Introduction
Two bacterial enteric pathogens, Shigella and enterotoxigenic Escherichia coli (ETEC), are considered prototypical disparate ends of the spectrum from the perspective of pathogenesis. Shigella is the paradigm of a locally invasive pathogen that invades and destroys gut mucosa, often resulting clinically in gross blood and mucus in diarrheal stools (dysentery). In contrast, ETEC represents one of the best examples of a non-invasive bacterial pathogen that does not anatomically damage intestinal mucosa but rather elicits intestinal secretion and diminished absorption consequent to the effects of the heat-stable and heat-labile enterotoxins that it produces. Enterotoxin-induced intestinal secretion can result in copious purging, overt dehydration, shock and death in frail young infant hosts.
Professor Philippe Sansonetti is widely recognized as the premier authority on the pathogenesis of disease caused by Shigella. His decades of research describing the interaction between this human host-restricted pathogen and the intestinal mucosa collectively comprise one of the most extraordinary contributions on bacterial pathogenesis. His discoveries and use of novel technologies have contributed widely to the field of microbial pathogenesis in general and have served as inspiration to generations of investigators. The Sansonetti laboratory at the Institut Pasteur served as a site to which the best and brightest came to study under his tutelage and upon their departure to establish their own independent research units elsewhere, Professor Sansonetti’s scientific progeny came to constitute a network that includes many of the world’s leading research microbiologists and cell biologists. This paper is meant to serve as a tribute to Philippe Sansonetti and to honor him as a colleague, collaborator and friend.
Shigella and Enterotoxigenic Escherichia coli
Whilst Shigella and ETEC exhibit highly divergent pathogenesis, they nevertheless share some important commonalities that are relevant to the development of practical, well-tolerated and efficacious vaccines. Shigella and ETEC are each recognized as one of the top four enteric pathogens that cause diarrheal illness in young children in developing countries and as leading causes of young child deaths (Kotloff et al., 2013;Liu et al., 2016). These two bacterial pathogens are also major etiologic agents of travelers’ diarrhea among persons from industrialized countries who visit developing regions of the world (Bourgeois et al., 2016;Hyams et al., 1991;Porter et al., 2017). Thus, a single vaccine that could target both these pathogens is highly desirable and would greatly enhance the vaccine’s cost-effectiveness, an important attribute in the modern era. These pathogens also share a major impediment for vaccine developers in that each exhibits broad serotype or antigenic heterogeneity. Thus, rational, evidence-based strategies must be articulated to achieve broad-spectrum protection against the antigenic heterogeneity that each pathogen displays.
Shigella
Shigella and ETEC are classified as members of the enterobacteriaceae and are phylogenetically indistinguishable. It has been argued by some, based on genomics, that Shigella species should be taxonomically included within E. coli (Pettengill et al., 2015)
However, counter arguments to keep Shigella as a distinct genus include the clinical manifestations of mucosally invasive disease and epidemiologic features including the small inoculum size capable of causing shigellosis (DuPont et al., 1989;Levine et al., 1973) and direct fecal oral contact spread as the main mode of transmission (Sahl et al., 2015;Strockbine and Maurelli, 2005). Despite high levels of genomic homology, a set of unique virulence factors encoded by each pathogen confers very different pathogenic mechanisms that result in distinct clinical outcomes (Table 1).
Table 1.
