Abstract
Opioids are an essential component of current clinical treatments for pain, but they also produce side effects that include abuse liability. Recent media attention surrounding the use of opioids in the United States has elevated the discussion of their benefits and drawbacks to one of national concern, leading to increased scrutiny of prescribing practices. Regulatory agencies have responded by recommending stricter limits on the amount and duration of opioid prescriptions for pain treatment; however, the relationship between pain states and the abuse-related effects of opioids is still not completely understood. Intracranial self-stimulation (ICSS) is one preclinical procedure that can be used to study the abuse-related effects of opioids in naïve subjects over the course of initial opioid exposure and in the context of inferred pain states. The goal of this review is to provide a summary of evidence from our laboratory using ICSS to study the modulation of opioid reward by pain states and examine these results in the context of related studies from other groups.
Keywords: intracranial self-stimulation, mu opioid receptor, antinociception
Introduction
The Opioid Crisis is the first nationwide Public Health Emergency not initiated by a natural disaster since the H1N1 Flu outbreak in 2009. Its origins, at least in part, can be traced to the 1990s, when clinical use of opioids began to escalate dramatically (Sehgal et al., 2012; Wilson-Poe and Moron, 2018). Prior to this, abuse liability had long been recognized as a limitation to the therapeutic utility of opioids. One factor that contributed to the sudden increase in opioid prescribing was the widespread citation of evidence from an in-patient study to suggest that the risk of iatrogenic addiction was low when initial opioid exposure occurred under medical supervision for the treatment of acute or chronic pain (Leung et al., 2017; Porter and Jick, 1980). A limitation of this study, however, was that opioid administration occurred exclusively in the hospital setting. In other words, the subjects in the initial study may not have been representative of all patients who receive opioids, which includes out-patient populations self-administering opioids for the treatment of pain without the direct supervision of a medical professional. More recent studies have sought to address this limitation and challenged the general perception that the risk of addiction for opioids is low when they are used for the treatment of pain (Boscarino et al., 2010; Manchikanti et al., 2010). For example, one study suggested that opioid prescriptions for as few as five days are associated with increased risk of prolonged opioid use (Shah et al., 2017). Furthermore, regulatory agencies increasingly recommend stricter limits on the amount and duration of opioid prescriptions for pain treatment (Dowell et al., 2016).
Although the perception of risk for abuse when opioids are used in the context of treating pain continues to fluctuate, the relationship between pain states and the abuse-related effects of opioids is still not completely understood. Despite the utility of large-scale epidemiological studies for examining patient outcomes following the prescription of opioids for the treatment of pain, it is difficult to make inferences about the mechanisms underlying a transition from therapeutic use to opioid addiction. To begin unpacking a complex question like this requires the contribution of multiple diverse studies designed to examine a variety of behavioral endpoints. Thus, several preclinical models have been developed. Although evidence from these models cannot be extrapolated to clinical conditions, it can begin to address questions about the interaction between reward-related and analgesic-like effects of opioids in the context of inferred pain states. Our group, which has an extensive history of using intracranial self-stimulation (ICSS) to examine the abuse-related effects of drugs (Negus and Miller, 2014), has developed procedures to incorporate inferred pain states into our study of opioid effects on ICSS behavior. The goal of the current review is to summarize evidence collected by our group using these procedures, to compare our findings to similar ICSS studies conducted by other groups, and to examine the cumulative evidence from ICSS studies of opioids in the context of inferred pain states for potential insight into our current understanding of the relationship between pain states and opioid reward.
ICSS Procedure
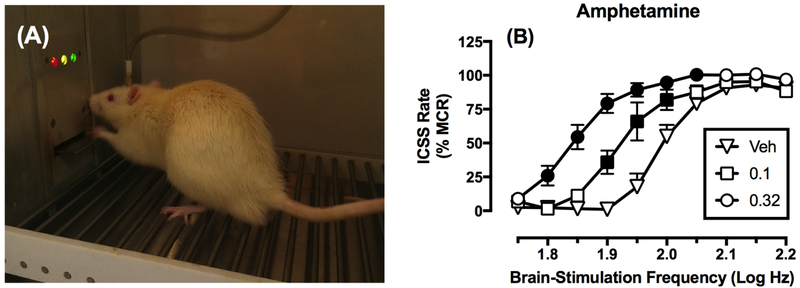
Intracranial self-stimulation (ICSS) has proven useful for examining the abuse potential of drugs (Carlezon and Chartoff, 2007; Kornetsky and Esposito, 1979; Negus and Miller, 2014; Wise, 1996). In ICSS procedures, a microelectrode targeting a brain-reward area is surgically implanted and the subject is trained to emit an operant response to receive pulses of electrical brain stimulation. For example, Figure 1A depicts a rat engaged in ICSS. The frequency or intensity can then be systematically manipulated in daily sessions from low to high levels that maintain low to high rates or probabilities for responding, respectively. The primary dependent measure in any ICSS procedure is one of operant responding. Although different ICSS methodologies have been developed and are used by other groups, the majority of experiments done in our group and thus primarily represented in the current review employ the “frequency-rate” procedure, in which increases in the frequency of brain stimulation maintain increasing rates of operant behavior (Negus and Miller, 2014). Once subjects have been trained to emit reliable baseline frequency-rate curves, drug effects can be evaluated for their effectiveness to shift behavior from that baseline.
