Abstract
Objective:
The potential long-term effects of childhood fluoxetine therapy on brain serotonin systems were studied using a nonhuman primate model, the rhesus monkey.
Method:
Juvenile male rhesus (1-4 years old, corresponding to 4-11 years of age in children) were treated orally with fluoxetine (2 mg/kg) or vehicle daily for two years and removed from treatment during the third year. Each treatment group was assigned an equal number of subjects with low and high transcription polymorphisms of the monoamine oxidase A (MAOA) gene. One year after discontinuation of treatment, positron emission tomography (PET) scans were conducted (N=8 treated, 8 control) using [11C]DASB to quantify serotonin transporter (SERT) in 16 cortical and subcortical regions.
Results:
Fluoxetine treated monkeys with the MAOA low transcription-polymorphism had significantly lower [11C]DASB binding potentials than controls. This finding was seen throughout the brain but was strongest in prefrontal and cingulate cortices. The MAOA*fluoxetine interaction was enhanced by binding potentials that were nonsignificantly higher in monkeys with the high transcription polymorphism.
Conclusions:
Juvenile fluoxetine treatment has residual post-treatment effects on brain SERT that depend on MAOA genotype. MAOA genotype may be important to consider when treating children with fluoxetine.
Keywords: PET, serotonin transporter, rhesus monkey, juvenile, fluoxetine, MAOA polymorphism
Introduction:
When psychoactive drugs are used in children, there is concern about possible effects on brain development and subsequent consequences for later brain function. The Selective Serotonin Reuptake Inhibitors (SSRIs) are among the drugs increasingly prescribed for children (1). Because serotonin plays many roles in brain development (2, 3) SSRI exposure of the immature brain at any stage of brain development could alter the developmental trajectory resulting in altered brain morphology, circuitry and function. A number of basic research studies have demonstrated residual effects on brain and behavior when SSRIs are administered prenatally, before weaning or during adolescence to rodents (4, 5). Additionally, there has been a surge in research on SSRI effects on offspring when SSRIs are used in human pregnancy (6, 7). However, residual effects of SSRI administration during postnatal development prior to puberty have been less studied.
Of interest to SSRI therapy in childhood is a study design which administers SSRIs chronically during the juvenile stage of brain development (after infancy, before puberty), terminates drug treatment, and then evaluates brain function in the young subjects for residual effects. Unfortunately, there is no literature with this design in children, although research is ongoing (8). While this study design has not been implemented in rodents, there are some relevant studies with juvenile/adolescent treatment and adult evaluation of serotonergic systems. Using a therapeutically relevant dose of fluoxetine (5 mg/kg) in juvenile rats, changes found after discontinuation of treatment included increased extracellular serotonin in prefrontal cortex in response to fluoxetine challenge (9), increased serotonin transporter (SERT) binding in frontal cortex detected with 3H paroxetine (10), and increased SERT binding in prefrontal cortex by [123I]beta-CIT PET (11). These studies are consistent with SERT changes in cortical areas. In each case, different results were found with dosing of adult rats.
Further information has become available in a nonhuman primate model, the rhesus monkey, using this study design and examining residual effects on SERT expression in brain. Shrestha et al. (12) treated juvenile rhesus with 3 mg/kg/d fluoxetine daily for one year. Eighteen months after dosing ended, shortly after puberty, PET studies of SERT found an increase in SERT binding detected by PET in neocortical regions.
We previously reported behavioral effects in juvenile rhesus treated with 2 mg/kg/d fluoxetine that included effects on sleep, social interaction, emotional response and attention during the dosing, some of which persisted post dosing (13). Importantly, our studies found interactions between fluoxetine and common genetic polymorphisms of the MAOA gene in determining behavior (14). MAOA is the principal catabolic enzyme for serotonin. In humans, MAOA polymorphisms have been found to interact with adverse childhood experiences (15), and with adult fluoxetine treatment (16). However, MAOA interaction with fluoxetine treatment in children has not been studied. Thus MAOA*fluoxetine interactions were included in studies of residual effects on SERT expression. Here, we hypothesized the occurrence of residual effects of juvenile fluoxetine on SERT binding, following on previous rodent and nonhuman primate studies, and also an interaction between MAOA and fluoxetine.
Methods and Materials:
Subjects:
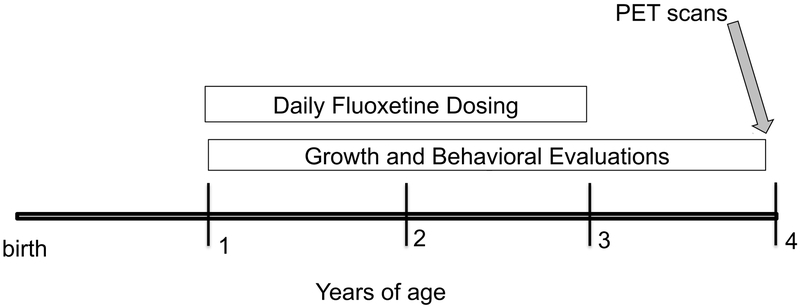
The scans were conducted in 16 male juvenile rhesus monkeys at 4.07±0.02 years of age. The subjects were prepubertal and had participated from one year of age in a study of juvenile fluoxetine effects (13) (see Figure 1 for project timeline). The oral daily fluoxetine treatment (2 mg/kg/day) had been administered from 1 to 3 years of age and discontinued for 12 months prior to PET scans. This fluoxetine dose produces plasma levels of the active agent that are within the range seen in children undergoing treatment and are not influenced by MAOA genotype (see Supplementary Materials). Prior to one year, the monkeys lived in outdoor group housing with their mothers, and during the study they were pair-housed with peers indoors in the same room (14). The subjects remained together under experimental protocols and were treated identically between the termination of the fluoxetine administration and the PET scans.