Differences and Similarities between Shigella and ETEC
| Shigella | ETEC | |
|---|---|---|
|
Taxonomic Classification; Genomic Content |
enterobacteriaceae; ~4Mb genome with ~220kb virulence plasmid |
enterobacteriaceae; ~4Mb genome with plasmid(s) encoding CFs and toxins |
| Strain Diversity in Relation to Key Antigens | Diversity based on LPS O-antigen serotypes | Diversity based colonization factors and toxins |
| Clinical Manifestations | Initial watery diarrhea followed by dysentery (blood + mucus in stool) | Profuse Watery diarrhea |
| Key Virulence Factors | Invasion mechanisms, secreted effectors and enterotoxins | Attachment factors and LT and/or ST toxins |
| Key Pathogenic Mechanism | Invasion, cell-to-cell spread, and induction of inflammation | Attachment and delivery of toxins (LT and/or ST) that promote GI ion transport dysregulation |
| Vaccine Development Strategies | Oral: Live attenuated Killed whole cell Parenteral: Conserved antigens O-Antigen conjugates (synthetic, non-synthetic) Combinations (GEMMA, Invaplex) |
Oral: Killed Whole Cell Multivalent Live vectored Live attenuated Parenteral: Conserved antigen |
| Vaccine Target Populations | Young children in developing countries Specific populations in developed countries (daycare centers and custodial institutions) Travelers including military personnel |
Young children in developing countries Travelers including military personnel |
The key virulence factors of Shigella are encoded on a large virulence plasmid that was characterized extensively by Sansonetti and colleagues (Sansonetti and Phalipon, 1996). Indeed, it was the seminal research of Sansonetti, working with the late Samuel B. Formal and Dennis Kopecko in the Walter Reed Army Institute of Research in Washington, D.C., that identified the virulence plasmid as responsible for host cell invasion capacity, the distinguishing pathogenic property of Shigella (Sansonetti et al., 1981;Sansonetti et al., 1982a;Sansonetti et al., 1982b;Sansonetti et al., 1983). Sansonetti and colleagues proceeded to characterize key regions of this plasmid that harbored genes encoding invasion capability, secreted effectors, and a unique cell-to-cell spread phenotype that utilized polymerization of host cell actin (Baudry et al., 1987;Bernardini et al., 1989;Hale et al., 1983;Maurelli et al., 1985).
ETEC
In contrast to the invasive phenotype of Shigella, ETEC cause disease by attaching to the small intestine via colonization factors (CFs) and elaborating heat labile (LT) and/or heat stable (ST) toxin(s) that cause dysfunction of ion transport and result in watery diarrhea (Levine, 1987). The genes encoding CFs and toxins are often located on plasmids in ETEC and isolates often harbor genes encoding more than one CF.
Protective antigens of Shigella, antigenic heterogeneity and vaccine strategies
Epidemiologic field studies and volunteer re-challenge studies document that an initial clinical episode of shigellosis confers circa 75% protection against clinical illness upon re-exposure to the homologous Shigella serotype. Other observations indicate that the protective immune responses are directed against the Shigella O antigens that define serotype (Levine et al., 2007). However, that fact that multiple serotypes of Shigella contribute to the overall global burden of disease make antigenic diversity an obstacle that hinders a simple vaccine development strategy. Epidemiologically important Shigella serotypes include S. sonnei and the 15 S. flexneri serotypes and subtypes. S. sonnei, consisting of a single serotype, is the most important strain in industrialized countries and accounts for ~23% isolates in less developed regions of the world. S. flexneri is the most important species globally and comprises 15 serotypes. In contrast, whereas S. dysenteriae has 15 serotypes and S. boydii includes 19 serotypes, they account collectively for only 10.4% of cases worldwide (Livio et al., 2014). Thus, a broadly protective Shigella vaccine must provide protection against the 16 epidemiologically important serotypes (Livio et al., 2014).
While many Shigella vaccine strategies have been pursued (reviewed in (Ashkenazi and Cohen, 2013;Barry et al., 2013;Levine et al., 2007;Mani et al., 2016), the current leading approaches include: 1) parenteral vaccines that deliver chemically purified or synthetic Shigella OPS antigens as conjugates to carrier proteins, genetic bioconjugates (Riddle et al., 2016) or as general outer membrane vesicles derived from Shigella serotypes (Launay et al., 2017) (Launay et al., 2017), or; 2) live attenuated vaccine strains administered as live oral vaccines.
Recently, Mullard, Phalipon, Sansonetti and colleagues have pursued a synthetic carbohydrate-based conjugate approach to Shigella vaccine development. A series of elegant technical advances facilitated the biochemical synthesis of Shigella O-antigen specific carbohydrate antigens that were conjugated to a protein carrier (Phalipon et al., 2006;Phalipon et al., 2009;van der Put et al., 2016). These vaccine candidates have been demonstrated to be safe and immunogenic in volunteers (Cohen et al., 2017) and are advancing to evaluation using the controlled human infection model.