Figure 1. Overview of ICSS procedure.
Panel A shows a male Sprague-Dawley rat in an operant conditioning chamber pressing a lever for electrical brain stimulation. Stimulation is delivered via a microelectrode that is implanted in the medial forebrain bundle and attached by a cable to an ICSS stimulator located outside the frame of the photograph. Panel B shows representative data collected using a “frequency-rate” ICSS procedure, in which brain-stimulation frequency is varied during daily behavioral sessions, and rates of responding are monitored during availability of each frequency. Drugs with abuse liability (e.g. amphetamine) typically produce leftward/upward shifts in ICSS frequency-rate curves across some range of doses. Abscissa: Log brain-stimulation frequency in Hz. Ordinate: ICSS response rate expressed as % Maximum Control Rate (%MCR), a transformation that normalizes data in each rat on each test day to maximum rates during baseline sessions. Filled points show rates significantly different from those after Vehicle (Veh) administration (p<0.05). All points show mean ± SEM from n=6 rats. Data adapted from Bauer et al. (2013).
Data from frequency-rate procedures may be analyzed in a variety of ways, which we have compared in detail previously (Negus and Miller, 2014). The predominant method, although not in use by our group, is “curve-shift analysis” that uses non-linear or linear regression to distill data for full frequency-rate curves down to two parameters that quantify the lateral position of the curve along the X-axis (e.g. EF50, Effective Frequency to maintain 50% of maximum rate) and peak of the curve along the Y-axis (e.g. Maximum Rate). Drug-induced changes in these two endpoints are often interpreted as evidence for drug effects on either reward-related sensitivity to brain stimulation (inferred from lateral shifts in the curve without changes in maximum rates) or general motor competence to perform the operant response (inferred from vertical increases or decreases in maximum rates). The less common method of analysis, utilized by our group and emphasized in this review, uses two-way analysis of variance to compare frequency-rate curves before and after drug treatment (with brain-stimulation frequency as one factor and drug dose or pretreatment time as a second factor). One strength of this approach, in comparison to curve-shift analysis, is that it can accommodate results from treatments that flatten frequency-rate curve slopes and make quantification of lateral shifts difficult or impossible. However, as with curve-shift analysis, drug-induced reward is inferred from increases in low ICSS rates maintained by low brain-stimulation frequencies. Conversely, decreases in high ICSS rates maintained by high frequencies could result from anhedonia (i.e. decreased sensitivity to brain stimulation, if maximum ICSS rates are retained) and/or motor impairment (if maximum rates are decreased).
As one example of drug effects on ICSS frequency-rate curves, Figure 1 shows data from male Sprague-Dawley rats with electrodes implanted in the medial forebrain bundle (Figure 1A), and compares the effects of increasing doses of amphetamine in the frequency-rate procedure (Figure 1B) (Bauer et al., 2013). Amphetamine, which promotes the release of dopamine from dopaminergic neurons such as those projecting from the ventral tegmental area to the nucleus accumbens, is a known drug of abuse, and it produces effects in the frequency-rate procedure that are typical of drugs with abuse liability: a dose-dependent leftward shift in the frequency-rate curve and increase in low ICSS rates maintained by low brain-stimulation frequencies. This pattern of drug effects is often referred to as “facilitation.” Facilitation in ICSS is characteristic of drugs of abuse. Conversely, drugs with very low abuse potential, such as the serotonin-selective releaser fenfluramine (Bauer et al., 2013), fail to produce facilitation of ICSS up to doses that decrease high rates of ICSS maintained by high brain-stimulation frequencies, a pattern of behavior referred to as “depression.” Failure to produce facilitation up to doses that produce depression in ICSS is characteristic of drugs with low abuse liability. The effectiveness of a drug to produce facilitation in this frequency-rate ICSS procedure is highly correlated with effectiveness to produce abuse-related effects in drug self-administration procedures, which serve as a cornerstone for preclinical assessment of abuse potential (Negus and Miller, 2014).
