Abstract
Disruptions in multiple-neurobiological-pathways and neuro-molecular processes have been widely implicated in the etiopathology of Alzheimer’s disease (AD), a complex, progressive and ultimately lethal neurological-disorder whose current incidence, both domestically-and-globally, is reaching epidemic proportions. While only a few percent of all AD cases appear to have a strong genetic- or familial component, the major-form of this disease, known as idiopathic- or sporadic-AD, displays a multi-factorial pathology, and represents one of the most complex and perplexing neurological disorders known. More effective and innovative pharmacological-strategies for the successful intervention and management of AD might be expected: (i) to arise from strategic-treatments that simultaneously address multiple-interrelated AD targets that are directed at the initiation, development and/or propagation of this disease; and (ii) those that target the ‘neuropathological core’ of the AD process at early- or upstream-stages of AD. This ‘Perspectives paper’ will review current-research involving microRNA (miRNA)-mediated, messenger-RNA (mRNA) targeted gene-expression pathways in sporadic-AD and address the potential-implementation of evolving anti-microRNA (AM) strategies in the amelioration and clinical-management of AD. This novel therapeutic approach: (i) incorporates a system involving the restoration of multiple miRNA-regulated mRNA-targets via the use of selectively-stabilized AM-species; and (ii) via implementation of synthetic AMs the abundance of only relatively small-families of miRNAs need be modulated or neutralized to re-establish neural-homeostasis in AD-affected brain. In doing so these strategic-approaches will jointly and interactively address multiple AD-associated processes such as the disruption of synaptic-communication, defects in amyloid-peptide clearance and amyloidogenesis, tau-pathology, deficits in neurotrophic-support, alterations in the innate-immune-response and the proliferation of neuroinflammatory-signaling.
Keywords: Alzheimer’s disease, microRNA (miRNA), messenger RNA (mRNA), miRNA-mRNA linkage analysis, miRNA-7, miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, miRNA-155
Overview - AD heterogeneity
Alzheimer’s disease (AD) is a complex, progressive and ultimately lethal neurodegenerative disorder whose primary risk factor is aging of the human-CNS. The longitudinal-course of AD progresses very differently amongst individuals and clearly evolves over different time scales-commensurate with an individual’s biomedical-history, biophysical- and hormonal-signature, and highly-interrelated patient-characteristics including lifestyle, genetic-constitution and predisposition [1–4]. Epidemiological-, genetic-, epigenetic-, neuroimaging-, neuropathological-, biomarker-, lifestyle-, clinical-, etiopathological- and ethnological-based-evidence continues to suggest that AD is a particularly heterogeneous disorder [1–10]. Therapeutic-interventions for AD combined with genome-editing, RNA-interference-technologies, pharmacotherapeutics and/or lifestyle including dietary-alterations: (i) may provide the most promising-approaches for modulating or repairing the primary molecular-genetic deficits associated with such age-related, and heterogeneous-neurodegenerative-pathologies; and (ii) may be the best-example-yet to which a tailoring of ‘personalized or precision medicine’ to each AD patient for disease-amelioration may be successfully applied [7–10].
One attractive therapeutic-strategy for AD intermediation would be an interventional therapy such as a pharmaceutical agent that: (i) is non-toxic at physiologically realistic doses; (ii) has relatively high stability in vivo, long acting and effective throughout the treatment-regimen; (iii) has a modifiable-structure that can increase or decrease intrinsic-stability to shorten or prolong its pharmacological-efficacy; (iv) is highly selective with minimal-toxicity and limited off-target effects; (v) is well-tolerated by the aged AD patient and their compromised-physiological condition especially with respect to nephrotoxicity, hepatotoxicity and neurotoxicity; (vi) has an intrinsic-capacity to neutralize multiple disease targets; (vii) as a treatment focuses on at least one molecular-genetic component lying upstream from the branching of a critical-pathological pathway relevant to the AD-process; and (viii) can be effectively delivered into the CNS using viral-mediated transfer, exosomes or related-packaging and drug-delivery techniques. Currently few drug-choices for AD possess all of these qualities; however, all 8 of these roles are fulfilled by microRNAs (miRNA) and anti-miRNAs (AMs). miRNAs and AMs are small 18–22 nucleotide (nt) highly soluble single-stranded non-coding (sncRNA) species whose stability can be greatly increased using 3’ adenylation, locked nucleic acids (LNAs; a bicyclic furanose unit locked in an RNA-mimicking sugar-conformation) or other RNA-chemical modification techniques that significantly-prolongs miRNA half-life and stability both in vitro and in vivo. Put simply AM ribonucleotide sequences are complementary to respective miRNA sequences and the creation of a miRNA-AM-hybrid reduces the abundance of that miRNA which can restore-homeostasis to the system under study [11–17].
