Abstract
Background
Leptospirosis is a neglected zoonosis of public health importance transmitted through contact with contaminated soil, water or urine of infected animals. In pigs the disease is characterized by abortion, still births and weak piglets. A cross-sectional study was conducted in May to July 2018 to estimate the sero-prevalence of leptospirosis and factors associated with seropositivity in slaughter pigs. A questionnaire was used to collect information on animal demographics. Serum was tested for anti-leptospiral antibodies using microscopic agglutination test (MAT) with a panel of 8 serovars. Sera were considered positive for sero-reactivity at a MAT titre ≥1:40 against at least one serovar. Chi-square tests were used to measure the strength of association between the MAT test result and exploratory variables.
Results
A total of 252 pig serum samples from seven slaughterhouses were tested for Leptospira antibodies by MAT. Of the 252 pigs sampled, 88.8% (244/252) were indigenous breeds; 55.6% (140/252) were female and 88.7% (220/252) were reared in extensive production systems. Eighty-three (32.9%; 83/252) sera samples tested positive on MAT against at least one serovar. Of the 8 serovars, the highest prevalence was recorded for serovar Lora 21.4% followed by Kenya 5.2%, Sokoine 3.6% and Grippotyphosa at 3.2%. Risk factors for leptospirosis seropositivity in pigs were: originating from farms with other types of livestock (OR 2.3; 95% CI 1.0–4.5) and mature pigs (OR 1.9; 95% CI 1.1–3.3).
Conclusion
This study demonstrates that there is a high prevalence of leptospirosis positive pigs at slaughter in a small-holder livestock keeping region of the Lake Victoria basin. The potential for cross species transmission of pathogenic serovars is highlighted as well as the potential for occupational exposure to slaughterhouse personnel. Improvements in husbandry practices (confinement and rodent control) and public health education among slaughterhouse workers and other high-risk groups is recommended.
Keywords: Leptospirosis, Occupational exposure, Slaughterhouse workers, Microscopic agglutination test
Background
Leptospirosis is a bacterial zoonotic disease caused by pathogenic serovars of the genus Leptospira which were historically divided into two species; pathogenic Leptospira interrogans and saprophytic Leptospira biflexa. However, genetic classification has grouped Leptospira spp. into eight pathogenic genomospecies (L. alexanderi, L. alstonii, L. borgpetersenii, L. interrogans, L. kirschneri, L. weilii, L. noguchii and L. santarosai) [1–4]. In addition, Leptospira have been further classified serologically into more than 250 pathogenic serovars [5, 6]. Leptospirosis is transmitted directly through contact with urine or body fluids of infected animals or indirectly through water or soil contaminated with urine from infected animals. Domestic animals including pigs harbor leptospires in the kidneys and genital tracts where they can persist for a long period of time with intermittent shedding in urine. This acts as a source of infection to humans and other animals [7–9]. The level of susceptibility varies within the domestic species and each serovar tends to be maintained in a particular animal species [10]. Animals can be infected with serovars maintained by the same animal species or other animal species in the same geographical location [2].
Porcine leptospirosis has been reported most often in South East Asia and South America due to the favorable weather conditions for environmental survival and transmission of leptospires [11]. In a serological survey in Colombia using the microscopic agglutination test (MAT) in different animal species, a seroprevalence of 55.9% in pigs was reported [7]. Another study in fattening pigs in 5 provinces in Vietnam reported an overall seroprevalence of 8.17% by MAT [12]. In regions where pig management practices include vaccination against leptospirosis, the overall seroprevalence has been on the decline [1, 2]. This decline has also been attributed to improved housing since it limits animal-environmental interaction [1, 2]. A study in pig farms in Greece reported a seroprevalence of 17.8% by MAT [13]. In Sicily Italy, a study of free-roaming semi-wild black swine demonstrated leptospires by PCR targeting the 16S rRNA gene with prevalence of 40% [14]. The higher prevalence was attributed to their wild living conditions [14]. More recent studies in Europe have reported an upward trend of Leptospira infections attributed to climatic changes that results in wetter conditions that promote prolonged survival of the Leptospira bacteria in the environment and change in the herd management practices from indoor intensive to extensive or semi-intensive with outdoor access aimed at improved animal welfare [15–17]. In Africa several prevalence studies have been carried-out providing evidence of occurrence of leptospirosis in animals. Several studies in Tanzania have reported on pig leptospirosis; a serological survey tested 100 pigs using the MAT test showed high percentage of pigs positive to L. kirschneri serovar Sokoine (41%) and to L. borgpetersenii serovar Kenya (27%) [18]. Another cross-sectional survey tested pig sera using MAT in Morogoro municipality, reported an overall prevalence of 4.42%. Of the positive samples this study reported high proportions against L. interrogans serovar Ballum at 47%, L. interrogans serovar Icterohaemorrhagiae at 41% and L. interrogans serovar Pomona at 12% [19]. Porcine leptospirosis results in economic losses in pig farms due to fetal death, abortion, infertility and birth of weak piglets, subfertility as evidenced by reduced litter sizes has also been reported [20].
Globally, human leptospirosis cases have been estimated at about one million cases annually [21] resulting in the loss of 2.9 million Disability-Adjusted Life Years (DALYs) per annum [22]. The International Leptospirosis Society further estimates the incidence of severe human leptospirosis at 350,000–500,000 cases annually though this is maybe an underestimate due to lack of a notification system or since notification is not mandatory in most countries [5, 23, 24]. The burden of human leptospirosis is expected to rise with demographic shifts and climate change that result in heavy rainfall and flooding [25–27] resulting in loss of man hours and costs associated with medication in cases of chronic sequelae [28–31].
Leptospirosis is listed as one of the priority zoonotic diseases in Kenya based on a five point scoring criteria using the One Health Zoonotic Disease Prioritization (OHZDP) tool [32]. Earlier studies in Kenya in livestock focused on cattle, sheep and goats. A study by D’Souza [33] reported a 41% prevalence by MAT in a nation-wide serological survey of bovine leptospirosis [33]. Another nation-wide serological survey in goats reported a sero-prevalence of 16.2% by MAT [34]. A serological survey in Nyandarua and Turkana showed Leptospira antibodies by MAT were present in both regions in livestock and humans [35]. In Nyandarua, the study reported a prevalence of 49% in cattle and 55% in sheep and goats while in Turkana there was a prevalence of 44% in cattle and 24% in sheep and goats [35]. The higher prevalence in Nyandarua was attributed to the wetter climatic conditions compared to Turkana that may promote the survival of Leptospira bacteria in the environment [35]. A study among slaughterhouse workers in western Kenya using a commercial ELISA kit reported a seroprevalence of antibodies to Leptospira at 13.4% [36]. Though data on the incidence of leptospirosis is lacking, increasing reports of outbreaks in several parts of the world suggest that it’s re-emerging as an important public health problem [24, 37]. There are no studies on leptospirosis in pigs in Kenya thus the role of pigs in the transmission of leptospirosis is not known. This study aimed to estimate the sero-prevalence of Leptospira antibodies, identify some of the circulating serovars and identify associated factors among the slaughter pigs.
