Abstract
The prevalence of cardiovascular diseases (CVD) is increased in subjects with post-traumatic stress disorder (PTSD). Vascular inflammation mediates CVD and may be assessed by18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging. In this pilot study, we investigated whether subjects with PTSD have enhanced vascular and systemic inflammation compared to healthy controls, as assessed by FDG PET imaging.
Methods
A prospective group of 16 subjects (9 PTSD and 7 controls, age 34±7) without prior history of CVD underwent FDG PET/CT imaging. The presence of PTSD symptoms at the time of the study was confirmed using PTSD checklist for DSM-5 (PCL5) questionnaire. Blood samples were collected to determine blood glucose, lipid and inflammatory biomarkers (tumor necrosis factor α, interleukin-1β, and interleukin-6) levels. FDG signal in the ascending aorta, amygdala, spleen and bone marrow was quantified.
Results
The two groups matched closely with regards to cardiovascular risk factors. The inflammatory biomarkers were all within the normal range. There was no significant difference in FDG signal in the aorta (target to background ratio: 2.40±0.29 and 2.34±0.29 for control and PTSD subjects, difference −0.6, 95% confidence interval of difference −0.38 – 0.26), spleen, bone marrow, or amygdala between control and PTSD subjects. There was no significant correlation between aortic and amygdala FDG signal. However, a significant positive correlation existed between amygdala, splenic, and bone marrow FDG signal.
Conclusion
This pilot, small study did not reveal any difference in vascular or systemic inflammation as assessed by FDG PET imaging between PTSD and healthy control subjects. Because of the small number of subjects, a modest increase in vascular inflammation, which requires larger scale studies to establish, cannot be excluded. The correlation between FDG signal in amygdala, spleen and bone marrow may reflect a link between amygdala activity and systemic inflammation.
Keywords: FDG, PET/CT, Inflammation, PTSD
Introduction
Post-traumatic stress disorder (PTSD), “the complex somatic, cognitive, affective, and behavioral effects of psychological trauma” (1), is associated with cardiovascular disease (CVD) and related mortality (2) (3). Both behavioral factors (smoking, alcohol abuse, and non-adherence to medications), and physiological mechanisms (autonomic dysregulation, hypertension, hypothalamic-pituitary-adrenal axis dysregulation, and inflammation) may contribute to this increased CVD prevalence and mortality. Although not a consistent finding, several studies have reported that PTSD is associated with systemic inflammation (4). Vessel wall inflammation may, at least in part, mediate the potential effects of systemic inflammation on the development of CVD in PTSD (5).
18F-fluorodeoxyglucose (FDG) uptake is increased in highly metabolic cells, including inflammatory cells. Accordingly, FDG PET has been used as a tool to detect vascular inflammation associated with atherosclerosis and systemic diseases (6, 7). It is reported that the aortic signal on FDG PET images correlates with the spleen and bone marrow metabolic activity, particularly in patients with acute coronary syndrome (8), potentially reflecting a link between vascular and systemic inflammation. Similarly, an association between vascular inflammation, as detected by FDG PET imaging, and metabolic activity in amygdala (a hyperactive brain region in patients with PTSD (9)) has been reported (10). Notably, the amygdala metabolic activity on FDG PET images predicted the risk of future CV events in this study (10). This led us to conduct a pilot FDG PET imaging study to investigate whether relative to healthy controls, subjects with PTSD and no established CVD have enhanced vascular and systemic inflammation in correlation with amygdala metabolic activity.
