Abstract
Female smokers are more likely to relapse than male smokers, but little is known about sex differences in nicotine withdrawal. Therefore, male and female rats were prepared with minipumps that contained nicotine or saline and sex differences in precipitated and spontaneous nicotine withdrawal were investigated. The intracranial self-stimulation (ICSS) procedure was used to assess mood states. Elevations in brain reward thresholds reflect a deficit in reward function. Anxiety-like behavior was investigated after the acute nicotine withdrawal phase in a large open field and the elevated plus maze test. The nicotinic receptor antagonist mecamylamine elevated the brain reward thresholds of the nicotine-treated rats but did not affect those of the saline-treated control rats. A low dose of mecamylamine elevated the brain reward thresholds of the nicotine-treated male rats but not those of the females. Mecamylamine also precipitated more somatic withdrawal signs in the nicotine-treated male than female rats. Minipump removal elevated the brain reward thresholds of the nicotine-treated rats for about 36 h but did not affect those of the saline-treated rats. There was no sex difference in the reward deficit during spontaneous nicotine withdrawal. In addition, the nicotine-treated male and female rats did not display increased anxiety-like behavior three to four days after minipump removal. In conclusion, these studies suggest that relatively low doses of a nicotinic receptor antagonist induce a greater reward deficit and more somatic withdrawal signs in male than female rats, but there is no sex difference in the reward deficit during spontaneous withdrawal.
Keywords: Nicotine, sex differences, estrous cycle, ICSS, reward deficit, somatic signs, withdrawal, rats
1. Introduction
Nicotine is the main psychoactive component in combustible cigarettes and e-cigarettes. Worldwide there are more than 1 billion people who smoke, and about 7 million people die each year from smoking (WHO, 2018). In the US, the use of combustible cigarettes is on the decline, but there has been a sharp increase in the use of e-cigarettes (Cullen et al., 2018).
Nicotine is mildly rewarding and enhances cognitive function (Henningfield et al., 1985; Rezvani and Levin, 2001). Prolonged nicotine use leads to the development of dependence and cessation of nicotine use leads to affective and somatic withdrawal signs, which play an essential role in the maintenance of nicotine use (Cook et al., 2004). People who quit smoking experience an intense craving for nicotine, anhedonia, cognitive impairment, and increased anxiety (Cook et al., 2017; Cook et al., 2015). There is evidence that female smokers are more likely to relapse than male smokers (Jackson et al., 1986; Smith et al., 2016; Tunstall et al., 1985). Nicotine addiction is a complex disorder, and many factors play a role in smoking and relapse. It has been hypothesized that females experience nicotine withdrawal as more aversive than males and that they are therefore more likely to relapse (O’Dell and Torres, 2014). This is supported by research with daily smokers that shows that women experience greater negative affect during nicotine withdrawal than men (Faulkner et al., 2018). However, another study with a large group of daily cigarette smokers found that only the level of nicotine dependence, and not sex, affects nicotine withdrawal (craving, irritability, frustration, anxiety, concentration, restlessness, depression, appetite, and insomnia)(Langdon et al., 2013). Other differences between male and female smokers could account for the higher relapse rate in females. Female smokers are more likely to be depressed than male smokers, and depression contributes to relapse (Bruijnzeel, 2012; Killen et al., 2002). Furthermore, smoking cessation treatments such as nicotine gum and the patch are less effective in diminishing withdrawal and relapse in females than males (Hatsukami et al., 1991; Perkins and Scott, 2008). Some smoking cessation treatments might be less effective in females than males because smoking in females is more reinforced by non-nicotine factors and less by nicotine (Perkins, 1996).
Animal studies have compared differences between males and females in nicotine withdrawal. Numerous studies show that female rats, compared to male rats, have higher plasma corticosterone levels during nicotine withdrawal (Gentile et al., 2011; Skwara et al., 2012; Torres et al., 2013). Furthermore, female rats have higher corticotropin-releasing factor (CRF) levels in the nucleus accumbens during nicotine withdrawal and display higher levels of anxiety-like behavior (Torres et al., 2013). In one study, a place conditioning procedure was used to compare nicotine withdrawal between male and female rats. A nicotinic acetylcholine receptor (nAChR) antagonist caused place aversion in the nicotine-treated male and female rats, but this effect was more pronounced in the female rats (O’Dell and Torres, 2014). Although place conditioning procedures do not directly measure the mood state of the animals, this outcome suggests that nicotine withdrawal is more aversive in female than male rats (O’Dell and Torres, 2014). Another study reported that there are no differences in spontaneous somatic nicotine withdrawal signs between male and female rats in a brightly-lit environment, however, females showed more somatic withdrawal signs than males in a dimly-lit environment (Hamilton et al., 2009).
The main goal of the present studies was to compare affective and somatic nicotine withdrawal signs between male and female rats. The reward deficit associated with nicotine withdrawal was investigated with the ICSS procedure, which provides a quantitative measure of the state of the brain reward system (Barr et al., 2002). In the present study, we determined the effect of sex on nicotine withdrawal by administering a wide range of doses of mecamylamine (precipitated withdrawal) to nicotine-treated rats and removing the nicotine pumps (spontaneous withdrawal). Sex differences in somatic withdrawal signs and anxiety-like behavior were also determined. Previous studies have investigated the effects of acute nicotine administration on locomotor activity and anxiety-like behavior, however, little is known about the effects of chronic nicotine administration on locomotor activity and anxiety-like behavior (Bruijnzeel et al., 2011; File et al., 1998). Therefore, we also investigated the effects of chronic nicotine administration on locomotor activity and anxiety-like behavior in male and female rats.
2. Materials and Methods
2.1. Animals
Wistar rats (males 200–225 g, females 175–200 g, Charles River, Raleigh, NC) were housed socially in a climate-controlled vivarium on a reversed 12 h light-dark cycle (light off at 7 AM). Food and water were available ad libitum. All animal procedures were performed in accordance with the University of Florida Institutional Animal Care and Use Committee as well as National Institutes of Health guidelines.
2.2. Drugs
Nicotine and mecamylamine were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Nicotine and mecamylamine were dissolved in sterile saline (0.9 % sodium chloride). Mecamylamine was administered subcutaneously (sc) in a volume of 1 ml/kg body weight. Nicotine doses are expressed as free base, and mecamylamine doses are expressed as salt.
2.3. Experimental design
In Experiment 1, male and female rats were prepared with ICSS electrodes and trained on the ICSS procedure. When the brain reward thresholds were stable (±10% over 5 days)(Bruijnzeel and Markou, 2003), the rats were prepared with osmotic minipumps that contained nicotine or saline (see Fig. S1 for a schematic overview). After at least seven days of nicotine administration, the effects of mecamylamine on brain reward thresholds and response latencies were investigated. The minipumps were removed on day 14, and the brain reward thresholds were determined 6, 12, 24, 36, 48, 72, and 96 h later. Three days after the removal of the minipumps, the rats were tested in the large open field test, and the following day (day 4) they were tested in the elevated plus maze test. The effect of the estrous cycle on the reward thresholds and response latencies was determined using ICSS data from the five test days before the implantation of the minipumps. The effect of the estrous cycle on brain reward thresholds and response latencies during spontaneous nicotine withdrawal was investigated at the 6 and 12 h time point. The brain reward thresholds of the nicotine-treated female rats were elevated after the administration of 3 mg/kg of mecamylamine. However, because only 2 of the 10 females were in estrus, the effect of the estrous cycle on precipitated nicotine withdrawal could not be determined. In Experiment 2, male and female rats were prepared with minipumps that contained nicotine or saline, and after 10 days mecamylamine-precipitated somatic withdrawal signs were counted (schematic overview, Fig. S1). Sex differences in the locomotor effects of chronic nicotine administration were determined in a large open field on day 6 and in a small open field on day 13. In both experiments, mecamylamine was administered subcutaneously (sc) 15 min before testing.