Early generations of live oral vaccines were shown to protect against natural exposure to wild type pathogens in field trials (Levine et al., 1976;Mel et al., 1965) and to protect vaccinated U.S. volunteers against experimental exposure to wild type organisms in the course of volunteer challenge studies (Coster et al., 1999;DuPont et al., 1972). The historical difficulty of this approach was in achieving the optimal balance of safety by attenuation, while retaining adequate immunogenicity to ensure protection.
Sansonetti and colleagues developed live attenuated Shigella vaccine strains based on their discovery of the role of VirG/IcsA in catalyzing cell-to-cell spread by polymerization of host cell actin (Bernardini et al., 1989). The first candidates were based on the fundamental mutation in icsA and included additional mutations in either iuc:iut, encoding the iron scavenging siderophore aerobactin production and transport (Fontaine et al., 1990;Sansonetti and Arondel, 1989) or ompB, encoding an important osmoregulatory protein (Sansonetti et al., 1991). One candidate, S. flexneri 2a vaccine strain SC602 was shown to induce protective efficacy against severe diarrhea and dysentery challenge in a controlled human infection model in North American volunteers (Coster et al., 1999;Katz et al., 2004). The narrow window of safety exhibited by this vaccine complicated its further clinical development. In clinical trials in U.S. volunteers, SC602 was highly reactogenic when administered in doses above 104 CFU. When tested in adults and children in Bangladesh, SC602 did not cause adverse reactions. However, it also failed to elicit immune responses (Rahman et al., 2011).
Investigators at the CVD pursued an alternative strategy based on inactivation of genes encoding critical enzymes in metabolic pathways, namely guaBA (Noriega et al., 1996) and genes encoding two newly discovered enterotoxins of Shigella. The observations that Shigella infection includes early clinical manifestations of watery diarrhea (Kinsey et al., 1976;Rout et al., 1975) and that live attenuated vaccine strains still caused watery diarrhea in volunteers prompted CVD investigators to identify enterotoxins that elicit secretory diarrhea. Shigella enterotoxin 1 (ShET1) is encoded by the set1A and B genes that are located on the chromosome of S. flexneri 2a and 2b strains (Fasano et al., 1995;Fasano et al., 1997;Livio et al., 2014;Noriega et al., 1995). Shigella enterotoxin 2 (ShET2) is encoded by sen, a gene found on the virulence plasmid of all Shigella strains (Nataro et al., 1995). The attenuation resulting from deletion of guaBA in S. flexneri 2a vaccine strain CVD 1204 and the importance of deletions in enterotoxin-encoding genes set and sen to achieve a higher level of safety in vaccine strain CVD 1208 were established in volunteer studies (Kotloff et al., 2004;Kotloff et al., 2007). CVD 1208S has been manufactured as a cGMP lot and is advancing to challenge studies.
Our strategy for constructing a vaccine that confers broad protection against Shigella infection is informed by epidemiologic studies that identified S. sonnei (single serotype) and S. flexneri serotypes 2a, 3a and 6 as the most prevalent serotypes isolated from young children with moderate-to-severe diarrhea (Livio et al., 2014) (Table 2). Inclusion of these 4 serotypes would provide direct protection against ~64% Shigella strains (Livio 2014). Recently we have included an additional attenuated S. flexneri strain of serotype 1b to the multivalent vaccine to broaden direct coverage up to ~72% of epidemiologically relevant, disease-associated, Shigella serotypes. However it is anticipated that based on cross reactivity among serotypes within the vaccine with other important serotypes that are not in the vaccine but that share O group or type antigens with the vaccine strains, it may be possible to protect against up to 89% of the epidemiologically relevant Shigella serotypes. (Levine et al., 2007;Noriega et al., 1999a). Live attenuated versions of each of these serotypes have been engineered with guaBA and sen deletions and demonstrated to be safe, immunogenic, and protective against wild type challenge in animal models (DeLaine et al., 2016). While no S. dysenteriae 1 strains were isolated in GEMS, the exceptional virulence of this serotype that expresses Shiga toxin, causes severe disease with complications, and has been responsible for epidemics and pandemics, argues for its inclusion either in a broadly protective vaccine or as a monovalent vaccine to be kept in a potential emerging pathogen stockpile. In the event of re-appearance and resurgence of S. dysenteriae 1, such a stockpile could help limit the spread of Shiga dysentery. A Shiga toxin-negative, attenuated derivative of S. dysenteriae 1 has been developed (Wu et al., 2011). Furthermore, we have shown that a mixed inoculum composed of two or three live attenuated strains of Shigella can induce immune responses to all components and provide protection against each component serotype (DeLaine et al., 2016;Noriega et al., 1999b). Immunological readouts include serum and mucosal antibody titers as well as antibody secreting cells (ASC) or antibodies in lymphocyte secretions (ALS) to serotype-specific LPS O-antigen. Recent studies have supported the use of functional assays including serum bactericidal (SBA) and opsonophagocytosis inhibition (OPA) assays as potential correlates of protection (Shimanovich et al., 2017).