microRNAs (miRNAs) in neurological disease
MicroRNAs (miRNAs) represent a unique, evolutionary conserved class of sncRNAs which act in the post-transcriptional regulation and expression of genetic information in higher eukaryotes [18–29]. Only about ~2,650 miRNAs are abundant in the human brain and retina, and these have a potential to regulate a transcriptome consisting of about ~22,000–27,000 messenger RNAs (mRNAs; this is the total number of mRNAs in an average eukaryotic cell, but not all mRNAs are either generated or expressed in any one specific cell type). The major mode of action of miRNAs is to function as negative regulators of mRNA-mediated gene expression by recognizing and binding to complementary ribonucleotide sequences in the 3’ untranslated region (3’-UTR) of target mRNAs and causing translational repression and/or target degradation [18–21]. Hence, small families of up-regulated miRNAs might be expected to down-regulate their target mRNAs and down-regulate the expression of the genetic information that these mRNAs encode. This capability of small families of up-regulated miRNAs to target and down-regulate multiple mRNAs and combinatorial miRNA-mRNA linked gene expression networks, which are altered in disease conditions makes them amongst the most interesting and potentially useful candidates for effective therapeutic intervention using AM therapeutic strategies.
Interestingly each miRNA can potentially target the mRNAs encoded by hundreds of genes simultaneously; most miRNAs display a tightly regulated expression pattern that is often tissue specific or even cell-type specific, emphasizing the importance of miRNAs in defining the space, time, and developmental stage of specific gene expression patterns [24–29]. Importantly, (i) the abundance, complexity, speciation and regulation of a cell’s endogenous miRNA pool is a critical event in the definition of cell identity and behavior, both under steady-state conditions, during development and aging and in health and disease; and (ii) other important components of the CNS including, ceramides and sphingosine and their phosphates may modulate gene expression signaling as AD progresses - these compounds also most likely involved in the regulation of gene expression in the CNS in other neurodegenerative disorders [28–31].
Up-regulated NF-kB-sensitive microRNAs in AD versus age- and gender-matched controls
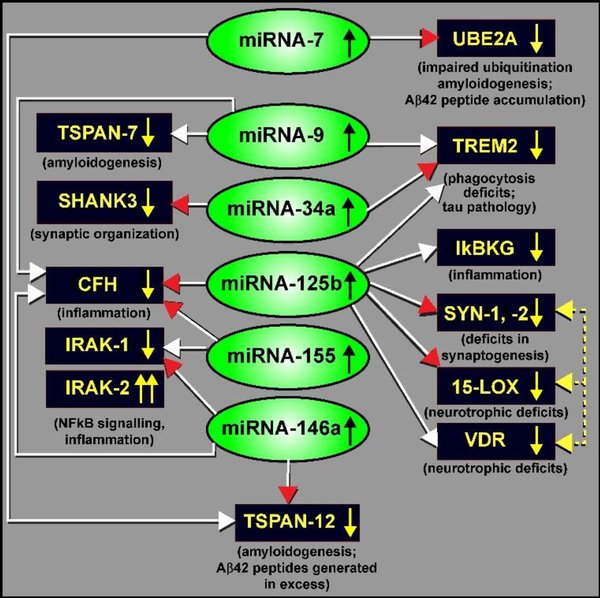
Our understanding of the regulation of the expression of human inflammatory-related microRNAs (miRNAs) in the CNS was advanced by experimental induction the pro-inflammatory transcription factor NF-kB (p50/p65) in human neuronal-glial (HNG) cell co-cultures in primary culture, and by subsequently analyzing the resulting induced miRNAs using a high-density microRNA array approach (capable of quantifying all 2650 human miRNA species). Up-regulated miRNA signals were further quantified and analyzed using LED-Northern micro-dot blots and RT-PCR [26–30]. NF-kB was induced using Aβ42 peptides, neurotoxic metal sulfates, TNFα and/or IL-1β, for example – these are known pro-inflammatory mediators observed to be up-regulated in AD brain tissues [30–33]. Selective NF-кB-DNA binding and translocation inhibitors, including the chelator and anti-oxidant pyrollidine dithiocarbamate (PDTC), the caffeic acid phenethyl ester CAPE and the polyphenolic and the polyphenolic resveratrol analog CAY10512 (trans-3,5,4’-trihydroxystilbene), reaffirmed the NF-кB sensitivity of a small family of up-regulated miRNAs in HNG cells. Certain of the most highly induced miRNA promoters of interest were next sequenced, analyzed for NF-kB-DNA binding sites using bioinformatics software, and verification of NF-kB-mediated regulation of these miRNAs was performed using pLightSwitch 3’-UTR luciferase reporter vector systems stably transfected into HNG cells in primary culture [21,26,27,34]. For example, the inducible pathogen-associated miRNA-125b and miRNA-146a were found to have multiple NF-kB-DNA binding sites in their upstream promoters [26]. Verified mRNA targets for miRNA-125b and miRNA-146a, including: (i) 15-lipoxygenase [15-LOX; a critical enzyme in neurotrophic support and the synthesis of neuroprotectin D1 (NPD1) from docosahexaenoic acid and other omega-3 fatty acids]; (ii) synapsin (SYN-1 and SYN-2; synaptic phosphoproteins that act as key modulators of neurotransmitter release across the presynaptic membrane of CNS synapses; down-regulated SYN-1 and SYN-2 proteins result in a deficit in synaptogenesis and disruption of normal synaptic signaling function); (iii) complement factor H (CFH; originally known as beta-1H globulin; a key soluble glycoprotein and regulator of complement activation in the innate-immune response); and (iv) tetraspanin-7 and tetraspanin-12 (TSPAN-7 and TSPAN-12; a βAPP-associated transmembrane protein whose deficits are associated with amyloidogenesis), suggested complex and highly interactive roles for the NF-кB (p50/p65) complex, miRNA-125b and miRNA-146a in the regulation of multiple mRNAs, and hence their expression, in human CNS neural cells [20,35]. These data further indicated that a small number of NF-кB-induced miRNAs have tremendous potential to contribute to or detract from the regulation of neurotrophic support, synaptogenesis, neuro-inflammation, innate-immune signaling and amyloidogenesis in stressed HNG cells and in AD affected tissues. Many of the pathologically up-regulated miRNAs in AD brain (including to date, miRNA-7, miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a and miRNA-155; based on the analysis of ~90 short PMI control and AD brain from female brains) and significantly down-regulated mRNAs (resulting in critical deficits in gene expression, as is observed in AD) and their neuropathological consequences are shown in the schematic-diagram in Figure 1 and are further described in the legend to this figure [35–38].