Results
Slaughter pig demographic characteristics
A total of 252 pigs were sampled from seven selected slaughterhouses. In total, 88.9% (244/252) of the pigs sampled were indigenous breeds. Female pigs accounted for 55.6% (140/252) of the pigs sampled and 88.7% (220/252) were sourced from farms practicing the extensive (tethering/free range) production system. The pigs were bought from small-scale farmers with 91.3% (230/252) of them owning less than 5 pigs. In 79.8% (197/247) of the farms, the pigs were reared with other types of livestock species (Table 1 and Additional file 1).
Table 1.
Characteristics of pigs, proportion of MAT results, sero-prevalence and associated odds ratios by their demographic characteristics, (Antibody titre cut-off > 40), Busia County, Kenya 2018 (n = 252)
| Variable | Variable categories | N (%) | MAT positive n (%) | MAT negative n (%) | Prevalence (95% CI) | Odds ratio (OR) 95% CI |
|---|---|---|---|---|---|---|
| Sex of pig | Female | 140 (44.4) | 49 (35.0) | 91 (65.0) | 35.0 (27.1–43.5) | 1.2 (0.7–2.2) |
| Male | 112 (55.6) | 34 (30.4) | 78 (69.6) | 30.3 (22.0–39.8) | Ref | |
| Age category | Mature | 110 (43.7) | 45 (40.9) | 65 (59.1) | 40.9 (31.6–50.7) | 1.9 (1.1–3.3) |
| Young | 142 (56.7) | 38 (26.8) | 104 (73.2) | 26.8 (19.7–34.8) | Ref | |
| Breed | Exotic/Crosses | 28 (11.1) | 10 (35.7) | 18 (64.3) | 35.7 (18.6–55.9) | 1.2 (0.5–2.8) |
| Indigenous | 224 (88.9) | 73 (32.6) | 151 (67.4) | 32.6 (26.6–39.2) | Ref | |
| Body condition score | Poor/emaciated | 45 (17.9) | 20 (44.4) | 25 (55.6) | 44.4 (29.6–60.0) | 1.7 (0.8–3.4) |
| Good/very good | 207 (8.2.5) | 63 (30.4) | 142 (69.6) | 30.4 (24.2–37.2) | Ref | |
| Herd size at the farm of origin | 5+ pigs | 22 (8.7) | 11 (50.0) | 11 (50.0) | 50.0 (28.2–71.8) | 2.2 (0.8–5.9) |
| < 5 pigs | 230 (91.3) | 72 (31.3) | 158 (68.7) | 31.3 (25.4–37.7) | Ref | |
| Production system | Extensive | 220 (88.7) | 71 (32.3) | 149 (67.7) | 32.3 (26.2–38.9) | 0.8 (0.4–1.9) |
| Intensive | 28 (11.3) | 12 (42.9) | 16 (57.1) | 42.9 (24.5–62.8) | Ref | |
| Other types of livestock | Yes | 197 (79.8) | 72 (36.5) | 125 (63.5) | 36.5 (29.8–43.7) | 2.3 (1.0–4.5) |
| No | 50 (20.2) | 10 (20.0) | 40 (80.0) | 20 (10.0–33.7) | Ref | |
| Presence of kidney white spots | Yes | 21 (8.3) | 7 (33.3) | 14 (66.7) | 33.3 (14.6–57.0) | 1.0 (0.3–2.8) |
| No | 231 (91.7) | 76 (32.9) | 155 (67.1) | 32.9 (26.9–39.4) | Ref | |
| Sub county of origin | Butula | 59 (23.4) | 19 (32.2) | 40 (67.8) | 32.2 (20.6–45.6) | 1.2 (0.3–5.9) |
| Funyula | 26 (10.3) | 7 (26.9) | 19 (73.1) | 26.9 (11.6–47.8) | 0.9 (0.2–5.3) | |
| Matayos | 81 (32.1) | 27 (33.3) | 54 (66.7) | 33.3 (23.2–44.7) | 1.3 (0.3–6.0) | |
| Nambale | 72 (28.6) | 26 (36.1) | 46 (63.9) | 36.1 (25.1–48.3) | 1.4 (0.4–6.8) | |
| (Butere/Matungu/Teso south) | 14 (5.6) | 4 (28.6) | 10 (71.4) | 28.6 (8.3–58.1) | Ref | |
| Slaughterhouse | ||||||
| Butula | Bumala | 48 (19.1) | 16 (33.3) | 32 (66.7) | 33.3 (20.4–48.4) | 0.7 (0.4–1.6) |
| Butula | 18 (7.1) | 3 (16.7) | 15 (83.3) | 16.7 (3.6–41.4) | ||
| Funyula | Funyula | 24 (9.5) | 9 (37.5) | 15 (62.5) | 37.5 (18.8–59.4) | 1.1 (0.4–3.1) |
| Matayos | Mundika | 74 (29.4) | 24 (32.4) | 50 (67.6) | 32.4 (22.0–44.3) | 0.9 (0.4–1.8) |
| Nambale | Mungatsi | 14 (5.6) | 6 (42.9) | 8 (57.1) | 42.9 (17.7–71.1) | Ref |
| Nambale | 59 (23.4) | 21 (35.6) | 38 (64.4) | 35.6 (23.6–49.1) | - | |
| Tanga-kona | 15 (6.0) | 4 (26.7) | 11 (73.3) | 26.7 (7.8–55.1) | - | |
Data are the number (%) pigs sampled, number (%) of leptospirosis positive pigs, number (%) of leptospirosis negative pigs, prevalence of leptospirosis seropositivity with their 95% confidence interval and odds ratios and their 95% confidence interval stratified by demographic characteristic and location. A pig serum sample was considered positive for leptospirosis when the endpoint titre was equal or more than 40 (MAT titre ≥1:40) against at least one serovar
Ref reference group
Serological analysis
Of the 252 sera tested by MAT, 32.9% (95% CI 27.2–39.1%) tested positive for Leptospira antibodies to at least one serovar using a cut-off titre > 40. The apparent sero-prevalence for specific serovars were 21.4% (54/252) for L. interrogans serovar Lora; 5.2% (13/252) for L. borgpetersenii serovar Kenya; 3.6% (9/252) for L. kirschneri serovar Sokoine; and L. interrogans serovar Bataviae (Table 2). The apparent seroprevalence in each subcounty was 37.5% (95% CI 18.8–59.4%) in Funyula; 35.2% (95% CI 25.3–46.1%) in Nambale; 32.4% (95% CI 22.0–44.3%) in Matayos; and 28.8% (95% CI 18.3–41.4%) in Butula.
Table 2.