Effects of Opioids in ICSS
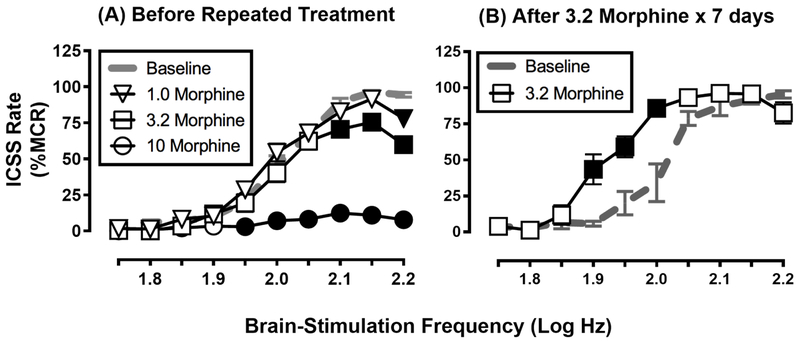
Amphetamine and other abused psychostimulants produce robust, dose-dependent facilitation of ICSS in drug-naïve subjects that persists even after regimens of repeated administration (Bauer et al., 2014; Johnson et al., 2018; Riday et al., 2012). Thus, one might predict that morphine, another drug of abuse that reliably engenders abuse-related effects in drug self-administration procedures, would produce effects in ICSS similar to amphetamine (i.e., dose-dependent facilitation). Contrary to this, morphine and other mu opioid receptor (MOR) agonists often produce a different profile of effects that is strongly influenced by the history of opioid exposure (Altarifi et al., 2013; Miller et al., 2015; Negus and Moerke, 2019; Reid, 1987). Specifically, morphine and other MOR agonists often fail to produce ICSS facilitation in opioid-naïve subjects, and instead produce primarily dose-dependent ICSS depression, although slight facilitation during initial exposure will sometimes emerge at later time points after depression has dissipated (see Negus and Moerke, 2019 for a review of opioid effects in ICSS). However, repeated daily treatment with morphine produces tolerance to ICSS depression and emergence of ICSS facilitation that becomes more pronounced and occurs earlier in the time course of drug effects (Altarifi et al., 2013; Legakis and Negus, 2018; Miller et al., 2015). Figure 2 shows data from male Sprague-Dawley rats with electrodes implanted in the medial forebrain bundle and compares the effects of morphine before and after treatment with 3.2 mg/kg/day morphine for seven days. Before repeated daily treatment, morphine primarily depressed ICSS (Figure 2A); however, following daily treatment, a dose of morphine that depressed ICSS in opioid-naïve animals (3.2 mg/kg) produced facilitation and the leftward shift in the frequency-rate curve typical for drugs of abuse (Figure 2B).
Figure 2. Repeated morphine exposure increases expression of morphine-induced ICSS facilitation.
Panel A shows that morphine failed to facilitate ICSS in 20 drug-naïve male Sprague-Dawley rats, and instead produced only dose-dependent ICSS depression. These rats were subsequently divided into three groups that received repeated daily treatment with saline (n=6), 1.0 mg/kg/day morphine (n=7), or 3.2 mg/kg/day morphine (n=7). Panel B shows that repeated treatment with 3.2 mg/kg/day morphine produced tolerance to ICSS depression and emergence of abuse-related ICSS facilitation at a test dose of 3.2 mg/kg morphine. Similar ICSS facilitation did not emerge after repeated treatment with saline or the lower dose of 1.0 mg/kg/day morphine (data not shown). For each panel, the abscissa shows brain-stimulation frequency in log Hz, and the ordinate shows ICSS rate expressed as a percent of the Maximum Control Rate (%MCR). All data show mean ± SEM, and filled points indicate significantly different from Baseline (p<0.05). Data adapted from Miller et al. (2015).