Figure 1.
Timeline. PET scans were conducted at the conclusion of a multi-year study of fluoxetine. Results from growth and behavioral assessments have been published (14, 52-54).
All procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council. The California National Primate Research Center (CNPRC) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Protocols for this project were approved prior to implementation by the UC Davis Institutional Animal Care and Use Committee.
Sixteen subjects were selected for PET at the end of the study from the larger study population of 32. The two experimental groups (fluoxetine and control, N=8/each) were balanced for MAOA-uVNTR polymorphisms (high and low transcription) at the time of group assignment (N=4 for each subgroup) in order to study MAOA*fluoxetine interactions. This polymorphism had previously been determined in the infants at 3 months of age as part of the CNPRC Biobehavioral Assessment program (BBA) (17). 5HTTLPR polymorphisms were also available from the BBA and selection balanced the experimental groups for 5HTTLPR LL and SL genotypes to prevent possible confounding. Finally, the selection process provided balance of other potential confounding factors including social cage of origin, birth and weaning weight, and mother’s age and parity.
Nonhuman primate studies strive to reduce laboratory animal usage while providing strong causal conclusions in a model of unique relevance to human problems. To accomplish this, we precisely control the independent and dependent variables (i.e. drug treatment and DASB measurement) under highly standardized protocols. In addition, we maintain animals in a controlled environment, in which a wide variety of potential covariates can be measured and carefully examined for potential influences. In this way, small sample nonhuman primate studies can lead to confident causal conclusions and contribute to a better understanding of human problems.
PET scans
PET Scan acquisition protocol:
Subjects were immobilized with ketamine (10 mg/kg i.v.), intubated for isoflurane anesthesia, and catheterized for i.v. fluid and tracer administration. The 90 min scan began 70 min after the initial immobilization, and the [11C]DASB dose was injected 10 sec after scan initiation. Ketamine-scan interval is important as ketamine binds SERT (18). Scans were conducted either in the morning (0830-1100) or afternoon (1230-1500). Technicians were blind to the treatment group of the animals during acquisition and processing of the scans.
Radiochemistry:
[11C]DASB was synthesized with cyclotron-produced [11C]CO2, according to Wilson and coworkers (19) with minor modifications (see Table S1). Sixteen doses of [11C]DASB were produced with >95% radiochemical purity, and a specific activity of 180±90 (mean±SD) GBq/umol at the end of synthesis and 78±32 GBq/umol at injection 21 to 32 minutes later.
PET scanning:
In vivo [11C]DASB PET imaging was performed using a microPET system with a spatial resolution of ~1.8 mm (20). Dynamic PET data were acquired beginning 5 seconds before radiotracer injection using 22 time-frames (6 × 30 s, 5 × 60 s, 6 × 300 s, 5 × 600s). Image reconstruction was carried out with the vendor-provided method (3-dimensional reprojection, or 3DRP) (21).
MRI acquisition protocol:
Magnetic resonance imaging (MRI) data were acquired within 5 weeks of PET scans for selection of regions of interest (ROIs) and co-registration with PET scans. ROI-based analyses were selected to derive the PET kinetic parameters to ensure their stability and reliability. The monkeys were immobilized with ketamine (10 mg/kg i.m.), intubated for isoflurane anesthesia, catheterized for i.v. fluids and positioned in the imaging coil (HD TRknee PA) of the scanner (GE 1.5T Signa HDx 16.0) using foam blocks. Whole brain anatomical images were acquired using a 3D T1-weighted FSPGR sequence (see Table S1 for parameter details).
MRI template registration:
Each MRI image was transformed to a template image previously used by Shrestha et al.(12) and applied to the DASB timeseries. T1 images were rotated and adjusted for inhomogeneity using N3BiasFieldCorrection, and aligned to the initial template image with the ANTS toolkit for registration of images (22), first using a 12-parameter affine transformation, then using a nonlinear registration (cross correlation metric, with weight 1 and radius 2). The transformation model was SyN with a step-size of 0.10. A gaussian regularizer with sigma 3 was applied to the similarity gradient. Up to 100,75,75, and 50 iterations were performed at successive levels of coarseness, with the final level being at full resolution. A whole-brain mask was applied to the template image during registration. Images were manually-inspected to ensure accurate co-registration. Finally, the mean of these images was computed to obtain a study-specific template image on which ROIs could be drawn.