Materials and Methods
Subjects
The study was performed under protocols approved by Yale University and VA Connecticut Healthcare System Institutional Review Board (IRB) committees. Sixteen subjects (9 PTSD and 7 healthycontrols) were recruited between April and September 2017 through advertisement and by contacting potentially interested subjects from a screening IRB protocol at our institution. Inclusion criteria were: (i) age between 21 and 50 years, (ii) clinical diagnosis of current (or no PTSD for the control group), iii) ability to read and write English and give informed consent. Exclusion criteria included: (i) any history of cardiovascular or cerebrovascular disease, (ii) incapacitating or life-threatening illness, (iii) systemic inflammatory disorders, including known HIV, (iv) eGFR <60 mL/min/1.73 m2, or history of kidney disease, (v) claustrophobia, and (vi) current pregnancy or intention to become pregnant during the study. Following written informed consent, the subjects’ medical, psychiatric and social history information, including cardiovascular risk factors and current treatment were collected and a physical exam was performed. The diagnosis of PTSD was confirmed using the Clinician-Administered PTSD Scale (CAPS-5) questionnaire, excluding other major psychiatric disorders. The persistence of PTSD symptoms at the time of imaging was evaluated using PTSD checklist for DSM-5 (PCL5) questionnaire, with a score of 23 or more considered diagnostic of persistent PTSD. Samples of blood were collected in all subjects to determine blood glucose level, lipid panel, creatinine and inflammatory biomarkers.
Imaging Data Acquisition
The subjects were advised to follow a low carbohydrate diet for 24 hours and fast for > 6 hours prior to the study. After verifying the blood glucose level (≤ 106 mg/dL in all subjects), a low dose non-contrast CT (17 mAs, 120 kVp) was obtained for attenuation correction and anatomic co-registration. Next, the subjects were injected with 340 ± 28 MBq of FDG and a 2 h-long dynamic PET acquisition was performed using a Biograph mCT PET/CT scanner (Siemens Medical Systems). This included an initial chest focused acquisition (1-bed position) to determine first-pass blood activity, followed by multi-pass, continuous 3 or 4- bed-position data acquisition covering the neck, chest and abdomen. PET images were reconstructed using ordered-Subset Expectation-Maximization algorithm (21 subsets, 2 iterations) with 2.036 × 2.036 × 2 mm3 voxel size with Point-Spread-Function and Time-of-Flight information incorporated, and using the CT data for attenuation correction. Imaging was interrupted after ~95 minutes at the request of the subject in one of the PTSD subjects. This subject was excluded from imaging data analysis. Brain MRI was performed on a separate day to identify the location of the amygdala. MR data were acquired on a 3.0T Trio ((Siemens Healthcare, Erlangen, Germany) with a T1-weighted gradient-echo (magnetization-prepared rapid gradient- echo, MP-RAGE) sequence yielding 1 mm3 isotropic resolution.
Image analysis
FDG uptake in the ascending aorta was quantified by two independent investigators (JT and HE) on images reconstructed from the final 30 minutes of the two-hour data acquisition, excluding areas of high signal originating from outside the vessel wall, as described (11). Blood pool activity (SUVmean) was measured in superior vena cava. For aortic FDG signal, maximal standardized uptake values measured on consecutive slices cranial to pulmonary arteries and covering the entire ascending aorta were averaged as SUVmax. Data were expressed as target-to-background ratio (TBR) by normalizing SUVmax to blood pool SUVmean. FDG signals in the spleen and bone marrow (expressed as SUVmax) were quantified by placing oval regions of interest over the spleen and lumbar vertebrae. The intraclass correlation coefficient (ICC) for combined SUV measurements was 0.98 (p<.0001), indicating an excellent interobserver agreement. Images were analyzed using OsiriX (Geneva, Switzerland). To assess the amygdala signal, FDG PET brain images were registered to the subject’s MRI image, which was in turn coregistered to the Montreal Neurological Institute template. Time-activity curves of the amygdala were extracted for quantitation of SUVmean.
Blood biomarker analysis
The concentrations of tumor necrosis factor (TNF)α, interleukin (IL)-1β, and IL-6 in the plasma were quantified by ELISA using dedicated kits (Quantikine ELISA Kit, R&D Systems) following the manufacturer’s instructions.
Statistical analysis
Continuous parametric variables are presented as mean ± SD. Categorical variables are presented as counts and percentages. Normal distribution of continuous data was tested with the D’Agostino-Pearson normality test. Continuous variables were compared using Student’s t test for normally distributed, and Mann- Whitney U test for non-normally distributed data (creatine). Chi-square test was used to compare dichotomous variables. Pearson’s correlation was used to test the association between two variables. A p-value below 0.05 was considered statistically significant. For sample size analysis, assuming a TBR of 2.36 ± 0.25 in normal subjects (11), 7 subjects in each group provide a two sided 80% power and an alpha of 5% to establish a 0.41 difference in the mean values of FDG signal between the two groups of subjects. Data were analyzed using GraphPad Prism 7, GraphPad StatMate 2, and IBM SPSS Statistics 24 (for ICC).
