Abstract
Toxoplasma gondii is a parasitic protist that can infect nearly all nucleated cell types and tissues of warm-blooded vertebrate hosts. T. gondii utilizes a unique form of gliding motility to cross cellular barriers, enter tissues, and penetrate host cells, thus enhancing spread within an infected host. However, T. gondii also disseminates by hijacking the migratory abilities of infected leukocytes. Traditionally this process has been viewed as a route to cross biological barriers such as the blood-brain barrier. Here we review recent findings that challenge this view by showing that infection of monocytes down-regulates the program of transendothelial migration. Instead, infection by T. gondii enhances Rho-dependent interstitial migration of monocytes and macrophages, which enhances dissemination within tissues. Collectively, the available evidence indicates that T. gondii parasites use multiple means to disseminate within the host, including enhanced motility in tissues and translocation across biological barriers.
Introduction
Intracellular pathogens exploit host cells as a protected niche that supports growth while avoiding immune detection. Some intracellular pathogens further co-opt host cells as vectors that enable dissemination. This strategy can allow the pathogen to gain access to tissue by invading and translocating through the cells that form epithelial and endothelial barriers. For example, enteric bacterial pathogens such as Shigella and Salmonella invade the intestine by translocating through epithelial M cells (Vazquez-Torres and Fang, 2000). Alternatively, intracellular pathogens can spread to more distal sites by infecting migratory leukocytes that routinely traffic throughout the host using lymphatic and circulatory vasculature as conduits to reach deep tissues. Dissemination via migrating infected leukocytes often functions through a Trojan horse mechanism in which infected leukocytes ferry intracellular pathogens across biological barriers. Such Trojan horse style spread can lead to devastating consequences, such as when infected blood phagocytes deliver pathogens across the blood-brain barrier (BBB) and into the immune-specialized central nervous system (CNS) compartment (Santiago-Tirado and Doering, 2017; Ueno and Lodoen, 2015). Leukocytes also migrate through interstitial tissue spaces; however, the role of this pathway in pathogen dissemination has not been extensively explored. Infected leukocytes may promote pathogen dissemination by carrying pathogens harbored as intracellular cargo as they migrate into tissues, enter lymphatic and circulatory vessels, or traverse vulnerable biological barriers.
Toxoplasma gondii is a parasitic protist that causes acute and chronic toxoplasmosis in many warm-blooded vertebrates (Dubey, 2007). In immunocompetent humans, acute toxoplasmosis is typically not life-threatening but leads to systemic parasite dissemination that results in a lifelong chronic infection focused in the CNS (Montoya and Liesenfeld, 2004). T. gondii infects a variety of migratory leukocytes and has been theorized to exploit infected leukocytes as Trojan horses to enable BBB penetration. However, recent work suggests that T. gondii exploits infected monocytes as shuttles to efficiently disseminate through tissues, rather than using leukocytes to cross endothelial barriers. Here we review the literature surrounding T. gondii dissemination to illustrate the diverse mechanisms by which intracellular pathogens can exploit host cells to promote dissemination into a variety of host niches.
Progression of toxoplasmosis
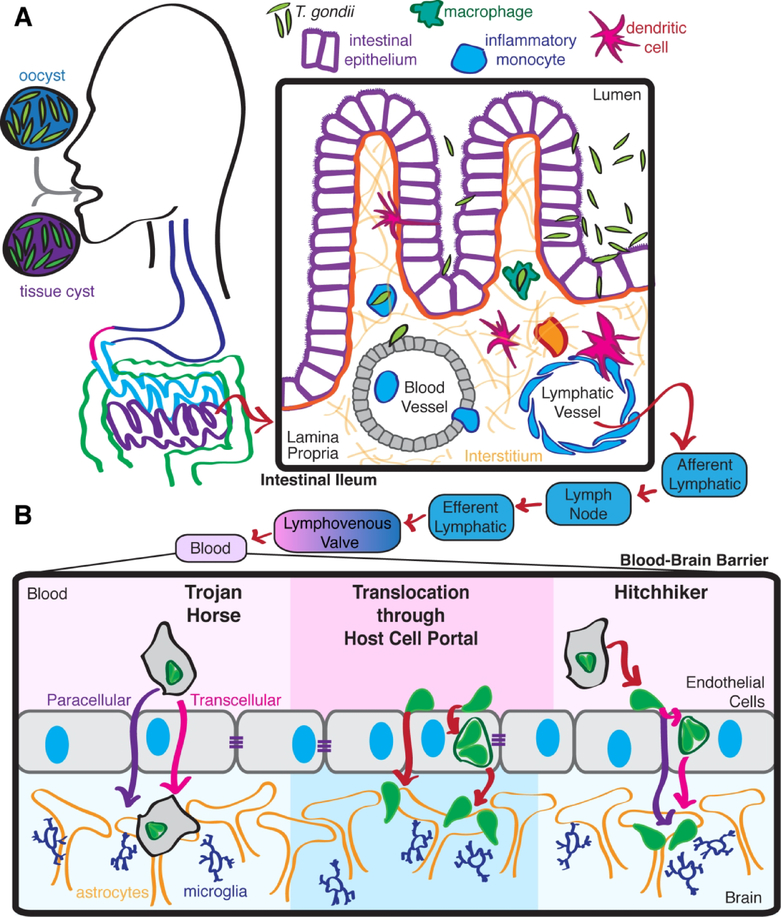
Toxoplasmosis is typically acquired when hosts ingest encysted parasites in the form of either bradyzoite tissue cysts or feline-shed oocysts (Hill and Dubey, 2002) (Figure 1A). Parasites emerge from either oocysts or tissue cysts within the stomach (Dubey et al., 1998). The freed parasites then travel into the small intestine and access the intestinal lamina propria by invading and replicating within or traversing through epithelial enterocytes, events that primarily occur in the ileum (Dubey et al., 2012; Dubey et al., 1997). Upon entering the intestinal lamina propria, parasites infect and replicate within a wide variety of cells, being most abundantly found within monocytes, macrophages and neutrophils (Dubey, 1997; Dubey et al., 1997; Gregg et al., 2013).