Figure 1.
A highly schematicized figure, taken from recent peer-reviewed publications in the up-regulated-miRNA-in-AD field - indicating a miRNA-regulated expression network involving a small family of inducible, NF-kB (p50-p65) controlled microRNAs (miRNAs; green ovals) that down-regulate the expression of multiple messenger RNA (mRNA) targets (black rectangles) of relevance to the onset, development and propagation of AD neuropathology [64,65]. This graphic figure summarizes the effects of 6 up-regulated miRNAs on the down-regulation of 12 of their messenger RNA (mRNA) targets. All 6 miRNAs or 12 mRNAs are brain-enriched or brain-specific RNA transcripts [29,36,37]. White arrowheads indicate bioinformatics-derived evidence [based primarily on the free energy of association EA, between the entire miRNA and the 3’-untranslated region (3’-UTR) of their target mRNA], and are generally free energies of association of less than −21 kcal/mol; (miRBase release 22.1; http://www.mirbase.org/; last accessed 25 April 2019; and TargetScan (TargetScanHuman release 7.2; http://www.targetscan.org/vert_72/; last accessed 25 April 2019; and/or other miRNA-mRNA bioinformatics algorithms); red arrowheads indicate both bioinformatics- and experimentally-derived evidence and verification that has appeared in the recent peer-reviewed scientific literature; experimental verification is based mainly on miRNA-mRNA binding studies and 3’-UTR-luciferase-reporter binding assay using pLightSwitch vectors transfected into human neuronal-glial (HNG) cell co-cultures; yellow dashed lines and yellow arrowheads indicate interrelated and/or coordinated gene expression; down-regulated target mRNAs (and hence down-regulated expression of multiple brain-enriched genes) appear to contribute, sometimes interactively, to impaired ubiquitination (UBE2A), deficits in phagocytosis (TREM2), amyloidogenesis (TSPAN-7, TSPAN-12), deficits in innate-immune signaling (CFH), stimulation of NF-kB-signaling and neuro-inflammation (IkBKG, IRAK-1; with a compensatory increase in IRAK-2 expression), altered synaptogenesis, synaptic structure and organization (SHANK3, SYN-1 and SYN-2) and deficits in neurotropic support (15-LOX, VDR). See text for further details. Because miRNAs typically have multiple mRNA targets only a few up-regulated miRNAs can explain the observed down-regulation of multiple mRNA targets; most of which reflect down-regulated brain-enriched gene expression patterns that in part characterize AD neuropathology [18,20,22,43,47]. Neutralization of miRNA activities using anti-microRNA (AM) therapeutic strategies may be of immense benefit in the clinical management of AD and other neurodegenerative disorders with an inflammatory component. Other miRNA-mRNA linked signaling circuits may be involved. This analysis was based on 90 female AD and age-matched control temporal lobe neocortex and/or hippocampal CA1 regions all having post-mortem intervals (PMIs) of 2.5 hrs or less [36; unpublished observations].
Down-regulated messenger RNAs (mRNAs) observed in AD brain
Multiple independent-investigators have reported a generalized down-regulation of gene expression in AD brain neocortex and hippocampus to about 50–66% of that what is found in healthy, age-and gender-matched controls [36,39–41]. These deficiencies in genetic-output include deficits in mRNAs encoding information necessary for homeostatic ubiquitination (UBE2A), phagocytosis (TREM2) and impairments in the end-stage clearance of amyloid from the cell with ensuing amyloidogenesis (TSPAN-7, TSPAN-12), down-regulation in neurotropic support including 15-lipoxygenase (15-LOX) and the vitamin D receptor (VDR), deficits in innate-immune signaling (CFH), ectopic stimulation of NF-kB-signaling and neuro-inflammation (IkBKG, IRAK-1; with a compensatory increase in IRAK-2 expression), and altered synaptogenesis, synaptic-structure and organization and the ability of synaptic-vesicles to be ‘released’ and ‘reabsorbed’ at the synapse (TSPAN-7, TSPAN-12, SHANK3, SYN-1 and SYN-2). What is particularly interesting is the overlapping-functions of groups of down-regulated genes whose brain functions are highly inter-related, such as TSPAN-7, TSPAN-12, SHANK3, SYN-1 and SYN-2 in synaptic-organization and signaling and in the coordinated down-regulation in the expression-of-genes involved in inter-neuronal trans-synaptic communication and neurotrophic support, including SYN-1, SYN-2, 15-LOX, VDR (Figure 1).