Effects of Pain Manipulations in ICSS
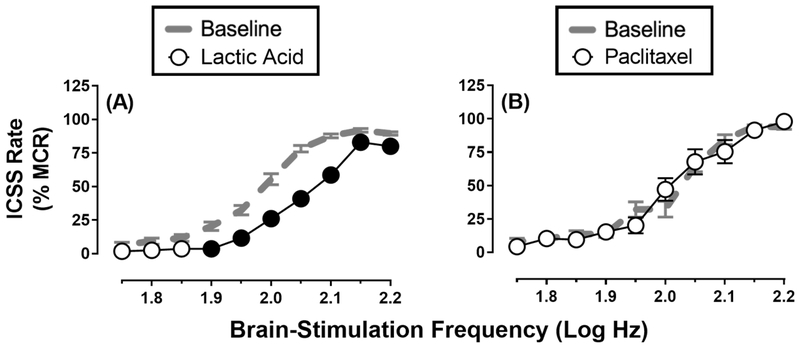
ICSS can also be used to examine the behavioral effects of various pain-related manipulations. Clinically relevant pain states often involve depression of behavior and mood, and preclinical assays have been developed in rodents to assess the expression and treatment of pain-related depression of unconditioned behaviors, such as feeding (Kwilasz and Negus, 2012), wheel running (Kandasamy et al., 2016; Stevenson et al., 2011) and nesting (Negus et al., 2015). Positively reinforced operant behaviors, such as ICSS, are also sensitive to depression by some pain manipulations. For example, intraperitoneal injection of diluted lactic acid can serve as an acute visceral pain stimulus to produce significant, transient (≤1 hr) rightward shifts in ICSS frequency-rate curves, as illustrated in Figure 3A (Altarifi and Negus, 2015; Altarifi et al., 2015; Brust et al., 2016; Negus et al., 2010; Pereira Do Carmo et al., 2009). This type of rightward and downward shift in ICSS frequency-rate curves may reflect a combination of anhedonia (i.e., decreased sensitivity to normally reinforcing stimuli) and/or motor impairment because even maximal ICSS rates were significantly decreased (Carlezon and Chartoff, 2007). Moreover, this pain-related depression of ICSS is associated with depression of mesolimbic dopamine release, a neurochemical correlate of anhedonia and motor impairment (Leitl et al., 2014a). However, regardless of the relative contributions of anhedonia vs. motor impairment, this ICSS depression was likely pain-related insofar as it could be completed blocked by clinically effective analgesics (Leitl et al., 2014a).
Figure 3. Effects of noxious stimuli on ICSS in rats.
Panel A shows that a visceral acute noxious stimulus depressed ICSS. ICSS frequency-rate curves were determined before (Baseline) or after (Lactic Acid) intraperitoneal administration of 1.8% lactic acid in 34 male Sprague-Dawley rats as described in Negus et al. (2012). Lactic acid was administered in a volume of 1 ml/kg immediately before ICSS evaluation. Panel B shows that a model of chronic chemotherapy-induced neuropathic pain was not sufficient to depress ICSS. ICSS frequency-rate curves were determined before (Baseline) and after (Paclitaxel) treatment with the chemotherapeutic paclitaxel in 16 male and female Sprague-Dawley rats as described in Legakis and Negus (2018). Paclitaxel was administered IP at a dose of 2.0 mg/kg every other day for four total injections (total of 8.0 mg/kg paclitaxel). Data show ICSS 29 days after initiation of paclitaxel treatment, a time when rats displayed sustained mechanical hypersensitivity to stimulation with von Frey filaments as a chronic pain-related behavior. For each panel, the abscissa shows brain-stimulation frequency in log Hz, and the ordinate shows ICSS rate expressed as a percent of the Maximum Control Rate (%MCR). All data show mean ± SEM, and filled points indicate significantly different from Baseline (p<0.05).
Intraplantar injection of complete Freund’s adjuvant (CFA), surgical paw incision, and intraplantar injection of formalin serve as more sustained pain manipulations that can depress ICSS for periods of hours (CFA), days (paw incision), or weeks (formalin) (Ewan and Martin, 2014; Leitl and Negus, 2016; Leitl et al., 2014b). As with the more transient effects produced by intraperitoneal injection of diluted lactic acid, ICSS depression produced by these more sustained pain states appears to reflect some combination of anhedonia and motor impairment, but more importantly, ICSS depression by all these manipulations can be alleviated by clinically effective analgesics.
However, this phenomenon does not appear to extend to all other putative chronic pain states. For example, spinal nerve ligation (SNL) is a model of neuropathic pain that can produce sustained hypersensitivity of hindpaw-withdrawal responses to tactile stimuli for weeks to months, but SNL did not depress ICSS in rats (Ewan and Martin, 2011a, 2012, 2014). Similarly, paclitaxel treatment is a widely-used model of chemotherapy-induced neuropathic pain that is often sufficient to produce mechanical hypersensitivity in rats, but it also failed to produce any depression of ICSS, as shown in Figure 3B (Legakis et al., 2018; Legakis and Negus, 2018).