Figure 1. Progression of toxoplasmosis.
(A) T. gondii infections are acquired when hosts ingest parasite cysts. Upon reaching the ileum, parasites released from the cysts traverse invade through intestinal epithelium and gain access to the lamina propria. (B) T. gondii then spreads systemically. Parasites may invade the CNS when extracellular T. gondii directly invade BBB endothelial cells or with the assistance of migratory infected leukocytes using Trojan horse or hitchhiker mechanisms.
T. gondii rapidly spreads from the lamina propria, first into secondary lymphoid tissues and then into distal organs. In murine infection models, Peyer’s patches and mesenteric lymph nodes are infected by two to three days post inoculation (Courret et al., 2006; Dubey, 1997; Dubey et al., 2012; Dubey et al., 1997; Gregg et al., 2013). After about one week, parasites will infect all distal organs, including the lungs, heart, spleen, and brain (Dubey, 1997; Dubey et al., 2012; Dubey et al., 1997). T. gondii rapidly appears within secondary lymphoid organs and the blood and spleens of infected mice (Courret et al., 2006; Dubey, 1997; Dubey et al., 2012; Dubey et al., 1997; Konradt et al., 2016), suggesting that hematogenous and lymphatic spread are both plausible routes for systemic dissemination. The intrinsic gliding motility of extracellular T. gondii (Sibley, 2010) and shuttling of intracellular parasites within migratory host cells likely both contribute to systemic dissemination.
Leukocyte migratory modes
Leukocytes are migratory immune cells and they respond both to environmental signals and the substratum to navigate their journeys. The migration of leukocytes was initially believed to universally depend upon adhesion mediated by transmembrane integrin proteins. However, more recent studies have conclusively shown that leukocytes can also migrate using a lowly-adhesive, entirely integrin-independent mode (Lammermann et al., 2008; Lämmermann and Germain, 2014). Both integrin-dependent and -independent migration are well-suited to promote T. gondii dissemination at various stages of infection.
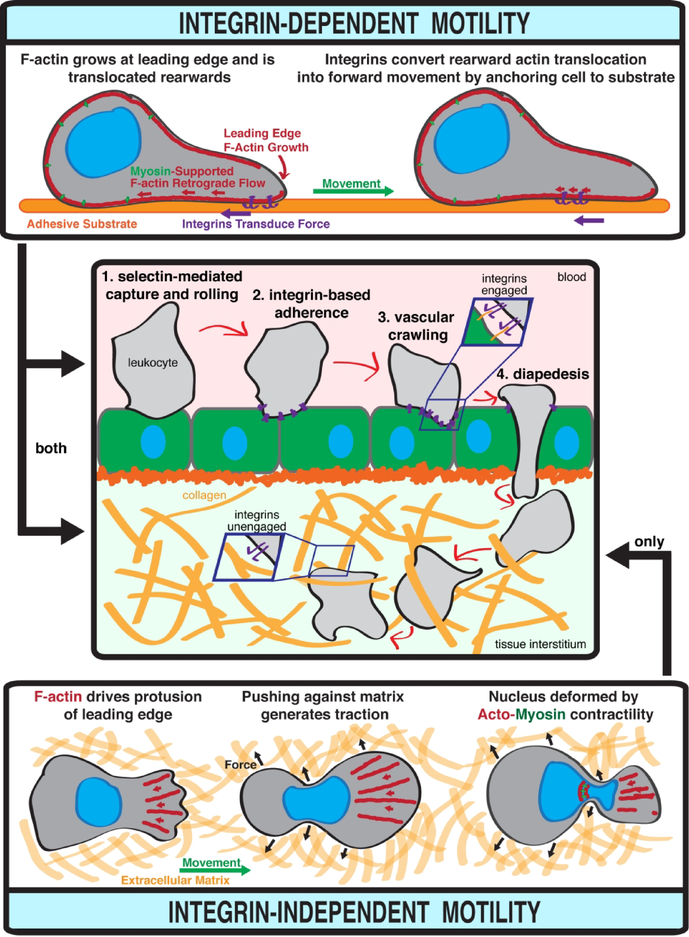
Integrin-dependent leukocyte migration was elucidated by studies that extensively used two-dimensional cell culture systems to model the extravasation of blood leukocytes through endothelium and into tissues (Figure 2). During this process, circulating leukocytes are captured onto endothelial surfaces when glycosylated transmembrane leukocyte adhesins including PSGL-1 and CD44 interact with the endothelial ligands P- and E-selectin (Kansas, 1996; Ley et al., 2007). This interaction is strengthened by shear stress imposed by blood flow (Marshall et al., 2003) and leads to leukocyte rolling over the endothelium. Leukocyte rolling is slowed and eventually arrested when activated leukocyte integrins engage with endothelial ligands such as ICAM-1 and VCAM-1 (Berlin et al., 1995; Chan et al., 2001; Ley et al., 2007). Arrested leukocytes then crawl over endothelial vasculature. Endothelial crawling presumably functions to identify preferred sites for endothelial traversal, which is termed diapedesis. Diapedesis occurs via both transcellular and paracellular routes (Ley et al., 2007) (Figure 2).
Figure 2. Leukocyte migration.