Seroprevalence of Leptospira serovars and serogroups by microscopic agglutination test (titer > 1:40) among 83 positive samples in Busia County, Kenya
| Genomospecies | Serogroup | Serovar | Positive (n) | Prevalence (%) | 95% CI |
|---|---|---|---|---|---|
| L. santarosai | Hebdomadis | Hebdomadis | 1 | 0.4 | 0.01–2.2 |
| L. interrogans | Bataviae | Bataviae | 9 | 4.8 | 1.7–6.7 |
| Pomona | Pomona | 2 | 0.8 | 0.1–2.8 | |
| Australis | Lora | 54 | 21.4 | 16.5–27.0 | |
| L. kirschneri | Grippotyphosa | Grippotyphosa | 8 | 3.2 | 1.4–6.2 |
| Icterohaemorrhagiae | Sokoine | 9 | 3.6 | 1.7–6.7 | |
| L. borgpetersenii | Ballum | Kenya | 13 | 5.2 | 2.8–8.7 |
| Sejroe | Sejroe | 1 | 0.4 | 0.01–2.2 |
Data are number of samples Leptospira positive (cut-off titer > 1:40), the serovar specific prevalence and their 95% confidence interval. MAT end-point titre was defined as the highest dilution at which ≥50% of leptospires were still agglutinate
Out of the 252 pigs tested, the apparent seroprevalence among female pigs was 35% (95% CI 27.1–43.5%) compared to 30.4% (95% CI 22.0–39.8%) among male pigs. According to age category, the apparent seroprevalence among mature pigs was 40.9% (CI 31.6–50.7%) compared to 26.8% (CI 19.7–34.8%) in young pigs while in exotic/cross breeds the apparent seroprevalence was 35.7% (95% CI 18.6–55.9%) compared to 32.6% (CI 26.6–39.2%) in indigenous pigs (Table 1). The level of seropositivity by slaughterhouse showed varied from 42.9% (6/14) in Mungatsi to 16.7% (3/18) in Butula slaughterhouse (Table 1). Of the 252 sera tested, 6.8% (17/252) had relatively high MAT titers (Table 3).
Table 3.
Frequency of MAT titer of pig sera by serovar in Busia County, Kenya (n = 109)
| Serogroup | Serovar | 1:20 | 1:40 | 1:80 | > 1:160 |
|---|---|---|---|---|---|
| Hebdomadis | Hebdomadis | 0 | 0 | 0 | 1 |
| Bataviae | Bataviae | 18 | 5 | 2 | 2 |
| Pomona | Pomona | 0 | 1 | 1 | 0 |
| Australis | Lora | 4 | 25 | 18 | 11 |
| Grippotyphosa | Grippotyphosa | 2 | 5 | 2 | 1 |
| Icterohaemorrhagiae | Sokoine | 5 | 7 | 1 | 1 |
| Ballum | Kenya | 10 | 8 | 4 | 1 |
| Sejroe | Sejroe | 0 | 0 | 1 | 0 |
Data are the number of samples Leptospira reactive (cut-off titer > 1:20) by serovar
Factors associated with Leptospira infection
There was no significant difference between seropositivity in female pigs compared to male pigs (OR 1.2, 95% CI 0.7–2.2) (Table 1). Mature pigs were significantly more likely to be seropositive compared to young pigs (OR 1.9, 95% CI 1.1–3.3). There was no association between breed and Leptospira seropositivity with exotic/cross breed pigs having OR 1.2 (95% CI 0.5–2.8) compared to indigenous breeds. The herd size at the farm of origin was not associated with Leptospira seropositivity with pigs being raised in a farm with more than 5 pigs having OR 2.2 (95% CI 0.8–5.9) compared to farms with 5 or less pigs. However, being raised with other livestock species was associated with Leptospira seropositivity (OR 2.3; 95% CI 1.0–4.5).
Discussion
This study reports an apparent seroprevalence (32.9%) of leptospirosis in slaughter pigs in local slaughterhouses in a small holder livestock production system in the Lake Victoria basin, western Kenya. The study raises occupational health concerns that slaughterhouse workers are at risk of exposure to leptospires during their daily work routine. This is further compounded by the poor use of personal protective equipment among the slaughterhouse workers in the study area [38].
To the best of our knowledge, this is the first study demonstrating anti-Leptospira antibodies in pigs in Kenya. In the East Africa region a few studies have been conducted in Tanzania on porcine leptospirosis. A serological survey among a 100 pigs in Morogoro using the MAT test showed a high percentage of pigs positive for L. kirschneri serovar Sokoine (41%); L. borgpetersenii serovar Kenya (27%) and L. kirschneri serovar Grippotyphosa (22%) with MAT cut-off set at titres > 1:20 [18]. The lower cut-off may explain the higher estimate reported. The detection of antibodies against serovars Sokoine, Kenya and Grippotyphosa in the former study is similar to our findings suggesting that these serovars are a common cause of leptospirosis in pigs in East Africa. The study by Kessy et al. [19], reported a sero-prevalence of 4.42% in pigs in Morogoro, which was much lower than the prevalence in our study. A study in Morogoro municipality on pig production reported that over 94% of farmers confine their pigs [39]. The reasons for confinement were to avoid conflict with neighbors for religious reasons and local government regulations. We hypothesize that confinement inadvertently protected the pigs from infections and the external environment and this could explain the lower prevalence reported [39]. The study by Kessy et al. [19], also used a higher cut-off (> 1:160) compared to a cut-off (> 1:40) in our study. While the lower cut-off results in a higher prevalence, it ensured both recent and chronic infections normally characterized by low antibodies titres were reported as positive, an approach common in prevalence studies [33]. At a cut-off > 1:160, our study shows sero-prevalence of 6.8% (17/252) which is only slightly higher than the 4.42% reported by Kessy et al., suggesting the infection rates in the two studies could be similar. Further, the high titers (> 1:160) could suggest some of the pigs sampled had active Leptospira infection.
Our study found the sero-prevalence among females (35%, 95% CI 27.1–43.5%) was slightly higher than among male pigs (30.3%, 95% CI 22.0–39.8%). This finding was similar to reports in fattening pigs in Vietnam, that showed the sero-prevalence among female pigs was higher compared to males [12]. Even though this association was not statistically significant, some studies have suggested venereal transmission occurs in leptospirosis and these could explain the higher prevalence in females [40]. Mature pigs (OR 1.9, 95% CI 1.1–3.9) compared to the young pigs were more likely to be seropositive, this finding compares with other studies that have suggested the difference to be attributed to increased exposure over time [12, 41].
Pigs being raised in a farm with other livestock species (OR 2.3; 95% CI 1.0–4.5) were more likely to be seropositive suggesting the possibility of transmission between multiple animal species [41]. A study on bovine leptospirosis in Brazil, reported co-grazing of cattle and other livestock species especially pigs was associated with bovine leptospirosis seropositivity [42]. Similar findings have been reported in three case reports of leptospirosis outbreaks in South Africa where infections occurred in mixed livestock species farming units [43]. In the first case report in Mpumalanga Province, 17% (9/52) of the pigs sampled in an intensively farmed 300-sow unit had positive titres by MAT. In the same farm, 52% (88/170) of the cattle and 1.3% (2/153) of the sheep tested positive. In the second case report in a 350-sow unit in the Bronkhorstspruit district, Gauteng Province reported 10.5% (4/38) of pigs sampled had high titres (median titre = 1/2560) to L. interrogans serovar Pomona. Cattle, sheep and dogs on the same farm were also infected with serovar Pomona. The third case report in Free State was an outbreak in a 250-cow Jersey herd with a 60-sow unit on the farm. At the herd level, 50% (104/204) cattle had MAT titres equal to and over 1:100 against serovars Icterohaemorrhagiae, Pomona and Bratislava. Pigs tested on the same farm showed positive titres in 45% (9/20) of the pigs against serovars Pomona and Bratislava [43]. A study in bovine leptospirosis in Tanga found that presence of sheep and goats on the farm were strongly associated with bovine seropositivity [44]. These reports support our findings that suggest an association between porcine seropositivity and the presence of other livestock species on the farm and this may be due to transmission between different livestock species.