The mechanism underlying the differential effects of pain manipulations in ICSS is unclear, but may be related to the relatively strong reinforcing effectiveness of ICSS or the weak behavioral depressant effects of commonly used neuropathic pain manipulations. Nonetheless, as other pain-related behavioral endpoints (e.g., tactile hypersensitivity) can be reliably observed even in the absence of pain-depressed ICSS, interactions between pain states and the effects of opioids can be examined in ICSS studies of both acute and chronic putative pain states.
Effects of Pain Manipulations + Opioids in ICSS
Modulation of abuse-related opioid effects by pain states has frequently been examined using both drug self-administration (Colpaert et al., 2001; Ewan and Martin, 2013; Kupers and Gybels, 1995) and conditioned-place-preference procedures (Lim et al., 2014; Narita et al., 2005; Niikura et al., 2008; Oe et al., 2004; Ozaki et al., 2003; Ozaki et al., 2002; Shippenberg et al., 1988). However, results from these studies have been mixed. The ICSS procedure provides an additional, complementary approach for studying the interaction between opioid effects and preclinical pain manipulations. One advantage of ICSS as compared to drug self-administration procedures for examining abuse-related effects of drugs is that the evolution of both abuse-related effects (ICSS facilitation) and abuse-limiting effects (ICSS depression) can be monitored during the earliest stages of drug exposure. In other words, no prior exposure to drug is required to train the rats in the ICSS procedure. Therefore, ICSS experiments can be performed in drug-naïve subjects, facilitating the examination of any potential change in the interaction between reward-related opioid effects and inferred pain states from the first opioid exposure over the course of a repeated treatment. For example, because opioids typically produce ICSS depression in opioid-naïve subjects, if pain states are protective against opioid reward, then one might predict that pain states would prevent or retard the emergence of opioid-induced ICSS facilitation during repeated morphine treatment. Two studies have tested this hypothesis, and both studies found that pain states failed to prevent the emergence of opioid-induced ICSS facilitation.
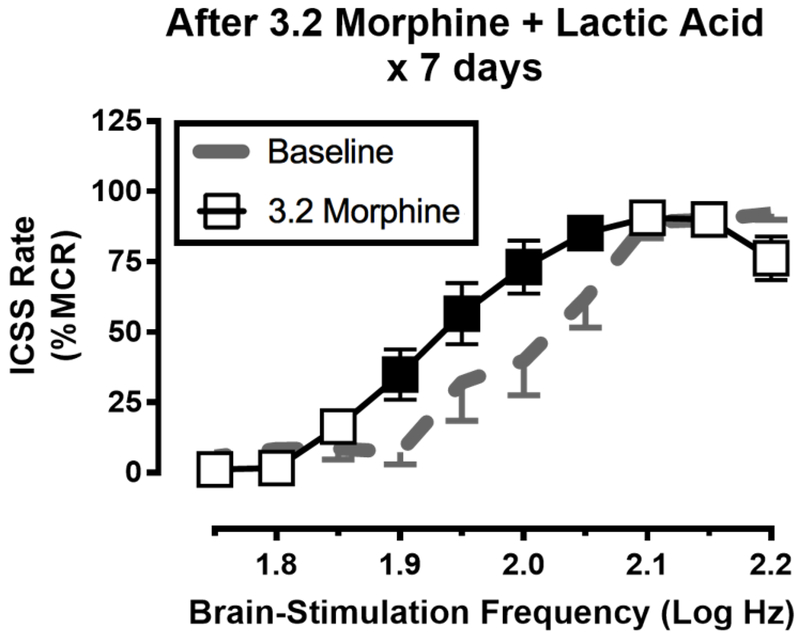
One of these studies compared the effects of repeated daily treatment with morphine or saline for seven days on ICSS in rats treated concurrently with either repeated intraperitoneal injections of dilute lactic acid (a transient but repeatable acute visceral pain stimulus) or repeated acid vehicle (a control, non-pain stimulus) (Miller et al., 2015). Effects of morphine (or saline vehicle) + lactic acid (or acid vehicle) on ICSS were evaluated during the repeated-treatment regimen to assess antinociceptive effectiveness of morphine during treatment. Additionally, effects of morphine alone were evaluated after treatment to determine effects of the treatment regimen on emergence of morphine-induced ICSS facilitation in the post-pain state. Repeated treatment with saline + acid vehicle for seven days did not alter ICSS, whereas repeated treatment with saline + lactic acid produced a repeatable and pain-related depression of ICSS (similar to that shown in Figure 3A). Neither repeated acid vehicle nor repeated lactic acid altered morphine effects determined after the treatment regimen, such that morphine produced only dose-dependent ICSS depression. Thus, repeated daily exposure to an acute pain state was not sufficient to alter morphine effects. In the morphine treatment groups, treatment with 3.2 mg/kg/day morphine + acid vehicle produced tolerance to ICSS depression and enhanced expression of ICSS facilitation as described above (see Figure 2B). Moreover, when 3.2 mg/kg/day morphine was administered daily with repeated lactic acid, morphine blocked lactic acid-induced ICSS depression (indicative of an antinociceptive morphine effect), but after conclusion of repeated treatment, morphine alone also produced ICSS facilitation (Figure 4). Thus, repeated morphine administration produced the therapeutically desirable effect of antinociception during