Abstract
Background.
Best practices to facilitate high-quality shared decision-making for lung cancer screening (LCS) are not well established. In our LCS program, patients are first referred to attend a free group education class on LCS, taught by designated clinician specialists, before a personal shared decision-making visit is scheduled.
Objective.
To evaluate class effectiveness in enhancing patient knowledge and shared decision–making about LCS.
Methods.
For quality improvement purposes, participants were asked to complete one-page surveys immediately before and after class to assess knowledge and decision–making capacity regarding LCS. To evaluate knowledge gained, we tabulated the distributions of correct, incorrect, unsure, and missing responses to eight true-false statements included on both pre- and post-class surveys and assessed pre-post differences in the number of correct responses. To evaluate decision-making capacity, we tabulated the distributions of post-class responses to items on decision uncertainty.
Results.
From June 2017 to August 2018, 680 participants completed both pre- and post-class surveys. Participants had generally poor baseline knowledge about LCS. The proportion who responded correctly to each knowledge-related statement increased pre- to post-class, with a mean difference of 0.9 (paired t-test, p<0.0001) in the total number of correct responses between surveys. About 70% reported having all the information needed to make a screening decision.
Conclusions.
Our results suggest that a well-designed group education class is an effective system-level approach for initially educating and equipping patients with appropriate knowledge to make informed decisions about LCS.
Keywords: lung neoplasms, mass screening, patient education, shared decision-making, quality improvement
Results from the National Lung Screening Trial (NLST) support current guidelines to screen asymptomatic high-risk smokers for lung cancer annually with low-dose computed tomography (LDCT) in the United States [1–4]. Along with potential benefits, lung cancer screening (LCS) involves potential harms, including false-positive findings and unnecessary invasive follow-up procedures [5]. Given the tradeoffs between benefits and harms, the importance of shared decision-making (SDM) prior to screening initiation has been emphasized, notably by the Centers of Medicare and Medicaid Services (CMS) requiring a documented face-to-face SDM visit as a prerequisite for LDCT screening coverage [6]. Best practices for educating individuals to facilitate high-quality SDM for LCS, however, are not yet well established.
Various strategies, including use of patient decision aids, clinician education, use of health navigators, and practice redesign to engage patients in their healthcare decisions, have been proposed to support SDM [7]. Nevertheless, growing evidence suggests that providers lack sufficient time and resources to enable potentially eligible patients to make fully informed decisions about LCS [8–11]. During pilot implementation of LCS in our healthcare system, we identified additional workflow issues from direct LCS referral by primary care physicians (PCPs), including referral of ineligible individuals and incomplete clinical documentation of eligibility [12].
To mitigate these quality issues and enhance patient engagement, PCPs now refer English- speaking individuals who are interested in being screened to first attend a free 60-minute standardized group education class on LCS. The class is taught by designated clinician specialists, primarily pulmonologists, responsible for navigating the initial steps of the screening process, from verifying and documenting eligibility to ordering baseline LDCT scans. Class attendance has become a standard practice prior to scheduling a personal SDM visit, as part of our LCS program.
In this context, we conducted a quality improvement evaluation to determine class effectiveness in increasing patient knowledge and supporting SDM regarding LCS. Our expectation was that after class attendance, patients would be better educated and equipped to decide whether to move forward with screening.
METHODS
Organizational Setting and Context
Kaiser Permanente Northern California (KPNC) is a fully integrated healthcare delivery system that currently serves over four million individuals, roughly a third of the population in its 23–county service area, at 21 hospital-based centers and 242 medical offices. Its membership is relatively stable, with particularly high retention in older adults, and representative of the insured population in Northern California except at the socioeconomic extremes [13]. In September 2012, its physician group practice, The Permanente Medical Group, formed a multidisciplinary working group tasked with developing and implementing a high-quality LCS program. Among its program goals is enabling eligible individuals to make an informed decision about LCS.