Another highly-illustrative example is regulation of the superfamily of tetraspanin (TSPAN) proteins (including TSPAN-7 and TSPAN-12 – whose expression are both significantly down-regulated in AD by up-regulated miRNA-7, miRNA-9 and miRNA-146a) [42,43]. TSPAN proteins are normally engaged in organizing cellular interactions, membrane trafficking, compartmentalization and the formation of complex protein networks known as ‘tetraspanin-webs’ with functions in neural-activity dependent synapse formation, function and plasticity – and are involved in both neurological and retinal degeneration [44–46]. Interestingly the post-synaptic organizing and scaffolding protein SHANK3 (down-regulated by increased miRNA-34a presence) and down-regulated TSPAN proteins may jointly contribute to neurodegenerative aspects of neuropsychiatric-illness [43,47]. Deficits in such highly inter-related, and functionally inter-connected, gene networks may be exploited when the same over-abundant miRNAs such as miRNA-9 and miRNA-125b target individual components of this complex-system (Figure 1). Hence anti-miRNA (AM) strategies may be used to advantage to knock down miRNA-regulated networks of small families of genes to tailor the desired pharmaco-therapeutic response and form a mechanistic basis for the personalization of treatments for progressive, age-related inflammatory-neurodegenerative disorders such as AD.
Anti-NF-kB strategies
Firstly, because all of these 6 potentially pathogenic miRNAs are up-regulated in abundance after the induction of the pro-inflammatory dimeric transcription factor NF-kB (p50/p65) complex, it may seem at first that inhibition of NF-kB could provide a novel and efficacious therapeutic strategy. There are currently about ~1000 NF-kB-inhibitors (such as IkB-phosphorylation, IkB-activation and NF-kB-translocation inhibitors, etc.,) available to neurological researchers and clinicians including a variety of natural- and synthetic-antioxidants, peptides, small RNA/DNA, microbial- and viral-proteins, antibodies and engineered-dominant-negative or constitutively-active-polypeptides that have direct anti-NF-kB activities [48-50; unpublished). Interestingly, the therapeutic-, preventative- and beneficial-anti-inflammatory effects of many natural products such as allicin, curcumin, ellagic acid, resveratrol and sulforaphane may, at least in part, be due to their ability to inhibit NF-kB-signaling [29,30,35,49,51,53–55].
There are however important limitations to the implementation of anti-NF-kB therapeutic-strategies due to: (i) the hepatotoxicity (liver toxicity), nephrotoxicity (renal toxicity) and neurotoxicity (toxicity in the CNS) of many anti-NF-kB compounds, especially in aged humans [48,49,52]; (ii) difficulties in drug-delivery and the selective targeting of NF-kB signaling-pathways within CNS compartments [49,53], and (iii) significant off-target effects for NF-kB inhibitors, many of which were recognized at least 13 years ago [48,49,52]. Related to these limitations, NF-kB is often a critical and pluripotent transcription factor that orchestrates many diverse physiological and pathological processes and responses, especially those involved in cell proliferation, inflammation and immunity, cell growth, apoptosis and necrosis, the expression of pathogenic viral genes, and cancer development and progression – thereby unwanted off-target effects may be of a genuine concern when strategically implementing anti-NF-kB compounds therapeutically [29,30,49,50,52–55]. The singular importance and significance of NF-kB in the generation of potentially pathogenic miRNA in cancer and neurodegenerative disease have been presented in a series of well-illustrated peer-reviewed papers from multiple laboratories [27–31,43,52–57,65].
Anti-microRNA (AM) strategies
There are multiple useful intrinsic properties, advantages and benefits in both the molecular structure and function of anti-miRNAs (AMs) which impart to them a significant potential for their successful therapeutic application as AM-based therapies in the human CNS. These include: (i) both miRNAs and AMs are non-toxic at physiologically realistic doses in human brain cells in primary culture; (ii) because AMs (and miRNAs) are typically less than ~1/375th the size of the smallest known single-stranded RNA virus (poliovirus, 7500 nt), miRNAs (at 18–22 nt) are probably too small to elicit a robust immune response in the host; (iii) the regulation of their stabilities, and hence functional longevity in the cell is extremely versatile; although many miRNAs and AMs are short-lived in the brain and retina, their structure can be chemically and/or physically modified to significantly increase their stability in vivo, and can be made to be long acting and effective throughout the treatment regimen; miRNA in the brain and retina can also be relatively unstable entities and this may be useful in limiting the efficacy of the AMA itself; (iv) just like antibody-protein recognition, miRNAs are highly selective with minimal toxicity and negligible off-target effects; (v) AMs can be designed so that their complementary anti-miRNA ribonucleotide sequence is either fully or only partly complementary to modulate the full AM effect; (vi) miRNAs are well tolerated by the aged AD patient and their often compromised physiological condition; (vii) miRNAs have an intrinsic capacity to neutralize multiple disease targets; (viii) depending on complementarity between the AM and the miRNA can ‘throttle down’ or completely negate the abundance and actions of a particular miRNA; (ix) a strategic approach of an AM targeting a specific miRNA could define a pharmacological treatment that focuses on at least one critical molecular-genetic component lying upstream from the branching of a critical pathological pathway relevant to the AD process; (x) an extremely high selectivity – based on complementary AM-miRNA pairing that is one of the most specific and selective links in human neurobiology; (xi) delivery of AMs via exosomes and viral vectors; (xii) especially in the brain and retina, many sncRNA and AM stabilities can be kept relatively short; indicating that the therapeutic effect of miRNAs or sncRNAs can be tailored to be self-limiting; and (xiii) if necessary, anti-NF-kB and AM strategies may be combined to achieve the desired pharmaco-therapeutic effect.