Table 2.
Strategy for Shigella serotype inclusion in a broadly protective vaccine based on Shigella Serotype Distribution in GEMS (Livio et al., 2014)
| Strain/ serotype |
% Total (GEMS) |
||
|---|---|---|---|
| S. sonnei | 23.7% | ||
| S. flexneri serotype | Type- and/or Group-antigen Expressed | ||
| Type- antigen | Group-antigen | ||
| 2a | 20.2% | II | 3,4 |
| 6 | 11.0% | VI | |
| 2b | 10.9% | II | 7,8 |
| 3a | 9.4% | 7,8 | |
| 1b | 7.5% | I | 6 |
| 4a | 2.9% | IV | 3,4 |
| 7a | 2.0% | VII | |
| X | 1.0% | 7,8 | |
| Y | 0.4% | 3,4 | |
| 5b | 0.3% | V | 7,8 |
| 1a | 0.3% | I | 3,4 |
| 3b | 0.1% | 3,4; 6 | |
| 4b | 0 | IV | 6 |
Bold: serotypes and antigens included in CVD vaccine;
Protective antigens of ETEC, antigenic heterogeneity and vaccine strategies
In contrast to the invasive phenotype of Shigella, ETEC cause disease by attaching to the small intestine via colonization factors (CFs) and elaborating heat labile (LT) and/or heat stable (ST) toxins which cause watery diarrhea. Epidemiological and volunteer studies support the protective capacity of antibodies that block colonization factors to prevent disease (Levine et al., 2019). Clinical isolates of ETEC express a multitude of different CFs. There are 7 major CFs that have been firmly associated with isolates that cause disease in young children including CFA/I, and CS1 through CS6 (Vidal et al., 2019). While a variety of minor CFs have been identified, with just a few exceptions (CS7 and CS17), convincing evidence of their association with disease has not been established through epidemiological studies or volunteer challenges (Vidal et al., 2019). Recent analysis identified one minor CF, CS14, as being both common and significantly associated with diarrheal disease (Vidal et al., 2019). Our strategy to provide broad coverage against ETEC includes antigens to induce colonization blocking immune responses against all the major CFs (i.e., CFA/I, CS1-CS6) plus CS14 (Table 3) (Levine et al., 2019). In addition, an antigen to induce LT-toxin neutralizing antibodies is included to cover a subset of LT-only ETEC strains that are associated with diarrhea in specific populations (Mansour et al., 2014;Steinsland et al., 2002).
Table 3.