Serological testing for leptospirosis using the gold standard MAT utilizes live cultures of Leptospira isolates of locally circulating serovars or serovars from reference laboratories [18]. As expected, our study demonstrated a higher prevalence against Leptospira serovars that have been previously isolated in the East African region. This is consistent with other research in the region [18]. The apparent prevalence of the locally isolated serovars were, Lora (21.4%), Kenya (5.2%), Sokoine (3.6%), and Grippotyphosa (3.2%) while apparent prevalence to the reference serovars were, Sejroe (0.4%), Pomona (0.8%), and Hebdomadis (0.4%). However, reference serovar Bataviae had a high apparent prevalence of 4.8%. Similarly the study by Kessy et al., in Morogoro, Tanzania reported a low prevalence of serovar Pomona [19].
The serovar Lora used in the MAT panel was isolated from rodents in Tanzania [18]. This high prevalence of a rodent adapted serovar suggests transmission between the rodent maintenance host and pigs in the study area. A spatial ecology study in the Western Kenya region has reported pigs spend 47% of the time outside the owners farm and roam many kilometers scavenging for food [45]. This free-range system increases the risk of the pig acquiring zoonotic infections including leptospirosis due to extensive interaction with the environment and other maintenance animal hosts [45, 46]. A study in southern Sweden among outdoor reared pigs similarly reported the highest prevalence of antibodies to a local mouse strain (mouse 2A) [47]. The role of rodents as a source of infection of leptospirosis among domestic pigs has also been suggested by research in Vietnam [12, 41]. Previous research in Kenya has demonstrated the presence of pathogenic leptospires in rodents in both urban (18.3%) [48] and rural settings (41.8%) [49]. The role of rodents in the epidemiology of leptospirosis in Kenya requires further investigation.
There was limited information on the circulating serovars in the region, thus the serovars included in the test panel were selected based on studies conducted in the East African region [18]. It is likely that there are other serovars circulating in the area that were not included in the panel and could have led to an underestimation of the prevalence. Further studies to isolate Leptospira spp. circulating in Kenya and the East African region which can be stocked in the reference laboratories will go a long way in increasing the understanding of leptospirosis in the region. The MAT is relatively serovar-specific but cross reactions occur between related serovars particularly those in the same serogroup thus overestimate the prevalence. There is also evidence of seasonality between the wet and dry months of the year with a higher prevalence being reported during the wetter months [47]; our study was conducted during the dry months in the Lake Victoria basin region. These limitations are likely to have resulted in the under estimation of the sero-prevalence in this study. Slaughterhouse sampling strategy also meant that we lacked sufficient data on husbandry practices to allow for assessment of more risk factors that could increase or reduce exposure of pigs to leptospires. The study was unable to determine the impact of leptospirosis on the pig population in Busia County such as infertility and abortions but this needs to be established to understand the potential economic losses associated with this disease.
A previous study in the same area reported the seroprevalence among slaughterhouse workers at 13.4% demonstrating that leptospirosis is an important zoonotic disease in this population [36]. A similar study in the Tanga region in Tanzania among different occupational groups reported seroprevalence of 19.4, 18.1 and 17.1% in livestock farmers, veterinary/meat inspectors and abattoir workers respectively [50]. Several risk factors among the high risk groups have been identified including hygiene practices (hand washing during and after work, wounds/injuries, and eating and smoking during slaughter operations) that lead to exposure to leptospires [38, 51, 52]. These findings demonstrated that a significant proportion of people working closely with livestock are exposed to pathogenic Leptospira. The findings from these studies not only demonstrate the risk for slaughterhouse workers, but also people who handle pigs. Public-health interventions against leptospirosis should therefore target not only the high risk occupational groups but also the general population [50, 53].
Conclusion
This study reports seroprevalence in slaughter pigs in slaughterhouses in western Kenya and highlights the potential occupational and public health risks. Prevention and control of the disease in pigs involves vaccination and a combination of effective strategies for farm biosecurity, good animal husbandry, and rodent control to prevent infection between the animal hosts and protect people [1, 38, 54]. Improvement in the husbandry practices (confinement and rodent control) and public health education among slaughterhouse workers and other high-risk groups on leptospirosis and the role of PPE use, personal hygiene might reduce the potential for transmission of leptospires.
Methods
Study area
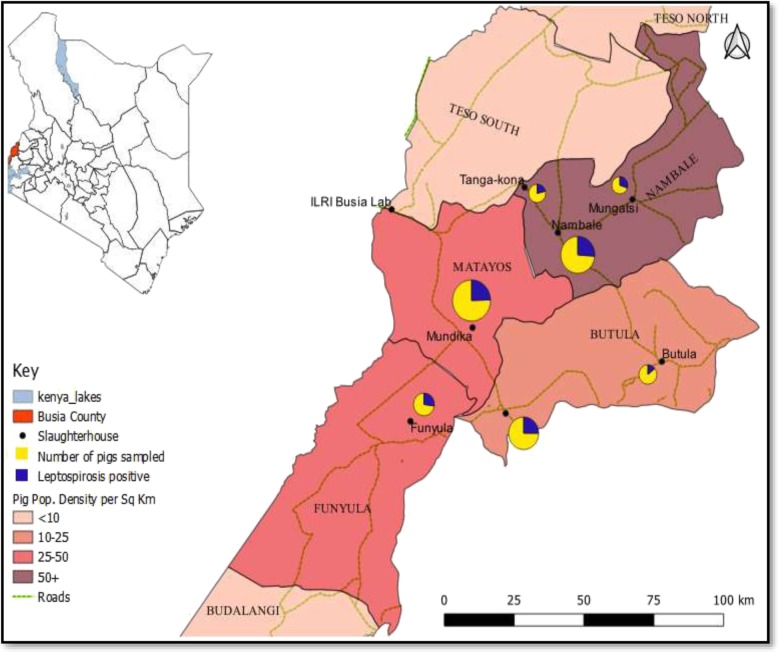
Busia County is located in the Lake Victoria Basin region on the border with Uganda and lies at latitude 0.4347°N and longitude 34.2422°E (Fig. 1). The county is predominantly rural with the main economic activity being crop and livestock subsistence agriculture. Pig production is an important economic activity in the study area with 26.2% of the national pig population found in western Kenya; of which 48,788 (55.5%) are in Busia County [55, 56]. Farmers in Busia County practice traditional free-ranging production systems where pigs are tethered or graze freely with only 4% of the pig rearing households providing improved housing [46]. The production system is also characterized by poor husbandry practices, biosecurity and disease control measures [45, 46].