Integrin-dependent motility enables transendothelial migration of leukocytes from the blood into tissues, and interstitial migration through tissues. Integrin-independent motility only functions in confined three-dimensional spaces such as tissue interstitium.
During the transendothelial migration (TEM) cascade, integrins fulfill two critical functions: 1) mediating the firm adherence required for leukocytes to maintain endothelial engagement in the presence of blood flow, and 2) serving as essential force transducers during crawling and endothelial traversal (Calderwood and Ginsberg, 2003) (Figure 2). The force that produces leukocyte crawling and endothelial traversal is primarily generated by the concerted action of actin polymerization at the leading edge of migrating leukocytes and myosin II contractility. Actin polymerization drives forward protrusion of the cell membrane, leading to rearward treadmilling of actin filaments. Myosin acts further back in the cell, pulling the cell cortex rearwards (Renkawitz and Sixt, 2010). The cytoskeletal protein talin serves as a universal adaptor that connects the actin cytoskeleton to transmembrane integrins engaged with external ligands (Calderwood and Ginsberg, 2003). This linkage of the leukocyte cytoskeleton to external ligands via integrins and talin is critical for converting the retrograde cytoskeletal forces into forward movement (Renkawitz and Sixt, 2010).
Contrasting the absolute requirement for integrin-mediated adhesion and force transduction for successful migration over and through endothelial vasculature, leukocytes can migrate independently of integrin functionality in confined three-dimensional spaces such as tissue interstitium. The idea that leukocytes could migrate independently of integrins was initially very controversial. However, the feasibility of integrin-independent leukocyte migration was elegantly shown by murine studies that demonstrated that genetic ablation of either the universal integrin adaptor talin or all relevant integrins heterodimers in dendritic cells (DCs) did not cause any defects in DC interstitial migration in vivo (Lammermann et al., 2008). Integrin-independent migration is not restricted to DCs, as further work has provided similar evidence for neutrophils (Lammermann et al., 2013) and T cells (Woolf et al., 2007).
The mechanistic basis of force generation during integrin-independent migration remains an area of active investigation. Several models have been proposed, including deformation-based movement, membrane flow, and polarized blebbing (Fackler and Grosse, 2008; Paluch et al., 2016; Renkawitz and Sixt, 2010). These models are generally unified in positing that integrin-independent migration can occur when the environment of a migrating cell eliminates the need for stable adhesion and traction to convert forces into movement. The interstitium meets this requirement by confining leukocytes in extracellular matrices so that passive disengagement is not possible (Renkawitz and Sixt, 2010). Lowly-adhesive interstitial migration can be driven solely by actin polymerization or the concerted action of actin polymerization and Rho/ROCK-activated myosin II contractility which assists the cell in squeezing through small pores (Lammermann et al., 2008; Lämmermann and Germain, 2014) (Figure 2).