Following U.S. Preventive Services Task Force (USPSTF) recommendations [2], pilot implementation of LCS workflow processes and tools at selected KPNC medical centers started in July 2014During the first pilot, when PCPs referred patients directly to LDCT screening, nearly a third of referred patients were ineligible, and clinical documentation of SDM was inadequate (unpublished data). These observations led to the next pilot that substantiated the use of clinical navigators to ensure patients meet eligibility criteria and make informed decisions. We also discovered that a meaningful, personalized SDM discussion about LCS often took longer than the time allotted in the 2016 CMS fee schedule for this visit (>15 minutes). This finding motivated the design and integration of a standardized patient education class within our regional LCS program, which was rolled out across medical centers between December 2016 and October 2017.
Patient Education Class
PCPs have since referred English-speaking patients who are interested in LCS to attend this class before a personal face-to-face SDM visit occurs. At each center, designated clinician specialists, of whom over 80% are pulmonologists, teach this class, hold personal SDM visits, and verify and document patient eligibility before ordering screening LDCT scans. Non-English-speaking patients are referred either to the class with an interpreter and provided an option for a same-day SDM visit with the instructing clinician specialist and interpreter, or an extended personal SDM visit with a clinician specialist and interpreter.
The class provides the opportunity for patients to decide whether LCS is right for them. Key aspects, including the eligibility criteria and potential benefits and harms, are presented. A risk assessment is illustrated for a hypothetical patient during class and then later personalized and discussed at the SDM visit if a patient chooses to continue with screening. The importance of smoking abstinence is stressed to encourage current smokers to quit. Patient education materials and a decision worksheet handout, all developed by our Regional Health Education department, are provided to support the learning process.
After the initial class rollout, we solicited feedback from the clinician specialists to refine the class curriculum and enrich the patient experience. We made revisions iteratively during Spring 2017. For example, a figure presenting statistics on benefits and harms was simplified with pictographs, absolute risks, and plain language to better communicate potential risks to patients [14].
Participants
This evaluation was determined as not research by the National Research Compliance Officer of the Kaiser Foundation Research Institute. Participants were class attendees from June 2017 to August 2018, who completed surveys administered immediately before and after the class. Herein, we limited evaluation to those who completed both pre- and post-class surveys, specifically to assess individual-level change in LCS knowledge resulting from class attendance. Among 856 participants over the 14-month evaluation period, 680 (79%) completed both surveys, 118 (14%) completed the pre-class survey only, 25 (3%) completed the post-class survey only, and 33 (4%) completed neither survey.
Survey Administration and Measures
We developed self-administered one-page pre- and post-class surveys to examine the impact and quality of the class on enhancing LCS knowledge and SDM. Facility staff sent completed surveys by secure electronic fax for centralized data management and analysis.
Pre-class survey.
The pre-class survey queried about class expectations, smoking history, and general attitudes and knowledge about LCS. To characterize attitudes, participants were asked to respond to ten statements, using a five-category Likert scale (strongly agree, agree, disagree, strongly disagree, undecided/not sure). These items were selected from work by Crothers et al. [15], Carter-Harris et al [16], and Cataldo et al.[17]. To measure knowledge, participants were asked to select the best response (true, false, or unsure) to eight statements used by Crothers et al. [15]. Four regarded potential benefits, three regarded potential harms, and one regarded eligibility for screening.
Post-class survey.
The post-class survey queried about decision–making capacity and knowledge regarding LCS, interest in LCS and quitting smoking, and demographic information (i.e., birth year, gender, and race/ethnicity). To measure decision–making capacity, participants were asked to respond to three statements about decision uncertainty from the Decision Conflict Scale, using a five-category Likert scale [18], and the yes-no question, “Do you feel you have all the information you need to make a decision?” Those who remained uncertain were asked to specify what else they needed to make their decision. To reassess knowledge, participants responded to the same eight statements included on the pre-class survey. To gauge interest in being screened or quitting smoking, participants were asked, “How willing are you to move forward with lung cancer screening?” and “How interested are you in talking about quitting smoking?”, using a five-level scale from “not willing at all” to “very willing”. Participants were also offered alternative choices of “not sure” or “I don’t currently smoke”, respectively, if they were undecided and/or former smokers.