2.4. Estrous cycle
Rats have a short estrous cycle that lasts 4–5 days and consists of 1 day of proestrus, 1–2 days of estrus, and 2–3 days of diestrus (Andersson et al., 2013). To determine the stage of the estrous cycle, vaginal samples were collected, and cytological assessments were performed (Marcondes et al., 2002; McLean et al., 2012). The vaginal samples were collected daily at 9 AM by inserting a disposable transfer pipette with 0.5 mL of sterile saline and then flushing and aspirating two to three times (Cora et al., 2015). A drop of the sample was placed on a glass slide, and the slides were air dried. The slides were stained with 1% Methylene Blue overnight (Biopharm Inc., Hatfield, AR) and rinsed with distilled water in the morning. After applying a drop of mounting medium, glass coverslips were placed on the slides. The samples were evaluated using a Leica DM2500 light microscope and a 10x or 20x objective. Samples that contained clusters of nucleated epithelial cells were labeled as proestrus. Samples that contained predominantly cornified squamous epithelial cells were labeled as estrus. Samples with mainly small, darkly stained leukocytes and sometimes cornified squamous epithelial cells were labeled as diestrus (McLean et al., 2012). It was determined if the estrous cycle affects spontaneous nicotine withdrawal. Because of the small number of animals at each stage and the lack of differences between proestrus and diestrus in addiction studies (Feltenstein and See, 2007; Fuchs et al., 2005), data from the proestrus and diestrus rats were combined (non-estrus) and compared with those of rats in estrus as described previously (Kerstetter et al., 2008; Nicolas et al., 2019). See figure S2 for representative images of the different stages of the estrous cycle.
2.5. Surgical procedures
2.5.1. Osmotic minipump implantation
Rats were anesthetized with isoflurane (1–3% isoflurane) and received minipumps (14-day pumps; Durect Corporation, Cupertino, CA, USA) filled with either saline or nicotine dissolved in saline. The nicotine concentration was adjusted to compensate for differences in body weight and to deliver a dose of 3.16 mg/kg/day nicotine free base.
2.5.2. Electrode implantation
Rats were anesthetized with isoflurane and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). The head of the rat was shaved from the area between the eyes to the back of the head, and a 1-cm incision was made. After cleaning the skull, 5 small holes were drilled, and 4 skull screws (0–80 × 1/16, Plastics One, Roanoke, VA, USA) were implanted. The fifth hole was used for the electrode. The coordinates for the electrodes were based on previous studies in which electrodes were implanted in the medial forebrain bundle of adult rats (Epping-Jordan et al., 1998; Qi et al., 2016). The electrodes (11 mm in length, Plastics One) were inserted in the medial forebrain bundle with the incisor bar set 5 mm above the interaural line (−0.5 AP, ±1.7 ML, −8.3 DV from dura). After at least 7 days of recovery, the rats were trained on a modified discrete-trial ICSS procedure (Bruijnzeel et al., 2009; Markou and Koob, 1992).
2.6. Behavioral tests
2.6.1. ICSS procedure
The operant conditioning chambers were housed in sound-attenuating chambers (Med Associates, Georgia, VT, USA). The operant conditioning chambers had a 5 cm wide metal response wheel that was centered on a sidewall, and a photobeam detector recorded every 90 degrees of rotation. Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA, USA). The rats were first trained to turn the wheel on a fixed ratio 1 (FR1) schedule of reinforcement. Each quarter turn of the wheel resulted in the delivery of a 0.5 s train of 0.1 ms cathodal square-wave pulses at a frequency of 100 Hz. After the acquisition of responding on this FR1 schedule, defined as 100 reinforcements within 10 minutes, the rats were trained on a discrete-trial current-threshold procedure. The discrete-trial current-threshold procedure is a modification of a task developed by Kornetsky and Esposito (Kornetsky and Esposito, 1979), and previously described in detail (Bruijnzeel and Markou, 2003, 2004). Each trial began with the delivery of a non-contingent electrical stimulus, followed by a 7.5 s response window during which the animal could respond to receive a second identical stimulus. A response during this 7.5 s response window was labeled a positive response, while the lack of a response was labeled a negative response. During the 2 s period immediately after a positive response, additional responses had no consequence. The inter-trial interval (ITI), which followed either a positive response or the end of the response window, had an average duration of 10 s (ranging from 7.5 s to 12.5 s). Responses that occurred during the ITI resulted in a further 12.5 s delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the ITI and delay periods induced by time-out responses were increased until the animals performed consistently at standard test parameters. The training was completed when the animals responded correctly to more than 90% of the non-contingent electrical stimuli. It took 1 to 2 weeks of training for most rats to meet this response criterion. The rats were then tested on the current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. Each test session consisted of four alternating series of descending and ascending current intensities starting with a descending sequence. Blocks of three trials were presented to the rats at a given stimulation intensity, and the intensity was altered systematically between blocks of trials by 5 μA steps. The initial stimulus intensity was set 30 μA above the baseline current-threshold for each animal. All the rats were tested at least 5 days on the current threshold procedure before the minipumps were implanted. Each test session typically lasted 30–40 minutes and provided two dependent variables for behavioral assessment (brain reward thresholds and response latencies). The brain reward threshold (μA) was defined as the midpoint between stimulation intensities that supported responding and stimulation intensities that failed to support responding. The response latency (s) was defined as the time interval between the beginning of the non-contingent stimulus and a positive response. A decrease in reward thresholds is indicative of the potentiation of reward function (Kornetsky and Esposito, 1979). Drugs that have sedative effects or induce motor impairments increase the response latency, and stimulants decrease the response latency (Igari et al., 2013; Liebman, 1985).
2.6.2. Somatic withdrawal signs
Rats were observed for 10 minutes in Plexiglas observation chambers (25 × 25 × 46 cm; L × W × H) with 0.5 cm of corncob bedding. The rats were habituated to the observation chamber for 5 minutes per day on 3 consecutive days before testing with mecamylamine. The rats received 2 mg/kg (sc) of mecamylamine and were placed in the observation chamber 15 min later. The rats received 2 mg/kg of mecamylamine because in previous studies we showed that this dose induces withdrawal signs in nicotine-treated male rats but not in saline-treated control rats (Bruijnzeel et al., 2010; Rylkova et al., 2008). The following somatic signs were recorded: body shakes, cheek tremors, escape attempts, gasps, genital licks, head shakes, ptosis, teeth chattering, writhes, and yawns (Malin et al. 1992; Rylkova et al. 2008). Ptosis was counted once per minute if present continuously. The total number of somatic signs was defined as the sum of the individual occurrences. For the statistical analyses, the signs were divided into the following categories: abdominal constrictions which included gasps and writhes; shakes included head shakes, and body shakes; ptosis; other signs occurred occasionally and included all other recorded signs.
