Abstract
Background
Learning and memory are impaired in schizophrenia. Some theories have proposed that one form of memory, habituation, is particularly impaired. Preliminary evidence suggests memory impairment is associated with failed hippocampal habituation in chronic schizophrenia patients. Here, we studied how abnormal habituation of the hippocampus is related to relational memory deficits in the early stage of psychosis.
Methods
We measured hippocampal activity in 62 patients with early psychosis and 70 healthy individuals using fMRI. Habituation was defined as the slope of fMRI signal change to repeated presentations of faces and objects. Relational memory ability was measured as the slope of preferential viewing during a face-scene pair eye movement task outside the scanner.
Results
Early psychosis patients showed impaired relational memory (p < 0.001) and less hippocampal habituation to objects (p = 0.01) than healthy control subjects. In the healthy control group, better relational memory was associated with faster anterior hippocampal habituation (faces, r = −0.28, p = 0.03). In contrast, early psychosis patients showed no brain-behavior relationship (r = 0.12, p = 0.40).
Conclusions
We found evidence for disrupted hippocampal habituation in the early stage of psychosis, along with an altered association between hippocampal habituation and relational memory ability. These results suggest neural habituation may provide a novel target for early cognitive interventions in psychosis.
Keywords: novelty, first episode, schizophrenia, relational memory, hippocampus, visual cortex
INTRODUCTION
Repetition is one of the most familiar memory tools. Repetition is often intentional (e.g., memorizing words by repeating them), but also shapes memory through automatic processes. As information is repeatedly encountered, a stimulus-specific reduction in neural activity—i.e., habituation—can be measured at both the behavioral and neural level (1–3). Habituation is a fundamental, highly conserved process through which repeated information is incorporated into memory (1; 4–8). Sustained processing, in contrast, may reflect continued attempts to incorporate information (9) or the continued processing of repeated information as if it were novel. Despite its ubiquity across the nervous system, substantial individual differences in habituation exist as early as infancy (10) and have been hypothesized to contribute to psychopathology (11).
Habituation deficits have long been observed in schizophrenia. As early as the 1960’s (12), numerous behavioral and electrophysiological studies have pointed to persistent habituation deficits in chronic schizophrenia patients (13–20). Early habituation studies hypothesized habituation deficits may underlie cognitive deficits in schizophrenia (12). One of the largest cognitive deficits is memory dysfunction (21–23); however, few studies have investigated whether habituation deficits are related to memory dysfunction in schizophrenia. Holt and colleagues (24) initially identified a deficit in hippocampal habituation to repeated faces in chronic schizophrenia patients. A recent study replicated and extended these findings, showing hippocampal habituation deficits were sustained and associated with poorer memory scores (25).
Memory impairments in schizophrenia are present early in the illness (22; 26–28), progress with illness duration (22; 28–30) and are strong predictors of functional outcome (31–33). Particularly striking are selective deficits in relational memory (34), a type of memory linked to hippocampal impairments (30; 35; 36). Although convergent evidence indicates habituation deficits exist in chronic schizophrenia, we do not know at what phase of illness they occur and whether they are linked to hippocampal-dependent relational memory performance.
Here we examined habituation and relational memory in the early stage of psychosis. Habituation was studied with functional magnetic resonance imaging (fMRI) signal change during passive viewing of faces and objects, while relational memory was studied with a face-scene viewing task. We defined habituation as the slope of fMRI signal change, and relational memory as the slope of change in preferential viewing of the matching face-scene pair (Figure 1). We hypothesized that neural habituation deficits exist in the early stage of psychosis, consistent with findings of existing memory deficits at the time of diagnosis (27; 28; 37). Because early hippocampal pathology is localized to the anterior hippocampus, we also hypothesized that habituation deficits are specific to the anterior hippocampus.
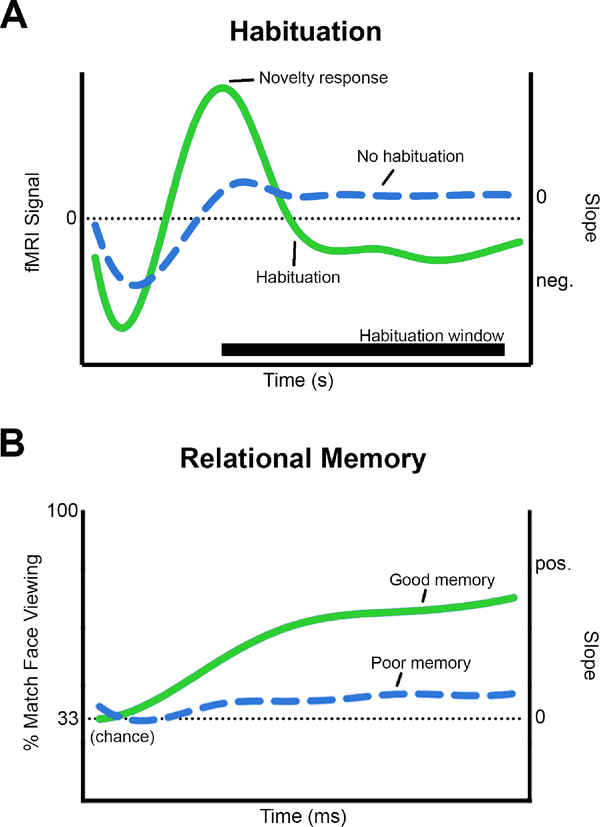
Figure 1.
We studied habituation and relational memory slopes (i.e., change over time). A. Habituation of the fMRI signal. The negative slope of the fMRI signal, during an a priori defined window of observation, was defined as habituation (green line). A sustained fMRI signal indicated impaired habituation (blue line). To accurately characterize habituation independent of novelty response differences, fMRI habituation slopes were adjusted for individual novelty response (b’). B. Preferential viewing of faces. The positive slope of preferential viewing (of a face previously paired with a background scene) was defined as relational memory (green line). A slope of zero indicated no preferential viewing and was defined as poor relational memory (blue line).