Integrins are expressed by all mammalian cells except erythrocytes (Hynes, 2002), and integrin ligands are ubiquitously found in essentially every body tissue (Humphries et al., 2006). Accordingly, leukocytes likely deploy both integrin-dependent and -independent migratory strategies during interstitial migration. Intracellular pathogens disseminating via a strategy that relies on the hijacking of migratory host cells could thus target integrin-dependent or - independent strategies to spread through tissues. Conversely, a Trojan horse strategy would require the migrating host cell to perform integrin-dependent TEM. Specifically targeting integrin-independent migration might benefit pathogens by targeting spread to deep tissue or enhancing rates of infected leukocyte entry into collecting lymphatics.
Leukocytes as Trojan horses
Because of the severe clinical consequences of toxoplasmic encephalitis (Montoya and Liesenfeld, 2004), much attention has been paid to the potential for migratory blood leukocytes to deliver T. gondii across the BBB and into the CNS.
Murine models indicate that following oral T. gondii infection, the vast majority of blood leukocytes that harbor parasites are CD11b+ cells (Courret et al., 2006). In mice, CD11b+ blood leukocytes include inflammatory CCR2+ Ly6Chi monocytes and patrolling CCR2− Ly6Clo CX3CR1hi monocytes (Geissmann et al., 2003). Whether T. gondii preferentially infects either subset remains unknown. However, the predominance of CD11b+ cells among parasitized blood leukocytes positions monocytes as the most appealing candidate Trojan horse for delivering T. gondii into the CNS. The best in vivo evidence supporting a role for infected monocytes in promoting T. gondii dissemination is that adoptive transfer of T. gondii-infected monocytes into mice hastens dissemination to the brain, when compared to transfer of extracellular parasites. Notably, this result may simply reflect that intracellular parasites enjoy enhanced protection from immune defenses such as complement attack while in the bloodstream. Alternatively, T. gondii infection might reprogram monocyte motility to promote dissemination. This idea is suggested by a collection of in vitro studies that showed that infection alters the rolling and crawling of monocytes interacting with endothelial monolayers or fibronectin-coated substrates (Cook et al., 2018; Harker et al., 2013; Ueno et al., 2014).
Somewhat perplexingly, these studies reported that infection inhibited integrin-mediated adherence in vitro, yet enhanced endothelial crawling in a manner sensitive to antibody blockade of integrin-ligand pairings (Cook et al., 2018; Harker et al., 2013). A separate study reported that T. gondii infection transiently increases de-adherence of peritoneal macrophages and J774 macrophages to the integrin ligands fibronectin, laminin, and collagen IV (Da Gama et al., 2004). Parasite traversal of the BBB within a monocyte Trojan horse would require completion of integrin-dependent TEM. Accordingly, perturbing integrin-mediated adherence in infected monocytes seems an inefficient strategy to advance dissemination across the BBB. One study reported that T. gondii infection did not significantly decrease monocyte TEM across a human umbilical vein endothelial cell barrier (Ueno et al., 2014), which suggests that inhibited integrin function is eventually overcome. Another study reported increased prevalence of infected CD45+/CD11bc+ cells in a pool of rat PBMCs following TEM across a model BBB, indicating that infection might enhance TEM in this system (Lachenmaier et al., 2011). However, we recently demonstrated that T. gondii infection profoundly decreased monocyte TEM across several in vitro models of peripheral and BBB endothelium (Drewry et al., 2019). Moreover, an in vivo analysis showed that T. gondii-infected monocytes adoptively transferred into mice were abundantly found in brain vasculature but failed to transmigrate across the BBB and into the brain parenchyma (Konradt et al., 2016). The parasitized monocytes found in the blood of T. gondii-infected mice thus do not seem optimally poised to ferry parasites across the BBB.
Hitchhiking with migrating leukocytes
Within hours of ingestion by a host, T. gondii encounters and infects a variety of migratory leukocytes in the intestinal lamina propria. The rapid spread of T. gondii through and out of the lamina propria compartment may be aided by infected leukocytes acting inadvertently as shuttles that carry intracellular parasites throughout the tissue interstitium, and possibly into lymphatic vessels. Travel of infected leukocytes through lymphatics would then deliver intracellular parasites to secondary lymphoid organs (Figure 1B). Although leukocytes are normally thought of as defenders for the host, by inadvertently promoting pathogen dissemination leukocytes are transformed into unwilling accomplices to parasite infection that enable access to new host niches and shield intracellular parasites from engulfment by tissue resident or recruited phagocytes.