Statistical Analysis
Participant characteristics were determined exclusively from survey data. Proportions of responses were tabulated for statements with categorical responses. To assess class impact on knowledge, we tabulated the number and proportion of correct and incorrect responses, along with unsure and missing responses, to the true/false statements, separately for pre- and post-class surveys. We further calculated and assessed mean differences in the number of correct responses to statements between surveys, with both unsure and missing responses counted as incorrect responses, using the paired t-test. Analyses were conducted using SAS 9.4 (SAS Institute, Cary NC).
RESULTS
Participant Characteristics and Attitudes
Participants were most commonly ages 55 to 80 years, male, white, and current smokers (Supplemental Table 1). Of the 544 who reported sufficient smoking history data to determine screening eligibility (smoking status, quantity, duration, and if applicable, time since quit), 59% were current smokers. Nearly half met USPSTF eligibility criteria on age and smoking history, of whom 51% were current smokers (Supplemental Table 1).
On the pre-class survey (Table 1), over 80% agreed or strongly agreed with wanting to be screened, wanting to follow their doctor’s recommendation, and believing they are at risk for lung cancer. Approximately two out of three agreed or strongly agreed that an abnormal CT scan would cause worry (67%) or that a normal CT scan would reduce worry (62%). Conversely, 83% disagreed or strongly disagreed that, if their CT scan was normal, they could continue to smoke without worry. About three out of four disagreed or strongly disagreed that they might put off screening owing to worry related to blame for having smoked (74%), or that if they have lung cancer, it is better not to know (74%). To a lesser extent, participants disagreed or strongly disagreed about concerns over potential harms, namely radiation exposure (59%) and additional tests following an abnormal scan (48%). Among those identified as screening-eligible, response patterns were similar, although for each statement, a slightly greater proportion either agreed/strongly agreed or disagreed/strongly disagreed (Supplemental Table 2).
Table 1.
Pre–class survey responses on attitudes regarding LCS (n=680)
| Response (%) | ||||||
|---|---|---|---|---|---|---|
| Statement | Strongly Agree | Agree | Disagree | Strongly Disagree | Undecided/ Not Sure | Not Reported |
| I want to be screened for lung cancer. | 46.3 | 34.6 | 0.9 | 0.4 | 15.4 | 2.4 |
| I want to follow my doctor’s recommendation. | 42.2 | 46.2 | 0.7 | 0.4 | 7.1 | 3.4 |
| I am at risk for lung cancer. | 38.5 | 42.8 | 2.4 | 0.2 | 12.8 | 3.4 |