METHODS AND MATERIAL
Participants
We studied 62 patients in the early stage of psychosis, who were defined as: patients with a non-affective psychotic disorder (schizophreniform disorder (n=43), schizophrenia (n=17), and schizoaffective disorder (n=2)) within the first 2 years following their index episode of psychosis (Table 1). To specifically target early pathology (38), the majority of patients were recruited during the initial months of illness (i.e., the average duration of psychosis was 7 months, ranging from less than 1 month to no more than 24 months). More than 80% of the patients were in the first episode of psychosis (i.e., meeting criteria A for schizophrenia at the time of study inclusion), and half of the sample were studied after their first hospitalization for psychosis. On average, patients reported prodromal symptoms for 1.5 years. Patients were recruited from the inpatient units and outpatient clinics of the Vanderbilt Psychiatric Hospital. More than 80% of the patient sample were treated with antipsychotic medication at the time of the study.
Table 1.
Participant characteristics.
| fMRI sample | Relational memory sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic | Healthy Control | Early Psychosis | Statistic | df | p | Healthy Control | Early Psychosis | Statistic | df | p |
| Age, years | 22 ± 2.9 | 22 ± 3.4 | 0.18 | 130 | 0.86 | 22 ± 2.9 | 22 ± 3.4 | 0.46 | 112 | 0.65 |
| Sex (% male) | 73% | 79% | 0.68 | 1 | 0.41 | 73% | 78% | 0.30 | 1 | 0.58 |
| Race (white/black/other) | 54/12/4 | 49/12/1 | 1.56 | 2 | 0.46 | 46/11/3 | 44/9/1 | 0.93 | 2 | 0.63 |
| Handedness (% right) | 91% | 97% | 1.54 | 1 | 0.22 | 90% | 96% | 1.59 | 1 | 0.21 |
| Participant education, years | 14.6 ± 1.9 | 13.6 ± 2.2 | 2.86 | 128 | 0.005* | 14.6 ± 1.9 | 13.6 ± 2.3 | 2.66 | 110 | 0.009* |
| Parental education, years | 15.0 ± 2.4 | 15.7 ± 2.6 | −1.59 | 127 | 0.11 | 14.8 ± 2.3 | 15.8 ± 2.7 | −1.36 | 109 | 0.23 |
| IQ, WTAR | 112 ± 10.8 | 105 ± 14.6 | 3.15 | 128 | 0.002* | 112 ± 10.5 | 105 ± 14.9 | 2.74 | 110 | 0.007* |
| Clinical | Mean | Range | Mean | Range | ||||||
| Ham-D | 11.5 ± 8.5 | 0 – 39 | 11.6 ± 8.7 | 0 – 39 | ||||||
| YMRS | 2.6 ± 4.7 | 0 – 20 | 2.7 ± 5.0 | 0 – 20 | ||||||
| PANSS – total | 68.0 ± 19.4 | 32 – 114 | 67.5 ± 20.5 | 32 – 114 | ||||||
| PANSS – positive | 17.9 ± 7.8 | 7 – 37 | 17.4 ± 7.9 | 7 – 37 | ||||||
| PANSS – negative | 17.0 ± 7.1 | 7 – 36 | 17.0 ± 7.4 | 7 – 36 | ||||||
| PANSS – general | 33.0 ± 8.8 | 16 – 59 | 33.1 ± 9.4 | 16 – 59 | ||||||
| CPZ | 299 ± 198 | 0 – 900 | 294 ± 203 | 0 – 900 | ||||||
| Duration of illness, months | 7.4 ± 5.8 | 0.9 – 24 | 7.3 ± 5.9 | 0.9 – 24 | ||||||
| Duration of prodrome, months | 18 ± 20.0 | 0 – 77 | 17 ± 18.3 | 0 – 77 | ||||||
| First episode, % | 84% | - | 83% | - | ||||||
| First hospitalization, % | 50% | - | 50% | - | ||||||
| Antipsychotic treatment | 84% | - | 82% | - | ||||||
Patients with early psychosis were compared to a group of 70 healthy control participants recruited from the surrounding community. All participants were assessed by a trained rater using the Structured Clinical Interview for the DSM-IV (SCID I-P) (39), and diagnoses were confirmed by a senior clinician (S.H.). Patients with early psychosis were assessed for current mood, anxiety, psychotic symptom severity, and intellectual function (see Supplementary Methods). There were no significant between-group differences in age, sex, race, handedness, or years of parental education (Table 1).
Exclusion criteria were age less than 16 or greater than 35, a history of significant head injury, major medical (i.e., HIV, cancer) or neurological illness, any contraindication for MRI scanning (e.g., pregnancy, metal implants, claustrophobia), current alcohol or substance abuse within the past month, estimated IQ < 75, and uncorrected vision deficits. Healthy control subjects were excluded for history of major mood or psychotic disorders, a first-degree relative with a psychotic illness, current substance abuse or dependence, or current psychotropic medication use.
This study was conducted in accordance with the Vanderbilt Human Research Protection Program and all participants provided written informed consent prior to study procedures. Participants received financial compensation for their time.
Experimental paradigm
Repetition task
Patients with early psychosis and healthy control participants completed a task designed to assess habituation to faces and objects. Participants viewed four 2-minute runs of a repeated neutral face (run 1 & 2) and a repeated neutral object (run 3 & 4). Images were presented for 500 ms followed by a 500 ms black screen. To promote and assess attention, a target detection task was included. Targets were small versions of the face or object images (25% of original size (40)) presented on 10% of trials. No targets were presented during the first 10 s of stimulus repetitions. Subjects were asked to press a button during each small image presentation. Target detection was high across both groups (detection mean > 93%; ps > 0.62; see Supplementary Results).