MRI ROI template.
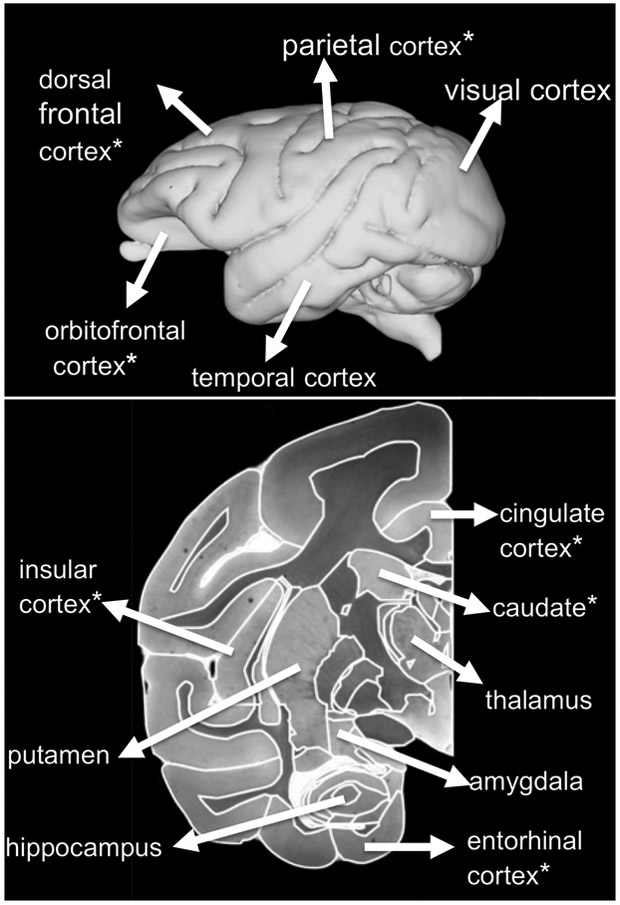
ROIs for target regions were manually traced on the study-specific T1 template image according to the Paxinos atlas (23). Target regions, shown in Table 1 and Figure 1, were selected to provide broad coverage throughout cortical and subcortical structures in the brain.
Table 1.
Binding potentials and ANOVA results for individual Regions of Interest
| Region of Interest | 1.Binding Potentiala |
2.MAOA*flx p valueb |
3.low-MAOA flx effect p valuec |
4.low-MAOA flx effect sized |
|---|---|---|---|---|
| CORTEX | ||||
| Dorsal Frontal Cortex | 0.19 | 0.0032 | 0.0048 | 3.23 |
| Enterorhinal/Piriform Cortex | 0.21 | 0.3461 | 0.6822 | ns |
| Posterior Cingulate/Precuneus | 0.21 | 0.0101 | 0.0107 | 2.51 |
| Visual Cortex | 0.22 | 0.0618 | 0.0594 | ns |
| Parietal Cortex | 0.24 | 0.0101 | 0.0091 | 2.32 |
| Orbital Frontal Cortex | 0.24 | 0.0101 | 0.0107 | 3.64 |
| Cingulate Cortex | 0.26 | 0.0016 | 0.0032 | 3.65 |
| Temporal Cortex | 0.29 | 0.0142 | 0.0294 | 2.51 |
| SUBCORTEX | ||||
| Hippocampus | 0.31 | 0.2430 | 0.2763 | ns |
| Caudate Nucleus | 0.46 | 0.0101 | 0.0107 | 2.62 |
| Amygdala | 0.60 | 0.3185 | 0.2785 | ns |
| Insular Cortex | 0.66 | 0.0130 | 0.0149 | 2.36 |
| Hindbrain/Ventral Brainstem | 0.72 | 0.1093 | 0.1781 | ns |
| Putamen | 0.74 | 0.0236 | 0.0594 | ns |
| Thalamus | 0.92 | 0.0716 | 0.0644 | ns |
| Midbrain/Dorsal Brainstem | 1.26 | 0.0957 | 0.0925 | ns |
ROIs were averaged on left and right side of brain; the mean of left and right averages was then computed
Interaction term from 2-way ANOVA, least squares model, FDR corrected
Pairwise comparison of fluoxetine and control within the low-MAOA subgroup; FDR correction
Cohen’s D effect size for fluoxetine effect within the low-MAOA subgroup; ns=not significant (p value ≤0.01 for significance)
PET analysis:
Dynamic PET data analysis was carried out in the PMOD 3.802 software. First, each subject’s dynamic PET images were co-registered to their T1-weighted MRI scan with a rigid-body transformation based on the mean DASB timeseries using ANTS (22), then transformed to a standard template space based on the warp-field estimated using the T1-weighted MRI image. The ROIs provided in the MRI-based template space were then used for regional analysis of PET data for estimation of serotonin transporter binding. Time activity curves were derived for each region. The binding potential (BP) was then measured with the cerebellum as the reference tissue using both a simple reference tissue model (SRTM), and the Logan reference model (12). The Logan model values, with start time 30 min were selected for statistical analysis.