Results
Subject characteristics
The two groups matched closely by age and sex, and with regards to cardiovascular risk factors, blood pressure, smoking history, lipid profile, and fasting blood glucose (Table 1). The subjects had minimal medication use in the week prior to the study (5 PTSD subjects and 4 controls), consisting mainly of analgesics and psychotropic agents. The index trauma (mainly sexual trauma, war-related trauma, and gun violence), dated back to 16 ± 13 years (range 1 to 30 years). Evaluation of PTSD symptoms around the timeof imaging showed a PCL-5 score of 33 ± 12 in PTSD subjects and 3 ± 4 in controls (p < 0.0001).
Table 1:
Clinical characteristics and laboratory data of the subjects
| Control (n = 7) | PTSD (n = 8) | p-value | |
|---|---|---|---|
| Age (years) | 34 ± 8 | 34 ± 7 | 0.94 |
| Male sex | 5(71) | 5 (63) | 0.71 |
| Current smoker | 3 (43) | 2(25) | 0.58 |
| BMI | 30 ± 3 | 31 ± 6 | 0.82 |
| Systolic BP (mmHg) | 132 ± 10 | 125 ± 12 | 0.24 |
| Diastolic BP (mmHg) | 83 ± 6 | 83 ± 10 | 0.95 |
| Total cholesterol (mg/dL) | 191 ± 45 | 210 ± 47 | 0.44 |
| LDL-C (mg/dL) | 121 ± 36 | 130 ± 43 | 0.69 |
| HDL-C (mg/dL) | 46 ± 10 | 49 ± 13 | 0.67 |
| Triglycerides (mg/dL) | 119 ± 44 | 160 ± 76 | 0.23 |
| Creatinine (mg/dL) | 0.56 ± 1.08 | 0.49 ± 1.01 | 0.39* |
| Glucose (mg/dL) | 95 ± 5 | 92 ± 11 | 0.50 |
| PCL-5 score | 3 ± 4 | 34 ± 12 | <0.0001 |
| Time since index event (year) | n/a | 15 ± 13 | n/a |
Values are mean ± SD or n (%). BMI: body mass index; BP: Blood pressure; LDL-C: low density lipoprotein- cholesterol; HDL-C: high density lipoprotein-cholesterol; PCL-5: PTSD checklist for DSM-5.
Mann- Whitney U test.
Comparison of arterial and systemic inflammation between PTSD and control groups
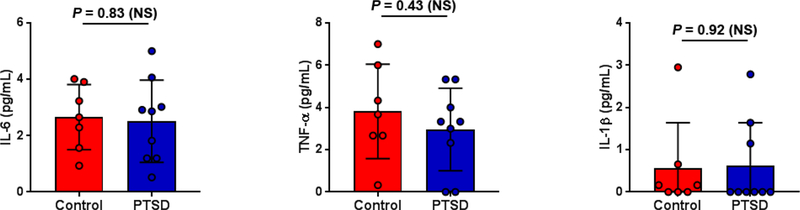
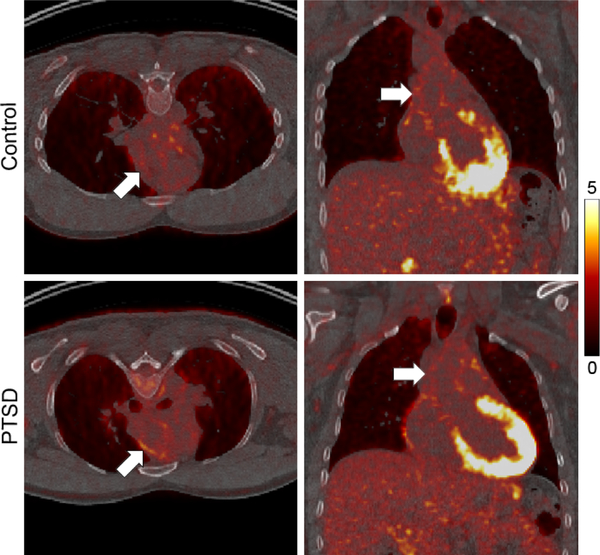
None of the subjects showed elevated inflammatory biomarkers (TNFα < 7 pg/mL, IL-1β: < 3 pg/mL, IL-6: < 5 pg/mL for all subjects, Figure 1). Examples of PET/CT images of the chest in subjects from the control and PTSD groups are presented in Figure 2. No difference in aortic TBR (or SUVmax) was observed between control and PTSD groups (2.40 ± 0.29 vs 2.34 ± 0.29, respectively, p = NS, 95% CI of difference −0.38 – 0.26, Table 2). Similarly, there was no difference between the two groups in FDG signal in the spleen, bone marrow, or amygdala (Table 2). Finally, there was no significant correlation between inflammatory biomarker levels and FDG signal in the amygdala or other tissues (data not shown).