Relational memory
Outside the scanner, eye movements were recorded to assess memory performance during a face-scene pair task (41; 42) (Supplementary Figure 1). During training, participants viewed 36 face-scene pairs, with each pair presented once per block over three training blocks. Participants were instructed to memorize face-scene pairs, as they would be tested on the pairings later. During testing, participants were shown three previously-seen faces superimposed over a previously-seen background scene for 10 s. All faces and backgrounds presented during testing were equally familiar. During Match trials, one of the three overlaid faces (Match face) had been previously paired with the background scene. During Non-Match trials, none of the three overlaid faces (Non-Match faces) had been previously paired with the background scene. Participants were instructed to try to remember which of the three faces had been paired with the background scene during training and to focus their eyes on it as quickly as possible, or to keep their eyes focused on the computer screen if no matching face was detected. Fixation durations for each element (three faces + background) were summed in 250 ms time bins (e.g., 0–250, 250–500, 500–750 ms, ... 1750–2000) and averaged across test trials. Because hippocampally-driven preferential viewing occurs rapidly (43), viewing was examined over the first 2 s of each trial. Relational memory performance was characterized as the change in the proportion of time spent viewing the Match face (slope). Slopes were corrected for between-subject differences in initial eye position (first 250 ms) following face presentation (44; 45). For further task details see Supplementary Methods. Sixty healthy control subjects and 54 patients with early psychosis completed the relational memory task.
Imaging data acquisition and processing
Imaging data were collected on a 3T Philips Intera Achieva magnetic resonance imaging (MRI) scanner located in the Vanderbilt University Institute for Imaging Science. A high-resolution T1-weighted fast field echo (FFE) structural scan and four 2.5-minute functional echo planar images (EPI) were acquired for each subject (see Supplementary Methods for details). Functional data were processed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and assessed for motion (46) and outliers (ART; Neuroimaging Informatics Tools and Resources Clearinghouse (NITRC)). Motion and outliers were similar between early psychosis and healthy control groups (ps > 0.16). Small images, outliers, and motion (rotation, translation, mean displacement) were entered into the first-level general linear model (GLM; 47) as regressors of no interest.
Data analysis
Regions of interest (ROIs)
Our goal was to test for hippocampal differences in early psychosis patients. Subject-specific ROIs were created using in-house automated multiatlas segmentation techniques (48; 49). Hippocampal multiatlas segmentations were manually divided into an anterior and posterior mask at the uncal apex (50; 51) by a trained rater (S.A.). To determine if an expanded network of regions implicated in the early course of psychotic illness (52–54) also showed habituation differences, we conducted secondary exploratory analyses in the striatum (caudate and putamen), visual cortex (calcarine cortex and occipital pole), fusiform face area (FFA), medial temporal lobe cortex (entorhinal cortex and parahippocampal gyrus (MTLC)), ventromedial prefrontal cortex (vmPFC), and amygdala. The FFA was defined in each subject using a localizer task (55) (Supplementary Figure 2; Supplementary Methods).
Habituation
Habituation was defined across two timescales: habituation to the stimulus visual properties (rapid); and habituation to stimulus category (slow). For rapid habituation (Figure 1), the habituation window was defined as the peak signal magnitude following the initial presentation of a stimulus and the volume in which the mean residual signal returned to baseline in the healthy control group. Healthy control signal peaked 8 s following stimulus onset, and returned to baseline by 18 s following stimulus onset, resulting in a 10 s habituation window. This window is consistent with prior fMRI studies of habituation in healthy subjects (56); additionally, this window did not include any small image presentations to minimize the effects of intervening images on habituation results (2).
To analyze slow changes in signal related to stimulus category, 10 s time bins (n = 12) were modeled using the first-level GLM, and residuals from the first-level GLM were entered into a second-level analysis. Novelty response was defined as signal in the first 10 s time bin, and habituation was defined as the slope of change in signal from the 1st to the 12th time bin for each run.
For each analysis, average timeseries for each ROI was extracted from participants’ residual data using MarsBar (57). Residual time courses were plotted for each subject and time course calculations were conducted using Matlab R2017b (The MathWorks Inc., Natick, MA). Signal in the left and right hemispheres were highly correlated across regions; to increase statistical power and minimize type I error, data were averaged across hemispheres.
Habituation slope
Habituation is highly dependent on novelty response—there is more opportunity for signal to attenuate over time if signal is initially high. Because we were interested in examining differences in rate of habituation independent of differences in novelty response, we calculated a normalized habituation slope (b′), corrected for novelty response differences, for each participant (44; 45; 58). Novelty response was defined as the peak signal magnitude following the initial presentation of a stimulus in the healthy control group (Figure 1). Habituation slope (b′) values were calculated for each participant using linear regression analysis (see Supplementary Methods for details). Adjusted habituation slopes were calculated separately for faces and objects.
Habituation over consecutive runs
Because faces were presented prior to objects, we explored effects of time on habituation differences by examining habituation over the course of the experiment (see Supplementary Methods and Results).
Statistical analysis
Linear mixed effects models tested for group differences in neural response to stimuli with stimulus type (face, object), region (anterior, posterior hippocampus), and group as fixed factors, and participant as a random factor. For consistency with prior analyses (25) the first run of each condition was used for novelty and habituation analyses, as neural response is minimal following the initial habituation run (Figure 2). Regional habituation greater than zero and novelty response greater than baseline was examined using one-sample t-tests (p = 0.05). Between-group differences in relational memory were tested by analysis of variance (ANOVA). Spearman correlations tested for associations between novelty response or habituation and relational memory function (p = .05). Spearman correlations test for monotonic rather than linear relationships between variables. Correlation coefficients were compared using z-scores (59). Statistical analyses were performed using SAS software v9.4 (SAS Institute Inc., Cary, NC).
Figure 2.