Statistical analysis:
A hierarchical approach was used for statistical analysis in this small cohort of 16 animals. Potential fluoxetine effects and MAOA*fluoxetine interactions were first examined by 2-way ANOVA for the mean of all ROIs. If significant drug or drug interaction effects were identified, secondary ANOVAs were conducted for each of the ROIs using FDR modified p-values. When significant MAOA*fluoxetine interaction effects were identified, slice analyses were conducted within the gene polymorphism subgroups to identify drug effects. These pairwise post hoc comparisons were also FDR corrected. Additionally, three parameters from the [11C]DASB PET protocol, time of scan (am/pm), activity injected (MBq) and DASB mass injected (nmol/kg), were entered as covariates to ensure that they were not confounders. All ANOVA analyses were conducted in JMP software (SAS Institute) using least squares or general linear models.
ANOVA analysis for 5HTTLPR effects and fluoxetine interactions were also conducted. Because the population contained the LL and SL, but not the SS, genotype, these analyses were considered exploratory.
Results:
Serotonin transporter (SERT) brain distribution
The individual ROIs examined are shown in Figure 2. Mean BP values for individual ROIs are shown in Table 1 (column 1) in ascending order. Values were lowest in neocortex with the higher values in subcortical structures. This pattern of SERT distribution was previously seen in nonhuman primate PET studies (18, 24, 25) as well as human studies using immunohistochemistry (26) and PET (27). The low BPs seen in neocortex (<0.5) have also been demonstrated in human neocortical areas using [11C]DASB (27).
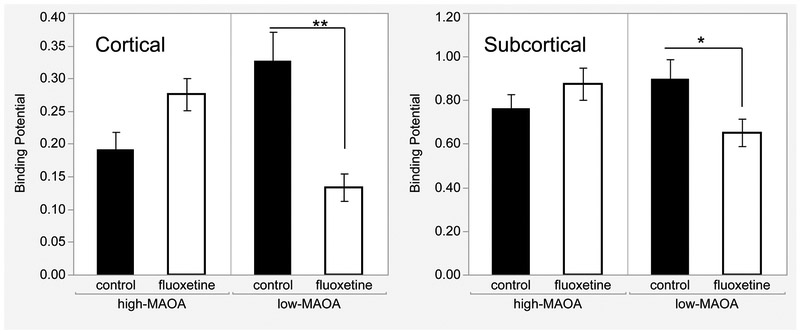
Figure 2.
MAOA*Fluoxetine interaction for Binding Potentials averaged across 9 cortical and 7 subcortical Regions of Interest. Mean and SEM shown, *p<0.05, **p<0.01, post hoc comparison. N=16, fluoxetine and control groups; N=4, MAOA-fluoxetine subgroups.
Prior fluoxetine led to lower SERT binding in subjects with low-MAOA transcription polymorphisms
When we analyzed the effects of early-life fluoxetine on SERT binding in this highly controlled population, there were no main effects of fluoxetine on the mean BP of all 16 ROIs, but the MAOA*fluoxetine interaction was significant (F(1,12)=10.86, p=0.0006) in the two-way ANOVA. The pattern of means contributing to the interaction were consistent across all regions (Figure 2), with BPs lower in than control in fluoxetine-treated subjects with low-MAOA genotypes, and higher than control in fluoxetine-treated subjects with high-MAOA genotypes. Statistical significance of the MAOA*fluoxetine interaction was not influenced when potentially confounding PET variables, i.e. activity injected, DASB mass injected, and time of scan were used as covariates. In secondary slice analyses, there was a fluoxetine effect in the low-MAOA group (F(1,12)=10,p=0.008) but not the high-MAOA group (F(1,12)=3.91,p=2.15,p=0.168).
Looking at individual ROIs, the interaction was significant for 7 of 9 cortical regions (excepting visual and entorhinal), but only 2 of 7 subcortical regions (caudate and putamen) (Table 1, column 2). For the slice post-hoc tests (Table 1, column 3), the fluoxetine effect in the low-MAOA subgroup (lower BPs) was significant for all ROIs showing the interaction, except for putamen.
In general, fluoxetine BPs were consistently higher than those of controls in the high-MAOA subgroup, but not statistically significant (ANOVA for ROI mean, p=.168, effect size D=1.04). For individual ROIs, only the cingulate cortex showed a significant fluoxetine effect in the high-MAOA subgroup (F(1,12)=9.07, p=0.011).
Effect sizes were also computed for the fluoxetine effect in the low-MAOA subgroup (Table 1 column 4). Effect sizes were greatest in prefrontal cortex (dorsal lateral prefrontal, orbitofrontal) and cingulate cortex (anterior), and lower in other cortical areas and the caudate.
In addition, two-way ANOVAs were conducted using fluoxetine and 5HTTLPR polymorphism genotype (SL,LL) as independent variables. No statistically significant main effects or interactions were found overall ROIs or for individual ROIs.