2.6.3. Large open field test
The large open field test can be used to assess anxiety-like behavior (Liebsch et al., 1998). The test was conducted as described previously (Bruijnzeel et al., 2016; Qi et al., 2016). The open field apparatus is a large arena measuring 120 × 120 × 60 cm (L × W × H) and is placed in a brightly lit (500 lux) room. The open field was divided into 16 zones (30 × 30 cm). The border zone consisted of the 12 border squares, and the center zone consisted of the 4 central squares. The arena is made of black high-density polyethylene panels that are fastened together and placed on a plastic bottom plate (Faulkner Plastics, Miami, FL) with gray laminate floor covering. The rats’ behavior was recorded with a camera mounted above the arena and analyzed manually with EthoVision XT 11.5 software (Noldus Information Technology, Leesburg, VA). The following behaviors were analyzed: total number of crossings, border crossings, center crossings, time in the border zone, and time in the center zone. The open field was cleaned with a Nolvasan solution between rats.
2.6.4. Elevated plus maze test
The elevated plus maze test is used to assess anxiety-like behavior and was conducted as described previously (Qi et al., 2016; Rylkova et al., 2009). The test setup consists of four black polypropylene arms (Coulbourn Instruments, Whitehall, PA). The two “open” arms had 0.5 cm ledges, and the two “closed” arms had 30 cm walls. The open arms were placed opposite of each other. The arms were 10 cm wide, 50 cm long, and were placed on 55 cm tall acrylic legs. Testing occurred in a quiet, brightly lit room. At the beginning of each test, the rats were placed in the center of the apparatus facing an open arm. Rats were allowed to explore the device for 5 min, and their behavior was recorded with a camera that was mounted above the maze. Behavior was scored automatically using EthoVision XT 11.5 software. The following behaviors were analyzed: total distance traveled, duration in the open and closed arms, and the number of open and closed arm entries. It was considered an open arm entry when the center of the rat was in one of the open arms. The apparatus was cleaned with a Nolvasan solution between rats.
2.6.5. Small open field test
The small open field test was conducted to assess locomotor activity (Bruijnzeel et al., 2016; Febo et al., 2003). The total distance traveled, and vertical beam breaks (i.e., rearing) were measured with an automated animal activity cage system in a dark room (AccuScan Instruments, Columbus, OH, USA). The system consisted of four animal activity cages made of clear acrylic (40 cm × 40 cm × 30 cm; L × W × H), with 16 equally spaced (2.5 cm) infrared beams across the length and width of the cage. One set of infrared beams was positioned 2 cm above the cage floor (horizontal activity beams), and another set of beams was placed 14 cm above the cage floor (vertical activity beams). All beams were connected to a VersaMax analyzer which sent information to a computer that displayed beam data through VersaDat software. The test setup was cleaned with a Nolvasan solution between animals.
2.7. Statistics
Data were analyzed with multi-factor ANOVAs appropriate for each experimental design, using IBM SPSS Statistics version 25 or GraphPad Prism version 7. Behavioral test data were analyzed using three-factor ANOVAs, with minipump content and sex as between-subjects factors and time or mecamylamine dose as the within-subjects factor. For all statistical analyses, significant interactions in the ANOVA were followed by Bonferroni’s post hoc tests to determine which groups differed from each other. P values less or equal to 0.05 were considered significant. Significant main effects and interactions are reported in the Results section.
3. Results
3.1. Experiment 1: Sex differences in ICSS and nicotine withdrawal
3.1.1. Precipitated withdrawal and ICSS (nicotine on board)
There were no differences in the absolute brain reward thresholds and response latencies between the male and female rats before the implantation of the minipumps (male thresholds 100.4 ± 7.4 μA, male latencies 3.3 ± 0.1 s, female thresholds 95.1 ± 5.3 μA, female latencies 3.1 ± 0.1 s). There was also no effect of the stage of the estrous cycle on the absolute brain reward thresholds and the response latencies during the 5 days before the implantation of the minipumps (Table 1). The administration of nicotine briefly decreased the brain reward thresholds (F1,31=11.08, p<0.01, Fig. 1A) and the response latencies F1,31=12.63, p<0.01, Fig. 1B). Furthermore, during these 5 days, the reward thresholds of the males slightly increased and those of the females slightly decreased (Days × Sex, F1,31=18.57, P<0.0001, Fig. S3A). There was no effect of sex on the response latencies (Fig. S3B). A separate ANOVA showed that on day 5 (test day before the onset of mecamylamine injections), there were no differences in the reward thresholds and response latencies between the nicotine-treated rats and the saline-treated control rats or between the male and female rats.
Table 1.
Absolute brain reward thresholds and response latencies during stages of the estrous cycle
| N | Thresholds (μA) | Latencies (s) | |
|---|---|---|---|
| Proestrus | 26 | 92.5 ± 4.5 | 3.1 ± 0.1 |
| Estrus | 21 | 93.9 ± 5.1 | 3.2 ± 0.1 |
| Diestrus | 43 | 97.4 ± 3.8 | 3.1 ± 0.1 |
Figure 1. Continuous nicotine administration lowers the brain reward thresholds and decreases the response latencies.
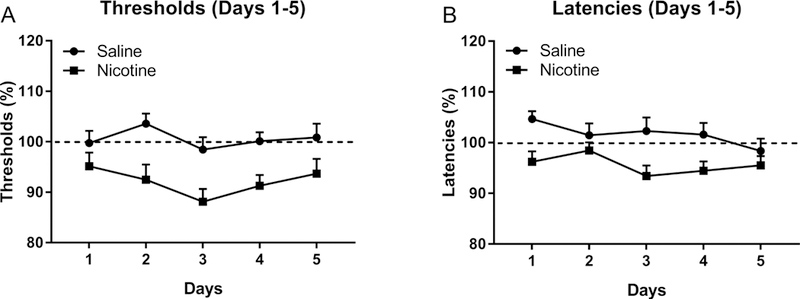
The rats were prepared with minipumps that contained nicotine or saline and the brain reward thresholds and response latencies were determined daily. N=8–10/group. Data are expressed as means ± SEM.
Mecamylamine increased the brain reward thresholds of the nicotine-treated rats, but not those of the saline-treated control rats (Nicotine treatment F1,31=8.64, P<0.01; Mecamylamine dose F3,93=10.31, P<0.0001; Mecamylamine dose × Nicotine treatment F3,93=5.15, P<0.001, Fig. 2A.). The effect of mecamylamine on the reward thresholds was greater in the nicotine-treated males than the nicotine-treated females (Sex × Nicotine treatment F1,31=4.77, P<0.05). The posthoc analysis indicated that 1 mg/kg of mecamylamine increased the brain reward thresholds of the nicotine-treated male rats but not those of the nicotine-treated female rats, but the 3 mg/kg dose had the same effect in the nicotine- treated males and females. Mecamylamine also increased the response latencies (Mecamylamine dose F3,93=8.9, P<0.0001, Fig. 2B). The effect of mecamylamine on the response latencies was greater in the nicotine-treated rats than the saline-treated control rats (Nicotine treatment F1,31=4.65, P<0.05; Mecamylamine dose × Nicotine treatment F3,93=7.70, P<0.0001). The sex of the rats did not affect the response latencies.
Figure 2. Sex differences in precipitated but not spontaneous nicotine withdrawal.