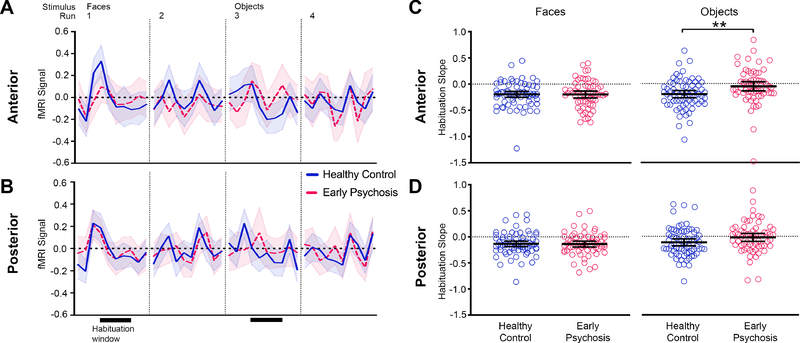
Hippocampal habituation to repeated faces and objects. Mean fMRI signal for the first 20 s of stimulus repetitions in each run is shown for the anterior (A) and posterior (B) hippocampus. Shaded area indicates the 95% confidence interval. Patients with early psychosis (red) and healthy control subjects (blue) show an initial novelty response to visual stimuli followed by a decrease in mean fMRI signal with stimulus repetition. Habituation slopes were calculated for a 10 s habituation analysis window, indicated by black bars below the fMRI signal (runs 1, 3). Slopes were corrected for differences in initial novelty response (b′). Dot plots of habituation slopes (b′) are shown for the anterior (C) and posterior (D) hippocampus. Black bars indicate mean habituation slope ± 95% confidence interval. Healthy control subjects had larger negative b′ slope values to repeated objects compared to early psychosis patients, indicating greater habituation of fMRI signal. Asterisks indicate between-group comparisons significance for habituation slopes. ** p = 0.01.
RESULTS
Novelty response
We first tested for novelty responses in the hippocampus. All hippocampal regions showed a novelty response greater than baseline for both faces and objects (Supplementary Results). Novelty responses were similar across groups (no main effect of group, p = 0.34).
Habituation
Rapid habituation
We examined the ability of the hippocampus to rapidly habituate over a 10 s window following the peak novelty response. Participants showed evidence for habituation to both faces and objects across hippocampal regions (one-sample t-test, ps ≤ 0.002, Supplementary Table 2, Supplementary Results). Habituation was faster for faces than objects (main effect of stimulus type, F 1,130 = 11.38, p = 0.001) and for anterior hippocampus compared to posterior hippocampus (main effect of region, F 1,130 = 8.54, p = 0.004). Overall, healthy control subjects showed greater habituation than early psychosis patients across hippocampal regions (main effect of group, F 1,130 = 3.77, p = 0.05; no group by region interaction, p = 0.18). However, groups differed in their habituation to faces and objects (group by stimulus type interaction, F 1,130 = 9.38, p = 0.003). Posthoc linear mixed analyses of faces and objects separately showed that group effects were driven by differences in habituation to objects—habituation to objects was greater in healthy control subjects compared to early psychosis patients (object, main effect of group, F 1,130 = 6.48, p = 0.01), while habituation to faces was similar across groups (face, main effect of group, F 1,130 = 0.01, p = 0.94).
Habituation over consecutive runs
To explore whether stimulus type effects may be related to presentation order (faces before objects), we tested for linear changes in habituation rate across the four consecutive runs. Hippocampal habituation rate decreased over consecutive runs (main effect of time, F 3,390 = 19.37, p < 0.001; no time by region interaction, p = 0.18), with early psychosis patients showing less habituation over time than healthy control subjects (group by time interaction, F 3,390 = 4.02, p = 0.006). Posthoc analysis of variance revealed the group by time effect was driven specifically by greater habituation in the healthy control group to the initial objects run (run 3) compared to early psychosis patients (F 1,130 = 6.10, p = 0.02, Supplementary Figure 3), while habituation was similar between groups across the remaining runs (ps ≥ 0.83).
Slow habituation
We next examined habituation over the full two minutes of stimulus exposure. Hippocampal habituation slopes were not significantly different from zero (Supplementary Table 2, Supplementary Results), indicating no habituation over two minutes. There was also little evidence for a hippocampal novelty response in the first time bin (Supplementary Table 1), suggesting a floor effect when averaging signal over 10 s time bins. Habituation did not differ by stimulus type, region, or group (ps ≥ 0.35).
Potential moderators
Hippocampal habituation in early psychosis patients was not predicted by medication status (chlorpromazine equivalent units), current psychosis symptoms, or state anxiety (ps ≥ 0.12, see Supplementary Results for further details).
Memory correlations
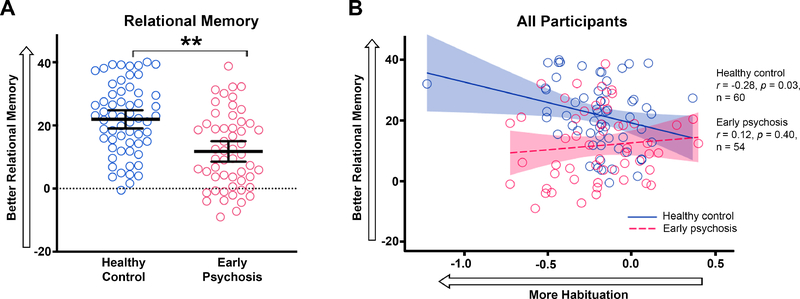
Healthy control subjects were better at identifying face-scene relational pairs than early psychosis patients (F 1,113 = 22.31, p < 0.001; Figure 3). In healthy control subjects, greater anterior hippocampal habituation to faces was correlated with better relational memory (r = −0.28, p = 0.03; Figure 3). Removal of an outlier healthy control subject did not significantly decrease the correlation (r = −0.25, p = 0.05). In contrast, early psychosis patients did not show the normal association between anterior hippocampal habituation and relational memory (r = 0.12, p = 0.40; significant between-group difference in correlational coefficients, z = −2.11, p = 0.03). Relational memory was not correlated with posterior hippocampal habituation or habituation to objects in either group (ps ≥ 0.09). Novelty response was not correlated with relational memory (ps > 0.18).