Concluding Comments
The utilization and implementation of anti-miRNA (AM) and related anti-sncRNA therapeutic treatment strategies in human neurological diseases such as AD are still in their infancy, given that the actual discovery of altered miRNA abundance, speciation and complexity in AD and their inducibility by oxidative stress was only a scant 12 years ago [56–58]. The first in vivo experiments using AM strategies in human brain cells in primary culture in vitro occurred only about 6 years ago [38]. Here we cite 5 relevant examples on the design and utilization of AM strategies and the potential usefulness of AM therapeutic approaches:
the efficient design of a specific AMs can help to improve their performance and potency towards the target miRNA by increasing, for instance, nuclease resistance and target affinity; the design of AM compounds including locked nucleic acid (LNA) oligonucleotides, that have recently proved their worth in silencing miRNAs is progressing and is being optimized in vivo [59];
expression of the integral membrane triggering receptor expressed in microglial cells (TREM2) and post-synaptic organizing protein SHANK3 has been shown to be down-regulated due to a significantly up-regulated miRNA-34a in AD and in stressed HNG cells in primary culture, and a specifically stabilized AM-34a was shown to both knock down miRNA-34a expression and restore normal TREM2 and SHANK3 expression and homeostasis in vivo [38,60];
the pro-inflammatory miRNA-146a has been shown to be significantly up-regulated in both sporadic AD neocortex and in stressed-HNG cells in primary-co-culture (Lukiw et al., 2008; Cui et al 2019); in preliminary experiments anti-miRNA-146a (AM-146a) based strategies using viral vector delivery (AAV) systems have been shown to regain miRNA-associated homeostasis in brain tissues and to improve cognitive performance in the aging 5xFAD murine model of AD [38,60; unpublished].
very recently an AM for miRNA-1 has been successfully used in the experimental treatment of middle cerebral artery occlusion (MCAO) in a rodent model - resulting in an improvement in neurological deficits and reduced infarction-volume, brain-edema and blood-brain barrier (BBB) permeability, indicating that AMs are able to protect the brain and provide a promising therapeutic approach after stroke and MCAO [61];
there is also recent evidence that miRNA-155 upregulated after traumatic brain injury (TBI) can be reduced to homeostatic-levels in the regulation of post-traumatic neuroinflammatory-responses and neurological-recovery using an AM-155 strategy, indicating that miRNA-155 may be a therapeutic target for post TBI-related neuro-inflammatory signaling [62–64];
Lastly, the implementation of successful AM-based treatment for AD patients may require a highly “individualistic” treatment strategy and clinical regimen based on multiple factors including highly interactive aspects of a particular patient’s epidemiology, multi-factorial pathological factors such as familial-genetic and medical history, disease-duration, disease-course, interceding-illness and the timing of the strategic miRNA-based intervention itself. For example, it has been recently shown that specific time-frames during the endrocrine-aging process, such as during the menopause-transition, may provide an optimal-window of opportunity for therapeutic-intervention to further prevent or delay the progression of AD [64,65]. Such ‘personalized AM-based therapeutic approaches’ would more effectively address the spectrum of specific miRNA, mRNA and gene expression deficits in individual AD-patients, including combinatorial and/or tailored multiple AM-therapeutic approaches that will take both clinician-focus and dedication, and probably individual-customization, to maximize the probability of success in the clinical management of the disease-course.