The intestinal lamina propria is heavily populated with CX3CR1+ macrophages that are continuously replenished by circulating inflammatory monocytes (Zigmond and Jung, 2013). Hence, following oral infection, T. gondii could target these incoming monocytes or the monocyte-derived CX3CR1+ macrophages to aid spread through and out of the lamina propria. Supporting this model, we recently showed that T. gondii infection enhanced monocyte migration through in vitro collagen matrices that model interstitium, and in vivo interstitial migration of splenic CX3CR1+ monocytes (Drewry et al., 2019). This result contrasts another model where infected neutrophils were theorized to passively promote local parasite spread through the intestinal lamina propria, with infection having no discernable impact on neutrophil motility (Coombes et al., 2013). T. gondii enhancement of monocyte and macrophage migration required the secreted parasite kinase ROP17 and was inhibited by blockade of Rho/ROCK signaling (Drewry et al., 2019). The Rho/ROCK-dependence of infected monocyte migration and corresponding inhibition of TEM suggest that T. gondii specifically activates integrin-independent migration in monocytes and macrophages (Drewry et al., 2019). Infected monocytes carrying hitchhiking parasites could efficiently navigate the confined spaces of tissue interstitium using integrin-independent migration, an appealing model given the disrupted integrin functionality observed in infected monocytes.
Extensive evidence suggests that T. gondii could also exploit infected DCs to promote tissue spread (Bhandage and Barragan, 2019). DCs are antigen presenting cells that primarily reside in peripheral tissues, where they acquire antigens that they subsequently travel to secondary lymphoid tissues to present to T cells (Randolph et al., 2005). As with monocytes, the best evidence for the ability of DCs to promote in vivo dissemination comes from experiments showing that adoptive transfer of T. gondii-infected DCs results in unusually rapid parasite dissemination to the brain (Lambert et al., 2006). T. gondii infection induces an in vitro hypermotility phenotype in DCs that includes enhanced migration across plastic transwell membranes and endothelial monolayers, increased crawling velocity and displacement on two-dimensional surfaces (Lambert et al., 2006), and enhanced migration through three-dimensional collagen matrix (Kanatani et al., 2015). The hypermotility of infected DCs correlates to rapid cytoskeletal changes including integrin redistribution (Weidner et al., 2013), requires GABA receptor signaling to a Cav1.3 voltage-dependent calcium channel (Bhandage and Barragan, 2019; Fuks et al., 2012; Kanatani et al., 2017), and can be induced by heterologous expression of a 14–3-3 protein (Weidner et al., 2016). DCs are not typically found in the blood in large numbers, and thus not surprisingly do not account for a meaningful portion of parasitized leukocytes in the blood during murine T. gondii infections (Courret et al., 2006). Accordingly, the DC hypermotility phenotype is most likely to be relevant to promoting parasite tissue dissemination, rather than direct traversal of endothelial barriers.
DCs do not only travel from tissues to lymph nodes via afferent lymphatics, but also exit lymph nodes via efferent lymphatics at low rates (Randolph et al., 2005). This route leads to the bloodstream via the lymphovenous valve (Randolph et al., 2017) (Figure 1B). As such, an infected leukocyte with robustly activated tissue migration could potentially deliver an intracellular parasite through tissues, into lymphatics, through a lymph node, and eventually into the blood stream (Figure 1). The plausibility of this model is supported by observations that T. gondii-infected DCs traffic to both mesenteric lymph nodes and the spleen in greater abundance than uninfected or LPS-activated DCs following adoptive transfer via intraperitoneal injection (Lambert et al., 2006). Upon entering the blood circulation, T. gondii could then penetrate endothelial barriers such as the BBB by using infected leukocytes as Trojan horses, or egress and directly engage a new niche as an extracellular parasite (Figure 1B).
Translocation through host cell portals
T. gondii traverses multiple biological barriers during natural infections, including the intestinal epithelium, BBB, and potentially the maternal-fetal barrier. T. gondii could penetrate these barriers as extracellular parasites taking a paracellular route between epithelial or endothelial cells, or as intracellular parasites carried within leukocyte Trojan horses trafficking across the barrier (Arora et al., 2017; Barragan and Sibley, 2002; Barragan and Sibley, 2003) (Figure 1B). However, recent studies suggest that direct invasion of BBB endothelial cells followed by expansive growth within this cellular compartment may be critical for successful CNS invasion by T. gondii. In this context, it is important to point out that prior studies that concluded that adoptive transfer of monocytes (Courret et al., 2006) or DCs (Lambert et al., 2006) enhanced infection of the brain did not distinguish between parasites present within leukocytes lodged in the vasculature vs. parasites that had migrated into the parenchyma (either extracellularly or within a host cell).