Figure 3.
Relational memory and habituation in the anterior hippocampus. A. The slope of preferential viewing of the correct face-scene pairing is shown for patients with early psychosis(red) and healthy control subjects (blue). Black bars indicate mean relational memory slope ± 95% confidence interval. Faster preferential viewing (positive slopes) indicate better relational memory. Patients with early psychosis had worse relational memory for trained face-scene pairs compared to healthy control subjects. B. In healthy control subjects, greater habituation in the anterior hippocampus was correlated with better relational memory performance. There was no relationship between habituation and relational memory in early psychosis patients. Asterisks indicate between-group comparisons significance for habituation slopes. * p ≤ 0.01.
Exploratory analysis
To determine whether habituation deficits in early psychosis were specific to the hippocampus, or rather, representative of neural processing deficits across a broader set of brain regions, we examined neural response in a set of brain regions commonly implicated in schizophrenia, including the striatum, visual cortex, FFA, MTLC, vmPFC, and amygdala. Exploratory regions significantly differed in their pattern of habituation and were analyzed separately (main effect of region, F 8,1031 = 37.31, p < 0.001; region by group interaction, F 8,1031 = 2.91, p = 0.003; Supplementary Results). Over the first 10 s of stimulus exposures, healthy control subjects showed greater habituation than early psychosis patients in two regions—the occipital pole and FFA (occipital pole, F 1,130 = 12.21, p < 0.001; FFA, F 1,130 = 6.44, p = 0.01). For both regions, group patterns were similar for faces and objects (no group by stimulus type interaction, ps ≥ 0.24). Habituation to faces in the occipital pole tended to be associated with better relational memory in healthy control subjects (r = −0.25, trend p = 0.06), but not early psychosis patients (r = 0.04, p = 0.76; Supplementary Figure 4).
DISCUSSION
We examined hippocampal habituation, or decrease in response, and its behavioral correlates in a group of early psychosis patients. We found two notable differences when comparing patients with healthy control subjects. First, early psychosis patients had less hippocampal habituation in response to repeated objects relative to healthy control subjects. Second, despite similar hippocampal habituation to faces across groups, the rate of face habituation was associated with differential behavioral performance on a relational memory task between groups. In healthy control subjects, greater anterior hippocampal habituation to faces was associated with better relational memory. In contrast, in early psychosis patients, there was no relationship between anterior hippocampal habituation to faces and relational memory performance. Brain-behavior associations were specific to the anterior hippocampus, consistent with evidence of preferential pathology in anterior hippocampus during the early phase of schizophrenia (60–62), and were not associated with current positive, negative, or general clinical symptoms, suggesting a trait-like relationship. Together, these findings suggest reduced habituation in the anterior hippocampus is associated with relational memory deficits in the early stage of psychosis.
Early psychosis patients showed failed habituation to repeated objects, but not faces. During an initial block of repeated faces, groups habituated similarly; however, in a later block of repeated objects, healthy control subjects continued to show the expected pattern of rapid hippocampal habituation (63–65) while early psychosis patients showed a sustained hippocampal response characteristic of failed habituation. One explanation may be that early disruptions in hippocampal processing are specific to non-social, but not social, information. However, a consistent literature has shown deficits in social cognition in schizophrenia (66; 67), making this explanation less likely. Alternatively, habituation deficits may be related to task order. For consistency with our previous studies (25), faces were always presented first. When modeling changes in the rate of habituation across the four consecutive runs, we found that early psychosis patients did not show the same pattern as healthy control subjects over time. Habituation rate is exquisitely sensitive to time and interval effects, making this explanation plausible. Habituation deficits in early psychosis patients may be associated with an inability to sustain rapid habituation over time and/or following changes in stimuli. However, further studies stringently controlling for time and stimulus type are needed.
To determine whether hippocampal habituation was associated with behavior, we examined the relationship between habituation patterns and performance on hippocampal-based relational memory task (68). Hippocampal habituation to faces was associated with better relational memory in healthy control subjects, but not early psychosis patients, suggesting a disrupted brain-behavior relationship. These findings are consistent with our prior findings of a disrupted relationship between habituation to faces and memory ability in chronic schizophrenia patients (25). We now extend prior findings to show that disruptions can be detected early in the illness at a younger age as deficits in object processing and are specific to the anterior hippocampus.
Deficits in hippocampal function are among the most consistent and replicable findings in schizophrenia. Hippocampal neurobiology is altered at the earliest stages of illness (52) with strong evidence for an imbalance in inhibitory/excitatory signaling originating in the anterior CA1 region during early illness (62; 69). In a previous study we found that chronic schizophrenia patients also show deficient hippocampal habituation to faces, and that hippocampal habituation to faces was associated with memory ability (25). Here, we extend these findings to show a specific relationship between anterior hippocampal habituation to faces and relational memory, a form of memory dependent on hippocampal integrity. In contrast, novelty response was not associated with relational memory, suggesting habituation may be a selective marker of hippocampal pathology.
We conducted an exploratory analysis to determine whether habituation deficits were specific to the hippocampus, or rather, reflected a broader neural processing deficit in patients. Of the nine additional regions investigated, two regions—the fusiform face area and occipital pole—also showed habituation deficits to both faces and objects in early psychosis patients. The occipital pole showed a similar brain-behavior relationship across groups as the anterior hippocampus. Because the hippocampus has strong reciprocal connections with the visual cortex, findings could result from either feedforward or feedback mechanisms. However, our data do not support a widespread feedforward effect originating with the hippocampus or visual cortex, as we did not find habituation deficits in other brain regions with strong hippocampal and visual processing connections (e.g., amygdala, parahippocampal cortex).