SERT binding potentials correlated with emotional responsiveness assessed post-dosing
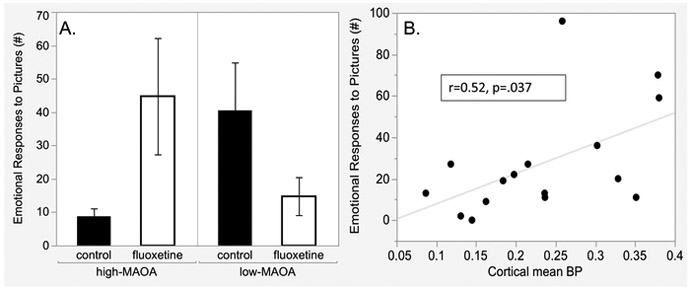
The MAOA*fluoxetine interaction pattern seen in PET data (Figure 3) was also seen in a previous publication (14) reporting behavioral assessment of Emotional Response to Pictures (Figure 3A) (see Supplementary Materials for description of this test). A moderate-size positive correlation (r=0.52, p=0.037) (Figure 3B) was found between the mean of cortical region BPs and the emotional response to pictures accounting for 25 % of the variance.
Figure 3.
Regions of Interest for PET scan registration. Asterisk (*) indicates regions with statistically significant BP MAOA-fluoxetine interactions and significant post-hoc comparisons of fluoxetine and control groups in the low-MAOA subgroup (see Table 1).
Discussion:
Fluoxetine effects on SERT depended on MAOA genotype
The major finding of this study was a decrease in SERT binding potential in low-MAOA genotype subjects previously treated with fluoxetine. The MAOA* fluoxetine interaction was strengthened by a trend toward increased SERT expression in high-MAOA subjects, significant in cingulate cortex. Our ability to detect this gene*drug interaction was enhanced by the all-male cohort because the MAOA gene is X-linked. This study reinforces MAOA as a highly sensitive “adaptability” or “plasticity” gene in primates with transcription polymorphisms (2, 28), and the value of considering MAOA polymorphism genotype as a covariate in studies of fluoxetine in childhood. In particular, the opposite direction of fluoxetine effects in low- and high- MAOA populations could lead to failure to identify effects if MAOA genotype is not taken into consideration.
These results generally support previous finding of Shrestha et al. (12) of long-term changes in SERT expression as detected with [11C]DASB PET after discontinuation of chronic fluoxetine treatment of juvenile rhesus at a therapeutically relevant dose. The fluoxetine effect in the two studies differed in that our effect was found in interaction with MAOA polymorphism genotype, which was not assessed by Shrestha et al. Further Shrestha et al. found increased expression in that cohort, while we found decreased SERT expression in the low-MAOA subgroup, and trend toward increased expression in the high-MAOA group. These two comparisons could be related; if the Shrestha et al. population was skewed toward high-MAOA subjects, the increased expression we saw in our high-MAOA subjects could have determined the direction of the fluoxetine effect. The effect size in our study indicates that statistical significance would be reached with a sample size of N=16 subjects per group with MAOA high expression polymorphisms. However, other comparisons between the studies could also be relevant; earlier age at initiation, longer treatment period, earlier age at assessment in our study. Our results are also consistent with rodent studies that demonstrated long-term changes in serotonin systems after juvenile and adolescent fluoxetine, in particular changes in SERT in cortical areas (9-11, 29, 30).
Of note, exploratory analysis found that 5HTTLPR polymorphisms were not associated with SERT BPs and did not interact with fluoxetine. While conclusions are limited because our sample did not include SS genotype subjects, other studies of young rhesus (18, 31) and adult humans (32-34) have also failed to find associations between 5HTTLPR genotype and SERT expression.
Behavioral relevance of MAOA*fluoxetine effects
The MAOA*fluoxetine effects on SERT were particularly interesting because a similar interaction was observed in prior behavioral testing of this cohort. Specifically, assessments of emotional response to pictures showed fluoxetine effects only in association with MAOA genotype. Emotional response was reduced by fluoxetine in the low-MAOA subgroup, but increased in the high-MAOA subgroup. Other behavioral assessments from the project (sleep, social behavior) also exhibited a MAOA*fluoxetine interaction in addition to fluoxetine main effects (Table S2, Supplementary Materials). Of note, in metabolomic studies of fibroblast samples collected during dosing, 4 metabolites showed a main effect of fluoxetine, but 11 showed MAOA*fluoxetine interaction, and 9 of these showed the same interaction pattern as the BP data reported here (35). The correlation between SERT expression and emotional responses in the post-dosing period seen here strengthens the interpretation that this interaction, seen in imaging and metabolomic endpoints, is relevant to brain function at the behavioral level.
Regional Patterns
The MAOA*fluoxetine interaction was seen with BPs integrated across all 16 brain regions assessed. The pattern of means for the interaction was the same in each individual region but size of mean differences varied. While it is difficult to say that the interaction was region specific, regional patterns did emerge. The interaction was stronger in neocortex and in specific frontal cortex ROIs than in subcortical regions. A possible explanation is relative regional distribution of the two MAO isoforms, MAOA and MAOB. Both isoforms can metabolize serotonin, as well as a variety of other endogenous monoamines, but, in general, MAOA is more highly expressed in cortex (26). MAOB is more prominent subcortical regions and generally expressed in astrocytes in cortex (36). Other possible explanations are distinct subregional serotonergic innervation patterns in some regions including hippocampus (37), and amygdala (38). Differential SSRI access to serotonin transporter across regions (39) is also possible. Density of SERT distribution does not appear to determine the strength of the interaction.