morphine + lactic acid treatment, but the presumptive pain state failed to block the emergence of morphine-induced ICSS facilitation after treatment. Intriguingly, a lower dose of 1.0 mg/kg/day morphine was sufficient to produce antinociception during treatment without promoting an increase in subsequent morphine-induced ICSS facilitation, suggesting that judicious use of low doses may be effective to produce antinociception without enhancing reward-related effects of morphine. Nonetheless, the major finding of this study was that repeated exposure to the lactic acid noxious stimulus was not sufficient to block the increase in abuse-related effects produced by concurrent repeated treatment with the higher dose of morphine.
Figure 4. Repeated morphine exposure increases expression of morphine-induced ICSS facilitation even if the morphine is administered in the context of a pain state.
Seven male Sprague-Dawley rats were treated daily with 3.2 mg/kg/day morphine followed by an intraperitoneal injection of 1.8% lactic acid. One day after this treatment regimen, rats received a test dose of 3.2 mg/kg morphine to determine if pain associated with the lactic acid injections would prevent tolerance to ICSS depression and emergence of ICSS facilitation by the test dose of morphine as shown in Figure 2. The lactic acid treatment did not block these effects, and morphine produced an abuse-related ICSS facilitation. The abscissa shows brain-stimulation frequency in log Hz, and the ordinate shows ICSS rate expressed as a percent of the Maximum Control Rate (%MCR). All data show mean ± SEM for n=7 male rats, and filled points indicate significantly different from Baseline (p<0.05). Data adapted from Miller et al. (2015).
The second of these studies compared the effects of repeated daily treatment with morphine or saline for seven days on ICSS in rats treated previously with vehicle (control) or paclitaxel (a model of chemotherapy-induced neuropathic pain) (Legakis and Negus, 2018). The paclitaxel treatment regimen (4 total injections of 2.0 mg/kg administered every other day for 7 days) produced tactile hypersensitivity that emerged during the week of paclitaxel treatment and was sustained for three weeks thereafter. Effects of ICSS were determined before paclitaxel or vehicle treatment (pre-paclitaxel baseline) and daily for four weeks during and after paclitaxel treatment to evaluate the impact of paclitaxel treatment on ICSS responding. Neither treatment with vehicle nor treatment with paclitaxel significantly altered ICSS responding in either male or female rats (see Figure 3B). The effects of daily treatment with morphine were evaluated on both tactile hypersensitivity (as a measure of morphine-induced antinociception) and on ICSS (as a measure of morphine reward) in the paclitaxel groups during the last week of the study. Morphine dose-dependently blocked tactile hypersensitivity, although repeated treatment produced modest tolerance to this antinociceptive effect. In studies of ICSS, initial morphine exposure produced ICSS depression in both male and female rats, although rewarding effects of initial morphine were stronger in female rats. This sex difference was eliminated with repeated morphine treatment, and paclitaxel treatment did not alter the increase in abuse-related effects of morphine that repeated morphine treatment produced in both male and female rats. Thus, the main findings of the study were the following: (1) treatment with paclitaxel produced tactile hypersensitivity but did not significantly alter ICSS responding in male or female rats, (2) paclitaxel treatment did not alter the effects of morphine to depress ICSS responding on initial exposure in male or female rats, and (3) repeated morphine produced tolerance to morphine antinociception but sensitization to morphine reward. Taken together, these two studies suggest that neither an acute pain stimulus (repeated intraperitoneal lactic acid; (Miller et al., 2015)) nor a sustained neuropathic pain stimulus (paclitaxel treatment; (Legakis and Negus, 2018)) was sufficient to prevent the emergence of morphine-induced ICSS facilitation in rats following repeated daily treatment with morphine.