Although habituation is a simple form of learning (70–72), ubiquitous across the nervous system, and highly conserved across species (73; 74), little is known about the underlying cellular and molecular processes (73; 75–77). An influential early model proposed that, as a stimulus is repeated, feedback inhibition promotes strong inhibition of continued novelty response, thus yielding habituation of neural response and a memory trace (78). The hippocampus may be uniquely organized to facilitate this type of response—the human hippocampus shows a strong novelty response (79–81) and strong interneuron-mediated suppression of firing to repeated information (82–84). In schizophrenia, there is compelling evidence for interneuron dysfunction in the hippocampus (85). Habituation differences may also result from interactions across neural systems (71). Schizophrenia has been described as a disorder of widespread connectivity deficits (89; 90), with evidence for degraded facial visual processing (86) and altered salience system activity (63; 87; 88), which may converge to increase hippocampal novelty response. Our current findings do not support altered salience interactions, as we did not find fMRI differences in the striatum. However, we do find habituation deficits in the ventral visual system, consistent with prior findings in chronic patients (25). Future work should investigate connectivity of hippocampal connections to disentangle within-hippocampus deficits from broader network dysfunction.
There are a variety of factors that influence habituation, including attention, anxiety, arousal, and saccadic suppression (3; 45; 91–93). It is unlikely our current findings are due to differences in attention, as target detection performance was high and did not differ between groups. Similarly, measures of trait anxiety were not correlated with habituation. Because the experiment did not include a measure of arousal at the time of the scan, it is possible that arousal during the scanning session differed between groups. However, we did not detect habituation differences in the striatum (94), rendering arousal differences less plausible. Eye movements during the habituation task may also alter habituation, as visual processing is suppressed during saccades (93); future studies should monitor eye movements during scanning.
Memory impairments in schizophrenia are substantial (95), progress with illness duration (28), and are strong predictors of functional outcome (31–33). We previously found that hippocampal habituation was disrupted in chronic schizophrenia and associated with memory impairment (25). Here we find that habituation is also disrupted in patients with early psychosis and associated with relational memory ability. Together, these findings further our understanding of the pathophysiology of schizophrenia and suggest that habituation may be useful as a marker of neurocognitive function and illness progression, although longitudinal studies are necessary.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Charlotte and Donald Test Fund, NIMH grant R01-MH70560 (Dr. Heckers), the Vanderbilt Psychiatric Genotype/Phenotype Project, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH).
Footnotes
FINANCIAL DISCLOSURES
The authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thompson RF, Spencer WA (1966): Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 73: 16–43. [DOI] [PubMed] [Google Scholar]
- 2.Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, et al. (2009): Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 92: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groves PM, Thompson RF (1970): Habituation: a dual-process theory. Psychol Rev. 77: 419–450. [DOI] [PubMed] [Google Scholar]
- 4.Pinsker H, Kupfermann I, Castellucci V, Kandel E (1970): Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 167: 1740–2. [DOI] [PubMed] [Google Scholar]
- 5.Turk-Browne NB, Yi DJ, Chun MM (2006): Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron. 49: 917–927. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ (2004): Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 42: 980–989. [DOI] [PubMed] [Google Scholar]
- 7.Schacter DL, Buckner RL (1998): Priming and the brain. Neuron. 20: 185–95. [DOI] [PubMed] [Google Scholar]
- 8.Wiggs CL, Martin A (1998): Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 8: 227–233. [DOI] [PubMed] [Google Scholar]
- 9.Turk-Browne NB, Scholl BJ, Chun MM (2008): Babies and brains: habituation in infant cognition and functional neuroimaging. Front Hum Neurosci. 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCall RB, Carriger MS (1993): A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev. 64: 57–79. [PubMed] [Google Scholar]
- 11.McDiarmid TA, Bernardos AC, Rankin CH (2017): Habituation is altered in neuropsychiatric disorders—A comprehensive review with recommendations for experimental design and analysis. Neurosci Biobehav Rev. 80: 286–305. [DOI] [PubMed] [Google Scholar]
- 12.Shakow D (1963): Psychological deficit in schizophrenia. Behav Sci. 8: 275–305. [DOI] [PubMed] [Google Scholar]
- 13.Freedman R (2010): The Madness Within Us, 1st ed. New York: Oxford University Press. [Google Scholar]
- 14.Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, et al. (1996): Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry. 53: 1114–21. [DOI] [PubMed] [Google Scholar]
- 15.Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M (1994): Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry. 36: 670–9. [DOI] [PubMed] [Google Scholar]
- 16.Braff DL, Swerdlow NR, Geyer MA (1995): Gating and habituation deficits in the schizophrenia disorders. Clin Neurosci. 3: 131–9. [PubMed] [Google Scholar]
- 17.Horvath T, Meares R (1979): The sensory filter in schizophrenia: a study of habituation, arousal, and the dopamine hypothesis. Br J Psychiatry. 134: 39–45. [DOI] [PubMed] [Google Scholar]
- 18.Geyer MA, Braff DL (1982): Habituation of the Blink reflex in normals and schizophrenic patients. Psychophysiology. 19: 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Bolino F, Manna V, Di Cicco L, Di Michele V, Daneluzzo E, Rossi A, Casacchia M (1992): Startle reflex habituation in functional psychoses: a controlled study. Neurosci Lett. 145: 126–8. [DOI] [PubMed] [Google Scholar]
- 20.Olincy A, Braff DL, Adler LE, Cadenhead KS, Calkins ME, Dobie DJ, et al. (2010): Inhibition of the P50 cerebral evoked response to repeated auditory stimuli: results from the Consortium on Genetics of Schizophrenia. Schizophr Res. 119: 175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo JY, Ragland JD, Carter CS (2019): Memory and cognition in schizophrenia. Mol Psychiatry. 24: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ (2009): Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology. 23: 315–336. [DOI] [PubMed] [Google Scholar]