CVD multivalent Shigella-ETEC Strategy
| Shigella Strain | Vaccine Name | ETEC Antigen(s) |
|---|---|---|
| S. sonnei | CVD 1233S | CS2, CS3 |
| S. flexneri 2a | CVD 1208S | CFA/I, LThA2B |
| S. flexneri 3a | CVD 1213 | CS1, CS5 |
| S. flexneri 6 | CVD 1215 | CS4, CS6 |
| S. flexneri 1b | CVD 1224 | CS14 |
| S. dysenteriae 1 | CVD1254 | StxB |
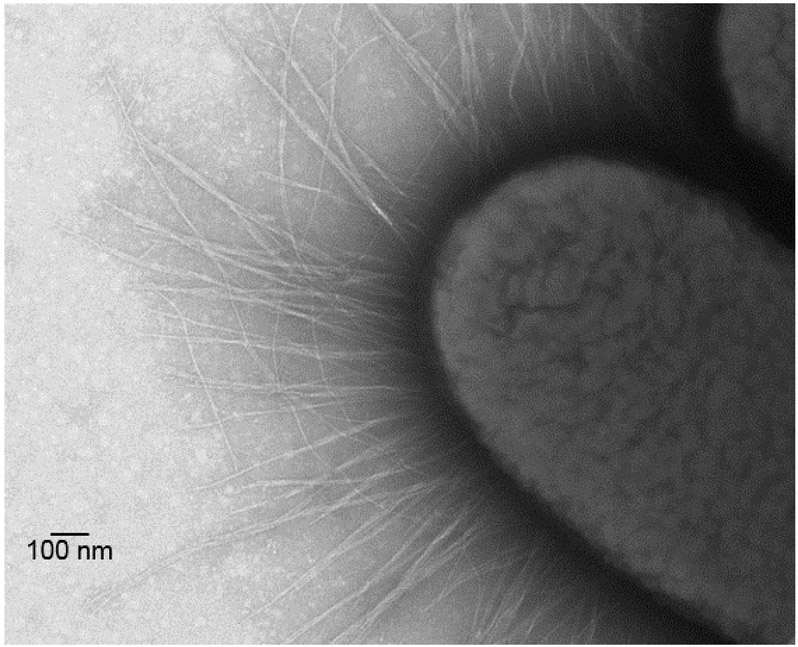
Specifically, we have engineered four live attenuated Shgella strains to constitutively express critical ETEC antigens (Table 3). This multivalent Shigella-ETEC approach was supported by a clinical trial wherein an earlier prototype Shigella-ETEC candidate consisting of S. flexneri 2a strain CVD 1208S harboring a stabilized plasmid encoding CFA/I and LThA2B, CVD 1208S(pCFA/I-LTB), was used to immunize volunteers. This vaccine was well tolerated but despite the use of a highly engineered plasmid system to ensure stable maintenance, the plasmid was lost from Shigella within the host GI tract. Plasmid loss was verified by characterization of bacteria shed in volunteer stool samples. Notwithstanding these disappointing results, a subset of volunteers shed the vaccine strain in which the plasmid was stably maintained in the Shigella live vector. Notably, those vaccinees mounted robust immune responses to both the Shigella live vector as well as ETEC antigens. This observation served as proof of principle that the combined Shigella-ETEC strategy could be effective if a more genetically-stabilized vaccine strain could be developed. To this end, we have now engineered live Shigella vaccine strains to express key ETEC antigens from chromosomal loci. The resultant Shigella-ETEC hybrid vaccine strains are remarkably stable, express morphologically correct fimbriae on the surface, elicit strong immune responses and confer protection against wild type challenge in animal models (Figure 1)(Barry et al., 2016).
Figure 1.
Transmission electron micrograph of S. flexneri 2a vaccine strain CVD 1208S expressing CFA/I fimbriae. CVD 1208S-CFA/I was suspended on a 300 mesh Formvar coated grid and stained with 2% ammonium molybdate. The image was examined using a JEOL electron microscope JEM-1200EX and is shown at 1500X; the scale bar represents 100 nm.
There is no good animal model that recapitulates ETEC disease; therefore in vitro assays are utilized to measure functional immune responses. Hemagglutination inhibition (HAI) is considered a proxy for inhibition of binding to human intestine (Baker et al., 2009;Cravioto et al., 1982) and HAI antibodies titers are one measure of functional responses to the ETEC component of these vaccine candidates.