Acknowledgements
The analytical, experimental and statistical work in this research report was presented in part at the Vavilov Institute of General Genetics autumn seminar series (Серия осенних семинаров) in Moscow RUSSIA November 2018 and at the Society for Neuroscience (SFN) Annual Meeting November 2018, San Diego CA, USA. Sincere thanks are extended to the late Drs. JM Hill (JMH; Louisiana State University), TPA Kruck (TPAK; University of Toronto), C. Bergeron (CB, University of Toronto) for helpful discussions on this research area and to F Culicchia, C Eicken, C Hebel, B. Krishnan, K Navel, and L. Wong for short postmortem interval (PMI) human and other mammalian brain tissues or extracts and data interpretation and to D Guillot for expert technical assistance. Thanks are also extended to the many neuropathologists, physicians and researchers of the US, Canada, Europe and Russia who have provided high quality, short postmortem interval (PMI) human CNS or extracted brain tissue fractions for scientific study. We would like to further thank the following 18 domestic and international brain banks, and their continuing cooperation, for access to high quality postmortem tissues and valuable analytical advice: the Autism Brain Net, Los Angeles CA, USA; the Harvard University/McLean Hospital Tissue Center, Boston MA, USA; Louisiana State University, New Orleans LA, USA; the Lomonosov Institute, Moscow State University, Moscow, Russian Federation; the National Disease Research Interchange, Philadelphia PA, USA; the National Institutes of Health NIH NeuroBioBank, comprised of tissues from the National Institute of Mental Health (NIMH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda MD USA; the Netherlands Brain Research Institute, Amsterdam, Netherlands; the New York State Institute for Basic Research, Staten Island NY, USA; the Oregon Health Sciences University, Portland OR, USA; the Southern Eye Bank, Metairie LA, USA; the University of California, Irvine CA, USA; the University of Kentucky Alzheimer’s disease Brain Bank, Lexington KY, USA; the University of Maryland Brain and Tissue Bank, Baltimore MD, USA; the University of Massachusetts, Worcester MA, USA; University of Pennsylvania School of Medicine, Philadelphia PA, USA, and the University of Toronto Brain Bank, Toronto ON, Canada. All authors agree on the content of this publication. Research on AM design and potential treatment strategies, metal neurotoxicity, human and murine microRNAs, small noncoding RNA (sncRNA), proinflammatory and pathogenic signaling in the Lukiw laboratory involving the innate-immune response, neuroinflammation and amyloidogenesis in AD, PrD and in other human neurological disorders was supported through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (RPB); the Louisiana Biotechnology Research Network (LBRN), the Alzheimer Association and NIH grants NEI EY006311, NIA AG18031 and NIA AG038834 (WJL).
Footnotes
Conflict of Interest Statement
Declaration of interest for all authors including financial and personal relationships with other people or organizations: none. We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. The experimental work in this paper was funded by the LSU Eye Center from Research to Prevent Blindness (RPB), the Louisiana Biotechnology Research Network (LBRN), the National Institutes of Health (NIH), Bethesda MD, USA and the Alzheimer Association Chicago IL, USA.
Ethics Statement
All acquisition, handling, experimental and analytical procedures involving postmortem human brain tissues were carried out in an ethical manner in strict accordance with the ethics review board policies at brain and tissue donor institutions and at the Louisiana State University (LSU) Health Sciences Center. Informed consent from next of kin was obtained at brain and tissue donor institutions for all tissue samples prior to autopsy and donation; coded postmortem brain tissue samples (containing no personal identifying information of the donors) were obtained from the 18 brain and tissue banks listed in the Acknowledgements section above. The ethical use of postmortem human brain tissues and their analyses were also carried out in strict accordance with the Institutional Biosafety Committee and the Institutional Review Board Committee (IBC/IRBC) ethical guidelines IBC#18059 and IRBC#6774 at the LSU Health Sciences Center, New Orleans LA 70112 USA.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Latta CH, Brothers HM, Wilcock DM (2015) Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience 302:103–11. doi: 10.1016/j.neuroscience.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane CA, Hardy J, Schott JM (2018). Alzheimer’s disease. Eur J Neurol. 25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 3.Rogaev EI (2018). Different pathways to neurodegeneration. Biochemistry (Mosc). 83:1007–1008. doi: 10.1134/S0006297918090018. [DOI] [PubMed] [Google Scholar]
- 4.Melis RJF, Haaksma ML, Muniz-Terrera G (2019). Understanding and predicting the longitudinal course of dementia. Curr Opin Psychiatry. 32:123–129.doi: 10.1097/YCO.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong A, Toledo JB, Honnorat N, Doshi J, Varol E, Sotiras A, et al. , (2017). Heterogeneity of neuroanatomical patterns in prodromal Alzheimer’s disease: links to cognition, progression and biomarkers. Brain. 140:735–747. doi: 10.1093/brain/aww319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blennow K, Zetterberg H (2018) Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 284:643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 7.Bhagwat N, Pipitone J, Voineskos AN, Chakravarty MM; Alzheimer’s disease neuroimaging initiative. (2019) An artificial neural network model for clinical score prediction in Alzheimer disease using structural neuroimaging measures. J Psychiatry Neurosci. 44:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz CL, Mosconi L, Scheyer O, Rahman A, Hristov H, Isaacson RS (2018). Precision medicine for Alzheimer’s disease prevention. Healthcare (Basel) 6(3). pii: E82. doi: 10.3390/healthcare6030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodes JF, Oakley CI, O’Keefe JH, Lu P, Galvin JE, Saif N, et al. , (2019). Alzheimer’s “Prevention” vs. “Risk Reduction”: transcending semantics for clinical practice. Front Neurol. 9:179. doi: 10.3389/fneur.2018.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaacson RS, Ganzer CA, Hristov H, Hackett K, Caesar E, Cohen R, et al. , (2018). The clinical practice of risk reduction for Alzheimer’s disease: A precision medicine approach. Alzheimers Dement. 14:1663–1673. doi: 10.1016/j.jalz.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kauppinen S, Vester B, Wengel J (2006) Locked nucleic acid: high-affinity targeting of complementary RNA for RNomics. Handbook of Exp Pharmacol. 173:405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, Baba T, Suzuki T (2009). Selective stabilization of mammalian microRNAs by 3’ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev. 23:433–438. doi: 10.1101/gad.1761509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi P, Lukiw WJ (2009). Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Rüegger S, Großhans H (2012) MicroRNA turnover: when, how, and why. Trends Biochem Sci. 37:436–46. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Pogue AI, Hill JM, Lukiw WJ (2014). MicroRNA (miRNA): sequence and stability, viroid-like properties, and disease association in the CNS. Brain Res. 1584:73–79. doi: 10.1016/j.brainres.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Alexandrov PN, Lukiw WJ (2016). Anti-microRNAs as novel therapeutic agents in the clinical management of Alzheimer’s disease. Front Neurosci. 10:59. doi: 10.3389/fnins.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exiqon-Qiagen, Germantown MD, USA; http://www.exiqon.com/lna-technology; last accessed 12 March 2019).
