent women with and without comorbid alcohol dependence. Alcoholism: Clinical and Experimental Research 25(7):1012–1017. [PubMed] [Google Scholar]
- National Institutes of Health (NIH). Opioid use disorder in pregnancy. 2017 Available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-HD-18-036.html.
- Olsen Y, and Sharfstein JM 2014. Confronting the stigma of opioid use disorder and its treatment. Jama 311(14):1393–1394. [DOI] [PubMed] [Google Scholar]
- Park EM, Meltzer-Brody S, and Suzuki J 2012. Evaluation and management of opioid dependence in pregnancy. Psychosomatics 53(5):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, and Adelson M 2006. Gender differences and pregnant women in a methadone maintenance treatment (MMT) clinic. Journal of Addictive Diseases 25(2):39–45. [DOI] [PubMed] [Google Scholar]
- Polak K, Haug NA, Drachenberg HE, and Svikis DS 2015. Gender considerations in addiction: Implications for treatment. Current Treatment Options Psychiatry 2(3):326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saia KA, Schiff D, Wachman EM, Mehta P, Vilkins A, Sia M, Price J, Samura T, DeAngelis J, Jackson C, et al. 2016. Caring for pregnant women with opioid use disorder in the USA: Expanding and improving treatment. Current Obstetrics and Gynecology Reports 5(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short VL, Hand DJ, MacAfee L, Abatemarco DJ, and Terplan M 2018. Trends and disparities in receipt of pharmacotherapy among pregnant women in publically funded treatment programs for opioid use disorder in the United States. Journal of substance abuse treatment 89:67–74. [DOI] [PubMed] [Google Scholar]
- Sigmon SC 2015. The untapped potential of office-based buprenorphine treatment. JAMA Psychiatry 72(4):395–396. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, and First MB 1992. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry 49(8):624–629. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). [March 9, 2018]; Physician and program data. 2018 Accessed. https://www.samhsa.gov/programs-campaigns/medication-assisted-treatment/training-materials-resources/physician-program-data.
- Svikis DS, Golden A, Huggins G, Pickens RW, McCaul ME, Velez M, Rosendale T, Brooner R, Gazaway P, Stitzer M, and Ball C 1997. Cost-effectiveness of treatment for drug-abusing pregnant women. Drug and Alcohol Dependence 45:105–113. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Lee JH, Haug NA, and Stitzer M 1997. Attendance incentives for outpatient treatment: Effects in methadone-and nonmethadone-maintained pregnant drug dependent women. Drug and Alcohol Dependence 48(1):33–41. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Silverman K, Haug NA, Stitzer M, and Keyser-Marcus L 2007. Behavioral strategies to improve treatment participation and retention by pregnant drug-dependent women. Substance Use & Misuse 42(10):1527–1535. [DOI] [PubMed] [Google Scholar]
- Thompson MP, and Kingree JB 1998. The frequency and impact of violent trauma among pregnant substance abusers. Addictive Behaviors 23(2):257–262. [DOI] [PubMed] [Google Scholar]
- Unger A, Jagsch R, Jones H, Arria A, Leitich H, Rohrmeister K, Aschauer C, Winklbaur B, Bawert A, and Fischer G 2011. Randomized controlled trials in pregnancy: Scientific and ethical aspects. Exposure to different opioid medications during pregnancy in an intra-individual comparison. Addiction 106(7):1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (HHS). [March 9, 2018]; Facing addiction in America: The surgeon general’s report on alcohol, drugs, and health. 2016 Accessed. https://addiction.surgeongeneral.gov/sites/default/files/executive-summary.pdf. [PubMed]
- Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, and Salihu HM 2014. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. Journal of Pregnancy 2014. [DOI] [PMC free article] [PubMed]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rousaville B, et al. 1992. The structured clinical interview for DSM- III-R (SCID). Archives of General Psychiatry 49(8):630–636. [DOI] [PubMed] [Google Scholar]