Fig. 1.
Map of the study area showing the locations of the slaughterhouses (pies), and the pig population densities (shading). The size of the pie indicates the number of pigs sampled with the dark-blue coloured wedge representing the number of leptospirosis positive pigs (Source: https://africaopendata.org/dataset/kenya-counties-shapefile 2019)
Study design
We conducted a cross sectional study involving pigs slaughtered at the licensed local slaughterhouses in urban centres within Busia County. The selected slaughterhouses were located in four subcounties (Nambale, Matayos, Butula, Funyula) where the pig population density was above 10 pigs per square kilometer [57].
Study population
A list of all the slaughterhouses in the four sub counties was obtained from the County Director of Veterinary Services. Slaughterhouses that operated for at least 5 days in a week and slaughter at least five pigs daily were selected. The slaughterhouses were ranked according to the number of pigs slaughtered using the monthly average calculated from slaughter data reports from April to August of the last 2 years (2016/2017). Seven slaughterhouses that ranked highest based on the monthly average were selected. The geo-coordinates of each of the selected slaughterhouses were recorded using a Global Positioning System (GPS) unit and assigned a unique identification number.
The study population was slaughter pigs presented between May and July 2018 at the 7 selected category C slaughterhouses (facilities that slaughter pigs to be consumed in the immediate locality) [58] in Busia County.
All pigs presented at the selected slaughterhouses during the study period and whose owners consented were eligible to be part of the study.
Sample size
A minimum sample size of 195 pigs was calculated using the Cochran formulae [59] with the following assumptions: seroprevalence of 4.42% [19], significance at p = 0.05, confidence level of 0.95 and design effect of 3.
Sampling procedures
Systematic random sampling method was used. The minimum sample of 195 pigs was divided proportionate to size among the seven selected slaughterhouses based on the monthly average of pigs slaughtered calculated using data from the April to August period of 2016 and 2017. In each slaughterhouse, the monthly average of pigs slaughtered was divided by the allocated sample to get the sampling interval (Kth). A random number (between 1 and Kth) was selected as the starting point by randomly picking a hand-written piece of paper from a bucket and the sampling interval used thereafter to continue with the sampling until the required number of pigs was reached. To avoid calculating the sampling interval every time, pigs presented for slaughter were listed continuously through-out the study period in each slaughterhouse. After identifying the pig from which to collect biological samples, consent was sort from the slaughterhouse worker/trader. When the pig trader agreed to participate in the study, he/she signed a consent form. If the pig trader declined the next pig on the list was selected and consent sort. Animal information was collected using a questionnaire that was labelled using a unique serial number.
Variables collected included; the locality where the pig was purchased, breed, sex, age category, body condition score, presence of other livestock species at the farm of origin, number of pigs at the farm of origin, production system at the farm of origin and presence of kidney lesions at post mortem.
Sample collection and processing
The identified pig was restrained using a snare, and 10 ml of whole blood was collected from the cranial vena cava using a 10 ml plain vacutainer tube and an 18-gauge needle (Becton, Dickinson and Company). The sample was labelled immediately with a unique number linking the sample to the questionnaire administered to the slaughterhouse worker/trader. All samples were allowed to clot at ambient temperature and transported in a cool box with ice packs to the ILRI Busia field laboratory. Serum was obtained from the clotted blood by centrifugation at 3000 RPM for 15 min and aliquoted into labelled cryogenic tubes and preserved at − 40 °C. The samples were couriered on dry ice to the Sokoine University of Agriculture, Pest Management Centre, Morogoro, Tanzania for serological analysis. The samples were maintained at − 20 °C during the period of serological analysis.
MAT antigens selection and preparation
Live Leptospira antigens representing eight serogroups recommended for use in Leptospira testing in the East Africa region were used for screening the collected sera [18]. Four reference serogroups Hebdomadis (Leptospira santarosai serovar Hebdomadis), Bataviae (L. interrogans serovar Bataviae), Sejroe (L. borgpetersenii serovar Sejroe) and Pomona (L. interrogans serovar Pomona) which had been initially sourced from the WHO Reference Laboratory at the Royal Tropical Institute (KIT), Amsterdam, Netherlands. The other four were local isolates belonging to serogroups Grippotyphosa (L. kirschneri serovar Grippotyphosa), Icterohaemorrhagiae (L. kirschneri serovar Sokoine), Australis (L. interrogans serovar Lora) and Ballum (L. borgpetersenii serovar Kenya) maintained at the Pest Management Centre, Morogoro, Tanzania (Table 4). The live antigens were grown in fresh Ellinghausen and McCullough medium-modified by Johnson and Harris (EMJH) (Difco-USA) supplemented with Leptospira enrichment and 5-fluorouracil for 5 to 7 days until they reached a density of 3 × 108 leptospires/ml based on the MacFarland scale, according to the guidelines of WHO/FAO/OIE Collaborating Centre for Reference and Research on Leptospirosis of the Royal Tropical Institute, Amsterdam, Netherlands. A loop-full of the stock culture was examined by dark field (DF) microscope to confirm the presence of viable leptospires and the absence of contamination.
Table 4.
Serovars of Leptospira spp. used as live antigens in the MAT panel
| Genomospecies | Serogroup | Serovar | Strain type |
|---|---|---|---|
| L. santarosai | Hebdomadis | Hebdomadis | Reference strain from KITa |
| L. interrogans | Bataviae | Bataviae | Reference strain from KITa |
| Pomona | Pomona | Reference strain From KITa | |
| Australis | Lora | Local isolate (Rodent) - Tanzania | |
| L. kirschneri | Grippotyphosa | Grippotyphosa | Local isolate (Cattle) - Tanzania |
| Icterohaemorrhagiae | Sokoine | Local isolate (Cattle) – Tanzania | |
| L. borgpetersenii | Ballum | Kenya | Local isolate (Rodent) – Tanzania |
| Sejroe | Sejroe | Reference strain from KITa |
aLeptospira strains from WHO/OIE Reference Laboratory - Royal Tropical Institute (KIT), Amsterdam, Netherlands, maintained at Sokoine University of Agriculture
Antibody detection
The MAT test was used to demonstrate Leptospira antibodies in pig sera as described in the manual for International Course on Laboratory Methods for the Diagnosis of Leptospirosis [60]. Briefly, 10 μl of the sera were mixed with 90 μl phosphate buffered saline (PBS) in U-bottom microtiter plates to obtain 100 μl (1:10 dilutions). Serial serum dilutions were prepared in subsequent wells with 50 μl of PBS by pipetting 50 μl of the sera-PBS mixture into them. An aliquot of 50 μl of the fully-grown serovars in EMJH medium was added to the sera (final dilution 1:20) in the microtiter plate wells and mixed gently and then incubated at 30 °C for 2 h. The serum antigen mixture was then visualized by DF microscopy for the presence of agglutination and the titres recorded. MAT end-point titre was defined as the highest dilution at which ≥50% of leptospires were still agglutinated [61]. A serum sample was considered positive for seroreactivity when the endpoint titre was equal or more than 40 (MAT titre ≥1:40) against at least one serovar. Positive samples were further diluted to titres of 1:5120 to detect the end point titres. This was compared to the negative control containing 50 μl PBS mixed with an equal volume of the antigen in liquid EMJH (dilution 1:2). Serial dilutions of serovar-specific hyper-immune rabbit serum was tested with each serovar and the end-point titre established; this was used as a positive control.