Figure 1.
Plasma inflammatory biomarker levels in control and PTSD groups. IL-6: interleukin-6; TNFα: tumor necrosis factor; IL-1β: interleukin-1β. NS: not significant.
Figure 2.
Examples of PET/CT images in subjects from the control and PTSD groups. Arrows point to the ascending aorta. Scale bar: SUV 0–5.
Table 2:
Quantification of FDG signal in different organs
| Control (n = 7) | PTSD (n = 8) | p | |
|---|---|---|---|
| Aortic wall (TBR) | 2.40 ± 0.29 | 2.34 ± 0.29 | 0.69 |
| Aortic wall (SUVmax) | 3.56 ± 0.33 | 3.30 ± 0.35 | 0.16 |
| Bone marrow (SUVmax) | 4.65 ± 0.93 | 4.13 ±0.66 | 0.24 |
| Spleen (SUVmax) | 3.27 ± 0.37 | 3.00 ± 0.45 | 0.21 |
| Amygdala (SUVmean) | 7.07 ± 0.81 | 7.01 ± 1.18 | 0.91 |
| Blood (SUVmean) | 1.50 ± 0.19 | 1.43 ± 0.21 | 0.51 |
Values are mean ± SD.
Relation of aortic FDG signal with the signal in other tissues
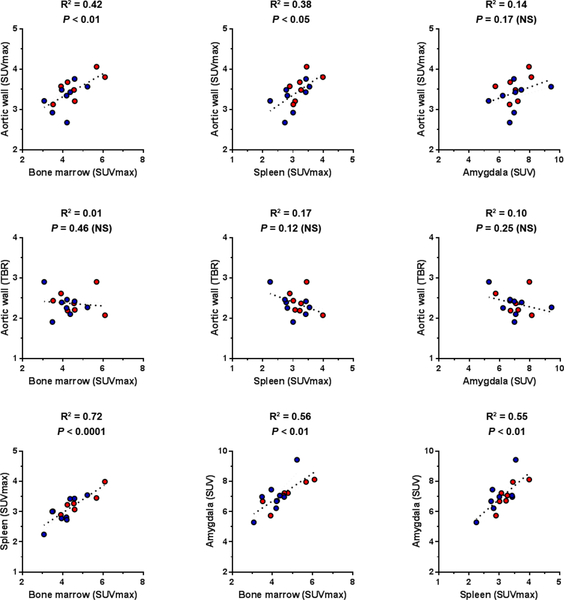
Using SUVmax to quantify FDG signal in the aorta, bone marrow and spleen, tracer uptake in all three organs correlated significantly with each other (Figure 3). No significant correlation existed between aortic TBR, and FDG signal in hematopoietic organs (bone marrow and spleen), whether reported as TBR or SUVmax (Figure 3 and Supplemental Table 2). Amygdala FDG signal significantly correlated with bone marrow and spleen, but not aortic FDG uptake, regardless of the methodology used to quantify the signal (Figure 3 and Supplemental Tables 2).
Figure 3.
Correlation between FDG signal in different organs. Control subjects are shown in red and PTSD subjects in blue circles. NS: not significant.