Notably, T. gondii actively invades into and egresses out of host cells in a process powered by a parasite actin-myosin motor (Drewry and Sibley, 2015; Sibley, 2010). Accordingly, T. gondii translocation into and through BBB endothelial cells would probably not be mediated by internalization driven by the host cell cytoskeleton as is observed with bacterial translocation through intestinal M cells (Vazquez-Torres and Fang, 2000). Instead, T. gondii may actively invade a barrier cell, replicate within that cell, and subsequently egress out into the brain parenchyma. A BBB invasion and replication model is supported by a study reporting that oral infection of mice with tissue cysts led to detection of replicated parasites within brain vasculature endothelial cells, which was followed by detection of parasite cysts in parenchyma regions near vasculature (Konradt et al., 2016). Extracellular parasites were detected in the blood in roughly equivalent quantity as cell-associated parasites (Konradt et al., 2016), which further supports the idea that extracellular T. gondii in the blood could seed CNS infections by directly invading BBB cells. A recent study assessing mice intraperitoneally challenged with T. gondii reported that parasite invasion of the brain, but not lung or spleen, was restricted by endothelial expression of a dominant negative EGFR expected to protect parasites from autophagy-mediated killing (Corcino et al., 2019). Intraperitoneal infection is not a physiological infection route for T. gondii and may prompt dissemination to progress in a different manner than in a natural infection initiated by oral ingestion of parasite cysts. However, if EGFR-mediated protection of parasites in BBB endothelial cells and enhanced brain burden also occurs following oral cyst inoculation, this would further support the idea that parasite development within BBB endothelial cells is a critical stage in successful parasite invasion of the CNS.
T. gondii may reach portals into new tissues such as lymphatic vessels or the BBB endothelium by migrating while extracellular using a substrate-dependent behavior termed gliding (Sibley, 2010). Alternatively, T. gondii could also use infected leukocytes to rapidly travel along standard leukocyte trafficking routes. This model would allow T. gondii to exploit hijacked migrating leukocytes as both shuttles for dissemination and shields against other host defenses, such as engulfment by activated phagocytes or complement attack in the blood. Upon reaching a target tissue, intracellular parasites could then egress from their host leukocyte and directly invade target endothelial cells (Figure 1B). It is unclear how parasites would optimize the timing of egress from migrating host leukocytes to occur in desirable locations. However, a recent study did report that adherence of infected leukocytes to endothelium triggered parasite egress from the host leukocytes (Baba et al., 2017).
Conclusions and future directions
The impressive ability of T. gondii to spread beyond its initial infection nidus in the gut and reach all host tissues including the CNS poses substantial risk to infected hosts. As part of this process, T. gondii infection clearly alters the migration of monocytes and DCs in vitro, which could function to promote tissue dissemination and barrier traversal by co-opting leukocyte migratory capacity. Early investigations into the potential for migrating leukocytes to promote T. gondii dissemination speculated that the parasite would exploit leukocytes as Trojan horses across biological barriers such as the BBB (Courret et al., 2006). DCs are not optimal candidates to fill this role, as infected DCs are exceedingly rare in the blood during murine infections (Courret et al., 2006). Blood monocytes are more frequently infected and hence better positioned to ferry parasites across the BBB. However, T. gondii infection profoundly impairs the ability of monocytes to transmigrate through in vitro endothelial barriers (Drewry et al., 2019). The in vivo relevance of this inhibited TEM phenotype is supported by reports that infected monocytes adoptively transferred into mice failed to cross into the brain parenchyma (Konradt et al., 2016). Accordingly, we consider T. gondii unlikely to exploit infected leukocytes as true Trojan horses across the BBB. Instead, we suggest that T. gondii is more likely to act as a hitchhiker that rides through tissues within infected leukocytes. Migrating infected leukocytes could carry parasites through the lymphatic system and eventually into the blood circulation, where parasites likely egress out of the leukocyte and confront barriers such as the BBB as extracellular parasites.
Future studies should establish more precisely how and when infected leukocytes promote T. gondii dissemination during natural infections initiated by oral ingestion of parasite cysts. Monocytes and DCs can clearly promote parasite dissemination when introduced into naïve mice via adoptive transfer (Courret et al., 2006; Lambert et al., 2006), but more work is needed to establish the steps where leukocyte migration aids dissemination during natural oral infections. Discriminating between parasites entering the vascular circulation of the CNS vs. crossing into the parenchyma will also be crucial. Recent studies reveal that T. gondii infection specifically upregulates interstitial migration of monocytes; however, the mechanism by which this occurs is only partially understood (Drewry et al., 2019). This pathway depends on the secreted parasite kinase ROP17 and host Rho signaling. Further studies are needed to define the substrates of ROP17 that drive this response. Intriguingly, very recent data indicate that ROP17 mediates translocation of several dense granule effector proteins across the parasitophorous vacuole membrane (Panas et al., 2019). Accordingly, ROP17 may promote enhanced migration by directly phosphorylating a host target, such as several known phospho-activated RhoGEFs (Hodge and Ridley, 2016), or by mediating the secretion of another parasite effector. In mice subcutaneously challenged with a hypervirulent type I T. gondii strain, ROP17-deficient parasites exhibited delayed dissemination kinetics that corresponded with a moderate enhancement of mouse survival (Drewry et al., 2019). Intriguingly, ROP17 deletion in the related type II T. gondii strain Pru has been shown to almost completely abrogate formation of brain cysts (Fox et al., 2016). Type II T. gondii also exhibit less robust extracellular motility than type I parasites (Barragan and Sibley, 2002). Accordingly, it is appealing to speculate that ROP17-dependent enhanced interstitial migration in infected monocytes and macrophages may be especially pivotal for advancing the dissemination of lowly-motile type II parasites. Monocytes and DCs may also be differentially exploited by T. gondii for dissemination purposes, as infection enhances integrin-dependent TEM in DCs (Lambert et al., 2006) but potently blocks TEM in monocytes (Drewry et al., 2019). Determining whether monocytes or DCs advance parasite dissemination at specific stages, or if particular subsets of monocytes or DCs are especially targeted by disseminating T. gondii remain key questions for future study.