| Having an abnormal result on the chest CT scan will cause me to worry. | 19.1 | 47.8 | 14.0 | 1.5 | 14.6 | 3.1 |
| I might put off having a lung scan because I worry about being blamed for having smoked. | 4.1 | 10.9 | 46.0 | 28.4 | 7.2 | 3.4 |
| I am worried about the additional tests that would be necessary if the scan found something. | 6.9 | 30.0 | 36.0 | 11.8 | 11.9 | 3.4 |
| I am worried about exposure to radiation from the CT scan. | 4.1 | 19.0 | 45.2 | 14.1 | 13.8 | 3.8 |
| A normal CT scan will make me less worried about developing lung cancer. | 16.2 | 45.7 | 16.6 | 3.7 | 12.2 | 5.6 |
| If my CT scan is normal, I can continue to smoke without worrying. | 1.8 | 4.0 | 37.8 | 45.2 | 7.1 | 4.3 |
| If I have lung cancer, it is better not to know. | 7.8 | 7.1 | 32.7 | 41.6 | 6.2 | 4.7 |
LCS Knowledge
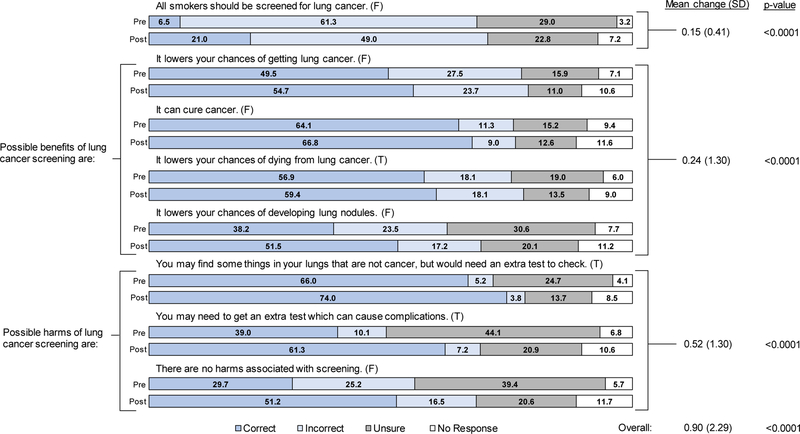
The proportion of correct responses to the eight statements used to assess LCS knowledge, both individually and combined, increased between surveys, suggesting an overall gain in knowledge from class attendance (Figure 1). On the pre-class survey, only 1% answered all statements correctly and 19% answered six or more statements correctly; on the post-class survey, corresponding proportions increased to 8% and 40%, respectively. About 51% provided at least one additional correct response on their post-class survey.
Figure 1. Pre- and post-class survey results on knowledge regarding LCS for 680 class participants.
Correct answer (either True [T] or False [F]) to each statement is indicated in parentheses.
After the class, however, only a fifth indicated that not all smokers should be screened for lung cancer (Figure 1). Participants also appeared to understand the potential harms better than the potential benefits of screening: 25% responded correctly to all four statements on potential benefits, while 43% responded correctly to all three statements on potential harms. Likewise, the mean pre-post difference in the number of correct responses was greater for statements about potential harms than benefits. Response patterns were similar for those identified as screening-eligible, with slightly larger pre-post increases in the proportion of correct responses to most statements (Supplemental Figure 1).
Decision-Making Capacity
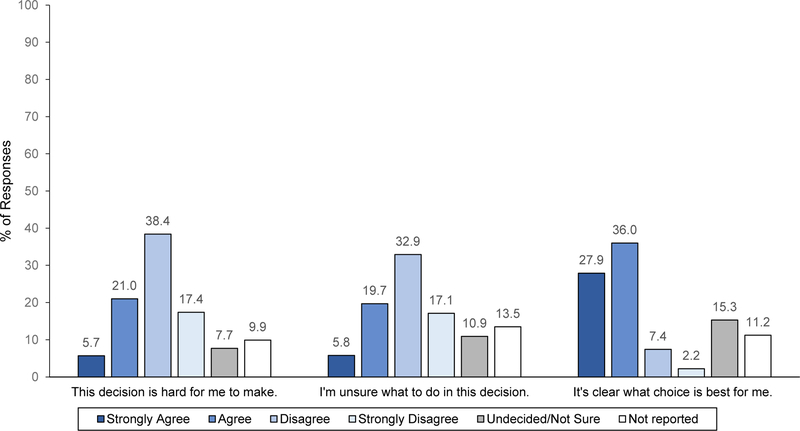
About 70% of participants reported having all the information needed to make a screening decision. Likewise, 64% agreed or strongly agreed that it was clear what choice was best for them (Figure 2). At least half disagreed or strongly disagreed that this decision was hard to make and that they were unsure of what to do. Response patterns were similar for those identified as screening-eligible (Supplemental Figure 2), among whom 78% reported having all the information needed to make a screening decision.