In contrast to the results described above, which used models of acute pain and chemotherapy-induced neuropathic pain in Sprague-Dawley rats, a different pattern of results has been described in studies using the SNL model of neuropathic pain in male Fischer 344 rats (Ewan and Martin, 2011a, b). In these studies, preliminary experiments were performed to determine the largest amounts of each drug that could be administered without decreasing the maximum response rate; thus, doses of drugs that did decrease the maximum response rate in drug-naïve subjects were not tested. In this strain of rats, morphine and other MOR agonists produced dose-dependent ICSS facilitation (as evidenced by differences in ΔEF50 values) in opioid-naïve subjects. As with paclitaxel-induced neuropathy, SNL produced sustained tactile hypersensitivity while failing to depress ICSS responding; however, SNL blocked morphine-induced ICSS facilitation and attenuated ICSS facilitation produced by the other MOR agonists heroin, methadone, fentanyl and hydromorphone. These studies did not evaluate effects of repeated MOR agonist treatment, so this study did not evaluate the degree to which the SNL model of neuropathic pain might attenuate the trajectory of increased MOR agonist reward with repeated MOR agonist treatment. Nonetheless, this study did suggest attenuation of MOR agonist reward in this surgical model of neuropathy.
Conclusion
The ICSS procedure has long been recognized as a useful procedure for evaluating the abuse potential of drugs, including opioid and non-opioid potential analgesics (Negus, 2013; Negus and Miller, 2014). It provides a unique opportunity to examine the effects of various putative pain states on abuse-related effects of opioids, because a measure of opioid reward can be evaluated in subjects that are opioid-naïve at the beginning of the study. The fact that ICSS allows for direct testing of hypotheses related to modulation of opioid reward in the context of putative pain states sheds further light on the perception that opioids administered in the context of a pain state represent a reduced or absent risk of subsequent abuse and dependence.
Preclinical data using the ICSS procedure with acute and chronic models of pain suggest that the presence of a pain state is not sufficient to prevent a change from initial depression of behavior by administration of MOR agonists to ICSS facilitation following repeated administration; however, presence of a pain state also does not appear to accelerate this transition (Ewan and Martin, 2011b; Legakis and Negus, 2018; Leitl and Negus, 2016; Miller et al., 2015). The only evidence suggesting that a pain-related manipulation might attenuate the abuse-related effects of opioids came from a series of studies using SNL-induced neuropathic pain (Ewan and Martin, 2011a, b). Importantly, these experiments did not test doses of opioids that depressed the maximum rate of ICSS responding in drug-naïve subjects, and the pain manipulation used was not itself sufficient to depress ICSS responding. However, the effectiveness of morphine and other MOR agonists to facilitate ICSS responding in control rats was attenuated in a dose-dependent manner in SNL rats, suggesting that the reinforcing effects of opioids were diminished in the SNL model of neuropathic pain.
Overall, results from ICSS suggest that regimens of repeated opioid treatment retain high abuse potential even when opioid exposure occurs in the context of an acute or chronic pain state. This approach complements other procedures for examining drug reinforcement in the presence of a putative pain state, including drug self-administration and conditioned place preference, and integration of evidence from all three procedures could prove useful for developing treatments that reduce the abuse liability of opioids without diminishing their effectiveness as analgesics. Nonetheless, these findings from ICSS suggest that addiction remains a significant risk even when opioids are used to treat pain, and that risk of addiction should be balanced against potential for analgesic benefits when prescribing opioid analgesics.