Acknowledgements
We are grateful to members of Sibley Lab, Mark Miller, Gwen Randolph, and Robyn Klein for fruitful conversations. Work in the Sibley Lab is supported by grants from the National Institutes of Health to LDS (AI034036). LLD was partially supported by an NSF GRFP Fellowship (DGE1143954). The authors declare there is no conflict of interest.
References
- Arora N, Sadovsky Y, Dermody TS, and Coyne CB. 2017. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 21:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Batanova T, Kitoh K, and Takashima Y. 2017. Adhesion of Toxoplasma gondii tachyzoite-infected vehicle leukocytes to capillary endothelial cells triggers timely parasite egression. Sci Rep 7:5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan A, and Sibley LD. 2002. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. The Journal of experimental medicine 195:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan A, and Sibley LD. 2003. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol 11:426–430. [DOI] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, and Butcher EC. 1995. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80:413–422. [DOI] [PubMed] [Google Scholar]
- Bhandage AK, and Barragan A. 2019. Calling in the CaValry—Toxoplasma gondii Hijacks GABAergic Signaling and Voltage-Dependent Calcium Channel Signaling for Trojan horse-Mediated Dissemination. Frontiers in Cellular and Infection Microbiology 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, and Ginsberg MH. 2003. Talin forges the links between integrins and actin. Nat Cell Biol 5:694–697. [DOI] [PubMed] [Google Scholar]
- Chan JR, Hyduk SJ, and Cybulsky MI. 2001. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. The Journal of experimental medicine 193:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JH, Ueno N, and Lodoen MB. 2018. Toxoplasma gondii disrupts beta1 integrin signaling and focal adhesion formation during monocyte hypermotility. J Biol Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Charsar BA, Han SJ, Halkias J, Chan SW, Koshy AA, Striepen B, and Robey EA. 2013. Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc Natl Acad Sci U S A 110:E1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcino YL, Portillo JC, and Subauste CS. 2019. Epidermal growth factor receptor promotes cerebral and retinal invasion by Toxoplasma gondii. Sci Rep 9:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, and Tardieux I. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Gama LM, Ribeiro-Gomes FL, Guimaraes U Jr., and Arnholdt AC. 2004. Reduction in adhesiveness to extracellular matrix components, modulation of adhesion molecules and in vivo migration of murine macrophages infected with Toxoplasma gondii. Microbes Infect 6:1287–1296. [DOI] [PubMed] [Google Scholar]
- Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, and Sibley LD. 2019. The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nature Microbiology In press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry LL, and Sibley LD. 2015. Toxoplasma Actin Is Required for Efficient Host Cell Invasion. mBio 6:e00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP 1997. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J Eukaryot Microbiol 44:592–602. [DOI] [PubMed] [Google Scholar]
- Dubey JP 2007. Toxoplasma Gondii. Toxoplasma Gondii 1–III. [Google Scholar]
- Dubey JP, Ferreira LR, Martins J, and McLeod R. 2012. Oral oocyst-induced mouse model of toxoplasmosis: effect of infection with Toxoplasma gondii strains of different genotypes, dose, and mouse strains (transgenic, out-bred, in-bred) on pathogenesis and mortality. Parasitology 139:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, and Speer CA. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 11:267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Shen SK, Kwok OC, and Blixt JA. 1997. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J Parasitol 83:870–882. [PubMed] [Google Scholar]
- Fackler OT, and Grosse R. 2008. Cell motility through plasma membrane blebbing. The Journal of cell biology 181:879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Rommereim LM, Guevara RB, Falla A, Hortua Triana MA, Sun Y, and Bzik DJ. 2016. The Toxoplasma gondii Rhoptry Kinome Is Essential for Chronic Infection. mBio 7:e00193–00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks JM, Arrighi RBG, Weidner JM, Kumar Mendu S, Jin Z, Wallin RPA, Rethi B, Birnir B, and Barragan A. 2012. GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii. PLoS pathogens 8:e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, and Littman DR. 2003. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 19:71–82. [DOI] [PubMed] [Google Scholar]
- Gregg B, Taylor BC, John B, Tait-Wojno ED, Girgis NM, Miller N, Wagage S, Roos DS, and Hunter CA. 2013. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect Immun 81:1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KS, Ueno N, Wang T, Bonhomme C, Liu W, and Lodoen MB. 2013. Toxoplasma gondii modulates the dynamics of human monocyte adhesion to vascular endothelium under fluidic shear stress. Journal of leukocyte biology 93:789–800. [DOI] [PubMed] [Google Scholar]