Figure 2.
Post-class results on decision-making capacity for 680 class participants
Interest in LCS and Quitting Smoking
On the post-class survey, almost 65% of participants reported being willing or very willing to undergo LCS, with only 8% being relatively less willing; the remaining reported being unsure or provided no response. Of the 340 participants who reported their interest level in quitting smoking, 63% expressed moderate to strong interest in quitting. Among those identified as screening-eligible, 73% reported being willing or very willing to undergo screening, and 53% expressed moderate to strong interest in quitting.
DISCUSSION
To our knowledge, we are the first to implement group education as a system-level strategy to initially educate and empower individuals in deciding whether to move forward with LCS.Before the class, participants had generally poor knowledge of the potential benefits and harms of screening. Their knowledge increased after the class, particularly about harms, although many still indicated that all smokers should be screened. We also found the class was effective in providing all the information needed for most participants to make a screening decision.
Several aspects of the class design likely contributed toward its overall effectiveness in enhancing LCS knowledge and decision-making capacity. First, a dedicated team of clinician specialists, most of whom are pulmonologists, have been trained to facilitate the class. Second, the curriculum has been structured and refined to ensure key messages are conveyed clearly. Lastly, patient education materials as decision aids are provided to help support SDM and enrich the patient experience.
Our data indicate the class had a similar impact on patient knowledge regarding LCS, relative to that of other decision aids or a personal counseling/SDM visit [15, 19], and point to potential areas of improvement. From focus group discussions of low-income adults who met the National Comprehensive Cancer Network (NCCN) LCS criteria, Crothers et al. found that decision aids were helpful in improving knowledge, especially about related harms, and that even after the focus groups, most participants (64%) still indicated that all smokers should be screened [15]. Whether the latter finding, from this and our study, reflects poor understanding about screening eligibility versus general opinion about access to screening cannot be discerned. However, it highlights the potential need to improve lung cancer risk communication with patients, specifically for health numeracy. Mazzone et al. likewise found that, prior to a personal counseling/SDM visit, most patients lacked the requisite understanding about the potential benefits and harms of screening to fully engage in SDM [19]. Patient knowledge improved substantially immediately after their visit, although it diminished slightly one month later.
In both studies, the knowledge gained was more impressive than what we observed [15, 19]. This difference may be explained in their inclusion of screening-eligible patients only, and specific to Mazzone et al.[19], one-on-one education with patients and use of more liberal criteria in assessing knowledge. Whether our patients gain further knowledge after their personal SDM visit is unknown, although the cumulative gain at that point (i.e., after class and SDM visit) could be equivalent to that reported by others.
Attitudes reported by class participants were comparable to participants in the focus groups conducted by Crothers et al. [15] Specifically, the majority (81% overall; 84% screening-eligible) agreed with the statement, “I want to be screened for lung cancer.” Some participants (15% overall; 15% screening-eligible), however, were still undecided after the class, suggesting that decision conflict is an inherent part of the decision-making process [20]. Further investigation is needed to understand if participants remain in decisional conflict, even after a SDM visit with a clinician specialist.
Additionally, most participants agreed with the statement, “I am at risk for lung cancer,” anddisagreed with the statement, “If my CT scan is normal, I can continue to smoke without worrying.” Participants expressed less concern over potential harms (i.e., radiation exposure and follow-up tests), possibly reflecting their limited understanding about them upfront. With a greater proportion of participants responding correctly to statements related to potential harms than benefits on the post-class survey, communication about harms appeared to be sufficient during the class. About 15% of participants agreed with the statements, “I might put off having a lung scan because I worry about being blamed for having smoked” and “If I have lung cancer, it is better not to know.” This finding highlights the importance of recognizing stigma, unease with uncertainty, locus control of health, and conflict between emotional versus cognitive decision-making. If left unaddressed, such concerns could negatively influence screening behavior and outcomes.