Our most advanced Shigella-ETEC candidate, S. flexneri 2a vaccine strain CVD 1208S-122, which expresses CFA/I and LThA2B from the chromosome, is safe and immunogenic in animal models and confers protective efficacy against wild type Shigella challenge (manuscript in preparation, VED abstract). This vaccine is currently being manufactured as a cGMP lot and a clinical development plan has been prepared. Anticipated positive outcomes from initial clinical studies will pave the way for testing combinations of the Shigella-ETEC hybrid strains that constitute a broadly protective vaccine against two important pathogens.
Epilogue
The pioneering work of Sansonetti and subsequent work by his multitude of collaborators and trainees have advanced the field of Shigella research and facilitated the development of novel interventions against this globally important human pathogen. Confirmation of the pathogenic impact of Shigella and ETEC on the most vulnerable populations has converged with advances in technology to result in an exciting era of vaccine development and the advancement of multiple novel vaccine strategies. Our combined Shigella-ETEC approach has the potential to expand protection to individuals at risk of disease by two important pathogens. All investigators who work on Shigella in the modern era owe a debt to Philippe Sansonetti for his many ground breaking discoveries and insights about this pathogen. These insights have paved the way for a new generation of Shigella vaccines.
Acknowledgements.
The authors gratefully acknowledge the electron microscopy performed by Kurt Hanevik. This work was supported by NIH U19 AI109776, R01 AI132257, U19 AI142725, and U54 AI57168.
References
- Ashkenazi S and Cohen D (2013) An update on vaccines against Shigella. Ther Adv Vaccines 1: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KK, Levine MM, Morison J, Phillips A, and Barry EM (2009) CfaE tip mutations in enterotoxigenic Escherichia coli CFA/I fimbriae define critical human intestinal binding sites. Cell Microbiol 11: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry EM, Pasetti MF, Sztein MB, Fasano A, Kotloff KL, and Levine MM (2013) Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroenterol Hepatol 10: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry EM, Wu T, Delaine B, Cunningham A, Grassel C, Hanevik K et al. Preclinical evaluation of combined Shigella-ETEC vaccine candidates. Abstracts of the 2016 Vaccines against Shigella and ETEC Conference. [Google Scholar]
- Baudry B, Maurelli AT, Clerc P, Sadoff JC, and Sansonetti PJ (1987) Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol 133: 3403–3413. [DOI] [PubMed] [Google Scholar]
- Bernardini ML, Mounier J, D'Hauteville H, Coquis-Rondon M, and Sansonetti PJ (1989) Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A 86: 3867–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois AL, Wierzba TF, and Walker RI (2016) Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 34: 2880–2886. [DOI] [PubMed] [Google Scholar]
- Cohen D, Atsmon J, Artaud C, Meron-Sudai S, Gougeon M-L, Bialik A et al. A phase I dose escalation study to assess the safety and immunogencity of the SF2a-TT15 conjugate vaccine against S. flexneri 2a in healthy adult volunteers (preliminary results). Abstracts of the 2017 Vaccines fro Enteric Diseases Meeting. [Google Scholar]
- Coster TS, Hoge CW, Van de Verg LL, Hartman AB, Oaks EV, Venkatesan MM et al. (1999) Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infection and Immunity 67: 3437–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravioto A, Scotland SM, and Rowe B (1982) Hemagglutination activity and colonization factor antigens I and II in enterotoxigenic and non-enterotoxigenic strains of Escherichia coli isolated from humans. Infect Immun 36: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLaine BC, Wu T, Grassel CL, Shimanovich A, Pasetti MF, Levine MM et al. (2016) Characterization of a multicomponent live, attenuated Shigella flexneri vaccine. Pathog Dis 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, and Gangarosa EJ (1972) Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis 125: 12–16. [DOI] [PubMed] [Google Scholar]
- DuPont HL, Levine MM, Hornick RB, and Formal SB (1989) Inoculum Size in Shigellosis and Implications for Expected Mode of Transmission. Journal of Infectious Diseases 159: 1126–1128. [DOI] [PubMed] [Google Scholar]