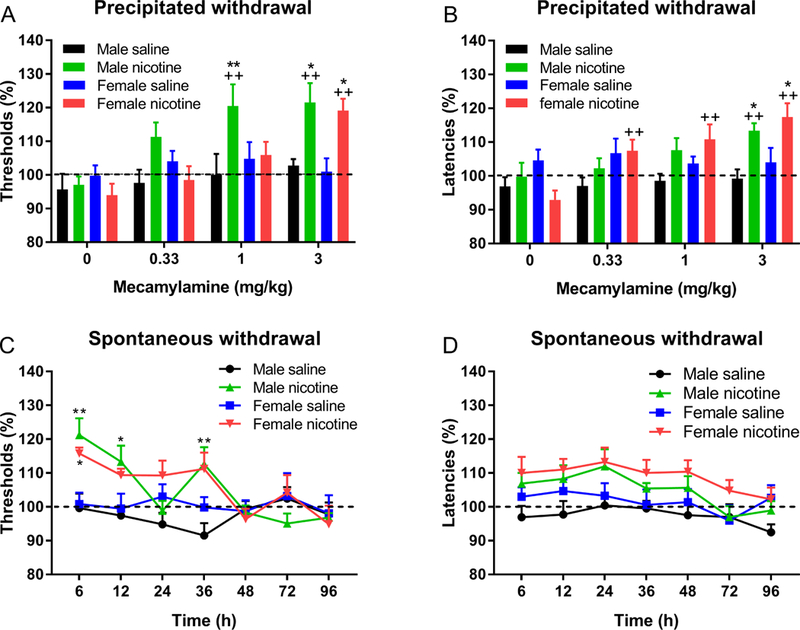
Rats were prepared with minipumps that contained nicotine or saline and the effects of mecamylamine-precipitated and spontaneous withdrawal on brain reward thresholds (A, C) and response latencies (B, D) were investigated. A, B) Asterisks indicate significantly different compared to rats of the same sex that received saline pumps and were treated with the same dose of mecamylamine. Plus signs indicate significantly different from rats in the same group (sex and pump content) and treated with vehicle (0 mecamylamine). C) Asterisks indicate significantly different from rats of the same sex and prepared with saline pumps. * p<0.05, ** ++ P<0.01. N=8–10/group. Data are expressed as means ± SEM.
3.1.2. Spontaneous withdrawal and ICSS (post pump removal)
Removal of the minipumps led to an elevation in brain reward thresholds of the nicotine-treated rats but not those of the saline-treated rats (Nicotine treatment: F1,31=9.05, P<0.01; Time: F6,186=5.61, P<0.0001; Nicotine treatment × Time: F6,186=6.68, P<0.0001, Fig. 2C). There was no effect of sex on the nicotine-withdrawal induced increase in brain reward thresholds. Removal of the minipumps led to an increase in the response latencies of the nicotine-treated rats but not in those of the saline-treated rats (Nicotine treatment: F1,31=7.69, P<0.01; Time: F6,186=5.202, P<0.0001, Fig. 2D). There was no effect of the stage of the estrous cycle on the elevations in brain reward thresholds or the increase in response latencies associated with nicotine withdrawal at the 6 or 12 h time point (estrus n=4, non-estrus n=6; Table S1). There was no significant difference in brain reward thresholds and response latencies between the 6 and 12 h time points in the female nicotine rats. When the ICSS data from these two time points were combined, there was no effect of the stage of the estrous cycle on the reward thresholds or response latencies during nicotine withdrawal (estrus n=8, non-estrus n=12).
3.1.3. Large open field test (post pump removal)
The large open field test was conducted 3 days after the removal of the minipumps. There was no effect of nicotine treatment on total crossings, crossings in the border or center zone, and time spent in the border or center zone. The total number of crossings was higher in females than males (F1,32=17.79, P<0.0001, Fig. 3A). The females had a higher number of crossings in the border zone (F1,32=16.47, P<0.0001) and the center zone (F1,32=9.90, P<0.01, Fig. 3A). Compared to the males, the females spent less time in the border zone (F1,32=5.96, P<0.05, Fig. 3B) and more time in the center zone (F1,32=6.13, P<0.05).
Figure 3. Sex differences in the large open field test.
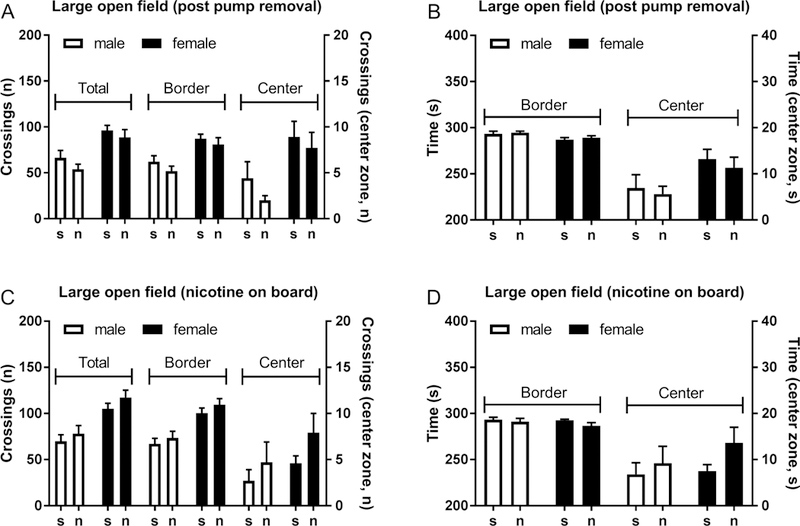
Rats were tested in the large open field 3 days after the removal of the nicotine and saline pumps (A, B) or 6 days after the implantation of the nicotine and saline pumps (C, D). The female rats traveled a greater distance in the open field than the male rats, but chronic nicotine administration or cessation of nicotine administration did not affect open field behavior. N=7–10/group. Data are expressed as means ± SEM.
3.1.4. Elevated plus maze test (post pump removal)
The elevated plus maze test was conducted 4 days after the removal of the minipumps. Prior treatment with nicotine decreased the total distance traveled in the elevated plus maze test (F1,31=12.21, P<0.01, Fig. 4A), but there was no effect of sex on the total distance traveled. The females made more entries in the open arms compared to the males (F1,31=11.37, P<0.01, Fig. 4B), but there was no sex difference in the number of closed arm entries (Fig. S4A). Open or closed arm entries were not affected by nicotine treatment. There was a strong trend towards the females have a higher percentage of open arm entries compared to the males (F1,31=4.092, P=0.0518, Fig. 4C). Sex or nicotine treatment did not affect the amount of time in the closed arms. However, prior nicotine treatment increased the amount of time on the open arms in the males (Nicotine treatment F1,31=6.277, P<0.05; Sex × Nicotine treatment F1,31=4.618, P<0.05, Fig S4B). Nicotine treatment increased the percentage of time on the open arms in the males, but not in the females (Sex × Nicotine treatment F1,31=6.11, P<0.05, Fig. 4D).
Figure 4. Decreased locomotor activity in the elevated plus maze in males and females after chronic nicotine administration.
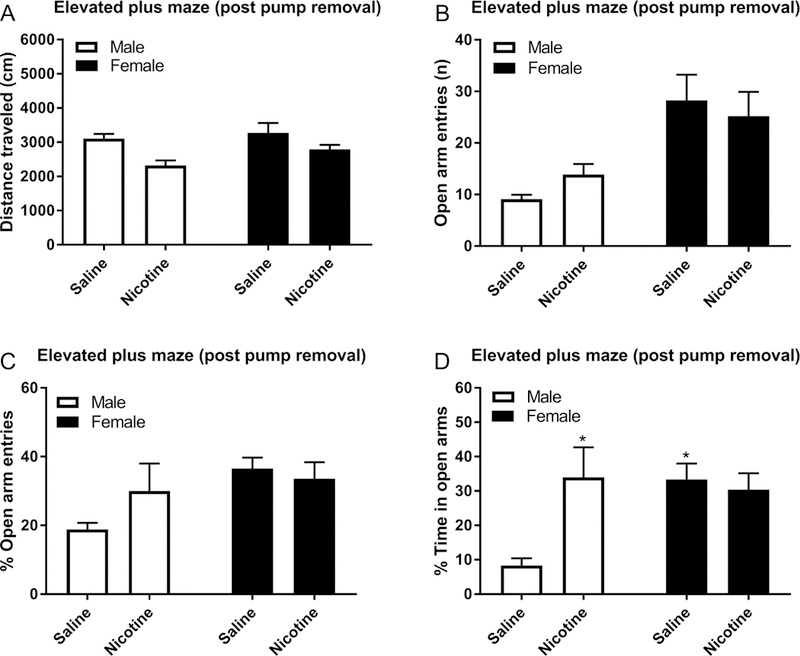
Rats were exposed to nicotine or saline for 14 days and tested on the elevated plus maze 4 days after removing the minipumps. Total distance traveled (A), the number of open arm entries (B), percentage open arm entries (C), and percentage time in open arms (D) were determined. Asterisks indicated a higher percentage of time on the open arms compared to male rats treated with saline. * p<0.05. N=8–10/group. Data are expressed as means ± SEM.