- 23.Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. (1991): Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 48: 618–24. [DOI] [PubMed] [Google Scholar]
- 24.Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, et al. (2005): Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 57: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 25.Williams LE, Blackford JU, Luksik A, Gauthier I, Heckers S (2013): Reduced habituation in patients with schizophrenia. Schizophr Res. 151: 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. (2012): Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 69: 562–71. [DOI] [PubMed] [Google Scholar]
- 27.Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN, et al. (2007): Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 33: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong K, Avery S, Blackford JU, Woodward N, Heckers S (2018): Impaired associative inference in the early stage of psychosis. Schizophr Res. 202: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams LE, Avery SN, Woolard AA, Heckers S (2012): Intact relational memory and normal hippocampal structure in the early stage of psychosis. Biol Psychiatry. 71: 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ongür D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S (2006): The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 63: 356–65. [DOI] [PubMed] [Google Scholar]
- 31.Green MF (1996): What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 153: 321–330. [DOI] [PubMed] [Google Scholar]
- 32.Green MF, Kern RS, Heaton RK (2004): Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr Res. 72: 41–51. [DOI] [PubMed] [Google Scholar]
- 33.Lepage M, Bodnar M, Bowie CR (2014): Neurocognition: Clinical and functional outcomes in schizophrenia. Can J Psychiatry. 59: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ragland JD, Ranganath C, Harms MP, Barch DM, Gold JM, Layher E, et al. (2015): Functional and Neuroanatomic Specificity of Episodic Memory Dysfunction in Schizophrenia: A Functional Magnetic Resonance Imaging Study of the Relational and Item-Specific Encoding Task. JAMA psychiatry. 72: 909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D (2004): Hippocampal activation during transitive inference in humans. Hippocampus. 14: 153–162. [DOI] [PubMed] [Google Scholar]
- 36.Zalesak M, Heckers S (2009): The role of the hippocampus in transitive inference. Psychiatry Res. 172: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartholomeusz CF, Proffitt TM, Savage G, Simpson L, Markulev C, Kerr M, et al. (2011): Relational memory in first episode psychosis: Implications for progressive hippocampal dysfunction after illness onset. Aust N Z J Psychiatry. 45: 206–213. [DOI] [PubMed] [Google Scholar]
- 38.Newton R, Rouleau A, Nylander A-G, Loze J-Y, Resemann HK, Steeves S, Crespo-Facorro B (2018): Diverse definitions of the early course of schizophrenia—a targeted literature review. npj Schizophr. 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First M, Spitzer RL, Gibbon M, Williams J (2002): Structured Clinical Interview for the DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). [Google Scholar]
- 40.Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T (2008): Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci. 11: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams LE, Must A, Avery S, Woolard A, Woodward ND, Cohen NJ, Heckers S (2010): Eyemovement behavior reveals relational memory impairment in schizophrenia. Biol Psychiatry. 68: 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheffield JM, Williams LE, Cohen N, Heckers S (2012): Relational memory in psychotic bipolar disorder. Bipolar Disord. 14: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannula DE, Ranganath C (2009): The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 63: 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montagu JD (1963): Habituation of the psycho-galvanic reflex during serial tests. J Psychosom Res. 52: 199–214. [DOI] [PubMed] [Google Scholar]
- 45.Avery SN, Blackford JU (2016): Slow to warm up: the role of habituation in social fear. Soc Cogn Affect Neurosci. 11: 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodward ND, Karbasforoushan H, Heckers S (2012): Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 169: 1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 2: 189–210. [Google Scholar]
- 48.Asman AJ, Landman BA (2011): Characterizing spatially varying performance to improve multi-atlas multi-label segmentation. Inf Process Med Imaging. 22: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asman AJ, Dagley AS, Landman BA (2014): Statistical label fusion with hierarchical performance models. Proc SPIE--the Int Soc Opt Eng. 9034: 90341E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L (2013): Long-axis specialization of the human hippocampus. Trends Cogn Sci. 17: 230–240. [DOI] [PubMed] [Google Scholar]
- 51.Strange B a, Witter MP, Lein ES, Moser EI (2014): Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 15: 655–669. [DOI] [PubMed] [Google Scholar]
- 52.Pantelis C, Yücel M, Bora E, Fornito A, Testa R, Brewer WJ, et al. (2009): Neurobiological markers of illness onset in psychosis and schizophrenia: The search for a moving target. Neuropsychol Rev. 19: 385–398. [DOI] [PubMed] [Google Scholar]
- 53.Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, Fusar-Poli P (2012): Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 36: 2325–2333. [DOI] [PubMed] [Google Scholar]
- 54.Dandash O, Pantelis C, Fornito A (2017): Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophr Res. 180: 48–57. [DOI] [PubMed] [Google Scholar]
- 55.Wong YK, Gauthier I (2010): A multimodal neural network recruited by expertise with musical notation. J Cogn Neurosci. 22: 695–713. [DOI] [PubMed] [Google Scholar]
- 56.Grill-Spector K, Henson R, Martin A (2006): Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn Sci. 10: 14–23. [DOI] [PubMed] [Google Scholar]
- 57.Brett M, Anton J-LL, Valabregue R, Poline J-B (2002): Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan Neuroimage. 16: abstract 497. [Google Scholar]
- 58.Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, et al. (2014): Amygdala habituation: a reliable fMRI phenotype. Neuroimage. 103: 383–90. [DOI] [PubMed] [Google Scholar]
- 59.Preacher K (2002): Calculation for the test of the differences between two independent correlation coefficients. Retrieved from http://quantpsy.org.