Statistical analysis
Data was stored using Microsoft Office Excel® 2013 (Microsoft Corp, Redmond, WA, USA) and analysed using Epi Info™7.1.4.0 (CDC, Atlanta, GA, USA) software. Proportions for categorical variables were calculated. The apparent prevalence estimates and their 95% CI were calculated using Epi Info7. Bivariate analysis to test for association between the independent and dependent variables was done, odds ratios (OR) and their 95% CI were reported. The independent variables included the breed, sex, age category, weight, body condition score, production system, other types of livestock reared at the farm of origin and presence of kidney white spots at postmortem and MAT test results as the outcome variable. Variables whose association with the outcome variable had a P-value of < 0.05 were considered to be statistically significant. The GPS information was used to map the location of the slaughterhouses using QGIS version 3.6.3 (ESRI, Redlands, California, USA) with base layers obtained from open data source (https://africaopendata.org/dataset/kenya-counties-shapefile). The number of sampled and positive cases by slaughterhouse were represented as pie charts on the map.
Supplementary information
Additional file 1. Busia slaughter pigs leptospirosis data on pig demographic and husbandry practices characteristics and microscopic agglutination test results.
Acknowledgements
We would like to thank the Kenya Field Epidemiology and Laboratory Training Program under the Ministry of Health - Kenya, the International Livestock Research Institute - Zoonosis in Livestock in Kenya Project- ZooLink and the Sokoine Pest Management Centre (SPMC), Sokoine University of Agriculture Morogoro, Tanzania for their support during the study. We are grateful to all the study participants for their cooperation and the county veterinary authority for the collaboration.
Abbreviations
- °C
Degrees Celsius
- CI
Confidence interval
- DALY’s
Disability-Adjusted Life Years
- DF
Dark field microscopy
- ELISA
Enzyme-linked Immunosorbent Assay
- EMJH
Ellinghausen, McCullough, Johnson and Harris
- FAO
Food Agricultural Organization
- GPS
Global Positioning system
- ILRI
International Livestock Research Institute
- KIT
Royal Tropical Institute
- L
Leptospira
- MAT
Microscopic Agglutination Test
- OHZDP
One Health Zoonotic Disease Prioritization
- OIE
World Organization for Animal Health
- OR
Odds Ratio
- PBS
Phosphate Buffered Saline
- PCR
Polymerase Chain Reaction
- RPM
Revolution per minute
- rRNA
Ribosomal Ribonucleic Acid
- SPMC
Sokoine Pest Management Centre
- WHO
World Health Organization
Authors’ contributions
JNN conceptualized the study, performed data curation, laboratory and statistical analysis, interpreted the results, and prepared the manuscript. EMF, CAO, MO assisted with refining the study design and implementation of the study and critically reviewed the manuscript. GFM and GGM assisted with laboratory analysis, interpretation of results, and reviewed the manuscript. EAJC assisted in conceptualization, assisted in statistical analysis and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council, the Department for International Development, the Economic & Social Research Council, the Medical Research Council, the Natural Environment Research Council and the Defence Science & Technology Laboratory, under the Zoonoses and Emerging Livestock Systems (ZELS) programme, grant reference BB/L019019/1. This work was part-funded by the Global Challenges Research Fund (GCRF) One Health Regional Network for the Horn of Africa (HORN) Project, from UK Research and Innovation (UKRI) and Biotechnology and Biological Sciences Research Council (BBSRC) (project number BB/P027954/1). It also received support from the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). We acknowledge the CGIAR Fund Donors (http://www.cgiar.org/funders). The work was also supported by the Kenya Field Epidemiology and Laboratory Training Program (K-FELTP); funding and support received from K-FELTP facilitated the first author in field data collection and biological sample analysis. The funders did not have any role in the design of the study, data collection, analysis and interpretation of results or in writing of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Ethics approval and consent to participate
Ethical clearance to conduct this research was obtained from the Institutional Research and Ethics Committee of Moi University (FAN: IREC 2042) and International Livestock Research Institute Animal Care and Use Committee (ILRI-IACUC 2017-04). Permission to access the slaughterhouses was granted by the county veterinary authority Busia County, Kenya. Written consent was obtained from the slaughterhouse workers to collect pig information and biological samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeremiah N. Ngugi, Email: jemiahngugi@gmail.com
Eric M. Fèvre, Email: Eric.Fevre@liverpool.ac.uk
Georgies F. Mgode, Email: gmgode@sua.ac.tz
Mark Obonyo, Email: drmarkobonyo@gmail.com.
Ginethon G. Mhamphi, Email: mhamphi@sua.ac.tz
Christina A. Otieno, Email: agaflo987@gmail.com
Elizabeth Anne Jessie Cook, Email: e.cook@cgiar.org.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12917-019-2159-3.
References
- 1.Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2. Australia: MediSci, Melbourne, Vic.; 1999. [Google Scholar]
- 2.Adler B. Leptospira and Leptospirosis, vol. 387. Springer; 2014.
- 3.Viana Mafalda, Mancy Rebecca, Biek Roman, Cleaveland Sarah, Cross Paul C., Lloyd-Smith James O., Haydon Daniel T. Assembling evidence for identifying reservoirs of infection. Trends in Ecology & Evolution. 2014;29(5):270–279. doi: 10.1016/j.tree.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerqueira GM, Picardeau M. A century of Leptospira strain typing. Infect Genet Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Leptospirosis Burden Epidemiology Reference Group (LERG). http://www.who.int/zoonoses/diseases/lerg/en/. Accessed 17 Mar 2017.
- 6.Evangelista KV, Coburn J. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiol. 2010;5(9):1413–1425. doi: 10.2217/fmb.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderón A, Rodríguez V, Máttar S, Arrieta G. Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop Anim Health Prod. 2014;46(2):427–432. doi: 10.1007/s11250-013-0508-y. [DOI] [PubMed] [Google Scholar]
- 8.Suepaul SM, Carrington CV, Campbell M, Borde G, Adesiyun AA. Seroepidemiology of leptospirosis in livestock in Trinidad. Trop Anim Health Prod. 2011;43(2):367–375. doi: 10.1007/s11250-010-9698-8. [DOI] [PubMed] [Google Scholar]
- 9.Allan KJ, Biggs HM, Halliday JE, Kazwala RR, Maro VP, Cleaveland S, Crump JA. Epidemiology of leptospirosis in Africa: a systematic review of a neglected zoonosis and a paradigm for ‘One Health’in Africa. PLoS Negl Trop Dis. 2015;9(9):e0003899. doi: 10.1371/journal.pntd.0003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis WA. Animal leptospirosis. Leptospira and leptospirosis. Springer; 2015. p. 99–137.