3.2. Experiment 2: Sex differences in somatic signs and locomotor effects of continuous nicotine administration
3.2.1. Somatic withdrawal signs (nicotine on board)
Mecamylamine precipitated a larger number of somatic signs (F1,26=13.56, P<0.001, Fig. 5), abdominal constrictions (F1,26=16.61, P<0.0001, Table 2), and occurrences of ptosis (F1,26=5.37, P<0.05, Table 2) in the nicotine-treated rats than the saline-treated control rats. The male rats treated with nicotine displayed more somatic signs (Sex F1,26=4.92, P<0.05; Sex × Nicotine treatment F1,26=6.44, P<0.05) and abdominal constrictions (Sex F1,26=16.61, P<0.0001; Sex × Nicotine treatment F1,26=10.80, P<0.01 ) than the female rats treated with nicotine. Furthermore, the males had more occurrences of ptosis (Sex, F1,26=6.22, P<0.05) and shakes (Sex, F1,26=6.22, P<0.01) compared to the female rats.
Figure 5. Sex differences in mecamylamine-precipitated somatic withdrawal signs.
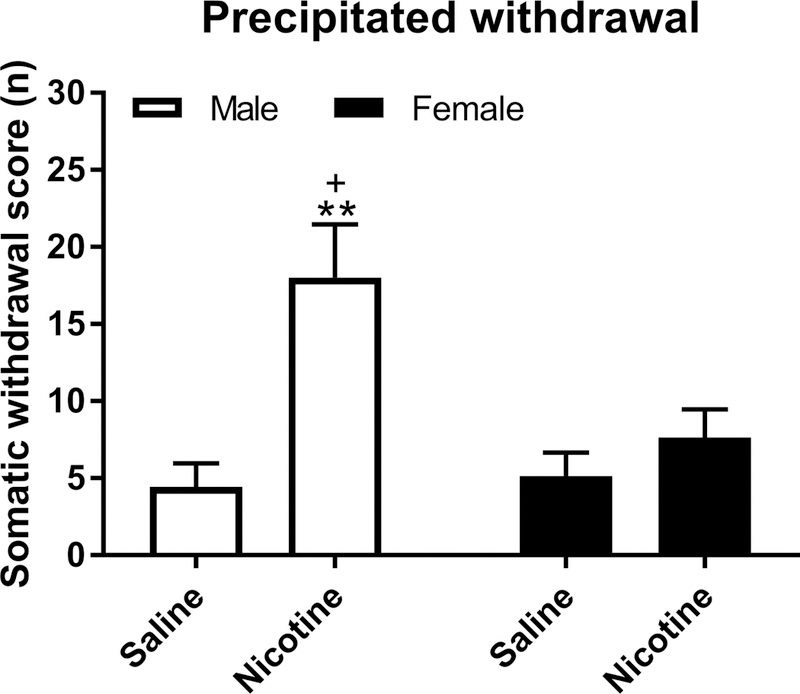
Rats were treated with nicotine for 10 days and then withdrawal was precipitated and somatic signs were counted. The total number of somatic signs are shown. The plus sign indicates more somatic signs compared to female rats that were prepared with nicotine pumps. Asterisks indicate more somatic signs compared to male and female rats that were prepared with saline pumps. + p<0.05, ** P<0.01. N=7–8/group. Data are expressed as means ± SEM.
Table 2.
Individual somatic withdrawal signs.
| N | Abdominal constrictions |
Ptosis | Shakes | Other | |
|---|---|---|---|---|---|
| Male saline | 7 | 1.0 ± 0.3 | 3.1 ± 1.3 | 0.3 ± 0.2 | 0.0 ± 0.0 |
| Male nicotine | 7 | 8.0 ± 1.8** | 9.3 ± 1.7+ | 0.1 ± 0.1 | 0.6 ± 0.4 |
| Female saline | 8 | 0.3 ± 0.2 | 2.0 ± 1.1 | 2.4 ± 1.0 | 0.5 ± 0.3 |
| Female nicotine | 8 | 1.0 ± 0.3 | 2.9 ± 1.7 | 3.1 ± 1.0 | 0.6 ± 0.4 |
Asterisks (p<0.01) indicate more abdominal constrictions compared to all other groups.
Plus sign (p<0.05) indicates more occurrences of ptosis compared to the female groups.
3.2.2. Small open field test (nicotine on board)
In the small open field test, there was an effect of sex and time on the distance traveled (Sex F1,26=26.17, P<0.0001; Time F2,52=267.09, P<0.0001, Fig. 6A, B). Chronic nicotine treatment had a larger effect on locomotor activity in the females than the males (Sex × Nicotine treatment F1,26=8.78, P<0.01) and the females were also more active at the beginning of the test than the males (Time × Sex F2,52=10.36, P<0.0001, Fig. 6A, B). The posthoc showed that female-nicotine rats traveled a greater distance than all other groups. There was an effect of sex on rearing activity, with the females showing more rearing activity than the males (Sex F1,26=14.63, P<0.001; Time F2,52=162.75, P<0.0001, Fig. 6C, D). Rearing activity was not affected by chronic nicotine treatment.
Figure 6. Chronic exposure to nicotine Increases locomotor activity in female rats in the small open field.
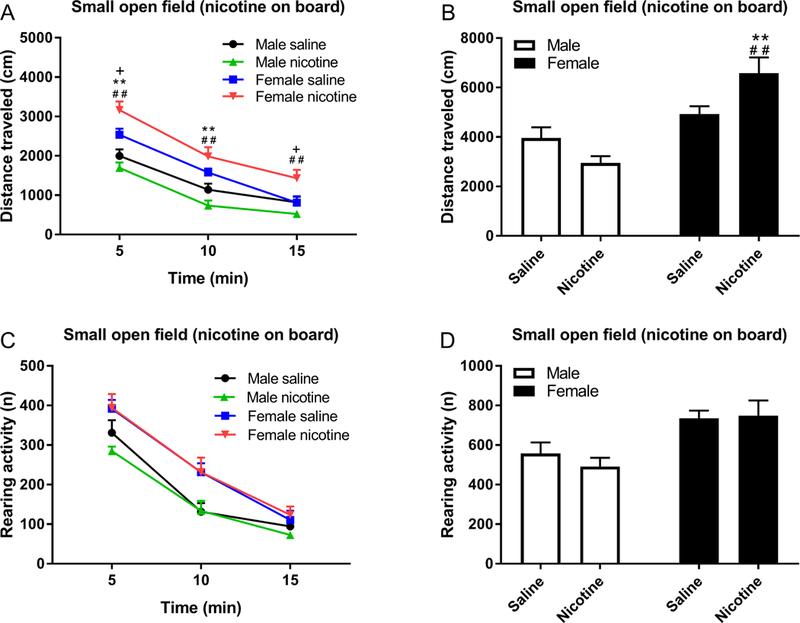
Rats were prepared with nicotine or saline pumps and locomotor activity (A, B) and rearing (C, D) was investigated on day 13. A) Pound signs indicate a larger distance traveled compared to rats in the male nicotine group, plus signs indicate a larger distance traveled compared to rats in the female saline group, and asterisks indicate a larger distance traveled compared to rats in the male saline group. **, ##, ++ P<0.01; + P<0.05. N=7–8/group. Data are expressed as means ± SEM.