- Fasano A, Noriega FR, Liao FM, Wang W, and Levine MM (1997) Effect of shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut 40: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Noriega FR, Maneval DR Jr., Chanasongcram S, Russell R, Guandalini S et al. (1995) Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Invest 95: 2853–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine A, Arondel J, and Sansonetti PJ (1990) Construction and Evaluation of Live Attenuated Vaccine Strains of Shigella-Flexneri and Shigella-Dysenteriae-1. Research in Microbiology 141: 907–912. [DOI] [PubMed] [Google Scholar]
- Hale TL, Sansonetti PJ, Schad PA, Austin S, and Formal SB (1983) Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun 40: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams KC, Bourgeois AL, Merrell BR, Rozmajzl P, Escamilla J, Thornton SA et al. (1991) Diarrheal disease during Operation Desert Shield. N Engl J Med 325: 1423–1428. [DOI] [PubMed] [Google Scholar]
- Katz DE, Coster TS, Wolf MK, Trespalacios FC, Cohen D, Robins G et al. (2004) Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect Immun 72: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey MD, Formal SB, Dammin GJ, and Giannella RA (1976) Fluid and electrolyte transport in rhesus monkeys challenged intracecally with Shigella flexneri 2a. Infect Immun 14: 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Pasetti MF, Barry EM, Nataro JP, Wasserman SS, Sztein MB et al. (2004) Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis 190: 1745–1754. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Simon JK, Pasetti MF, Sztein MB, Wooden SL, Livio S et al. (2007) Safety and Immunogenicity of CVD 1208S, a Live, Oral DeltaguaBA Deltasen Deltaset Shigella flexneri 2a Vaccine Grown on Animal-Free Media. Hum Vaccin 3. [DOI] [PubMed] [Google Scholar]
- Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Scire AS et al. (2017) Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 22: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM (1987) Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis 155: 377–389. [DOI] [PubMed] [Google Scholar]
- Levine MM, Barry EM, and Chen WH (2019) A roadmap for enterotoxigenic Escherichia coli vaccine development based on volunteer challenge studies. Hum Vaccin Immunother 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, DuPont HL, Formal SB, Hornick RB, Takeuchi A, Gangarosa EJ et al. (1973) Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis 127: 261–270. [DOI] [PubMed] [Google Scholar]
- Levine MM, Gangarosa EJ, Barrow WB, and Weiss CF (1976) Shigellosis in custodial institutions. V. Effect of intervention with streptomycin-dependent Shigella sonnei vaccine in an institution with endemic disease. Am J Epidemiol 104: 88–92. [DOI] [PubMed] [Google Scholar]
- Levine MM, Kotloff KL, Barry EM, Pasetti MF, and Sztein MB (2007) Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol 5: 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C et al. (2016) Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME et al. (2014) Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 59: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Wierzba T, and Walker RI (2016) Status of vaccine research and development for Shigella. Vaccine 34: 2887–2894. [DOI] [PubMed] [Google Scholar]
- Mansour A, Shaheen HI, Amine M, Hassan K, Sanders JW, Riddle MS et al. (2014) Pathogenicity and phenotypic characterization of enterotoxigenic Escherichia coli isolates from a birth cohort of children in rural Egypt. J Clin Microbiol 52: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli AT, Baudry B, D'Hauteville H, Hale TL, and Sansonetti PJ (1985) Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun 49: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mel DM, Terzin AL, and Vuksic L (1965) Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ 32: 647–655. [PMC free article] [PubMed] [Google Scholar]
- Nataro JP, Seriwatana J, Fasano A, Maneval DR, Guers LD, Noriega F et al. (1995) Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun 63: 4721–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FR, Liao FM, Formal SB, Fasano A, and Levine MM (1995) Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. J Infect Dis 172: 1408–1410. [DOI] [PubMed] [Google Scholar]
- Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, and Levine MM (1999a) Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun 67: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, and Levine MM (1999b) Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun 67: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FR, Losonsky G, Lauderbaugh C, Liao FM, Wang JY, and Levine MM (1996) Engineered deltaguaB-A deltavirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity, and potential efficacy as a mucosal vaccine. Infect Immun 64: 3055–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettengill EA, Pettengill JB, and Binet R (2015) Phylogenetic Analyses of Shigella and Enteroinvasive Escherichia coli for the Identification of Molecular Epidemiological Markers: Whole-Genome Comparative Analysis Does Not Support Distinct Genera Designation. Front Microbiol 6: 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalipon A, Costachel C, Grandjean C, Thuizat A, Guerreiro C, Tanguy M et al. (2006) Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: implications for the development of a chemically defined glycoconjugate vaccine. J Immunol 176: 1686–1694. [DOI] [PubMed] [Google Scholar]
- Phalipon A, Tanguy M, Grandjean C, Guerreiro C, Belot F, Cohen D et al. (2009) A synthetic carbohydrate-protein conjugate vaccine candidate against Shigella flexneri 2a infection. J Immunol 182: 2241–2247. [DOI] [PubMed] [Google Scholar]
- Porter CK, Olson S, Hall A, and Riddle MS (2017) Travelers' Diarrhea: An Update on the Incidence, Etiology, and Risk in Military Deployments and Similar Travel Populations. Mil Med 182: 4–10. [DOI] [PubMed] [Google Scholar]
- Rahman KM, Arifeen SE, Zaman K, Rahman M, Raqib R, Yunus M et al. (2011) Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine 29: 1347–1354. [DOI] [PubMed] [Google Scholar]
- Riddle MS, Kaminski RW, Di PC, Porter CK, Gutierrez RL, Clarkson KA et al. (2016) Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: a Single-Blind, Randomized Phase I Study. Clin Vaccine Immunol 23: 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout WR, Formal SB, Giannella RA, and Dammin GJ (1975) Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology 68: 270–278. [PubMed] [Google Scholar]
- Sahl JW, Morris CR, Emberger J, Fraser CM, Ochieng JB, Juma J et al. (2015) Defining the phylogenomics of Shigella species: a pathway to diagnostics. J Clin Microbiol 53: 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P and Phalipon A (1996) Shigellosis: from molecular pathogenesis of infection to protective immunity and vaccine development. Res Immunol 147: 595–602. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ and Arondel J (1989) Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine 7: 443–450. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Arondel J, Fontaine A, d'Hauteville H, and Bernardini ML (1991) OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine 9: 416–422. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, D'Hauteville H, Formal SB, and Toucas M (1982a) Plasmid-mediated invasiveness of “Shigella-like” Escherichia coli. Ann Microbiol (Paris) 133: 351–355. [PubMed] [Google Scholar]
- Sansonetti PJ, Hale TL, Dammin GJ, Kapfer C, Collins HH Jr., and Formal SB (1983) Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun 39: 1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ, Kopecko DJ, and Formal SB (1981) Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun 34: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ, Kopecko DJ, and Formal SB (1982b) Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun 35: 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL et al. (2017) Functional and Antigen-Specific Serum Antibody Levels as Correlates of Protection against Shigellosis in a Controlled Human Challenge Study. Clin Vaccine Immunol 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinsland H, Valentiner-Branth P, Perch M, Dias F, Fischer TK, Aaby P et al. (2002) Enterotoxigenic Escherichia coli infections and diarrhea in a cohort of young children in Guinea-Bissau. J Infect Dis 186: 1740–1747. [DOI] [PubMed] [Google Scholar]
- Strockbine NA and Maurelli AT (2005) Shigella In Bergey’s Manual of Sytematic Bacteriology, Volume 2 Brenner D, Krieg N, and Staley J (ed.) New York: Springer, pp. 811–823. [Google Scholar]
- van der Put RM, Kim TH, Guerreiro C, Thouron F, Hoogerhout P, Sansonetti PJ et al. (2016) A Synthetic Carbohydrate Conjugate Vaccine Candidate against Shigellosis:Improved Bioconjugation and Impact of Alum on Immunogenicity. Bioconjug Chem 27: 883–892. [DOI] [PubMed] [Google Scholar]
- Vidal RM, Muhsen K, Tennant SM, Svennerholm AM, Sow SO, Sur D et al. (2019) Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 13: e0007037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Grassel C, Levine MM, and Barry EM (2011) Live attenuated Shigella dysenteriae type 1 vaccine strains overexpressing shiga toxin B subunit. Infect Immun 79: 4912–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]