The class offers an invaluable opportunity to not only educate smokers about lung cancer screening, but also reinforce the numerous health benefits of smoking abstinence. Although the class has been designed for screening-eligible adults, all referred patients are permitted to attend regardless of their eligibility status. Over half of all participants reported being current smokers, substantiating the importance of incorporating and evaluating the effectiveness of referral to smoking cessation interventions as part of the screening process [21].
Among the most cited barriers by PCPs in conducting SDM are patients’ competing health priorities and time constraints [8, 10, 11]. In fact, most physicians indicate that they are unlikely to engage in SDM with patients if the discussion exceeds five minutes [10]. Given these known provider- level barriers, a group education class represents a system-level approach for shifting responsibilities from PCPs to dedicated specialists in facilitating more efficient and high-quality SDM for LCS. This approach seems especially well-suited for a healthcare system like ours that serves a large population over a wide geographical area, where centralizing LCS at a single medical center is not feasible.
Our findings are reassuring in that the class has been effective in enhancing LCS knowledge and decision-making capacity, particularly among screening-eligible participants, as well as fostering interest in smoking cessation. These findings are robust, originating from survey data on an ethnically diverse sample of 680 class participants over a 14-month period. However, the extent to which class attendance might inadvertently impose an initial patient–level barrier to screening (due to lack of transportation, inability to take time off from work or other commitments) is unknown, even with the class offered at no additional cost to health plan members.
Additional caveats should be noted. The mean difference in the number of correct responses to all statements used to assess knowledge between surveys is likely underestimated, due to slightly higher non- response to the post-class than pre-class survey. With only cross-sectional data collected, knowledge retention, subsequent screening initiation, and smoking cessation among class participants could not beassessed. Attitudes toward LCS were also not reassessed post-class; however, Crothers et al. found no substantial change in attitudes after their focus group discussions [15]. Participants were asked to report only basic demographic information, precluding a comprehensive analysis of whether the class was equally effective across levels of education or health literacy. Lastly, our evaluation may lack generalizability, being specific to insured English-speaking individuals from a single healthcare system.
Accumulating evidence indicates that providers lack sufficient time and resources to adequately counsel screening-eligible patients and that patients commonly misunderstand key aspects about LCS, calling for system-level approaches to improve the SDM process [8–11, 22]. Our evaluation suggests that a well-designed group education class is an effective and efficient means to initially educate and equip patients with sufficient knowledge to facilitate informed decision-making regarding LCS.
Supplementary Material
ACKNOWLEDGEMENTS
We recognize and appreciate the collective efforts of the following individuals who have contributed in developing, implementing, and supporting the LCS program at Kaiser Permanente Northern California:Sherry Andrews, MD (Pulmonary Medicine); Simon Ashiku, MD (Thoracic Surgery); Paramjeet Atwal, MD (Pulmonary Medicine); Elisa Avik, MD (Pulmonary Medicine); Ada Bajada, NP (Cardiology); Manuel Ballesca, MD (Internal Medicine); Anitha Channabasaviah, MD (Pulmonary Medicine); Carter Chang, MD (Internal Medicine); James Chang, MD (Regional Administration); Irene Chen, MD (Regional Administration); James Chen, MD (Radiology); Jayne Chu, MD (Pulmonary Medicine); Gloria Cruz (Quality and Operations Support); Theresa Curran, RCP/RRT (Respiratory Care Services); Thomas Dailey, MD (Pulmonary Medicine); Veena Devarakonda, MD (Pulmonary Medicine); Samjot Dhillon, MD (Pulmonary Medicine);Pradeep Doddamreddy, MD (Pulmonary Medicine); Laura Eberhard, MD (Pulmonary Medicine); Louis Eldeson, MD (Internal Medicine); Lois Eldridge-Lee (Regional Imaging); Renee Fogelberg, MD (Regional Health Education); Theodore Fong, MD (Pulmonary Medicine); Jennifer Fresco, MD (Pulmonary Medicine); John Gasman, MD (Pulmonary Medicine); Ali Ghias, MD (Pulmonary Medicine); Clarisse Glen, MD (Internal Medicine); Harjinder Gogia, MD (Pulmonary Medicine); Nancy Goler, MD (Patient Outreach Support); Amit Gupta, MD (Pulmonary Medicine); Sachin Gupta, MD (Pulmonary Medicine); Roberta Heck, NP (Internal Medicine); Jasper Ip, MD (Pulmonary Medicine); Archana Jayakumar, MD (Pulmonary Medicine); Brandon Johnson (Healthcare Operations); Cindy Kanegai, MD (Pulmonary Medicine); Nour Karzoun, MD (Pulmonary Medicine); Michael Kotton, MD (Interventional Radiology); Petey Laohaburanakit, MD (Critical Care Medicine); Todd Lasman, MD (Pulmonary Medicine); Peter Le, MD (Pulmonary Medicine); Theodore Lee, MD (Radiology); Steven Levine, MD (Information Technology); Sooraj Maharjan (Quality and Operations Support); Gregory Marelich, MD (Pulmonary Medicine); Jonathan Mates, MD (Radiology); Eric Mebane, MD (Internal Medicine); Raghu Midde, MD (Pulmonary Medicine); Monica Minguillon, MD (Pulmonary Medicine); Tomio Miyai, MD (Pulmonary Medicine); Lauran Mizock, MD (Internal Medicine); Greg Mogel, MD (Radiology); Jeremy Murdock, MD (Pulmonary Medicine); Daniel Navarro, MD (Imaging Informatics); Todd Osinski, MD (Radiology); Swapna Parikh, MD (Pulmonary Medicine); Ashish Patel, MD (Thoracic Surgery); Ryan Pavlovich, MD (Pulmonary Medicine); Burgess Peck, NP (Internal Medicine); Charles Poon, MD (Pulmonary Medicine); Sridhar Prasad, MD (Pulmonary Medicine); Paul Radosevich, MD (Interventional Radiology); David Rapko, MD (Internal Medicine); Usha Rao, MD (Pulmonary Medicine); Kuruganti Reddy, MD (Pulmonary Medicine); John Rego, MD (Radiology); Syed Safdar, MD (Pulmonary Medicine); Gurpartap Sahota, MD (Pulmonary Medicine); Debora Sawyer, MD (Outpatient Quality); Walter Shakespeare, DO (Pulmonary Medicine); Kelvin Shiu, DO (Pulmonary Medicine); Nirupam Singh, MD (Pulmonary Medicine); Darshan Sonik, MD (Pulmonary Medicine); Saroja Sripathi, MD (Pulmonary Medicine); Barbara Stumpf, MD (Pulmonary Medicine);Haiyan Sun (Quality and Operations Support); Sophie Taylor (Quality Management); Michael Thornton, MD (Pulmonary Medicine); Kanti Uppal, MD (Thoracic Surgery); Felix Vergara, MD (Internal Medicine); and Kiran Zachariah, MD (Pulmonary Medicine).
This work was supported in part by a career development award to L.C.S. from the National Cancer Institute (K07 CA188142). Preliminary results were published in abstract form and presented at the 2018 American Thoracic Society International Conference in San Diego, CA.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICTS OF INTEREST
All authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H et al. Development of The American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of The American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg. 2012;144(1):25–32.doi: 10.1016/j.jtcvs.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. doi: 10.7326/m13-2771. [DOI] [PubMed] [Google Scholar]
- 3.Wender R, Fontham ET, Barrera E Jr., Colditz GA, Church TR, Ettinger DS et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–17. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10(2):240–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW et al. Benefits and harms of CT screening for lung cancer: a systematic review. Jama. 2012;307(22):2418–29. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomgraphy (LDCT) (CAG-00439N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed 09/19/2018.