Potential pathways for MAOA*fluoxetine developmental interactions that could result in altered SERT expression.
In one scenario low-MAOA genotype and fluoxetine treatment could jointly elevate brain serotonin levels during treatment; low-MAOA by decreasing degradation of serotonin, and fluoxetine by blocking serotonin uptake. Two rodent models, prenatal MAOI treatment (40), and MAOA knock out mice (41), have shown that elevated cerebral serotonin during brain development is associated with decreased SERT density in adults. Unfortunately we do not know whether the low-MAOA polymorphism is associated with lower MAOA activity and elevated serotonin in juvenile primates; this is not the case in adult humans (37). However, methylation of the MAOA promotor region is associated with variability in MAOA activity (42) and susceptibility to methylation differs between high and low-MAOA polymorphisms (43) thus providing a potential epigenetic link between MAOA genotype, MAOA activity and serotonin elevation. In the case of our study, environmental conditions during development could leave epigenetic marks on the regulatory region of MAOA that would differ between high- and low-MAOA genotypes and subsequently influence serotonin and SERT expression.
A second scenario involves interacting influences on structural brain development. Two rodent studies that demonstrated increased SERT expression after developmental SSRI treatment attributed the effects to altered structural maturation of the serotonin system (10, 11). Fluoxetine is well known for its ability to activate BDNF/TrkB pathways and alter brain structural maturation (41). A role for MAOA and MAOA polymorphisms in structural brain development has also been proposed (44). During juvenile brain development, it could be hypothesized that MAOA and fluoxetine act together on pathways that alter brain connectivity of late developing cortical circuits like the developing frontal-cingulate-striatal circuit (45, 46). Connectivity in this circuit is sensitive to interaction between MAOA genotype and childhood abuse (47) and is sensitive to fluoxetine (48).
Limitations and Significance
Limitations to generalization of the results of our study include the all-male population, the treatment during the juvenile developmental period, age of the post-treatment assessment (limited to one maturational stage), and the specific housing and maintenance conditions for the animals. With regard to the finding of a MAOA*fluoxetine interaction, the MAOA polymorphisms are known to be sensitive to adverse environments. Also, the MAOA gene is X-linked and females have a double allele expression (low/low, high/high, low/high). These two factors could make it unlikely to detect the MAOA*fluoxetine interaction in a group of boys and girls with an array of early environmental experiences. With regard to PET data, the brain regions studied are large. An effect on a subregion of a complex structure like the hippocampus could be diluted in region-wide data. Further, the results of SERT expression studies with [11C]DASB can vary with the PET data processing approach used (49). With regard to sample size, the highly controlled environment needed to detect effects in small samples can be seen as exaggerating effect sizes (50). Finally, despite the value of the nonhuman primate model, translation to human concerns requires careful consideration of species differences. While there are many parallels in the higher processing regions of the brain, there are also differences (51). In spite of these limitations, our demonstration that an animal’s genotype can define how early-life drug treatment results in lasting alterations in receptor binding has far-reaching implications. Pharmacogenetics may be an aspect of precision medicine particularly valuable in assuring the safe and effective use of psychoactive drugs in children. Application of pharmacogenetics to pediatric psychopharmacology should include followup for effects detected after discontinuation of treatment.
Supplementary Material
Figure 4.
A. Emotional response to pictures after discontinuation of fluoxetine treatment. Emotional responses were postures, vocalization and movements elicited by viewing a series of neutral, social and aversive pictures (14). See Supplementary Materials for description of the Picture-elicited Emotional Response test. B. Correlation between emotional responses and cortical region BPs. N=16, fluoxetine and control groups, N=4 MAOA-fluoxetine subgroups. Mean and SEM shown, *p<0.05.
Acknowledgments:
Supported by NIH awards HD065862 (Mari Golub, PI), and OD011107 (Harris Lewin, PI). NIH had no role in conduct, analysis or interpretation of the study.
The authors acknowledge and thank Stal Shrestha for providing the initial template and ROI maps for derivation of the study specific template and ROIs, Chris Machado for initial ROI area selection, the UCDavis Center for Molecular and Genetic Imaging for synthesis of the [11C]DASB (Dave Kukis) and analysis of the PET scan data (Zach Harmany, Yimeng Dou), CNPRC staff for conducting the PET scans and School of Veterinary Medicine staff for conducting the MRI scans.