3.2.3. Large open field test (nicotine on board)
The females traveled a greater distance (more crossings) in the large open field than the males (F1,30=21.28, P<0.0001, Fig. 3C) and this was not affected by chronic nicotine-treatment. The females traveled a greater distance than the males in the border zone (F1,30=25.31, P<0.0001), and the males and females traveled the same distance in the center zone (Fig. 3C). The males and the females spent the same amount of time in the border zone and center zone and the time they spent in the two zones was not affected by nicotine treatment (Fig. 3D).
4. Discussion
The main goal of the present study was to determine sex differences in the effects of precipitated and spontaneous nicotine withdrawal on emotional states in rodents. This study showed that a low dose of mecamylamine (1 mg/kg) elevated the brain reward thresholds of nicotine-treated male rats, but not those of nicotine-treated female rats. There was no difference in mecamylamine-induced elevations in brain reward thresholds between nicotine-treated males and females when a high dose of mecamylamine (3 mg/kg) was used. Furthermore, there was no difference in the elevations in brain reward thresholds between nicotine-treated male and female rats during spontaneous withdrawal. A relatively low dose of mecamylamine (2 mg/kg) also induced more somatic withdrawal signs in nicotine- treated male rats than female rats. Thus, a low dose of mecamylamine precipitates a greater deficit in reward function and more somatic withdrawal signs in nicotine-treated male than female rats, but there is no sex difference in the reward deficit when a high dose of mecamylamine is used or during spontaneous nicotine withdrawal.
In the present study, the effects of precipitated and spontaneous nicotine withdrawal on reward function were investigated. Our findings suggest that there are no sex differences in impairments in reward function between males and females after the administration of a high dose of mecamylamine and during spontaneous withdrawal. This is in line with a study in human smokers that showed that withdrawal signs are affected by the level of dependence but not by the sex of smokers (Langdon et al., 2013). Another study, with hundreds of adolescent smokers, also found no differences in the type or frequency of withdrawal symptoms (craving, nervous and tense, restless, irritable, hungry, poor concentration, sad, sleep problems) between males and females (Rojas et al., 1998). The present studies are not in line with a conditioned place aversion study in which nicotine-treated female rats displayed more place aversion compared to nicotine-treated male rats (O’Dell and Torres, 2014). In the study mentioned above, nicotine and mecamylamine doses were not reported; therefore, it is not known if differences between our study and the place aversion study were due to dose differences. One difference between the ICSS paradigm and the place conditioning procedure is that the ICSS procedure directly measures the state of the brain reward system while the place conditioning procedure measures the behavioral response to a previous experience. Interestingly, many studies have shown that females are better at avoiding and escaping places where they have had an aversive experience (Beatty and Beatty, 1970; Dalla and Shors, 2009; Saavedra et al., 1990). Therefore, the difference between the males and females in the place aversion procedure might not reflect a greater deficit in reward function during nicotine withdrawal, but better avoidance behavior in the females. It might also be possible that different neuronal networks mediate place aversion and elevations in ICSS thresholds.
Before investigating the effects of nicotine and nicotine withdrawal on brain reward thresholds, it was determined if the stage of the estrous cycle affects the brain reward thresholds. There were no differences in the brain reward thresholds between the different stages of the estrous cycle (proestrus, estrus, or diestrus). This is in line with previous work that has shown that the stage of the estrous cycle does not affect reward thresholds in the ICSS procedure (Rao and Desiraju, 1990; Stratmann and Craft, 1997). Furthermore, the stage of the estrous cycle did not affect the elevations in brain reward thresholds associated with spontaneous nicotine withdrawal. This is in line with previous studies that have shown that the stage of the estrous cycle does not affect nicotine withdrawal (Hamilton et al., 2009). It should be noted that in the present study the group sizes were relatively small (estrus n=4, non-estrus n=6), and therefore it cannot be ruled out that with larger group sizes an effect of the estrous phase on nicotine withdrawal might have been observed.
It was also investigated if the rats display increased anxiety-like behavior in the large open field test and the elevated plus maze test after cessation of nicotine administration. In the large open field test, the female rats traveled a greater distance than the male rats and also spent more time in the center of the open field, but there was no effect of prior nicotine exposure. This suggests that exposure to nicotine does not have a long term effect of anxiety-like behavior in the large open field test. In the elevated plus maze, the females traveled a greater distance than the males, made more entries in the open arms, and spent more time in the open arms. Prior exposure to nicotine did not affect the percentage of open arm entries, which suggest that prior nicotine exposure did not increase-anxiety-like behavior. We also found that the female control rats and nicotine-treated female rats spent the same amount of time on the open arms. The male control rats spent less time on the open arms than the nicotine-treated male rats. It was somewhat surprising that the male control rats spent less time on the open arms than the nicotine-treated male rats. This pattern of results suggest that the male control rats might have been somewhat stressed during the elevated plus maze test, which limits the interpretation of sex differences in this test.
Many studies suggest that rodents display increased anxiety-like behavior immediately after cessation of nicotine administration, but some studies also suggest that prior nicotine exposure has no effect on anxiety-like behavior or reduces anxiety-like behavior (Chae et al., 2008; Damaj et al., 2003; Irvine et al., 2001; Kota et al., 2008; Morud et al., 2018; Stoker et al., 2008). In the present study, we did not observe the increase in anxiety-like behavior after the cessation of nicotine administration that other studies reported (Chae et al., 2008; Damaj et al., 2003; Irvine et al., 2001). It cannot be ruled out that this is related to the time point at which we investigated anxiety-like behavior in the elevated plus maze test. The rats were tested in the elevated plus maze 4 days after the removal of the minipumps while nicotine withdrawal-induced anxiety-like behavior is most severe during the first few days after cessation of nicotine administration (Damaj et al., 2003). Damaj et al., (2003) reported increased anxiety-like behavior in the elevated plus maze in male mice 2 and 3 days after the cessation of nicotine administration, but not at later time points. Furthermore, other studies reported an increase in anxiety-like behavior in rats 1 to 3 days after cessation of nicotine administration (Chae et al., 2008; Irvine et al., 2001). Therefore, it might have been possible that in the present study, an increase in anxiety-like behavior could have been observed at an earlier time point.
In the present study, we found that low doses of mecamylamine induced more affective and somatic withdrawal signs in male than female rats. One possible explanation for this differential sensitivity to mecamylamine in nicotine-treated male and female rats could be that nicotine administration induces a faster or larger increase in nAChRs in male than female rats. This is supported by studies that showed that male smokers have higher levels of β2 containing nAChR levels than female smokers, while there are no differences in β2 nAChR levels between males and females who do not smoke (Cosgrove et al., 2012). An animal study showed that once a day nicotine administration to rats leads to an upregulation of nAChRs in males but not in females (Koylu et al., 1997). However, chronic continuous administration of nicotine, like in the present study, increases nAChR levels in female rodents (Hoegberg et al., 2015; Marks et al., 1985). Therefore, it is unlikely that the current nicotine exposure protocol did not lead to an increase in nAChR levels in females.