- 18.Guo H, Ingolia NT, Weissman JS, Bartel DP (2010). Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Towler BP, Jones CI, Newbury SF (2015). Mechanisms of regulation of mature miRNAs. Biochem Soc Trans. 43, 1208–1214. doi: 10.1042/BST20150157. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Jaber VR, LeBeauf A, Sharfman NM, Lukiw WJ (2019). microRNA-34a (miRNA-34a) mediated down-regulation of the post-synaptic cytoskeletal element SHANK3 in sporadic Alzheimer’s disease (AD). Front Neurol. 10:28. doi: 10.3389/fneur.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharjee S, Zhao Y, Dua P, Rogaev EI, Lukiw WJ (2016). microRNA-34a-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration. PLoS One. 11(3):e0150211. doi: 10.1371/journal.pone.0150211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartel DP (2018). Metazoan MicroRNAs. Cell. 173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwakawa HO, Tomari Y (2015). The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 25: 651–665. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Jonas S, Izaurralde E (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16: 421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 25.Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y, Ren J (2019). Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol Life Sci. 76:441–451. doi: 10.1007/s00018-018-2940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukiw WJ, Zhao Y, Cui JG (2008) An NF-kB-sensitive miRNA-146a-mediated inflammatory circuit in AD and in stressed human brain cells. J Biol Chem. 283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, Percy ME, Tarr MA, Lukiw WJ (2009). Characterization of an NF-kB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg Biochem. 103:1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ (2010). Differential regulation of IRAK-1 and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer’s disease. J Biol Chem. 285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukiw WJ (2012). NF-κB-regulated, pro-inflammatory miRNAs in Alzheimer’s disease. Alzheimers Res Ther. 4:47. doi: 10.1186/alzrt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong W, Dong S, Yuan J, Li J, Liu J, Xu X (2012). miRNA-146a promotes proliferation and migration of rat vascular smooth muscle cells in vitro in a NF-κB-dependent manner. Nan Fang Yi Ke Da Xue Xue Bao. 32:270–273. [PubMed] [Google Scholar]
- 31.Czubowicz K, Jęśko H, Wencel P, Lukiw WJ, Strosznajder RP (2019). The role of ceramide and sphingosine-1-phosphate in Alzheimer’s disease and other neurodegenerative disorders. Mol Neurobiol. doi: 10.1007/s12035-018-1448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, Ho RC (2018). IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 8: 12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen XN, Niu LD, Wang YJ, Cao XP, Liu Q, Tan L, et al. , (2019). Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry. pii: jnnp-2018–319148. doi: 10.1136/jnnp-2018-319148. [DOI] [PubMed] [Google Scholar]
- 34.LightSwitch Assay, Switchgear Genomics (an Active Motif Company), Menlo Park CA USA; https://switchgeargenomics.com/resources/science-technology; last accessed 14 March 2019.