- 11.Desvars A, Cardinale E, Michault A. Animal leptospirosis in small tropical areas. Epidemiol Infect. 2011;139(2):167–188. doi: 10.1017/S0950268810002074. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Khong NV, Xuan HN, Nghia VB, Nguyen-Viet H, Grace D. Sero-prevalence of specific Leptospira serovars in fattening pigs from 5 provinces in Vietnam. BMC Vet Res. 2017;13(1):125. doi: 10.1186/s12917-017-1044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burriel A, Dalley C, Woodward MJ. Prevalence of Leptospira species among farmed and domestic animals in Greece. Vet Rec. 2003;153(5):146–148. doi: 10.1136/vr.153.5.146. [DOI] [PubMed] [Google Scholar]
- 14.Vitale M, Vitale F, Di Marco V, Currò V, Vesco G, Caracappa S. Polymerase chain reaction method for leptospirosis, analysis on samples from an autochthon swine population in Sicily, Italy. Rev Cubana Med Trop. 2005;57(1):25–27. [PubMed] [Google Scholar]
- 15.Habus J, Persic Z, Spicic S, Vince S, Stritof Z, Milas Z, Cvetnic Z, Perharic M, Turk N. New trends in human and animal leptospirosis in Croatia, 2009–2014. Acta Trop. 2017;168:1–8. doi: 10.1016/j.actatropica.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Bertelloni F, Turchi B, Vattiata E, Viola P, Pardini S, Cerri D, Fratini F. Serological survey on Leptospira infection in slaughtered swine in North-Central Italy. Epidemiol Infect. 2018;146(10):1275–1280. doi: 10.1017/S0950268818001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertelloni Fabrizio, Cilia Giovanni, Turchi Barbara, Pinzauti Paolo, Cerri Domenico, Fratini Filippo. Epidemiology of leptospirosis in North-Central Italy: Fifteen years of serological data (2002–2016) Comparative Immunology, Microbiology and Infectious Diseases. 2019;65:14–22. doi: 10.1016/j.cimid.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Mgode GF, Machang’u RS, Mhamphi GG, Katakweba A, Mulungu LS, Durnez L, Leirs H, Hartskeerl RA, Belmain SR. Leptospira serovars for diagnosis of leptospirosis in humans and animals in Africa: common Leptospira isolates and reservoir hosts. PLoS Negl Trop Dis. 2015;9(12):e0004251. doi: 10.1371/journal.pntd.0004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessy MJ, Machang’u RS, Swai ES. A microbiological and serological study of leptospirosis among pigs in the Morogoro municipality, Tanzania. Trop Anim Health Prod. 2010;42(3):523–530. doi: 10.1007/s11250-009-9455-z. [DOI] [PubMed] [Google Scholar]
- 20.Boqvist S, Thu HTV, Vågsholm I, Magnusson U. The impact of Leptospira seropositivity on reproductive performance in sows in southern Viet Nam. Theriogenology. 2002;58(7):1327–1335. doi: 10.1016/S0093-691X(02)00971-8. [DOI] [PubMed] [Google Scholar]
- 21.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, Goris MG, Stein C, Ko AI, Abela-Ridder B. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl Trop Dis. 2015;9(10):e0004122. doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed SA, Sandai DA, Musa S, Hoe CH, Riadzi M, Lau KL, Tang TH. Rapid diagnosis of leptospirosis by multiplex PCR. Malays J Med Sci. 2012;19(3):9. [PMC free article] [PubMed] [Google Scholar]
- 24.De Vries SG, Visser BJ, Nagel IM, Goris MG, Hartskeerl RA, Grobusch MP. Leptospirosis in Sub-Saharan Africa: a systematic review. Int J Infect Dis. 2014;28:47–64. doi: 10.1016/j.ijid.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Stern EJ, Galloway R, Shadomy SV, Wannemuehler K, Atrubin D, Blackmore C, Wofford T, Wilkins PP, Ari MD, Harris L. Outbreak of leptospirosis among adventure race participants in Florida, 2005. Clin Infect Dis. 2010;50(6):843–849. doi: 10.1086/650578. [DOI] [PubMed] [Google Scholar]
- 26.Sejvar J, Bancroft E, Winthrop K, Bettinger J, Bajani M, Bragg S, Shutt K, Kaiser R, Marano N, Popovic T. Leptospirosis in “eco-challenge” athletes, Malaysian Borneo, 2000. Emerg Infect Dis. 2003;9(6):702. doi: 10.3201/eid0906.020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz AR, Buchholz AE, Hinson K, Park SY, Effler PV. Leptospirosis in Hawaii, USA, 1999–2008. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dechet A, Parsons M, Rambaran M, Mohamed-Rambaran P, Florendo-Cumbermack A, Persaud S, Baboolal S, Ari M, Shadomy S, Zaki S. Leptospirosis outbreak following severe flooding: a rapid assessment and mass prophylaxis campaign; Guyana, January-February 2005. PLoS One. 2012;7(7):e39672. doi: 10.1371/journal.pone.0039672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouveia EL, Metcalfe J, De Carvalho ALF, Aires TS, Villasboas-Bisneto JC, Queirroz A, Santos AC, Salgado K, Reis MG, Ko AI. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14(3):505. doi: 10.3201/eid1403.071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotez PJ. Forgotten people, forgotten diseases. Washington, DC: George Washington University and Sabin Vaccine Institute ASM Press; 2008. [Google Scholar]
- 31.Cruz LS, Vargas R, Lopes AA. Leptospirosis: a worldwide resurgent zoonosis and important cause of acute renal failure and death in developing nations. Ethn Dis. 2008;19(1 Suppl 1):S1. [PubMed] [Google Scholar]
- 32.Munyua P, Bitek A, Osoro E, Pieracci EG, Muema J, Mwatondo A, Kungu M, Nanyingi M, Gharpure R, Njenga K. Prioritization of zoonotic diseases in Kenya, 2015. PLoS One. 2016;11(8):e0161576. doi: 10.1371/journal.pone.0161576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’souza CF. Occurrence of bovine leptospirosis in Kenya: University of Nairobi; 1983. (Thesis). http://erepository.uonbi.ac.ke:8080/handle/11295/24510. Accessed 5 Mar 2019.
- 34.Wanyangu S, Angolio A, Wamwayi H. Further serological evidence for caprine leptospirosis in Kenya. East Afr Agric Forestry J. 1993;59(2):137–143. doi: 10.1080/00128325.1993.11663189. [DOI] [Google Scholar]
- 35.Macharia SM. A comparative sero-epidemiological survey for the prevalence of Leptospira antibodies in domestic animals and man in Nyandarua and Turkana districts of Kenya: University of Nairobi; 1989. (Thesis). http://erepository.uonbi.ac.ke:8080/handle/11295/14490. Accessed 5 Mar 2019.