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Bachmann CJ, Aagaard L, Burcu M, Glaeske G, Kalverdijk LJ, Petersen I, et al. (2016): Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005-2012. Eur Neuropsychopharmacol, 26(3): 411–419. [DOI] [PubMed] [Google Scholar]
- 2.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF (2017): Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience, 342: 212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordquist N, Oreland L (2010): Serotonin, genetic variability, behaviour, and psychiatric disorders--a review. Ups J Med Sci, 115(1): 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivier JD, Blom T, Arentsen T, Homberg JR (2011): The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: A review. Prog Neuropsychopharmacol Biol Psychiatry,35(6): 1400–1408. [DOI] [PubMed] [Google Scholar]
- 5.Bowman MA, Daws LC (2019): Targeting Serotonin Transporters in the Treatment of Juvenile and Adolescent Depression. Front Neurosci, 13: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchison SM, Masse LC, Pawluski JL, Oberlander TF (2018): Perinatal selective serotonin reuptake inhibitor (SSRI) effects on body weight at birth and beyond: A review of animal and human studies. Reprod Toxicol, 77: 109–121. [DOI] [PubMed] [Google Scholar]
- 7.Rotem-Kohavi N, Oberlander TF (2017): Variations in Neurodevelopmental Outcomes in Children with Prenatal SSRI Antidepressant Exposure. Birth Defects Res, 109(12): 909–923. [DOI] [PubMed] [Google Scholar]
- 8.Bottelier MA, Schouw ML, Klomp A, Tamminga HG, Schrantee AG, Bouziane C, et al. (2014): The effects of Psychotropic drugs On Developing brain (ePOD) study: methods and design. BMC Psychiatry, 14: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klomp A, Hamelink R, Feenstra M, Denys D, Reneman L (2014a): Increased response to a 5-HT challenge after discontinuation of chronic serotonin uptake inhibition in the adult and adolescent rat brain. PLoS One, 9(6): e99873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegerer V, Moll GH, Bagli M, Rothenberger A, Ruther E, Huether G (1999): Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. J Child Adolesc Psychopharmacol, 9(1): 13–24; discussion 25–16. [DOI] [PubMed] [Google Scholar]
- 11.Bouet V, Klomp A, Freret T, Wylezinska-Arridge M, Lopez-Tremoleda J, Dauphin F, et al. (2012): Age-dependent effects of chronic fluoxetine treatment on the serotonergic system one week following treatment. Psychopharmacology (Berl), 221(2): 329–339. [DOI] [PubMed] [Google Scholar]
- 12.Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, et al. (2014): Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry, 171(3): 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golub MS, Hogrefe CE, Sherwood RJ, Turck CW (2018): Fluoxetine Administration in Juvenile Monkeys: Implications for Pharmacotherapy in Children. Front Pediatr, 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golub MS, Hogrefe CE, Bulleri AM (2016): Regulation of emotional response in juvenile monkeys treated with fluoxetine: MAOA interactions. Eur Neuropsychopharmacol, 26(12): 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. (2006): MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry, 11(10): 903–913. [DOI] [PubMed] [Google Scholar]
- 16.Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW (2005): Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology, 30(9): 1719–1723. [DOI] [PubMed] [Google Scholar]
- 17.Karere GM, Sullivan E, Kinnally EL, Capitanio JP, Lyons LA (2012): Enhancing genotyping of MAOA-LPR and 5-HTT-LPR in rhesus macaques (Macaca mulatta). J Med Primatol, 41(6): 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian BT, Fox AS, Oler JA, Vandehey NT, Murali D, Rogers J, et al. (2009): Serotonin transporter binding and genotype in the nonhuman primate brain using [C-11]DASB PET. Neuroimage, 47(4): 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S (2000): Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of (11)C-labeled 2-(phenylthio)araalkylamines. J Med Chem, 43(16): 3103–3110. [DOI] [PubMed] [Google Scholar]
- 20.Tai C, Chatziioannou A, Siegel S, Young J, Newport D, Goble RN, et al. (2001): Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Phys Med Biol, 46(7): 1845–1862. [DOI] [PubMed] [Google Scholar]
- 21.Goertzen AL, Bao Q, Bergeron M, Blankemeyer E, Blinder S, Canadas M, et al. (2012): NEMA NU 4-2008 comparison of preclinical PET imaging systems. J Nucl Med, 53(8): 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011): A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3): 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxinos G, X-F H, Petrides M, Toga A, The Rhesus Monkey Brain in Stereotaxic Coordinates 2nd Edition2008, San Diego, California: Academic Press. [Google Scholar]
- 24.Yokoyama C, Yamanaka H, Onoe K, Kawasaki A, Nagata H, Shirakami K, et al. (2010): Mapping of serotonin transporters by positron emission tomography with [11C]DASB in conscious common marmosets: comparison with rhesus monkeys. Synapse, 64(8): 594–601. [DOI] [PubMed] [Google Scholar]
- 25.Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, et al. (2006): Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci, 26(17): 4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, et al. (2013): Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab, 33(6): 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. (2003): Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab, 23(9): 1096–1112. [DOI] [PubMed] [Google Scholar]