To our knowledge, this is the first study that compared precipitated somatic nicotine withdrawal signs between males and females. Several previous studies examined spontaneous somatic withdrawal signs in males and females (Hamilton et al., 2009; Torres et al., 2013). One study found more somatic nicotine withdrawal signs in male than female rats, but this effect did not reach statistical significance (Torres et al., 2013). Another study found that the expression of somatic withdrawal signs in males and females is dependent on the lighting conditions (Hamilton et al., 2009). In a brightly-lit environment, the males and females had the same number of somatic withdrawal signs, and in a dimly-lit environment, the females displayed more somatic withdrawal signs. There are strong similarities between somatic nicotine withdrawal and somatic opioid withdrawal signs. A slightly modified opioid withdrawal scale is used to determine nicotine withdrawal, but overall fewer nicotine withdrawal signs are counted than opioid withdrawal signs (Malin et al., 1992). Many studies have also reported increased precipitated opioid withdrawal signs in male compared to female rats and mice (Craft et al., 1999; Kest et al., 2001; Radke et al., 2013). Taken together, these studies suggest that there are of sex differences in somatic nicotine withdrawal signs and whether the males or females show more signs might depend on the test conditions.
Previous studies have shown that acute nicotine administration affects locomotor activity (Bruijnzeel et al., 2011; Clarke and Kumar, 1983), but little is known about the effects of chronic nicotine administration on locomotor activity in male and female rats. In the present study, the rats were chronically treated with saline or nicotine and then tested in a small and large open field. The females traveled a greater distance than the males in the small and large open field, which is in line with previous studies that have shown that females are more active in open field tests (Bruijnzeel et al., 2019; Gray, 1971). Interestingly, the effects of chronic nicotine administration on locomotor activity were dependent on the sex and test conditions. Chronic nicotine infusion increased locomotor activity in female rats in the small open field but not in the large open field and did not affect activity in male rats in the small and large open field. These findings suggest that females, even after prolonged exposure, do not develop tolerance to the stimulant-like effects of nicotine. It is not completely clear why the effects of nicotine in females are dependent on the test environment. Both tests were conducted under the same lighting conditions (brightly lit environment) and the rats had not been exposed to the experimental setups before. One possible explanation is that the rats were more fearful in the large open field than the small open field and this might have suppressed the locomotor enhancing effects of nicotine in the large open field. Another possible difference between the two tests was that the animals were tested in the small open field one week after they had been tested in the large open field. It might be possible that more prolonged exposure to nicotine contributed to the larger nicotine-induced increase in locomotor activity in the female rats in the small open field test.
One potential point of concern is that in the present studies the sex differences were mainly observed during mecamylamine-precipitated withdrawal and there is little clinical evidence for precipitated withdrawal in smokers. One clinical study reported that oral mecamylamine does not precipitate nicotine withdrawal in smokers (Eissenberg et al., 1996). However, it has also been shown that mecamylamine leads to higher levels of intravenous nicotine self-administration in humans with a history of smoking (Rose et al., 2003). It was suggested that smokers treated with mecamylamine had higher levels of nicotine intake because mecamylamine prevents nicotine from diminishing withdrawal symptoms (Rose et al., 2003). In our previous work, we also found that mecamylamine increases nicotine self-administration in nicotine dependent rats (Geste et al., 2019). A study with nicotine-treated and untreated rhesus monkeys showed that the nicotine-treated monkeys are more sensitive to the effects of mecamylamine in a drug discrimination study (Cunningham et al., 2014). It was suggested that this was due to mecamylamine-induced nicotine withdrawal. Overall, these findings suggest that there are some similarities and discrepancies in findings with mecamylamine in nicotine dependent rodents and smokers. Some of these discrepancies might be due to the route of mecamylamine administration (oral vs subcutaneous), level of dependence, or metabolism of mecamylamine in humans versus rodents. Another concern is that in the present study, sex differences in nicotine withdrawal were observed after the administration of mecamylamine, but not after the removal of the minipumps. Rats excrete mecamylamine in the urine, and there are sex differences in renal clearance of drugs (Soldin et al., 2011). In addition to this, sex differences in the effect of mecamylamine on antinociception and reward function have been observed (Chi and de Wit, 2003; Chiari et al., 1999). These findings suggest that sex differences in renal clearance and sensitivity to mecamylamine could have contributed to the outcome of this study.
In conclusion, the present studies indicate that chronic nicotine administration leads to nicotine dependence in male and female rats and increased activity in female rats in a small open field. Nicotine- treated male rats are more sensitive to a low dose of mecamylamine than nicotine-treated female rats. However, a high dose of mecamylamine and removal of the minipumps leads to a similar increase in brain reward thresholds in the nicotine-treated male and female rats. The present findings suggest that there are sex differences in the expression of nicotine withdrawal in rats, but these sex differences are mainly observed after the administration of low doses of mecamylamine.
Supplementary Material
Highlights.
A low dose of mecamylamine precipitates nicotine withdrawal in males but not females
There is no sex difference in reward deficits during spontaneous nicotine withdrawal
The stage of the estrous cycle does not affect the brain reward threshold of rats
Chronic nicotine administration only increases locomotor activity in female rats
There is no increase in anxiety 3–4 days after cessation of nicotine administration
Acknowledgments:
This work was supported by a NIDA/NIH and FDA Center for Tobacco Products (CTP) grant (DA042530) to AB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. ST was supported by a visiting fellowship from the Chinese Scholarship Council (201608430251).