Discussion
Here, we present the results of a pilot study to evaluate vascular and systemic inflammation by FDG PET imaging in subjects with PTSD in comparison with healthy controls. Our study showed no difference in aortic, bone marrow, or spleen FDG signal between the two groups. Similarly, the blood inflammatory biomarkers, TNFα, IL-1β and IL-6, were within the normal range and comparable in both groups. Furthermore, while there was no correlation between FDG signal in the aorta and amygdala, FDG signal in the bone marrow, spleen and amygdala correlated significantly with each other.
PTSD is associated with increased risk of CVD and related morbidity and mortality. The exact mechanism of this association is not known, but the increased prevalence of CV risk factors, e.g., HTN, hyperlipidemia and smoking, probably contributes to this elevated risk (2). A recent meta-analysis of the studies on the association between CVD in PTSD concluded that the presence of PTSD is associated with a hazard ratio (HR) of 1.55 [95% CI, 1.34–1.79] for coronary heart disease, potentially independent of traditional CV risk factors. When corrected for comorbid depression the HR estimate decreased to 1.27 (95% CI, 1.08–1.49) (2). Given the role of inflammation in cardiovascular pathology, it is reasonable to assume that systemic or vascular inflammation may mediate the effect of PTSD on CVD. Indeed, several clinical studies have concluded that proinflammatory cytokines (e.g., IL-6, TNFα and IL-1β) are elevated in PTSD (4, 12). Interestingly, in our pilot study of a small number of relatively young subjects with minimal comorbidity, none of the subjects showed elevated levels of blood inflammatory biomarkers. A recent report based on 13 subjects concluded that perceived stress on psychometric analysis (Perceived Stress Scale (13)) correlates modestly with amygdala activity and arterial inflammation (10). However, a causal relationship between PTSD and systemic or vascular inflammation has not been established. Indeed, many of aforementioned studies have included older subjects with considerable co-morbidity which may have confounded the results, despite efforts aimed at accounting for these on multi-variable analysis. Our prospective pilot study in a well- evaluated, albeit small, group of PTSD subjects with no CV co-morbidity and few CV risk factors, did not show any increased systemic or vascular inflammation, as assessed by FDG PET and blood biomarkers, relative to controls.
Despite its well-known limitations, FDG PET is often used as a tool to detect vascular inflammation (6, 7), and efforts are underway to standardize the imaging and image analysis protocols (14). Our sample size analysis was based on reported ascending aorta TBR values for normal subjects (2.36 ± 0.25) and patients at increased CVD risk (2.80 ± 0.31). To put these numbers into perspective, for patients with known CVD, the TBR is reported to be 2.97 ± 0.59 (11). The 95% CI of the difference in aortic FDG signal between normal subjects and those with PTSD in our pilot study (−0.38–0.26) indicates that at most, PTSD in the absence of major CV risk factors, such as hypertension and smoking, has a modest effect on aortic FDG signal.
In the context of acute coronary syndrome (ACS), a setting associated with enhanced systemic inflammation, the bone marrow and spleen metabolic activity measured by FDG PET correlate with aortic FDG signal and blood levels of C-reactive protein (8, 15). A similar correlation is reported between the bone marrow/spleen metabolic activity and amygdala activity in retrospective analysis of FDG PET/CT studies performed for clinical indications, mainly for cancer screening (10). Notably, in our relatively healthy subjects (except for PTSD), we did not detect any association between FDG signal in amygdala and aorta, and the association between aorta and hematopoietic organs depended on the methodology used to evaluate the FDG signal. However, we did indeed detect a correlation between FDG signal in amygdala, bone marrow and spleen in the whole cohort of subjects, regardless of the quantification methodology. This suggests that unlike the previously reported association with vascular inflammation (10), a relationship between amygdala and bone marrow/splenic FDG signal may be present in a broad setting. The underlying cause of this interesting relationship, which may reflect the presence of a brain-systemic inflammation axis, merits further evaluation.