- 7.Lowenstein LM, Deyter GMR, Nishi S, Wang T, Volk RJ. Shared decision-making conversations and smoking cessation interventions: critical components of low–dose CT lung cancer screening programs. Transl Lung Cancer Res. 2018;7(3):254–71. doi: 10.21037/tlcr.2018.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triplette M, Kross EK, Mann BA, Elmore JG, Slatore CG, Shahrir S et al. An Assessment of Primary Care and Pulmonary Provider Perspectives on Lung Cancer Screening. Ann Am Thorac Soc. 2018;15(1):69–75. doi: 10.1513/AnnalsATS.201705-392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner AT, Malo TL, Margolis M, Elston Lafata J, James S, Vu MB et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med. 2018. doi: 10.1001/jamainternmed.2018.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberth JM, McDonnell KK, Sercy E, Khan S, Strayer SM, Dievendorf AC et al. A national survey of primary care physicians: Perceptions and practices of low-dose CT lung cancer screening. Prev Med Rep. 2018;11:93–9. doi: 10.1016/j.pmedr.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanodra NM, Pope C, Halbert CH, Silvestri GA, Rice LJ, Tanner NT. Primary Care Provider and Patient Perspectives on Lung Cancer Screening. A Qualitative Study. Ann Am Thorac Soc. 2016;13(11):1977–82. doi: 10.1513/AnnalsATS.201604-286OC. [DOI] [PubMed] [Google Scholar]
- 12.Gould MK, Sakoda LC, Ritzwoller DP, Simoff MJ, Neslund-Dudas CM, Kushi LH et al. Monitoring Lung Cancer Screening Use and Outcomes at Four Cancer Research Network Sites. Ann Am Thorac Soc. 2017;14(12):1827–35. doi: 10.1513/AnnalsATS.201703-237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon NP. How does the adult Kaiser Permanente membership in Northern California compare with the larger community? [Google Scholar]
- 14.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103(19):1436–43. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crothers K, Kross EK, Reisch LM, Shahrir S, Slatore C, Zeliadt SB et al. Patients’ attitudes regarding lung cancer screening and decision aids: a survey and focus group study. Ann Am Thorac Soc. 2016;13(11):1992–2001. doi: 10.1513/AnnalsATS.201604-289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter-Harris L, Slaven JE 2nd, Monohan P, Rawl SM. Development and Psychometric Evaluation of the Lung Cancer Screening Health Belief Scales. Cancer Nurs. 2017;40(3):237–44. doi: 10.1097/ncc.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cataldo JK, Slaughter R, Jahan TM, Pongquan VL, Hwang WJ. Measuring stigma in people with lung cancer: psychometric testing of the cataldo lung cancer stigma scale. Oncol Nurs Forum. 2011;38(1):E46–54. doi: 10.1188/11.Onf.E46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 19.Mazzone PJ, Tenenbaum A, Seeley M, Petersen H, Lyon C, Han X et al. Impact of a Lung Cancer Screening Counseling and Shared Decision-Making Visit. Chest. 2017;151(3):572–8. doi: 10.1016/j.chest.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Reyna VF, Nelson WL, Han PK, Pignone MP. Decision making and cancer. Am Psychol. 2015;70(2):105–18. doi: 10.1037/a0036834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minnix JA, Karam-Hage M, Blalock JA, Cinciripini PM. The importance of incorporating smoking cessation into lung cancer screening. Transl Lung Cancer Res. 2018;7(3):272–80. doi: 10.21037/tlcr.2018.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heffner JL, Krebs P, Johnson H, Greene PA, Klein DE, Feemster LC et al. Smokers’ inaccurate beliefs about the benefits of lung cancer screening. Ann Am Thorac Soc. 2018;15(9):1110–3. doi: 10.1513/AnnalsATS.201804-259RL [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.