- 28.Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R (2009): Vulnerability genes or plasticity genes? Mol Psychiatry, 14(8): 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klomp A, Tremoleda JL, Wylezinska M, Nederveen AJ, Feenstra M, Gsell W, et al. (2012b): Lasting effects of chronic fluoxetine treatment on the late developing rat brain: age-dependent changes in the serotonergic neurotransmitter system assessed by pharmacological MRI. Neuroimage, 59(1): 218–226. [DOI] [PubMed] [Google Scholar]
- 30.Klomp A, Vaclavu L, Meerhoff GF, Reneman L, Lucassen PJ (2014): Effects of chronic fluoxetine treatment on neurogenesis and tryptophan hydroxylase expression in adolescent and adult rats. PLoS One, 9(5): e97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinnally EL, Tarara ER, Mason WA, Mendoza SP, Abel K, Lyons LA, et al. (2010): Serotonin transporter expression is predicted by early life stress and is associated with disinhibited behavior in infant rhesus macaques. Genes Brain Behav, 9(1): 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalbitzer J, Frokjaer VG, Erritzoe D, Svarer C, Cumming P, Nielsen FA, et al. (2009): The personality trait openness is related to cerebral 5-HTT levels. Neuroimage, 45(2): 280–285. [DOI] [PubMed] [Google Scholar]
- 33.Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, et al. (2007): Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol Psychiatry, 62(4): 327–331. [DOI] [PubMed] [Google Scholar]
- 34.Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, et al. (2007): Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm (Vienna), 114(5): 635–639. [DOI] [PubMed] [Google Scholar]
- 35.Su SY, Hogrefe-Phi CE, Asara JM, Turck CW, Golub MS (2016): Peripheral fibroblast metabolic pathway alterations in juvenile rhesus monkeys undergoing long-term fluoxetine administration. Eur Neuropsychopharmacol, 26(7): 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitt P, Pintar JE, Breakefield XO (1982): Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci U S A, 79(20): 6385–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale E, Pehrson AL, Jeyarajah T, Li Y, Leiser SC, Smagin G, et al. (2016): Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectr, 21(2): 143–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asan E, Steinke M, Lesch KP (2013): Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol, 139(6): 785–813. [DOI] [PubMed] [Google Scholar]
- 39.Kim E, Howes OD, Kim BH, Chon MW, Seo S, Turkheimer FE, et al. (2017): Regional Differences in Serotonin Transporter Occupancy by Escitalopram: An [(11)C]DASB PK-PD Study. Clin Pharmacokinet, 56(4): 371–381. [DOI] [PubMed] [Google Scholar]
- 40.Whitaker-Azmitia PM, Zhang X, Clarke C (1994): Effects of gestational exposure to monoamine oxidase inhibitors in rats: preliminary behavioral and neurochemical studies. Neuropsychopharmacology, 11(2): 125–132. [DOI] [PubMed] [Google Scholar]
- 41.Evrard A, Malagie I, Laporte AM, Boni C, Hanoun N, Trillat AC, et al. (2002): Altered regulation of the 5-HT system in the brain of MAO-A knock-out mice. Eur J Neurosci, 15(5): 841–851. [DOI] [PubMed] [Google Scholar]
- 42.Shumay E, Logan J, Volkow ND, Fowler JS (2012): Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics, 7(10): 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shumay E, Fowler JS (2010): Identification and characterization of putative methylation targets in the MAOA locus using bioinformatic approaches. Epigenetics, 5(4): 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naoi M, Maruyama W, Shamoto-Nagai M (2018): Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis. J Neural Transm (Vienna), 125(1): 53–66. [DOI] [PubMed] [Google Scholar]
- 45.Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. (2008): Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry, 13(3): 313–324. [DOI] [PubMed] [Google Scholar]
- 46.Rubia K (2013): Functional brain imaging across development. Eur Child Adolesc Psychiatry, 22(12): 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart H, Lim L, Mehta MA, Curtis C, Xu X, Breen G, et al. (2018): Altered Functional Connectivity of Fronto-Cingulo-Striatal Circuits during Error Monitoring in Adolescents with a History of Childhood Abuse. Front Hum Neurosci, 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang CH, Chen MC, Lu J (2015): Effect of antidepressant drugs on the vmPFC-limbic circuitry. Neuropharmacology, 92: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norgaard M, Ganz M, Svarer C, Frokjaer VG, Greve DN, Strother SC, et al. (2019): Optimization of preprocessing strategies in Positron Emission Tomography (PET) neuroimaging: A [(11)C]DASB PET study. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. (2013): Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci, 14(5): 365–376. [DOI] [PubMed] [Google Scholar]
- 51.Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MF (2014): Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron, 81(3): 700–713. [DOI] [PubMed] [Google Scholar]
- 52.Golub MS, Hackett EP, Hogrefe CE, Leranth C, Elsworth JD, Roth RH (2017): Cognitive performance of juvenile monkeys after chronic fluoxetine treatment. Dev Cogn Neurosci, 26: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golub MS, Hogrefe CE (2016): Sleep disturbance as detected by actigraphy in pre-pubertal juvenile monkeys receiving therapeutic doses of fluoxetine. Neurotoxicol Teratol, 55: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golub MS, Hogrefe CE, Bulleri AM (2016): Peer social interaction is facilitated in juvenile rhesus monkeys treated with fluoxetine. Neuropharmacology, 105: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.