Limitations
Our study included relatively young subjects with longstanding and persistent PTSD symptoms (PCL- 5 score 34 ± 12), but sufficiently functional to enroll and participate in this study. This may have led to selection bias. We cannot rule out that aging and development of cardiovascular risk factors with chronic PTSD might have additional effects that are not detectable in younger subjects. Most of our subjects were not treated by pharmacotherapy, indicating that their PTSD symptoms were well-controlled with supportive measures. While this excludes the potential confounding effect of such therapy on vascular and systemic inflammation, we cannot extrapolate our findings to patients with poorly controlled PTSD symptoms. For image analysis, we relied on the most commonly used methodology (SUVmean, SUVmax, TBR) to quantify and report the FDG signal in each organ, acknowledging that there are inconsistencies and considerable variation in the robustness of such analytic methodologies for different organs. As discussed above, becauseof the small number of subjects included in this pilot study, a small increase (< 20% in TBR) in vascular inflammation cannot be excluded. Finally, our study is focused on PTSD, which is a chronic disease. As such, it does not provide any information on the effects of acute stress on vascular or systemic inflammation.
Conclusions
This pilot study did not reveal any significant differential vascular or systemic inflammation, as assessed by FDG PET imaging between PTSD and control subjects. Because of the small number of subjects, a modest increase in vascular inflammation, which requires larger scale studies to establish, cannot be excluded. FDG signal in the spleen and bone marrow correlated with FDG uptake in the amygdala, potentially reflecting a relation between amygdala activity and systemic inflammation.
New Knowledge Gained
There are at most modest differences in vascular or systemic inflammation as detected by FDG PET imaging between PTSD and control subjects with minimal associated cardiovascular risk factors. However, there is a correlation between FDG signal in the spleen and bone marrow, and FDG uptake in the amygdala, potentially reflecting a relation between amygdala activity and systemic inflammation.
Supplementary Material
Funding Sources
This work was supported by the VA National Center for PTSD, NIAAA (K01AA024788), and NIMH (R01MH110674).
Abbreviations:
- CAPS-5
Clinician-Administered PTSD Scale
- CT
Computed tomography
- CV/CVD
cardiovascular/cardiovascular disease
- ELISA
Enzyme-linked immunosorbent assay
- FDG
18F-fluorodeoxyglucose
- PCL5
PTSD checklist for DSM-5
- PET
Positron emission tomography
- PTSD
Post-traumatic stress disorder
- SUV
Standardized uptake value
- TBR
Target-to-background ratio
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures
No potential conflicts of interest relevant to this article exist. MMS is a consultant for Bracco Research USA.
References
- (1).van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. Am J Psychiatry 1996;153:83–93. [DOI] [PubMed] [Google Scholar]
- (2).Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. American heart journal 2013;166:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Edmondson D, von Kanel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 2017;4:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015;2:1002–12. [DOI] [PubMed] [Google Scholar]
- (5).Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014;11:443–57. [DOI] [PubMed] [Google Scholar]
- (7).Sadeghi MM. (18)F-FDG PET and vascular inflammation: time to refine the paradigm? J Nucl Cardiol 2015;22:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JHF et al. Splenic Metabolic Activity Predicts Risk of Future Cardiovascular Events: Demonstration of a Cardiosplenic Axis in Humans. JACC: Cardiovascular Imaging 2015;8:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007;164:1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).van der Valk FM, Verweij SL, Zwinderman KAH, Strang AC, Kaiser Y, Marquering HA et al. Thresholds for Arterial Wall Inflammation Quantified by (18)F-FDG PET Imaging: Implications for Vascular Interventional Studies. JACC Cardiovascular imaging 2016;9:1198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Mellon SH, Gautam A, Hammamieh R, Jett M, Wolkowitz OM. Metabolism, Metabolomics, and Inflammation in Posttraumatic Stress Disorder. Biological psychiatry 2018;83:866–75. [DOI] [PubMed] [Google Scholar]
- (13).Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- (14).Gholami S, Salavati A, Houshmand S, Werner TJ, Alavi A. Assessment of atherosclerosis in large vessel walls: A comprehensive review of FDG-PET/CT image acquisition protocols and methods for uptake quantification. J Nucl Cardiol 2015;22:468–79. [DOI] [PubMed] [Google Scholar]
- (15).Kim EJ, Kim S, Kang DO, Seo HS. Metabolic Activity of the Spleen and Bone Marrow in Patients With Acute Myocardial Infarction Evaluated by 18F-Fluorodeoxyglucose Positron Emission Tomograpic Imaging. Circulation: Cardiovascular Imaging 2014;7:454–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.