- 36.Cook EAJ, de Glanville WA, Thomas LF, Kariuki S, Bronsvoort BMC, Fèvre EM. Risk factors for leptospirosis seropositivity in slaughterhouse workers in western Kenya. Occup Environ Med. 2017;74(5):357–365. doi: 10.1136/oemed-2016-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vijayachari P, Sugunan A, Shriram A. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557–569. doi: 10.1007/s12038-008-0074-z. [DOI] [PubMed] [Google Scholar]
- 38.Cook EAJ, de Glanville WA, Thomas LF, Kariuki S, de Clare Bronsvoort BM, Fèvre EM. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health. 2017;17(1):14. doi: 10.1186/s12889-016-3923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henjewele CJ. Health risks and biosecurity measures in pig production in Urban and Peri-Urban areas of Morogoro Municipality, Tanzania (Doctoral dissertation, Sokoine University of Agriculture). 2015.
- 40.Lilenbaum W, Varges R, Brandão F, Cortez A, De Souza S, Brandão PE, Richtzenhain LJ, Vasconcellos SA. Detection of Leptospira spp. in semen and vaginal fluids of goats and sheep by polymerase chain reaction. Theriogenology. 2008;69(7):837–842. doi: 10.1016/j.theriogenology.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Boqvist S, Chau B, Gunnarsson A, Engvall EO, Vågsholm I, Magnusson U. Animal-and herd-level risk factors for leptospiral seropositivity among sows in the Mekong delta, Vietnam. Prev Vet Med. 2002;53(3):233–245. doi: 10.1016/S0167-5877(01)00263-X. [DOI] [PubMed] [Google Scholar]
- 42.Lilenbaum W, Souza G. Factors associated with bovine leptospirosis in Rio de Janeiro, Brazil. Res Vet Sci. 2003;75(3):249–251. doi: 10.1016/S0034-5288(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 43.Gummow B, Myburgh J, Thompson P, Van Der Lugt J, Spencer B. Three case studies involving Leptospira interrogans serovar pomona infection in mixed farming units: case report. J S Afr Vet Assoc. 1999;70(1):29–34. doi: 10.4102/jsava.v70i1.747. [DOI] [PubMed] [Google Scholar]
- 44.Schoonman L, Swai ES. Herd-and animal-level risk factors for bovine leptospirosis in Tanga region of Tanzania. Trop Anim Health Prod. 2010;42(7):1565–1572. doi: 10.1007/s11250-010-9607-1. [DOI] [PubMed] [Google Scholar]
- 45.Thomas LF, de Glanville WA, Cook EA, Fèvre EM. The spatial ecology of free-ranging domestic pigs (Sus scrofa) in western Kenya. BMC Vet Res. 2013;9(1):46. doi: 10.1186/1746-6148-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagira JM, Kanyari PW, Maingi N, Githigia SM, Ng'ang'a JC, Karuga JW. Characteristics of the smallholder free-range pig production system in western Kenya. Trop Anim Health Prod. 2010;42(5):865–873. doi: 10.1007/s11250-009-9500-y. [DOI] [PubMed] [Google Scholar]
- 47.Boqvist S, Eliasson-Selling L, Bergström K, Magnusson U. The association between rainfall and seropositivity to Leptospira in outdoor reared pigs. Vet J. 2012;193(1):135–139. doi: 10.1016/j.tvjl.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Halliday JE, Knobel DL, Allan KJ, Bronsvoort BMC, Handel I, Agwanda B, Cutler SJ, Olack B, Ahmed A, Hartskeerl RA. Urban leptospirosis in Africa: a cross-sectional survey of Leptospira infection in rodents in the Kibera urban settlement, Nairobi, Kenya. Am J Trop Med Hyg. 2013;89(6):1095–1102. doi: 10.4269/ajtmh.13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wainaina M, Bett B, Ontiri E, Picozzi K, Agwanda B, Strand T, Grace D, Lundkvist Å, Lindahl J. Leptospira bacteria detected in rodents in Tana River and Garissa counties of Kenya. Infect Ecol Epidemiol. 2018;8(1):1547093. [Google Scholar]
- 50.Schoonman L, Swai E. Risk factors associated with the seroprevalence of leptospirosis, amongst at-risk groups in and around Tanga city, Tanzania. Ann Trop Med Parasitol. 2009;103(8):711–718. doi: 10.1179/000349809X12554106963393. [DOI] [PubMed] [Google Scholar]
- 51.Abiayi E, Inabo H, Jatau E, Makinde A, Sar T, Ugbe D, Kumbish P, Okewole P. Knowledge, attitudes, risk factors and practices (KARP) that favor Leptospira infection among abattoir workers in North Central Nigeria. Asian J Epidemiol. 2015;8(4):104–113. doi: 10.3923/aje.2015.104.113. [DOI] [Google Scholar]
- 52.Tubiana S, Mikulski M, Becam J, Lacassin F, Lefèvre P, Gourinat A-C, Goarant C, D'Ortenzio E. Risk factors and predictors of severe leptospirosis in New Caledonia. PLoS Negl Trop Dis. 2013;7(1):e1991. doi: 10.1371/journal.pntd.0001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirambo MM, Mgode GF, Malima ZO, John M, Mngumi EB, Mhamphi GG, Mshana SE. Seroposotivity of Brucella spp. and Leptospira spp. antibodies among abattoir workers and meat vendors in the city of Mwanza, Tanzania: a call for one health approach control strategies. PLoS Negl Trop Dis. 2018;12(6):e0006600. doi: 10.1371/journal.pntd.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.FAO . Animal production and health, pig sector. 2012. [Google Scholar]
- 56.County Government. Busia county: county CIDP. 2013. https://busiacounty.go.ke/wp-content/uploads/2019/05/Busia-CIDP-2018-2022-Cabinet-Approved.pdf. Accessed 8 Mar 2017.
- 57.Thomas LF, Bishop RP, Onzere C, Mcintosh MT, Lemire KA, de Glanville WA, Cook EAJ, Fèvre EM. Evidence for the presence of African swine fever virus in an endemic region of Western Kenya in the absence of any reported outbreak. BMC Vet Res. 2016;12(1):192. doi: 10.1186/s12917-016-0830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.GoK GK. Meat control (local slaughterhouse) regulations, 2010. 2010. [Google Scholar]
- 59.Cochran WG. Sampling techniques-3. 1977. [Google Scholar]
- 60.Hartskeerl RA, Smits HL, Korver H, Goris MG, Terpstra WJ, Fernández C. International course on Laboratory methods for the diagnosis of leptospirosis: Royal Tropical Institute; 2001 (Book - Laboratory manual used at the Sokoine University of Agriculture, Pest Management Centre, Morogoro, Tanzania)
- 61.Goris MG, Hartskeerl RA. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr Protoc Microbiol. 2014;12E:15.11-12E. doi: 10.1002/9780471729259.mc12e05s32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Busia slaughter pigs leptospirosis data on pig demographic and husbandry practices characteristics and microscopic agglutination test results.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].