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson H, Rehm S, Stanislaus D, Wood CE, 2013. Scientific and regulatory policy committee (SRPC) paper: assessment of circulating hormones in nonclinical toxicity studies III. female reproductive hormones. Toxicologic pathology 41, 921–934. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG, 2002. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol.Sci. 23, 475–482. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Beatty PA, 1970. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. Journal of comparative and physiological psychology 73, 446. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, 2012. Tobacco addiction and the dysregulation of brain stress systems. Neurosci.Biobehav.Rev. 36, 1418–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H, 2010. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 212, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Knight P, Panunzio S, Xue S, Bruner MM, Wall SC, Pompilus M, Febo M, Setlow B, 2019. Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A, 2003. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50, 20–28. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A, 2004. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology 47, 572–579. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S, 2009. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol.Psychiatry 66, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Qi X, Guzhva LV, Wall S, Deng JV, Gold MS, Febo M, Setlow B, 2016. Behavioral Characterization of the Effects of Cannabis Smoke and Anandamide in Rats. PLoS One 11, e0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Rodrick G, Singh RP, Derendorf H, Bauzo RM, 2011. Repeated pre-exposure to tobacco smoke potentiates subsequent locomotor responses to nicotine and tobacco smoke but not amphetamine in adult rats. Pharmacol.Biochem.Behav. 100, 109–118. [DOI] [PubMed] [Google Scholar]
- Chae Y, Yeom M, Han J-H, Park H-J, Hahm D-H, Shim I, Lee H-S, Lee H, 2008. Effect of acupuncture on anxiety-like behavior during nicotine withdrawal and relevant mechanisms. Neuroscience letters 430, 98–102. [DOI] [PubMed] [Google Scholar]
- Chi H, de Wit H, 2003. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcoholism: Clinical and Experimental Research 27, 780–786. [DOI] [PubMed] [Google Scholar]
- Chiari A, Tobin JR, Pan H-L, Hood DD, Eisenach JC, 1999. Sex differences in cholinergic analgesia I: a supplemental nicotinic mechanism in normal females. Anesthesiology 91, 1447–1454. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R, 1983. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br.J.Pharmacol. 80, 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Lanza ST, Chu W, Baker TB, Piper ME, 2017. Anhedonia: Its Dynamic Relations With Craving, Negative Affect, and Treatment During a Quit Smoking Attempt. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco 19, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB, 2015. Anhedonia as a component of the tobacco withdrawal syndrome. Journal of abnormal psychology 124, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D, 2004. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine.Tob.Res. 6, 39–47. [DOI] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, Travlos G, 2015. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicologic pathology 43, 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, Krishnan-Sarin S, Staley JK, Picciotto MR, O’Malley SS, 2012. Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Archives of General Psychiatry 69, 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft R, Stratmann J, Bartok R, Walpole T, King S, 1999. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology 143, 1–7. [DOI] [PubMed] [Google Scholar]
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA, 2018. Notes from the field: Use of electronic cigarettes and any tobacco product among middle and high school students— United States, 2011–2018. Morbidity and Mortality Weekly Report 67, 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, Moerke MJ, McMahon LR, 2014. The discriminative stimulus effects of mecamylamine in nicotine-treated and untreated rhesus monkeys. Behav Pharmacol 25, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Shors TJ, 2009. Sex differences in learning processes of classical and operant conditioning. Physiology & behavior 97, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR, 2003. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J.Pharmacol.Exp.Ther. 307, 526–534. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Griffiths RR, Stitzer ML, 1996. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology 127, 328–336. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A, 1998. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393, 76–79. [DOI] [PubMed] [Google Scholar]
- Faulkner P, Petersen N, Ghahremani DG, Cox CM, Tyndale RF, Hellemann GS, London ED, 2018. Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology 235, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Gonzalez-Rodriguez LA, Capo-Ramos DE, Gonzalez-Segarra NY, Segarra AC, 2003. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem 86, 405–412. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE, 2007. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug and Alcohol Dependence 89, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM, 1998. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav.Neurosci. 112, 1423–1429. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE, 2005. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 179, 662–672. [DOI] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME, 2011. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull 85, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geste JR, Levin B, Wilks I, Pompilus M, Zhang X, Esser KA, Febo M, O’Dell L, Bruijnzeel AW,Relationship between nicotine intake and reward function in rats with intermittent short versus long access to nicotine. Nicotine & Tobacco Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, 1971. Sex differences in emotional behaviour in mammals including man: endocrine bases. Acta psychologica 35, 29–46. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, Grunberg NE, 2009. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacology Biochemistry and Behavior 92, 51–59. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, McBride C, Pirie P, Hellerstedt W, Lando H, 1991. Effects of nicotine gum on prevalence and severity of withdrawal in female cigarette smokers. J Subst Abuse 3, 427–440. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, Jasinski DR, 1985. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. Journal of Pharmacology and Experimental Therapeutics 234, 1–12. [PubMed] [Google Scholar]
- Hoegberg BG, Lomazzo E, Lee NH, Perry DC, 2015. Regulation of a402a5 nicotinic acetylcholinergic receptors in rat cerebral cortex in early and late adolescence: sex differences in response to chronic nicotine. Neuropharmacology 99, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW, 2013. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 39, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE, 2001. Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol.Biochem.Behav. 68, 319–325. [DOI] [PubMed] [Google Scholar]
- Jackson PH, Stapleton JA, Russell MA, Merriman RJ, 1986. Predictors of outcome in a general practitioner intervention against smoking. Prev Med 15, 244–253. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE, 2008. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology 198, 63–75. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, 2001. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacology Biochemistry and Behavior 70, 149–156. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Varady A, Kraemer HC, 2002. Do men outperform women in smoking cessation trials?: Maybe, but not by much. Exp Clin Psychopharmacol 10, 295. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, 1979. Euphorigenic drugs: effects on the reward pathways of the brain. Fed.Proc. 38, 2473–2476. [PubMed] [Google Scholar]
- Kota D, Martin B, Damaj M, 2008. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology 198, 201–210. [DOI] [PubMed] [Google Scholar]
- Koylu E, Demirgören S, London ED, Pöǧün S, 1997. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life sciences 61, PL185–PL190. [DOI] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ, 2013. Anhedonia and anxiety sensitivity: Prospective relationships to nicotine withdrawal symptoms during smoking cessation. Journal of studies on alcohol and drugs 74, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman JM, 1985. Anxiety, anxiolytics and brain stimulation reinforcement. Neurosci.Biobehav.Rev. 9, 75–86. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Montkowski A, Holsboer F, Landgraf R, 1998. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res 94, 301–310. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB, 1992. Rodent model of nicotine abstinence syndrome. Pharmacol.Biochem.Behav. 43, 779–784. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP, 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62, 609–614. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF, 1992. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 51, 111–119. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC, 1985. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J.Pharmacol.Exp.Ther. 235, 619–628. [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA, 2012. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of visualized experiments: JoVE 67, e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morud J, Strandberg J, Andrén A, Ericson M, Söderpalm B, Adermark L, 2018. Progressive modulation of accumbal neurotransmission and anxiety-like behavior following protracted nicotine withdrawal. Neuropharmacology 128, 86–95. [DOI] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You Z-B, McCarthy MM, Shaham Y, Ikemoto S, 2019. Incubation of cocaine craving after intermittent access self-administration: sex differences and estrous cycle. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, 2014. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology 76 Pt B, 566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, 1996. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol 4, 166. [Google Scholar]
- Perkins KA, Scott J, 2008. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine & Tobacco Research 10, 1245–1251. [DOI] [PubMed] [Google Scholar]
- Qi X, Guzhva L, Yang Z, Febo M, Shan Z, Wang KK, Bruijnzeel AW, 2016. Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Eur Neuropsychopharmacol 26, 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Holtz NA, Gewirtz JC, Carroll ME, 2013. Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology 227, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DN, Desiraju T, 1990. Comparative assessment of pedal pressing rates of self-stimulation of hypothalamus and midbrain with both square wave and sine wave stimulus parameters. Indian J Physiol Pharmacol 34, 162–170. [PubMed] [Google Scholar]
- Rezvani AH, Levin ED, 2001. Cognitive effects of nicotine. Biol.Psychiatry 49, 258–267. [DOI] [PubMed] [Google Scholar]
- Rojas NL, Killen JD, Haydel KF, Robinson TN, 1998. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 152, 151–156. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, 2003. Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacology Biochemistry and Behavior 76, 307–313. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW, 2008. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides 42, 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylkova D, Shah HP, Small E, Bruijnzeel AW, 2009. Deficit in brain reward function and acute and protracted anxiety-like behavior after discontinuation of a chronic alcohol liquid diet in rats. Psychopharmacology (Berl) 203, 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra MA, Abarca N, Arancibia P, Salinas V, 1990. Sex differences in aversive and appetitive conditioning in two strains of rats. Physiology & behavior 47, 107–112. [DOI] [PubMed] [Google Scholar]
- Skwara AJ, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME, 2012. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behav Brain Res 234, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA, 2016. Sex/gender differences in smoking cessation: a review. Prev Med 92, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A, 2008. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JA, Craft RM, 1997. Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend 46, 31–40. [DOI] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE, 2013. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Frontiers in psychiatry 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall CD, Ginsberg D, Hall SM, 1985. Quitting smoking. International journal of the addictions 20, 1089–1112. [DOI] [PubMed] [Google Scholar]
- WHO, 2018. WHO global report on trends in prevalence of tobacco smoking 2000–2025. Geneva. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