- 35.Lukiw WJ, Alexandrov PN (2012). Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol Neurobiol. 46:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ (2002). Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 70: 462–473. [DOI] [PubMed] [Google Scholar]
- 37.Burmistrova OA, Goltsov AY, Abramova LI, Kaleda VG, Orlova VA, Rogaev EI (2007). MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11). Biochemistry (Mosc). 72, 578–582. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Bhattacharjee S, Jones BM, Dua P, Alexandrov PN, Hill JM, et al. , (2013). Regulation of TREM2 expression by an NF-кB-sensitive miRNA-34a. Neuroreport 24: 318–323. doi: 10.1097/WNR.0b013e32835fb6b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsberg SD, Alldred MJ, Che S (2012). Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol Dis. 45:99–107. doi: 10.1016/j.nbd.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Bhattacharjee S, Jones BM, Hill J, Dua P, and Lukiw WJ (2014). Regulation of neurotropic signaling by the inducible, NF-kB-sensitive miRNA-125b in Alzheimer’s disease (AD) and in primary human neuronalglial (HNG) cells. Mol. Neurobiol. 50:97–106. doi: 10.1007/s12035-013-8595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Bhattacharjee S, Dua P, Alexandrov PN, and Lukiw WJ (2015). microRNA-based biomarkers and the diagnosis of Alzheimer’s disease. Front. Neurol. 6:162. doi: 10.3389/fneur.2015.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seipold L, Saftig P (2016). The emerging role of tetraspanins in the proteolytic processing of the amyloid precursor protein. Front Mol Neurosci. 9:149. doi: 10.3389/fnmol.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaber V, Zhao Y, Lukiw WJ (2017). Alterations in micro RNA-messenger RNA (miRNA-mRNA) coupled signaling networks in sporadic Alzheimer’s disease (AD) hippocampal CA1. J Alzheimers Dis Parkinsonism. 7(2). pii: 312. doi: 10.4172/2161-0460.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Termini CM, and Gillette JM (2017). Tetraspanins function as regulators of cellular signaling. Front. Cell Dev. Biol. 5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saint-Pol J, Eschenbrenner E, Dornier E, Boucheix C, Charrin S, Rubinstein E (2017). Regulation of the trafficking and the function of the metalloprotease ADAM10 by tetraspanins. Biochem Soc Trans. 45:937–944. doi: 10.1042/BST20160296. [DOI] [PubMed] [Google Scholar]
- 46.Murru L, Moretto E, Martano G, Passafaro M (2018). Tetraspanins shape the synapse. Mol Cell Neurosci. 91, 76–81. doi: 10.1016/j.mcn.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Alexandrov PN, Zhao Y, Jaber V, Cong L, Lukiw WJ (2017). Deficits in the proline-rich synapse-associated Shank3 protein in multiple neuropsychiatric disorders. Front Neurol. 8:670. doi: 10.3389/fneur.2017.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilmore TD, Herscovitch M (2006). Inhibitors of NF-kB signaling: 785 and counting. Oncogene. 25:6887–6899. [DOI] [PubMed] [Google Scholar]
- 49.Kaur U, Banerjee P, Bir A, Sinha M, Biswas A, Chakrabarti S (2015). Reactive oxygen species, redox signaling and neuro-inflammation in AD: the NF-κB connection. Curr Top Med Chem. 15:446–457. [DOI] [PubMed] [Google Scholar]
- 50.Yu L, Li L, Medeiros LJ, Young KH (2017). NF-κB signaling pathway and its potential as a target for therapy in lymphoid neoplasms. Blood Rev. 31:77–92. doi: 10.1016/j.blre.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khuda-Bukhsh AR, Das S, Saha SK (2014). Molecular approaches toward targeted cancer prevention with some food plants and their products: inflammatory and other signal pathways. Nutr Cancer. 66:194–205. doi: 10.1080/01635581.2014.864420 [DOI] [PubMed] [Google Scholar]
- 52.Hong JT (2017). NF-kB as a mediator of brain inflammation in AD. CNS Neurol Disord Drug Targets. doi: 10.2174/1871527316666170807130011. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Ding J, Yang J, Guo X, Zheng Y (2018). MicroRNA roles in the nuclear factor kappa B signaling pathway in cancer. Front Immunol. 9:546. doi: 10.3389/fimmu.2018.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Che J, Stark LA (2018). Crosstalk between NF-κB and nucleoli in the regulation of cellular homeostasis. Cells. 7, (10). pii: E157. doi: 10.3390/cells7100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendiola AS, Cardona AE (2018). The IL-1β phenomena in neuro-inflammatory diseases. J Neural Transm (Vienna). 125:781–795. doi: 10.1007/s00702-017-1732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lukiw WJ (2007) Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport. 18:297–300. [DOI] [PubMed] [Google Scholar]
- 57.Lukiw WJ, Pogue AI. (2007). Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 101:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schipper HM, Maes OC, Chertkow HM, Wang E (2007). MicroRNA expression in Alzheimer blood mononuclear cells. Gene Regul. Syst. Biol. 1: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lima JF, Cerqueira L, Figueiredo C, Oliveira C, Azevedo NF (2018). Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol. 15:338–352. doi: 10.1080/15476286.2018.1445959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Jaber V, Lukiw WJ (2017). Secretory products of the human GI tract and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol 7:318. doi: 10.3389/fcimb.2017.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talebi A, Rahnema M, Bigdeli MR (2019). Effect of intravenous injection of antagomiR-1 on brain ischemia. Mol Biol Rep. doi: 10.1007/s11033-018-04580-y [DOI] [PubMed] [Google Scholar]
- 62.Li YY, Alexandrov PN, Pogue AI, Zhao Y, Bhattacharjee S, Lukiw WJ (2012). miRNA-155 upregulation and complement factor H deficits in Down’s syndrome. Neuroreport 23: 168–173. doi: 10.1097/WNR.0b013e32834f4eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henry RJ, Doran SJ, Barrett JP, Meadows VE, Sabirzhanov B, Stoica BA, et al. , (2019). Inhibition of miRNA-155 limits neuro-inflammation and improves functional recovery after experimental traumatic brain injury in mice. Neurotherapeutics 16:216–230. doi: 10.1007/s13311-018-0665-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lukiw WJ (2012). NF-кB-regulated micro RNAs (miRNAs) in primary human brain cells. Exp Neurol. 235:484–90. doi: 10.1016/j.expneurol.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, et al. , (2018). Increased Alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLoS One. 13, e0207885. doi: 10.1371/journal.pone.0207885. [DOI] [PMC free article] [PubMed] [Google Scholar]