- Hill D, and Dubey JP. 2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clinical Microbiology and Infection 8:634–640. [DOI] [PubMed] [Google Scholar]
- Hodge RG, and Ridley AJ. 2016. Regulating Rho GTPases and their regulators. Nature Reviews Molecular Cell Biology 17:496–510. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, and Humphries MJ. 2006. Integrin ligands at a glance. J Cell Sci 119:3901–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687. [DOI] [PubMed] [Google Scholar]
- Kanatani S, Fuks JM, Olafsson EB, Westermark L, Chambers B, Varas-Godoy M, Uhlen P, and Barragan A. 2017. Voltage-dependent calcium channel signaling mediates GABAA receptor-induced migratory activation of dendritic cells infected by Toxoplasma gondii. PLOS Pathogens 13:e1006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani S, Uhlen P, and Barragan A. 2015. Infection by Toxoplasma gondii Induces Amoeboid-Like Migration of Dendritic Cells in a Three-Dimensional Collagen Matrix. PLoS ONE 10:e0139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS 1996. Selectins and their ligands: current concepts and controversies. Blood 88:3259–3287. [PubMed] [Google Scholar]
- Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, and Hunter CA. 2016. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nature Microbiology 1:16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenmaier SM, Deli MA, Meissner M, and Liesenfeld O. 2011. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. Journal of neuroimmunology 232:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Hitziger N, Dellacasa I, Svensson M, and Barragan A. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cellular microbiology 8:1611–1623. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, and Germain RN. 2013. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, and Sixt M. 2008. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453:51–55. [DOI] [PubMed] [Google Scholar]
- Lämmermann T, and Germain RN. 2014. The multiple faces of leukocyte interstitial migration. Seminars in immunopathology 36:227–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, and Nourshargh S. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. Immunology 7:678–689. [DOI] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, and Zhu C. 2003. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423:190–193. [DOI] [PubMed] [Google Scholar]
- Montoya JG, and Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. [DOI] [PubMed] [Google Scholar]
- Paluch EK, Aspalter IM, and Sixt M. 2016. Focal Adhesion-Independent Cell Migration. Annu Rev Cell Dev Biol 32:469–490. [DOI] [PubMed] [Google Scholar]
- Panas MW, Ferrel A, Naor A, Tenborg E, Lorenzi HA, and Boothroyd J. 2019. Translocation of dense granule effectors across the parasitophorous vacuole membrane in Toxoplasma-infected cells requires the activity of ROP17, a rhoptry protien kinase. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, and Swartz MA. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature reviews. Immunology 5:617–628. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Ivanov S, Zinselmeyer BH, and Scallan JP. 2017. The Lymphatic System: Integral Roles in Immunity. Annu Rev Immunol 35:31–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz J, and Sixt M. 2010. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO reports 11:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado FH, and Doering TL. 2017. False friends: Phagocytes as Trojan horses in microbial brain infections. PLoS Pathog 13:e1006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD 2010. How apicomplexan parasites move in and out of cells. Curr Opin Biotechnol 21:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N, Harker KS, Clarke EV, McWhorter FY, Liu WF, Tenner AJ, and Lodoen MB. 2014. Real-time imaging of Toxoplasma-infected human monocytes under fluidic shear stress reveals rapid translocation of intracellular parasites across endothelial barriers. Cellular microbiology 16:580–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N, and Lodoen MB. 2015. From the blood to the brain: avenues of eukaryotic pathogen dissemination to the central nervous system. Curr Opin Microbiol 26:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, and Fang FC. 2000. Cellular routes of invasion by enteropathogens. Curr Opin Microbiol 3:54–59. [DOI] [PubMed] [Google Scholar]
- Weidner JM, Kanatani S, Hernández-Castañeda MA, Fuks JM, Rethi B, Wallin RPA, and Barragan A. 2013. Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cellular microbiology 15:1735–1752. [DOI] [PubMed] [Google Scholar]
- Weidner JM, Kanatani S, Uchtenhagen H, Varas-Godoy M, Schulte T, Engelberg K, Gubbels MJ, Sun HS, Harrison RE, Achour A, and Barragan A. 2016. Migratory activation of parasitized dendritic cells by the protozoan Toxoplasma gondii 14–3-3 protein. Cellular microbiology 18:1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, and Alon R. 2007. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nature Immunology 8:1076–1085. [DOI] [PubMed] [Google Scholar]
- Zigmond E, and Jung S. 2013. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol 34:162–168. [DOI] [PubMed] [Google Scholar]