- 60.Sauras R, Keymer A, Alonso-Solis A, Díaz A, Molins C, Nuñez F, et al. (2017): Volumetric and morphological characteristics of the hippocampus are associated with progression to schizophrenia in patients with first-episode psychosis. Eur Psychiatry. 45: 1–5. [DOI] [PubMed] [Google Scholar]
- 61.Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, et al. (2017): Progressive Decline in Hippocampal CA1 Volume in Individuals at Ultra-High-Risk for Psychosis Who Do Not Remit: Findings from the Longitudinal Youth at Risk Study. Neuropsychopharmacology. 42: 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heckers S, Konradi C (2002): Hippocampal neurons in schizophrenia. J Neural Transm. 109: 891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murty VP, Ballard IC, Macduffie KE, Krebs RM, Adcock RA (2013): Hippocampal networks habituate as novelty accumulates. Learn Mem. 20: 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee ACH, Scahill VL, Graham KS (2008): Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb Cortex. 18: 683–696. [DOI] [PubMed] [Google Scholar]
- 65.Pedreira C, Mormann F, Kraskov A, Cerf M, Fried I, Koch C, Quiroga RQ (2010): Responses of human medial temporal lobe neurons are modulated by stimulus repetition. J Neurophysiol. 103: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF (2013): Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 170: 334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green MF, Horan WP, Lee J (2015): Social cognition in schizophrenia. Nat Rev Neurosci. 16: 620–631. [DOI] [PubMed] [Google Scholar]
- 68.Hannula DE, Ryan JD, Tranel D, Cohen NJ (2007): Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J Cogn Neurosci. 19: 1690–1705. [DOI] [PubMed] [Google Scholar]
- 69.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. (2013): Imaging Patients with Psychosis and a Mouse Model Establishes a Spreading Pattern of Hippocampal Dysfunction and Implicates Glutamate as a Driver. Neuron. 78: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pilz PKD, Arnold SW, Rischawy AT, Plappert CF (2014): Longterm-habituation of the startle response in mice is stimulus modality, but not context specific. Front Integr Neurosci. 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson RF (2009): Habituation: A history. Neurobiol Learn Mem. 92: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson DA, Linster C (2008): Neurobiology of a simple memory. J Neurophysiol. 100: 2–7. [DOI] [PubMed] [Google Scholar]
- 73.Giles AC, Rankin CH (2009): Behavioral and genetic characterization of habituation using Caenorhabditis elegans. Neurobiol Learn Mem. 92: 139–46. [DOI] [PubMed] [Google Scholar]
- 74.Schmid S, Wilson DA, Rankin CH (2015): Habituation mechanisms and their importance for cognitive function. Front Integr Neurosci. 8: 419–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glanzman DL (2011): Olfactory habituation: fresh insights from flies. Proc Natl Acad Sci U S A. 108: 14711–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glanzman DL (2009): Habituation in Aplysia: The Cheshire Cat of neurobiology. Neurobiol Learn Mem. 92: 147–154. [DOI] [PubMed] [Google Scholar]
- 77.Ramaswami M (2014): Network plasticity in adaptive filtering and behavioral habituation. Neuron. 82: 1216–1229. [DOI] [PubMed] [Google Scholar]
- 78.Sokolov EN (1963): Higher nervous functions; the orienting reflex. Annu Rev Physiol. 25: 545–580. [DOI] [PubMed] [Google Scholar]
- 79.Fried I, MacDonald KA, Wilson CL (1997): Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 18: 753–765. [DOI] [PubMed] [Google Scholar]
- 80.Kreiman G, Fried I, Koch C (2002): Single-neuron correlates of subjective vision in the human medial temporal lobe. Proc Natl Acad Sci. 99: 8378–8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kreiman G, Koch C, Fried I (2000): Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 3: 946–53. [DOI] [PubMed] [Google Scholar]
- 82.Rainer G, Miller EK (2000): Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron. 27: 179–189. [DOI] [PubMed] [Google Scholar]
- 83.Wilson FAW, Rolls ET (1993): The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Exp Brain Res. 93: 367–382. [DOI] [PubMed] [Google Scholar]
- 84.Arriaga M, Han EB (2017): Dedicated Hippocampal Inhibitory Networks for Locomotion and Immobility. J Neurosci. 37: 9222–9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heckers S, Konradi C (2015): GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 167: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Norton D, Ongur D, Heckers S (2008): Inefficient face detection in schizophrenia. Schizophr Bull. 34: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barkus C, Sanderson DJ, Rawlins JNP, Walton ME, Harrison PJ, Bannerman DM (2014): What causes aberrant salience in schizophrenia? A role for impaired short-term habituation and the GRIA1 (GluA1) AMPA receptor subunit. Mol Psychiatry. 19: 1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapur S (2003): Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 160: 13–23. [DOI] [PubMed] [Google Scholar]
- 89.McGlashan TH, Hoffman RE (2000): Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 57: 637–648. [DOI] [PubMed] [Google Scholar]
- 90.Avery SN, Rogers BP, Heckers S (2018): Hippocampal Network Modularity Is Associated With Relational Memory Dysfunction in Schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 3: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patacchioli FR, Taglialatela G, Angelucci L, Cerbone A, Sadile AG (1989): Adrenocorticoid receptor binding in the rat hippocampus: strain-dependent covariations with arousal and habituation to novelty. Behav Brain Res. 33: 287–300. [DOI] [PubMed] [Google Scholar]
- 92.Leussis MP, Bolivar VJ (2006): Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 30: 1045–64. [DOI] [PubMed] [Google Scholar]
- 93.Berman RA, Cavanaugh J, McAlonan K, Wurtz RH (2017): Neural Circuits: A circuit for saccadic suppression in the primate brain. J Neurophysiol. 117: 1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazarus M, Chen J-F, Urade Y, Huang Z-L (2013): Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol. 23: 780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aleman A, Hijman R, de Haan EHF, Kahn RS (1999): Memory impairment in schizophrenia: A meta-analysis. Am J Psychiatry. 156: 1358–1366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.