ICSS is useful for testing modulation of opioid reward by putative pain states
Initial depression of ICSS with opioids switches to facilitation with repeated administration
Emergence of ICSS facilitation is not prevented by acute or chronic pain states
Opioids maintain abuse potential even with exposure during treatment for pain
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers R01NS07015, T32DA007027].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Altarifi AA, Negus SS, 2015. Differential tolerance to morphine antinociception in assays of pain-stimulated vs. pain-depressed behavior in rats. Eur J Pharmacol 748, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS, 2013. Abuse-related effects of mu-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol 24, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS, 2015. Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther 352, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS, 2013. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168, 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS, 2014. The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology (Berl) 231, 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, Stewart WF, 2010. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction 105, 1776–1782. [DOI] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, Jones SR, Martin TJ, Bohn LM, 2016. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9, ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Chartoff EH, 2007. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2, 2987–2995. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W, 2001. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain 91, 33–45. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 65, 1–49. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2011a. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology 114, 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2011b. Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids. Anesthesiology 115, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2012. Intracranial self-stimulation of the paraventricular nucleus of the hypothalamus: increased faciliation by morphine compared to cocaine. Anesthesiology 116, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2013. Analgesics as reinforcers with chronic pain: Evidence from operant studies. Neurosci Lett 557 Pt A, 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2014. Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg 118, 854–862. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Selley DE, Negus SS, 2018. Amphetamine maintenance differentially modulates effects of cocaine, methylenedioxypyrovalerone (MDPV), and methamphetamine on intracranial self-stimulation and nucleus accumbens dopamine in rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM, 2016. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods 263, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, 1979. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc 38, 2473–2476. [PubMed] [Google Scholar]
- Kupers R, Gybels J, 1995. The consumption of fentanyl is increased in rats with nociceptive but not with neuropathic pain. Pain 60, 137–141. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS, 2012. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Bigbee JW, Negus SS, 2018. Lack of paclitaxel effects on intracranial self-stimulation in male and female rats: comparison to mechanical sensitivity. Behav Pharmacol 29, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Negus SS, 2018. Repeated Morphine Produces Sensitization to Reward and Tolerance to Antiallodynia in Male and Female Rats with Chemotherapy-Induced Neuropathy. J Pharmacol Exp Ther 365, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Negus SS, 2016. Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion and [INCREMENT]9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behav Pharmacol 27, 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA Jr., Banks ML, Negus SS, 2014a. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology 39, 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA Jr., Negus SS, 2014b. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain 10, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PTM, Macdonald EM, Stanbrook MB, Dhalla IA, Juurlink DN, 2017. A 1980 Letter on the Risk of Opioid Addiction. N Engl J Med, United States, pp. 2194–2195. [DOI] [PubMed] [Google Scholar]
- Lim G, Kim H, McCabe MF, Chou CW, Wang S, Chen LL, Marota JJ, Blood A, Breiter HC, Mao J, 2014. A leptin-mediated central mechanism in analgesia-enhanced opioid reward in rats. J Neurosci 34, 9779–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Ailinani H, Pampati V, 2010. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 13, 401–435. [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS, 2015. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol 23, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T, 2005. Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology 30, 111–118. [DOI] [PubMed] [Google Scholar]
- Negus SS, 2013. Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 42, 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL, 2014. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66, 869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ, 2019. Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides 112, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC, 2010. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL, 2015. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 156, 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC, 2012. Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340, 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Narita M, Narita M, Nakamura A, Okutsu D, Ozeki A, Kurahashi K, Kobayashi Y, Suzuki M, Suzuki T, 2008. Direct evidence for the involvement of endogenous beta-endorphin in the suppression of the morphine-induced rewarding effect under a neuropathic pain-like state. Neurosci Lett 435, 257–262. [DOI] [PubMed] [Google Scholar]
- Oe K, Narita M, Imai S, Shibasaki M, Kubota C, Kasukawa A, Hamaguchi M, Yajima Y, Yamazaki M, Suzuki T, 2004. Inhibition of the morphine-induced rewarding effect by direct activation of spinal protein kinase C in mice. Psychopharmacology (Berl) 177, 55–60. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Miyoshi K, Suzuki T, 2003. Suppression of the morphine-induced rewarding effect and G-protein activation in the lower midbrain following nerve injury in the mouse: involvement of G-protein-coupled receptor kinase 2. Neuroscience 116, 89–97. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T, 2002. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J Neurochem 82, 1192–1198. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS, 2009. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Jick H, 1980. Addiction rare in patients treated with narcotics. N Engl J Med 302, 123. [DOI] [PubMed] [Google Scholar]
- Reid LD, 1987. Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs In: Bozarth MJ, (Ed), Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag, Berlin, pp. 391–420. [Google Scholar]
- Riday TT, Kosofsky BE, Malanga CJ, 2012. The rewarding and locomotor-sensitizing effects of repeated cocaine administration are distinct and separable in mice. Neuropharmacology 62, 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal N, Manchikanti L, Smith HS, 2012. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 15, ES67–92. [PubMed] [Google Scholar]
- Shah A, Hayes CJ, Martin BC, 2017. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006–2015. MMWR Morb Mortal Wkly Rep 66, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A, 1988. Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa-but not of mu-receptor agonists. Pain 35, 179–186. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ, 2011. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav 98, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Poe AR, Moron JA, 2018. The dynamic interaction between pain and opioid misuse. Br J Pharmacol 175, 2770–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 1996. Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19, 319–340. [DOI] [PubMed] [Google Scholar]